Abstract
The ferric uptake regulators (Fur) form a large family of bacterial metal-activated DNA-binding proteins that control a diverse set of genes at the transcriptional level. Mycobacterium tuberculosis, the causative agent of tuberculosis, expresses two members of the Fur family, designated FurA and FurB. Although both belong to the same family, they share only approximately 25% sequence identity and as a consequence, they differ significantly in some of their key biological functions. FurA appears to be a specialized iron-dependent regulator that controls the katG gene, which encodes for a catalase-peroxidase involved in the response of M. tuberculosis to oxidative stress. KatG is also the key mycobacterial enzyme responsible for the activation of the first-line tuberculosis drug Isoniazid. FurB in contrast requires Zn2+ rather than Fe2+, to bind to its target sequence in regulated genes, which include those involved in Zn2+-homeostasis. Recent biochemical, crystallographic and spectroscopic data have now shed light on the activation and metal discrimination mechanisms in this protein family.
Keywords: Metal uptake, regulator, ferric, zinc, Mycobacterium tuberculosis
1. Introduction
Mycobacterium tuberculosis, a Gram-positive bacteria and a widespread human pathogen, resides mainly in alveolar macrophages within the lungs of infected individuals. M. tuberculosis has infected up to one third of the world's population but only a small proportion manifests the disease, tuberculosis (TB). TB is most often associated with poor socio-economic conditions and/or co-infections such as HIV. In 2006, it was estimated that there were approximately 9.2 million new cases of TB, with a death toll of more than 1.5 million people [1]. Even though efficacious short-course chemotherapy (DOTS) and the Bacille Guerin-Calmette (BGC) vaccine are now available for a large proportion of the populations of developed countries, the threat remains, as the incidence of multi-drug resistance is steadily increasing in M. tuberculosis [2].
Like all human pathogens, M. tuberculosis must contend with limited metal availability in order to survive in the human body. Iron and zinc are essential trace elements, however, both are toxic at elevated concentrations [3, 4]. As the sequestration of iron is part of the non-specific mammalian immune response metal uptake and regulation of metal ion concentrations are central to host-pathogen interactions [5]. Metal ion homeostasis in prokaryotes is generally maintained at the level of transcription by various metal dependent regulator proteins [3, 5, 6]. The M. tuberculosis genome contains genes encoding for four of these regulators belonging to two different families [7]. IdeR (for Iron-dependent Regulator) and SirR (for staphyloccocal iron regulatory repressor) belong to the Diphtheria toxin Repressor (DtxR) family. FurA and FurB are members of the second, the ferric uptake regulator (Fur) family.
IdeR is an essential protein in M. tuberculosis and plays the central role as an iron-dependent regulator which controls a large cohort of genes encoding proteins required for iron-uptake and storage [8, 9]. A series of crystal structures of holo-IdeR and IdeR in complex with DNA have shown that this protein undergoes a conformational change upon Fe2+-binding and most likely binds its target as a double dimer [10–12]. SirR has originally been identified in Staphylococcus epidermidis [13] as a 25 kDa protein with approximately 33% sequence identity to IdeR. In S. epidermidis SirR (SirRse) controls the three-gene operon sitABC encoding for an ABC transporter system that has been suggested to be involved in iron-uptake [13, 14]. Furthermore a pseudo-palindromic region in the operator/promoter sequence of sitABC has been shown to be specifically recognized by Fe2+- or Mn2+-activated SirRse [13]. The biological role of the SirR homologue in M. tuberculosis, however, has yet to be determined.
Fur was originally identified in E. coli where it acts as global regulator influencing the expression of close to 100 genes [15, 16]. Members of the Fur family affect the expression of an array of genes, not only associated with iron acquisition and storage, but also those involved in intermediary metabolism as well as responses to acidic environments and oxidative stress. Moreover, they directly or indirectly control the expression of a variety of genes associated with virulence in Gram-positive and negative bacteria [6, 16–19]. In the initial working model it was suggested that Fur is activated by ferric iron and then binds to its operator, a 19 base pair pseudo-palindrome called Fur-Box [16, 20]. Binding to the operator region of a regulated gene blocks access of the RNA-polymerase thereby repressing the transcription of the downstream gene(s). More recent studies, however, found that the regulation exerted by members of the Fur family is far more intricate than initially perceived. Several homologues, including Fur from E. coli (Furec) contain at least one tightly bound Zn2+-ion that is believed to serve a structural rather than a regulatory role [21–23]. Moreover, certain members of the Fur family are activated by divalent transition metals other than Fe2+, which include zinc, nickel and manganese. Accordingly, such members have now been named Zur [24], Nur [25] and Mur [26] which connotes that these family members are capable of selectively binding to a specific divalent transition metal other than Fe2+ to activate their DNA binding function. Furthermore, Fur can also act as a positive regulatory factor, rather than as a classical repressor. While different mechanisms for this type of regulation have been proposed, in a number of bacterial pathogens, Fur homologues directly repress the expression of non-coding small RNAs named RHyhB in E. coli [27] and V. cholerae [28], PrrF in P. aeruginosa [29] and NrrF in Neisseria meningitidis [30] that in turn repress the expression of certain genes. Under iron-starvation conditions RyhB is rapidly expressed and stabilized by the RNA-chaperone Hfq. The single-stranded RNA in the RNA-Hfq complex then pairs with the complementary messenger RNA which becomes susceptible to rapid degradation [31].
2. Discussion
2.1. Biological role of FurA and FurB in M. tuberculosis
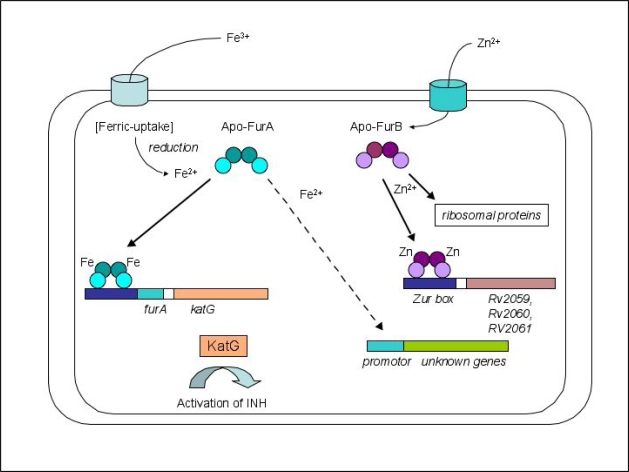
The furA gene is located immediately upstream of the katG gene encoding a catalase-peroxidase. KatG is involved in the oxidative stress response and a significant virulence factor of M. tuberculosis [32]. FurA is co-expressed along with KatG and it auto-represses its expression by binding to a unique sequence upstream of the furA gene [33]. Although there are indications that this protein is also involved in the regulation of other genes [32], the biological role of FurA, in contrast to the role of most members of the family appears to be more specialized (Figure 1). FurA could represent the metal-dependent peroxide sensor similar to the peroxide regulon repressor PerR that has been extensively characterized in B. subtilis [34]. Both proteins share a sequence identity of approximately 28% (Figure 2). PerRbs adopts a similar fold to Furpa and contains one structural Zn2+- and one regulatory Fe2+/Mn2+-binding site [35]. Once activated and bound to its target DNA-sequence the regulator senses oxidative stress by iron-catalyzed histidine oxidation that leads to the loss of DNA-binding activity [36]. FurA is of particular biomedical importance as it controls a protein central to the contemporary TB therapy. One of the more efficacious therapeutics for this disease is Isoniazid (isonicotinic acid hydrazide = INH), which is able to traverse the complex lipid membrane of M. tuberculosis, entirely by passive diffusion. While unmodified INH is not toxic to the pathogen it becomes activated by the mycobacterial catalase KatG, which modifies INH into a range of reactive intermediates including NAD+ and NADP+ adducts. These then act as potent inhibitors of the NADPH-dependent enoyl acyl carrier protein reductase (InhA) of the fatty acid synthase type II [37, 38]. InhA is an essential enzyme in the mycolic acid synthesis [39]. INH-resistant M. tuberculosis strains isolated from patients predominantly have mutations in the katG gene leading to a catalase-peroxidase with reduced or abolished catalytic activity [38]. In addition, mutations were also found in the inhA gene as well as various other genes including some in the furA gene [38, 40]. FurA has attracted increased interest as a potentially novel drug target for the development of inhibitors that could enhance KatG levels possibly boosting INH potency.
Figure 1.
Schematic representation of the regulatory functions of M. tuberculosis FurA and FurB, respectively. Full arrows refer to proven repression, dashed arrows refer to proposed activation pathways. Note, that the structural Zn2+-site present in both FurA and FurB has been omitted for clarity.
Figure 2.
Sequence alignment of Furec with FurA, PerRbs together with Zurec and FurB (performed with ClustalW2 [45]). Residues of the putative regulatory binding site identified in the crystal structure of FurB are depicted in green, residues of the putative structural binding site are red [43]. The proposed binding residues for the second, regulator binding site in PerRbs His37, Asp85, His91, His 93 and Asp104 are shown in blue [46]. Secondary structure assignment is based on the FurB crystal structure: blue rods refers to α-helix, and violet arrows to β-strands. The last line shows sequence conservation: '*' denote conserved residues, '.' and ':' indicate similar residues in the alignment.
The biological role of M. tuberculosis FurB has only recently been examined. Elegant biological and biochemical studies [41, 42] as well as biophysical and structural analyses [43] strongly suggested that FurB represents the authentic Zinc uptake regulator (Zur) in M. tuberculosis (Figure 1). The furB gene is co-transcribed with its upstream gene (Rv2358), which encodes another zinc-dependent regulator. In the absence of Zn2+ the gene product of Rv2359 represses the expression of both genes [41]. FurB is responsible for repressing at least 32 genes a number of which have been implicated with zinc homeostasis. For instance, the triplet gene cluster Rv2059-Rv2061c encodes for two components of an ABC-transport system, Rv2059 shows homology with the TroA superfamily, Rv2060 is similar to a membrane protein part of an ABC-type Mn2+/Zn2+ transport system [42, 43]. However, since the two reading frames of Rv2059 and 2060 overlap it is not clear if functional proteins are expressed from these genes [42]. In addition, FurB controls five genes encoding ribosomal proteins three of which containing a putative zinc-binding motif. Based on comparative genomics data these ribosomal proteins have also been suggested to be involved in zinc homeostasis [44]. While FurB plays an important biological role as the genuine zinc-dependent repressor no phenotype difference was observed between wild-type and a M. tuberculosis strain in which the zur gene was deleted [42].
Although FurA and FurB belong to the same protein family they only share a sequence identity of approximately 25%. Thus, while it is likely that they have a similar overall structure, certain key elements in their respective architectures are likely to differ considerably. For instance, some of the distinct structural details discriminate in divalent metal binding while others are critical to the recognition of DNA sequence motifs in the operator of regulated genes.
2.2. Crystal structure of FurB
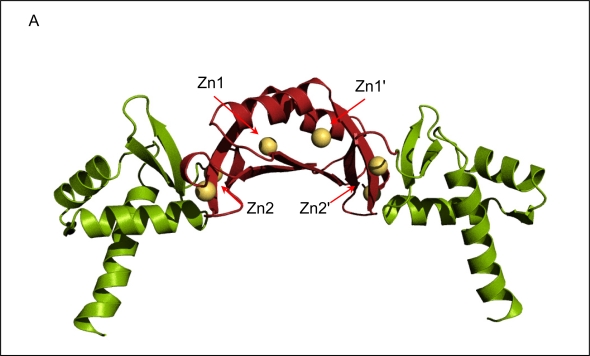
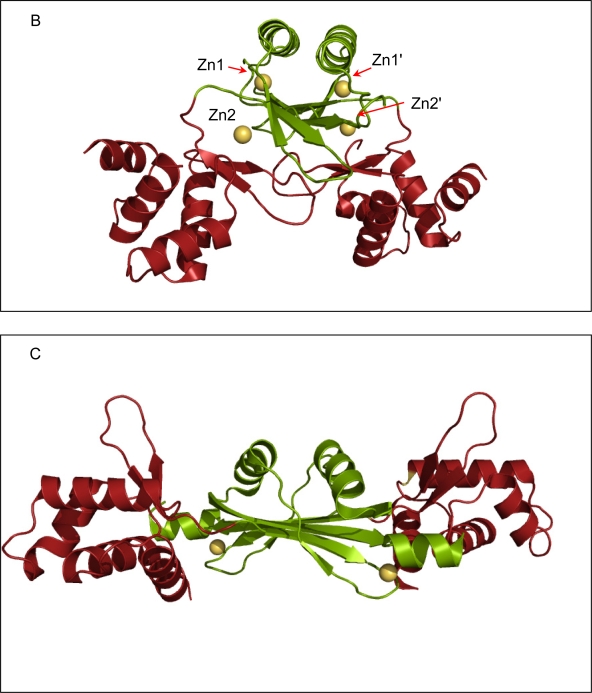
The crystal structure of FurB in complex with Zn2+ determined at a resolution of 2.7 Å revealed the familiar two-domain structure with the N-terminal DNA-binding domain composed of a three-helix bundle followed by a short antiparallel β-sheet, and the C-terminal metal-binding and dimerization domain [43]. Although the overall fold is similar to the first crystal structure of another member of the Fur-family (Fur from P. aeruginosa, Furpa [47]) there are noteworthy differences in domain orientation as well as metal binding sites. Overall, the individual domains of FurB are very similar with the exception that the DNA-binding domain of FurB lacks the N-terminal helix that is present in Furpa and Furec [48]. However, the relative orientations of their two domains are very different (Figure 3a-c). The FurB homodimer adopts a much more open conformation where the DNA-binding domains are further separated and the DNA-recognition helices (helix 3, residues 45–58 in FurB in Figure 2) are almost collinear to each other. While the amino-acid sequence of the DNA binding helix is well conserved within the Fur family there are sufficient differences to enable DNA sequence specific recognition. The mobility of DNA-binding domains with respect to the dimer interface is also highlighted by the crystal structure of apo-PerRbs which shows a similar fold but a distinctively different domain orientation [46]. It should be noted that such domain motions can also be caused by packing effects as have been observed previously in the crystal structures of apo- and holo-DtxR [49].
Figure 3.
Ribbon diagrams of the crystal structure of Fur homologues. All three proteins are dimers with the N-terminal DNA-binding domain depicted in green and the C-terminal dimerization domain shown in red. All Zn2+-ions are shown as golden spheres. (a) FurB in complex with Zn2+ solved at 2.7 Å resolution, the putative regulatory sites are labeled Zn1 and Zn1', the structural sites are labeled Zn2 and Zn2', respectively. The labels for the third site were omitted for clarity [43]. (b)Furpa complexed with Zn2+ [47]. (c)apo-PerRbs with its putative structural Zn2+-site [46]. All Figures were produced with PyMol [50].
2.3. Metal binding sites and metal selectivity
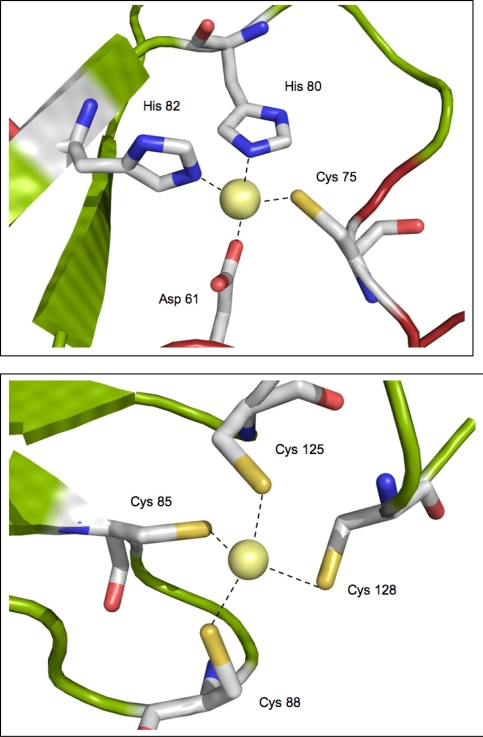
Three Zn2+-binding sites were identified in the crystal structure of FurB, two of which were further characterized by EXAFS measurements in solution. The biological role of the third Zn2+-site is not clear and it seems likely that this site may represent a crystallization artifact. The first metal site depicted in Figure 4a, revealed a Zn2+-ion tetrahedrally coordinated by Asp61 (corresponding to Thr69 in Furec), Cys75 (Thr83), His80 and His82, respectively (His88 and His90). Further spectroscopiC analysis showed that this site can be readily exchanged against Co2+-ions with the same coordination sphere [43], hence suggesting it represents the lower-affinity regulatory site used to switch the DNA-binding capability of FurB to on. It should be noted that this site corresponds roughly to the originally denoted putative structural site in Furpa [47]. However, more recent data indicate that this assignment based on crystallographic and spectroscopic data may not represent the biologically relevant state [18].
Figure 4.
Close-up of the two metal binding sites in the crystal structure of FurB: (a) putative regulatory Zn2+-site. (b) putative structural Zn2+-site. The residue numbers refer to the sequence given in Figure 1.
The sequence comparison shows that the residues of this site depicted in green in the sequence alignment (Figure 2) are only partially conserved, presumably because the different members of the Fur family have evolved to recognize different metals, possibly at different concentrations, thereby fine-tuning the regulatory networks in each organism. It is noteworthy that both Asp 61 and Cys75 in FurB are not conserved in FurA, rather they are changed to arginine residues, which cannot serve as ligands for divalent transition metals. Thus, it is likely that in FurA the framework of the overall Fur fold provides a chemically different metal environment better suited for Fe2+ than Zn2+. Compared to zinc ions, ferric iron prefers octahedral coordination with O,N ligands rather than tetrahedral coordination with S-containing ligands [51]. Considering that the FurA sequence shows a number of histidine and aspartic acid residues in the vicinity a preferred Fe2+-binding site can be realized without major structural changes. The residues that have been suggested to constitute the second, regulatory binding site in PerRbs (depicted in italic and blue/green in Figure 2) are in fact highly conserved in FurA (His37, Asp85, His91,His93 and Asp104, numbering according to PerRbs). Thus, it is possible, that FurA employs a similar mechanism to function as a peroxide sensor in M. tuberculosis.
The second metal binding site in FurB depicted in Figure 4b represents a regular tetrahedral coordination of the four cysteine residues Cys85, Cys88, Cys125 and Cys128. ZnS4 clusters in this arrangement have been observed in various proteins and are typical for structural Zn2+-sites [51, 52]. The notion of a structural Zn2+-site that ties the N-terminus to the core of the dimer was further supported by a series of experiments [43]. First of all it was not possible to completely remove the Zn2+ from FurB without precipitating the protein sample presumably due to (partial) unfolding. Secondly, microPIXE analysis [53] showed approximately one zinc atom per monomer and thirdly, the same tetrahedral ZnS4 environment was observed in EXAFS studies performed using a protein solution that had not been incubated with any additional metal. Moreover, a similar structural Zn2+ site was also revealed in the recent crystal structure of apo-PerRbs [46]. Even though the sequence alignment appears to indicate that all cysteine resides in this cluster are highly conserved between Furec, FurA, Zurec and FurB (depicted in red in Figure 2) it should be noted that a series of mutational and spectroscopic experiments point to slightly different Zn2+ surroundings for the structural sites ranging from Zn(S)2(O/N)2 in Furec to Zn(S)3(O/N) in Zurec [21, 54]. This metal site may be structurally less well conserved than deduced from sequence alone. However, preliminary EXAFS analysis on apo-FurA confirms a single Zn2+-ion tightly bound in a structural binding site. (Lucarelli, Pohl, Meyer-Klaucke, unpublished data). It is therefore likely that FurA and FurB share the same structural Zn2+-spot.
3. Conclusions
The past several years have seen significant progress in understanding the control of gene expression by metal dependent transcriptional regulators in M. tuberculosis and other bacterial pathogens. FurB has been identified as the Zinc uptake regulator (Zur) [42], while FurA controls the production of the catalase-peroxidase KatG, an essential enzyme in INH therapy of TB treatment [32]. FurA could thus represent the functional homologue of the peroxide sensor PerR. In general the mechanisms governing gene repression are reasonably well understood. By contrast, the full cohort of genes (activated and repressed) controlled by these Fur family members, and other metal-dependent regulatory proteins have not yet been identified, nor have the mechanisms associated with their activation been fully elucidated. Although metal binding and activation mechanism have now been examined in greater detail for a significant number of Fur family members, including FurA and FurB, their exact mode of DNA-binding and in particular the structural basis of specific target recognition, remains unknown. Further knowledge about the architecture and function of Fur-operator complexes will certainly enhance future structurally driven drug design efforts.
Acknowledgments
We would like to thank Drs. Alain Baulard (Institute Pasteur, Lille), Santina Russo and Anuschka Pauluhn (Paul Scherrer Institut) for their ongoing support and many valuable discussions. We are grateful to the anonymous reviewers for their comments that significantly improved the manuscript. This work was generously supported by a short-term fellowship to DL from the Boehringer Ingelheim Fonds and in part by a grant from the National Institutes of Health (NIAID R37AI15940) to MLV.
References
- 1.WHO. Report on tuberculosis: global tuberculosis control-surveillance, planning, financing . Vol. 2008 World Health Organization; Geneva, Switzerland: [Google Scholar]
- 2.Zignol M, Hosseini MS, Wright A, Weezenbeek CL, Nunn P, Watt CJ, Williams BG, Dye C. Global incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 2006;194:479–485. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- 3.Nelson N. Metal ion transporters and homeostasis. EMBO J. 1999;18:4361–4371. doi: 10.1093/emboj/18.16.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe DK, Morby AP. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003;27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]
- 5.Agranoff D, Krishna S. Metal ion transport and regulation in Mycobacterium tuberculosis. Front. Biosci. 2004;9:2996–3006. doi: 10.2741/1454. [DOI] [PubMed] [Google Scholar]
- 6.Braun V. Iron uptake mechanisms and their regulation in pathogenic bacteria. Int. J. Med. Microbiol. 2001;291:67–79. doi: 10.1078/1438-4221-00103. [DOI] [PubMed] [Google Scholar]
- 7.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez GM, Smith I. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol. Microbiol. 2003;47:1485–1494. doi: 10.1046/j.1365-2958.2003.03384.x. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez G. Control of iron metabolism in Mycobacterium tuberculosis. Trends. Microbiol. 2006;14:320–327. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Pohl E, Holmes RK, Hol WG. Crystal structure of the iron-dependent regulator (IdeR) from Mycobacterium tuberculosis shows both metal binding sites fully occupied. J. Mol. Biol. 1999;285:1145–1156. doi: 10.1006/jmbi.1998.2339. [DOI] [PubMed] [Google Scholar]
- 11.Wisedchaisri G, Holmes RK, Hol WG. Crystal structure of an IdeR-DNA complex reveals a conformational change in activated IdeR for base-specific interactions. J. Mol. Biol. 2004;342:1155–1169. doi: 10.1016/j.jmb.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 12.Feese MD, Ingason BP, Goranson-Siekierke J, Holmes RK, Hol WG. Crystal structure of the iron-dependent regulator from Mycobacterium tuberculosis at 2.0-A resolution reveals the Src homology domain 3-like fold and metal binding function of the third domain. J. Biol. Chem. 2001;276:5959–5965. doi: 10.1074/jbc.M007531200. [DOI] [PubMed] [Google Scholar]
- 13.Hill PJ, Cockayne A, Landers P, Morrissey JA, Sims CM, Williams P. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect. Immun. 1998;66:4123–4129. doi: 10.1128/iai.66.9.4123-4129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrissey J, Cockayne A, Hill P, Williams P. Molecular cloning and analysis of a putative siderophore ABC transporter from Staphylococcus aureus. Infect. Immun. 2001;68:6281–6288. doi: 10.1128/iai.68.11.6281-6288.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 16.Hantke K. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 2001;4(2):172–177. doi: 10.1016/s1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 17.Vasil ML, Ochsner UA. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 1999;34:399–413. doi: 10.1046/j.1365-2958.1999.01586.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 19.Vasil ML. How we learnt about iron acquisition in Pseudomonas aeruginosa: a series of very fortunate event. Biometals. 2007;20:587–601. doi: 10.1007/s10534-006-9067-2. [DOI] [PubMed] [Google Scholar]
- 20.Bagg A, Neilands JB. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacquamet L, Aberdam D, Adrait A, Hazemann JL, Latour JM, Michaud-Soret I. X-ray absorption spectroscopy of a new zinc site in the fur protein from Escherichia coli. Biochemistry. 1998;37:2564–2571. doi: 10.1021/bi9721344. [DOI] [PubMed] [Google Scholar]
- 22.Althaus EW, Outten CE, Olson KE, Cao H, O'Halloran TV. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999;38:6559–6569. doi: 10.1021/bi982788s. [DOI] [PubMed] [Google Scholar]
- 23.Zheleznova EE, Crosa JH, Brennan RG. Characterization of the DNA- and metal-binding properties of Vibrio anguillarum fur reveals conservation of a structural Zn(2+) ion. J. Bacteriol. 2000;182:6264–6267. doi: 10.1128/jb.182.21.6264-6267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patzer SI, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 25.Ahn BE, Cha J, Lee EJ, Han AR, Thompson CJ, Roe JH. Nur, a nickel-responsive regulator of the Fur family, regulates superoxide dismutases and nickel transport in Streptomyces coelicolor. Mol. Microbiol. 2006;59:1848–1858. doi: 10.1111/j.1365-2958.2006.05065.x. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Mireles E, Wexler M, Sawers G, Bellini D, Todd JD, Johnston AW. The Fur-like protein Mur of Rhizobium leguminosarum is a Mn(2+)-responsive transcriptional regulator. Microbiology. 2004;150:1447–1456. doi: 10.1099/mic.0.26961-0. [DOI] [PubMed] [Google Scholar]
- 27.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis BM, Quinones M, Pratt J, Ding Y, Waldor MK. Characterization of the small untranslated RNA RyhB and its regulon in Vibrio cholerae. J. Bacteriol. 2005;187:4005–4014. doi: 10.1128/JB.187.12.4005-4014.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, Vasil ML. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl. Acad. Sci. USA. 2004;101:9792–9797. doi: 10.1073/pnas.0403423101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellin JR, Goswami S, Grogan S, Tjaden B, Genco CA. A novel fur-and iron-regulated small RNA, NrrF, is required for indirect fur-mediated regulation of the sdhA and sdhC genes in Neisseria meningitidis. J. Bacteriol. 2007;189:3686–3694. doi: 10.1128/JB.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massé E, Salvaila H, Desnoyersa G, Arguin M. Small RNAs controlling iron metabolism. Curr. Opin. Microbiol. 2007;10:140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Pym AS, Domenech P, Honore N, Song J, Deretic V, Cole ST. Regulation of catalase-peroxidase (KatG) expression, isoniazid sensitivity and virulence by furA of Mycobacterium tuberculosis. Mol. Microbiol. 2001;40:879–889. doi: 10.1046/j.1365-2958.2001.02427.x. [DOI] [PubMed] [Google Scholar]
- 33.Sala C, Forti F, Di Florio E, Canneva F, Milano A, Riccardi G, Ghisotti D. Mycobacterium tuberculosis FurA autoregulates its own expression. J. Bacteriol. 2003;185:5357–5362. doi: 10.1128/JB.185.18.5357-5362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mongkolsuk S, Helmann JD. Regulation of inducible peroxide stress responses. Mol. Microbiol. 2002;45:9–15. doi: 10.1046/j.1365-2958.2002.03015.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Helmann JD. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J. Biol. Chem. 2006;281:23567–23578. doi: 10.1074/jbc.M603968200. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 37.Marrakchi H, Laneelle G, Quemard A. InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Microbiology. 2000;146:289–296. doi: 10.1099/00221287-146-2-289. [DOI] [PubMed] [Google Scholar]
- 38.Vilchèze C, Jacobs WRJ. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu. Rev. Microbiol. 2007;61:35–50. doi: 10.1146/annurev.micro.61.111606.122346. [DOI] [PubMed] [Google Scholar]
- 39.Timmins GS, Deretic V. Mechanisms of action of isoniazid. Mol. Microbiol. 2006;62:1220–1227. doi: 10.1111/j.1365-2958.2006.05467.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Yue J, Yang YP, Zhang HM, Lei JQ, Jin RL, Zhang XL, Wang HH. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J. Clin. Microbiol. 2005;43:5477–5482. doi: 10.1128/JCM.43.11.5477-5482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canneva F, Branzoni M, Riccardi G, Provvedi R, Milano A. Rv2358 and FurB: two transcriptional regulators from Mycobacterium tuberculosis which respond to zinc. J. Bacteriol. 2005;187:5837–5840. doi: 10.1128/JB.187.16.5837-5840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maciag A, Dainese E, Rodriguez GM, Milano A, Provvedi R, Pasca MR, Smith I, Palu G, Riccardi G, Manganelli R. Global analysis of Mycobacterium tuberculosis Zur (FurB) regulon. J. Bacteriol. 2006;189:730–740. doi: 10.1128/JB.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucarelli D, Russo S, Garman E, Milano A, Meyer-Klaucke W, Pohl E. Crystal structure and function of the zinc uptake regulator FurB from Mycobacterium tuberculosis. J. Biol. Chem. 2007;282:9914–9922. doi: 10.1074/jbc.M609974200. [DOI] [PubMed] [Google Scholar]
- 44.Panina E, Mironov A, Gelfand M. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. USA. 2003;100:9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin M, Blackshields G, Brown N, Chenna R, McGettigan P, McWilliam H, Valentin F, Wallace I, Wilm A, Lopez R, et al. ClustalW and ClustalX version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 46.Traoré DA, El Ghazouani A, Ilango S, Dupuy J, Jacquamet L, Ferrer JL, Caux-Thang C, Duarte V, Latour JM. Crystal structure of the apo-PerR-Zn protein from Bacillus subtilis. Mol. Microbiol. 2006;61:1211–1219. doi: 10.1111/j.1365-2958.2006.05313.x. [DOI] [PubMed] [Google Scholar]
- 47.Pohl E, Haller JC, Mijovilovich A, Meyer-Klaucke W, Garman E, Vasil ML. Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Mol. Microbiol. 2003;47:903–915. doi: 10.1046/j.1365-2958.2003.03337.x. [DOI] [PubMed] [Google Scholar]
- 48.Pecqueu L, D'Autréaux B, Dupuy J, Nicolet Y, Jacquamet L, Brutscher B, Michaud-Soret I, Bersch B. Structural changes of Escherichia coli ferric uptake regulator during metal-dependent dimerization and activation explored by NMR and X-ray crystallography. J. Biol. Chem. 2006;281:21286–21295. doi: 10.1074/jbc.M601278200. [DOI] [PubMed] [Google Scholar]
- 49.Pohl E, Holmes RK, Hol WG. Motion of the DNA-binding domain with respect to the core of the diphtheria toxin repressor (DtxR) revealed in the crystal structures of apo- and holo-DtxR. J. Biol. Chem. 1998;273:22420–22427. doi: 10.1074/jbc.273.35.22420. [DOI] [PubMed] [Google Scholar]
- 50.DeLano, W.L. The PyMOL graphics systems, 2002; http:///www.pymol.org
- 51.Harding MM. Geometry of metal-ligand interactions in proteins. Acta Crystallogr. D. Biol. Crystallogr. 2001;57:401–411. doi: 10.1107/s0907444900019168. [DOI] [PubMed] [Google Scholar]
- 52.Auld DS. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- 53.Garman EF, Grime GW. Elemental analysis of proteins by microPIXE. Prog. Biophys. Mol. Biol. 2005;89:173–205. doi: 10.1016/j.pbiomolbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Outten CE, Tobin DA, Penner-Hahn JE, O'Halloran TV. Characterization of the metal receptor sites in Escherichia coli Zur, an ultrasensitive zinc(II) metalloregulatory protein. Biochemistry. 2001;40:10417–10423. doi: 10.1021/bi0155448. [DOI] [PubMed] [Google Scholar]