Abstract
Neuroinflammation is a complex integration of the responses of all cells present within the CNS, including the neurons, macroglia, microglia and the infiltrating leukocytes. The initiating insult, environmental factors, genetic background and age/past experiences all combine to modulate the integrated response of this complex neuroinflammatory circuit. Here, we explore how these factors interact to lead to either neuroprotective versus neurotoxic inflammatory responses. We specifically focus on microglia and astrocytic regulation of autoreactive T cell responses.
Keywords: Microglia, TREM, Antigen-presentation
1. Introduction: what is inflammation?
Throughout the body, direct injury to a tissue induces immediate local inflammatory responses followed by systemic recruitment of immune cells [1,2]. The degree and extent of inflammation is a function of the interplay between (a) the initiating insult (pathogen and/or tissue trauma), (b) the local stromal cells and (c) the peripheral immune system [1,2]. A successful inflammatory response not only eliminates any invading pathogens, but it actively promotes wound healing and angiogenesis [1]. By contrast, chronic and/or progressive inflammatory disease can result from a failure to remove or resolve the initiating insult or from the dysregulated injury response of either the affected tissue or the recruited immune system [1].
Although the CNS is an immune privileged site, (reviewed in [3]), inflammatory reactions can and do occur within the CNS [4]. Indeed, neuroinflammation is now recognized to be a prominent feature of many classic neurodegenerative diseases including multiple sclerosis, Alzheimer's disease, Parkinson's disease, narcolepsy and even autism [5,6]! However, in all of these disorders, the role that neuroinflammation may be playing in wound healing (i.e., in neurorepair/neuroprotection) has received less attention than the role it likely plays in cytodestruction (i.e., neurodegeneration) [4,7]. Furthermore, in chronic or remitting/relapsing neurodegenerative disorders, inflammation is unlikely to be playing a purely beneficial versus detrimental function [3]. These distinctions are not of merely academic importance. Several recent clinical trials have tested the efficacy of different types of immune therapies for treating Alzheimer's disease (AD) and multiple sclerosis (MS) [8-10]. In the AD clinical trial, the goal was to direct the immune system to targeted destruction of amyloid plaques [8]. In the multiple sclerosis trial, the goal was to prevent T cell infiltration into the CNS [11]. Surprisingly, both trials were halted for unexpected forms of CNS inflammation. In the Alzheimer's trial, approximately 6% of the patients developed encephalitis. As yet there is debate as to the efficacy of inducing an auto-amyloid responses and even whether the induction of encephalitis was in the final analysis detrimental or beneficial. In the MS trial, three patients succumbed to a usually benign viral infection (PML) in a manner previously associated with immune deficiency diseases such as AIDs.
In this review, we will discuss the consequences (neuroprotection versus neurodestruction) of the very different types of interactions that can occur between the resident cells of the CNS and the peripheral immune system following tissue injury or pathogen encounter. We will focus specifically on how astrogliosis and microgliosis affect neuronal survival. Lastly, we will review the often overlooked role of genetics in dramatically altering the ultimate consequences of gliosis and CNS inflammation.
2. Neuroinflammation: the players
When discussing inflammation, the immediate focus generally turns to the “professional” immune system, specifically macrophages, granulocytes and lymphocytes. However, most cells in the body can and do contribute toward inflammatory responses following injury and/or pathogenic insults [1,2]. The initial responses of stromal cells throughout the body play a strong role in directing the subsequent responses of the “professional” immune system cells [1,2].
3. Neurons as inflammatory response cells?
For most of the last century, CNS neurons were inappropriately viewed as exempt and/or incapable of performing these immune directing functions. Recent data clearly refutes this view. For example, NF-κB is an inducible transcription factor that initiates the subsequent expression of several inflammatory molecules [12,13]. Several studies now indicate that neuronal expression of NF-κB is induced by a variety of insults, including TNF and mechanical injury and that this neuronal induction is associated with subsequent neuronal expression of inducible nitric oxide synthase (INOS) and superoxide dismutase (SOD) [14-17].
Even more striking, separate studies now suggest the inconoclastic view that CNS neurons have the potential to directly regulate the effector functions of CNS-infiltrating T cells in the absence of “professional” antigen-presenting cells (macrophages, microglia, dendritic cells and B cells) [18]. Traditional dogma indicates that T cell effector function is driven primarily by antigen-specific interactions with cells that express major histocompatibility complex molecules (MHC) [2]. For example, the T cell receptor (TCR) that mediates T cell recognition of specific antigens cannot bind nor recognize free antigens. TCRs only recognize antigens that are found within the binding cleft of MHC. Therefore, MHC class II expressing cells (macrophages, microglia, dendritic cells and B cells) must first capture whole proteins and process them into antigenic peptides. These peptides (antigens) are then incorporated into the binding cleft of MHC class II and transported to the cell surface. These MHC class II expressing cells are referred to as antigen-presenting cells (APCs). APCs can promote robust pro-inflammatory T cell effector functions, T cell inactivation (anergy), or even immunosuppressive T regulatory cell effector functions (Treg function). The outcome of antigen-presentation is dependent not only on the presentation of antigen from within MHC, but also by several additional co-stimulatory factors expressed by APCs [19]. However, since CNS neurons rarely if ever express high levels of classic MHC class II, they cannot act as APCs and regulate T cell effector function in an antigen-specific manner.
Suggestively, a new study by Liu et al. reveals the potential for neurons to induce antigen-independent proliferation of CD4+T cells previously activated by “professional” APCs [18]. In these studies, the authors provide suggestive evidence indicating that MHC class II negative neurons can stimulate T cell proliferation entirely by co-stimulatory pathways, namely the B7:CD28 and transforming growth factor (TGF)-β1:TGF-β receptor signaling pathways. In contrast to T cell activation classically induced by peripheral immune cells, this form of neuronally induced T cell activation induces the development of immunosuppressive regulatory T cell effector function (Treg T cell function). The authors thus suggest that activated T cells entering the CNS may be deactivated by encounters with neurons independent of their antigenic specificity! These data while intriguing were primarily based on in vitro co-cultures of T cells with cerebellar neurons cultured from post-natal day 7 mice. Therefore, it will be of interest whether similar results will be obtained with studies using other neuronal populations and whether neuronal induction of Treg phenotype is related to the age, activity or health status of the neurons.
The extent of direct cognate interactions between neurons and lymphocyte is largely unexplored and thus likely underestimated. In a series of studies, Zipp and colleagues have revealed the potential for TRAIL-expressing T cells to directly promote neuronal calcium responses and even neuronal apoptosis via neuronally expressed TRAIL receptors [20]. As yet it is completely unexamined how the interactions described by Zipp and colleagues versus by Liu et al. may be coordinately regulated or even simultaneously cross-regulate final T cell function and neuronal viability [18]!
While direct neuronal: T cell interactions are still incompletely characterized and their biological relevance still debated, neuronal regulation of gliosis has been widely examined and is recognized to play a major role in eliciting protective versus destructive inflammatory responses [21-24]. Indeed astrocytes and microglia appear highly specialized to detect and to respond to neuronal health and activity.
4. Astrocytes
Astrocytes are one of the two primary types of macroglia [21-23]. They comprise nearly 35% of the total CNS cell population and like microglia are found in all regions of the CNS. Histologically, astrocytes can be visualized by immunolabeling with antisera specific for glial fibrillary acidic protein (GFAP), S100b or the astrocyte specific glutamate transporters, GLT1 and GLAST [25]. Interestingly, GFAP immunoreactivity is best visualized in heavily fixed tissues [26]. Under fixation conditions commonly used, many investigators only detect the most robustly GFAP+ cells, and fail to visualize many astrocyte populations such as grey matter astrocytes. Thus in the inflammatory literature, many investigators have subsequently viewed GFAP immunoreactivity as a marker of reactive gliosis.
With these caveats in mine, astrogliosis and astroglial scar formation (usually monitored only by increased GFAP immunoreactivity) have been found to be nearly as common a response to CNS injury and dysfunction as microgliosis [23]. As yet, the adaptive consequences of astroglial scar formation are still a subject of debate. Astrocytic scar formation can encyst and wall off infected and/or damaged CNS tissue and thus prevent healthy CNS tissue from being exposed to degenerative agents. However, astroglial scar formation also impedes axonal regeneration and neurorepair.
5. Why focus on astrogliosis when discussing neuroinflammation?
In the healthy, uninjured CNS, astrocytes perform numerous functions absolutely essential for neuronal function. For example, astrocytes play a critical role in modulating glutamate levels in the extracellular space contributing both to the functional neuronal synapse and to the prevention of glutamate-induced excitotoxic cell death of neurons [23,27,28]. In addition, astrocyte endfeet are a key component of the blood brain barrier (BBB) [29-31]. In vitro data suggest that astrocyte interactions with the cerebrovasculature endothelium play a key role in the induction and maintenance of the tight junctions characteristic of the intact BBB. Furthermore, astrocyte production of pro-inflammatory cytokines such as TNFα play key roles in facilitating leukocyte extravasation from the bloodstream across the BBB into the CNS parenchyma [29-32]. Lastly, it is largely unexplored to what extent the many homeostatic functions of astrocytes such as the buffering of pH and glutamate are significantly altered by their cellular transition into “reactive” astrocytes. To restate, it is largely undeciphered how much of the detrimental effects of gliosis is due to decreased activity of their normal neuronal-support activities and how much is due to their production of cytotoxic factors.
6. Why the focus on microgliosis?
All tissues in the body are populated by tissue macrophages. Microglia are the heterogeneous population of macrophages that populate all regions of the CNS. Microglia comprise approximately 15% of the total CNS cell population [3]. Unlike neurons and macroglia (astrocytes and oligodendrocytes), which are of neuroectodermal origin, all types of microglia are of mesenchymal origin. Microglial activation is one of the earliest features of nearly any change in neuronal physiology and often precedes overt astrogliosis. As yet there is substantial debate as to whether microglial activation is by necessity always neurotoxic because it is primarily designed for pathogen defense. However, several clues suggest that microglia do play intrinsic functions required for normal CNS function and development [33]. For example, viable mammalian mutants spontaneously lacking microglia have not been reported. Indeed throughout evolution, the CNS has apparently co-evolved with a resident macrophage population [33-35]. In drosophila, normal CNS development is dependent on the presence of a hemocyte-derived macrophage population to clear the cellular debris associated with normal programmed cell death [34].
7. Is microgliosis another name for macrophage infiltration into the CNS?
Histologically, CNS-resident microglia are indistinguishable from blood-derived macrophages that acutely infiltrate the CNS in response to pathogenic insult [24]. Both myeloid cell types express classic macrophage markers (Iba-1, F4/80, and mac-1) and both cell types are inducible for MHC and co-stimulatory molecules (B7.1, B7.2, and CD40) required for antigen-dependent activation of T cells. However, more than 15 years ago, two seminal studies demonstrated that CNS resident microglia are not identical to macrophages that acutely infiltrate the CNS.
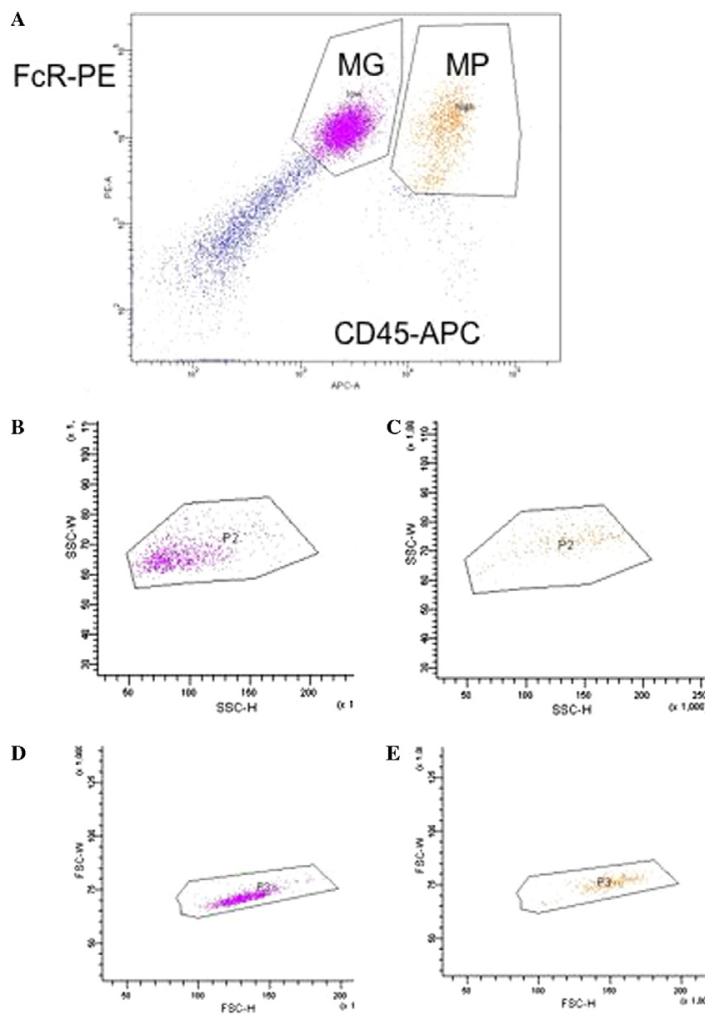
In the first set of studies, Hickey and Kimura observed that parenchymal microglia could be distinguished from peripheral immune cells, perivascular microglia, pericytes, menigneal and choroid plexes macrophages by one key characteristic [36]. Parenchymal microglia were rarely or only slowly replaced by bone marrow derived cells while all the other macrophage and immune cell populations were rapidly replaced by bone marrow derived cells within a few weeks. In the second set of studies, Sedgwick and colleagues discovered that in contrast to peripheral macrophages, parenchymal microglia expressed much lower levels of CD45, a protein tyrosine phosphatase expressed by all nucleated cells of hemopoietic lineage [37]. This difference in protein expression is difficult to reliably quantify histologically, but is quite evident when microglia and macrophages are isolated from CNS tissue and analyzed by flow cytometric analysis (Fig. 1).
Fig. 1.
Flow cytometric analysis of microglia and macrophages isolated from LPS injected murine CNS. (A) Microglia (MG) are defined as FcR+, CD45 low cells, macrophages (MP) are defined as FcR+, CD45 high cells. (B) depicts the side scatter (SSC-H, SSC-W) of just the microglia defined in (A). (C) Depicts the side scatter (SSC-H, SSC-W) of just the macrophages defined in (A). (D) Depicts the forward scatter (FSC-H, FSC-W) of just the microglia defined in (A). (E) depicts the forward scatter (FSC-H, FSC-W) of just the macrophages defined in (A).
The differential expression of CD45 between microglia and all other immune cells likely reflects significant biological consequences for CNS inflammatory responses. CD45 is also an inhibitory receptor for CD22, a molecule expressed by B cells and CNS neurons [38]! The higher levels of CD45 expressed by peripheral immune cells suggests that neurons have a greater potential to inhibit acutely infiltrating macrophages than CNS resident microglia!
With the continued innovations in flow cytometry, it is now possible to largely distinguish CD45 low/intermediate microglia from CD45 high peripheral macrophages based solely on refined forward and side scatter parameters (i.e., based solely on flow cytometric analysis of light scattering caused by cell size and cell granularity and not on expression of CD45). However, it must be cautioned that the ability to distinguish CNS resident microglia from acutely infiltrating macrophages by light scatter is not absolute (Fig. 1). These flow cytometric based observations are consistent with previous reports by Guilian and colleagues that microglia and macrophages isolated from CNS tissue can be distinguished by their differential size and density of cell processes [39]. These FACs and EM detectable differences in morphology of CNS-resident microglia and CNS-infiltrating macrophages are evident in ex vivo analysis. However, it must be stressed that in histological sections morphology is an unreliable marker of the recent source of a cell. For example, mature dendritic cells injected into the CNS can display a stellate morphology similar to parenchymal microglia [26].
8. Glial heterogeneity: implications for inflammation and neuronal health
Histologically, astrogliosis and microgliosis are monitored using a very limited palate of biomarkers coupled with imprecise gross characterizations of changes in cellular morphology. Consequently, there is a propensity in the literature to consider all forms of gliosis as essentially identical, varying only in intensity of cellular response. However, both molecular and functional studies have clearly indicated that both astrocytes and microglia are heterogeneous populations of cells [40-42]. For example, astrocytes differ in their electrophysiological responses and their expression of glutamate transporters as a function of brain region. However, because few markers other than GFAP are used to characterize astrocyte activation and astrogliosis in vivo, the implications of these types of observations for CNS health and function are still largely unexplored. Much more is know about microglial heterogeneity, in part because a large array of reagents able to characterize macrophage phenotypes have already been generated and characterized by studies on the peripheral immune system.
Recent experiments indicate that the homeostatic function and balance of the different microglia and macrophage populations within the CNS parenchyma may be altered by mechanical and ischemic damage may in ways not seen after an autoimmune or viral insult to the CNS. Using irradiation bone marrow chimeric mice, bone marrow derived cells have been found to take up long-term residence near the site of injury in mice with facial axotomies or ischemic damage [43,44]. This response is in stark contrast to what occurs in healthy mice or in mice after EAE, or virally induced demyelinating disease [36,37,45]. In these situations, bone marrow derived cells fail to contribute to the parenchymal microglial population. While the bone marrow derived parenchymal cells in the ischemia studies have been noted to reduce their expression of CD45, additional studies will be needed to explore if these cells are phenotypically different than other parenchymal microglia.
Characterizations of new molecules regulating macrophage and dendritic cell activation provide important examples of peripheral immune characterizations lead to new insights about the consequences of microglial heterogeneity. For example, Colonna and colleagues recently identified a new orphan family of receptors termed triggering receptor expressed on myeloid cells (TREMs) that is expressed by both macrophages and immature dendritic cells [46]. In our ongoing gene profiling studies, we found that not all microglia express similar levels of on of the TREM family members: TREM-2 [47]. TREM-2 is expressed at much higher levels per cell and by a greater percentage of microglia in the entorhinal cortex than in the hypothalamus. Indeed the lowest levels of expression and lowest percentage of positive cells was detected in brain regions with leaky or incomplete BBB. While the endogenous ligand for this receptor has not been published, Daws et al. have described a TREM-2 binding activity expressed by astrocyte cell lines [48]. These data imply that at least some forms of astrocytes may express the endogenous TREM-2 ligand and trigger TREM-2 mediated activation of microglia [48].
9. What are the potential consequences of differential TREM-2 expression?
Although the ligands for the TREM-2 have not been identified, several investigators have used antibody mediated cross-linking of the receptors to trigger TREM-2 mediated intracellular signaling. Using this method, several reports suggest that TREM-2 triggers cells to increase MHC class II expression, and thus to acquire APC function for CD4+T cells [49]. Taken together with our previous expression studies, these data imply that a subset of microglia may have a higher potential to acquire APC function and to activate CD4+T cells. The question then arises: why is expression high in regions such as the entorhinal cortex?
When we examined the inflammation-regulated expression of TREM-2, we initially observed that TREM-2 expression is dramatically down-regulated by bacterial signals such as LPS [47]. In contrast, more recently, we have found that TREM-2 expression is robustly induced in neuropathologies associated with abundant neurodegeneration and necrosis. Does this mean that high TREM-2 expression is neurotoxic? Studies following human families lacking functional TREM-2 suggest otherwise.
Individuals in which this receptor pathway is disrupted by genomic deletions develop a disease called Nasu-Hakola's disease [50]. Affected individuals lacking this microglial expressed molecule develop early onset cognitive dementia in their 20s and die by their early 40s. Since TREM-2 is expressed by only microglia within the CNS, these data clearly demonstrate that microglia and TREM-2 triggered functions are essential for normal function of CNS neurons. Considered with data presented in earlier sections of this review, it is tempting to speculate that microglial presentation to T cells is a necessary component to maintain optimal function of selected CNS neurons. If true, the induction of TREM-2 in degenerative diseases may not be in of itself maladaptive, but an attempt to recruit T cell mediated neuroprotection.
10. Can inflammation be neuroprotective?
Recently, several studies have demonstrated that not all forms of CNS inflammation are necessarily detrimental for CNS function. For example, less CNS regeneration is observed after mechanical trauma and more severe dysfunction results after cytokine induced injury in mice deficient for T cells or antigen-presenting cells [51-53]. As yet the mechanism of T cell mediated protection is being explored. However, both in vitro and in vivo studies suggest the likely contributions of T cell-produced neurotrophins.
Neurotrophins such as BDNF, NT-3, NT-4 have demonstrated potential to promote neuronal development and regeneration both in vitro and in vivo. In particular, BDNF is particularly potent in promoting neuronal survival and regeneration following mechanical injury [54]. Suggestively, human and rodent lymphocytes can be triggered to produce BDNF in vitro [55], while in vivo, BDNF-positive lymphocytes can be found within some human MS lesions [55,56]. The neuroprotective potential for lymphocytic production of these factors is further implied by the studies of Aharoni et al. [57]. In these studies, the authors induce demyelinating EAE and then treat their mice with Glatiramer acetate (GA), more commonly known as the MS therapeutic, Copaxone. GA was already known to reduce the clinical severity and histopathology of EAE. Aharoni et al. demonstrated that in GAs effectiveness correlated in part with lymphocytic production of BDNF.
11. What determines whether T cells are neuroprotective versus neurodestructive?
Many factors are known to regulate T cell effector function. The most significant are the nature and activation state of the antigen-presenting cell [1]. A recent set of experiments have revealed that in at least one model, CD4+T cell mediated neuroprotection is dependent on microglia acting as antigen-presenting cells [58]. Motoneuron degeneration following facial axotomy is associated with microglial activation and CNS-infiltration of macrophages and T cells [59]. This inflammation was initially speculated to be at best benign but more likely a contributing cause of motoneuron degeneration. Surprisingly, in the absence of CD4+T cells, motoneuron degeneration was found to be much more rapid and severe [51]. Using bone marrow chimeric mice, animals were generated in which either only microglia or only hematogenous immune cells could act as antigen-presenting cells [58]. Strikingly, microglia were not able to initiate the protective CD4+T cell response. Initiation of the T cell response was entirely dependent on hematogenous macrophages. While macrophages could initiate the response and did infiltrate the CNS, antigen-presentation by hematogenous macrophages was insufficient to support CD4+T cell mediated neuroprotection of motoneurons. However, once T cell activation was initiated by peripheral macrophages, protection of motoneurons following facial axotomy was absolutely dependent on antigen-presentation by microglia activated by local neurodegenerative signals [58].
12. What prevents microglia from eliciting neuroprotective T cell responses during MS and other destructive neuroinflammatory disorders?
Destructive CNS autoimmunity is not easily induced. At least two factors must converge: environmental cues and genetic background [1,5]. For example, T cell mediated CNS demyelination in rodents can only be induced on specific genetic backgrounds. Even in susceptible rodent strains, the presence of myelin-specific T cells is by itself insufficient to induce autoimmunity. Destructive autoimmunity requires the use of strong adjuvants such complete Freund's adjuvant containing heat-killed tuberculous mycobacterium coupled with pertussis toxin treatment.
Outside of the experimental laboratory, the onset of is not associated with such robust adjuvant treatment. However, adjuvant stimulation can be provided by common environmental agents. Stromal cells throughout the body (including astrocytes) and immune cells (including microglia) express pattern recognition receptors that bind evolutionarily conserved elements on pathogens [1,5]. For example, the Toll-like receptors (TLRs) recognize cellular components of gram negative and gram positive bacterial cell walls (TLR4 and TLR2), double stranded RNA (TLR3), unmethylated prokaryotic DNA (TLR9), flagellin (TLR5). These pattern recognition receptors have the demonstrated potential to promote production of pro-inflammatory cytokines (IFNg, TNFa) and reactive oxygen species not only in peripheral cells but also in astrocytes and microglia.
In the presence of strong adjuvants, antigen-presentation by peripheral macrophages and dendritic cells is entirely sufficient to drive destructive autoimmunity [36]. Under these conditions, antigen-presentation by microglia appears to play only a minor immunomodulatory role.
13. Genetic background modifies response to environmental agents
The clinical symptoms, kinetics, severity and histopathology of EAE are highly dependent on the type of adjuvant and the genetic background of the treated animal [60]. For example, the same antigenic peptide from the myelin oligodendrocyte glycoprotein (MOG)92-106, has been used to immunize SJL/J and A.SW mice. These two different mouse strains express the same MHC class II haplotype (H-2s) but have different propensities for generating CD4+T cell phenotypes [61]. SJL/J mice lack NK1.1 T cells and are more prone to generate Th1 CD4+T cell responses (IL-2, IFNc producing T cells), while A.SW mice have a normal complement of NK1.1 T cells and are more prone to generate Th2 CD4+T cell responses (IL-4, IL-10 producing, T cells). Immunizing SJL/J mice with MOG in the presence of complete Freud's adjuvant (CFA; the oil and water immersion of killed mycobacterium tuberculosis) leads to a relapsing remitting form of EAE. By contrast, immunizing A.SW mice with the same protocol leads to a primary progressive forms of EAE. Addition of pertussis toxin (PT) treatment to the immunization protocol failed to alter the long term disease progression observed in the SJL/J mice, but did convert clinical disease from a primary progressive to a secondary progressive form in A.SW mice.
Histologically, the identical causative agents induced very different pathologies in the two mouse strains [61]. In SJL/J mice, T cell infiltration was accompanied by only mild demyelination. In the A.SW mice, neutrophil infiltration with only limited T cell infiltration occurred yet large areas of demyelination developed. In immunized A.SW mice without PT treatment, immunoglobin deposition was detected in the CNS and high titers of MOG antibodies were detected in the serum.
14. Adjuvants, cytokines and the subsequent consequences of astrocyte activation
In the animal models just described, astrogliosis is prominent as judged by global increases in GFAP expression and changes in astrocyte morphology. Largely due to its co-incidence with destructive autoimmunity, the observed astrogliosis is usually assumed to contribute to the inflammation associated neurotoxicity. Consistent with this assumption, reactive astrocytes in vitro do produce neurotoxic molecules such as NO, reactive oxygen species and TNFa. Bethea and colleagues have also shown that inhibiting NF-κB expression in astrocytes was sufficient to promote increased neuronal survival following mechanical injury [12]. Furthermore, activated myelin-specific T cells reduce astrocytic expression of GLAST, one of the two primary glutamate transporters used by astrocytes to buffer and protect CNS neurons from exposure to high glutamate levels [62]. Thus, activated T cells can decrease neuronal viability by inhibiting primary neuroprotective astrocyte functions.
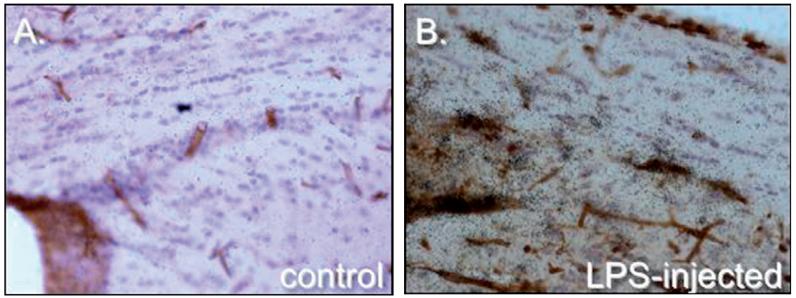
In contrast, many pro-inflammatory mediators produced by both T cells and microglia promote neuroprotective astrocyte responses. Specifically, inflammation associated prostaglandin production is demonstrated to be directly as neurotoxic in in vitro assays. However, in the EAE models just described, activated microglia apparently limit T cell proliferation and APC function of infiltrating immune cells via production of high levels of prostaglandins [63,64]. In addition, the high levels of prostaglandins produced by microglia stimulates increased astrocytic uptake of glutamate thus increased neuroprotection [65]. Similarly, intracerebral injection of LPS leads to a rapid activation of microglia, macrophage infiltration into the CNS, and robust induction of TNFα, NO, and MIP-2. However, we find that it also induces rapid and sustained induction of ceruloplasmin and TLR3 by astrocytes (Fig. 2). Bsibsi et al. find that in contrast to TLR3 induced responses in macrophages, TRL3 induces astrocytic expression of anti-inflammatory cytokines including interleukin-9 (IL-9), IL-10, and IL-11 and the promotion of neuronal survival in organotypic slice assays [66]. Ceruloplasmin is a copper binding protein and thus buffers the metal co-factor required for several dismutase enzymes. Thus, it is likely that increased production by astrocytes during inflammation serves to limit production of free radicals implicated in neurotoxicity.
Fig. 2.
Induction of ceruloplasmin in the corpus callosum by intracerebral injection of LPS. Brain sections depict ceruloplasmin expression in healthy adult mouse brain (A) and 24 h following LPS injection (B). Ceruloplasmin expression is visualized by 33P labeled riboprobes (dark emulsion grains), cell nuclei are visualized by hematoxylin (blue) and areas of inflammation are visualized by increased labeling of microglia, macrophages and blood vessels with tomato lectin (in brown).
15. Summary
Inflammation is a complex mixture of neurotoxic and neuroprotective responses. Some putatively neurotoxic responses such as prostaglandin production also serve to limit and terminate ongoing pro-inflammatory T cell responses. The gliotic reactions of both microglia and astrocyte are thus complex mixtures of pro- and anti-inflammatory responses aimed at defending neuronal function from pathogens, while simultaneously promoting neurorepair and termination of these defense reactions. For most of us, for most of our lives, these opposing responses remain in sufficient balance to maintain CNS function. However, age as well as neurodegenerative disorders such as Alzheimer's disease lead to alterations in the basal levels of microglial and astrocyte activation. In part these alterations are inherent to the glia, but they may also in part reflect changes in neuronal health (for example, neuronal expression of CD22, CD200, fractalkine [3]). As yet it is difficult to predict how these alterations will alter glial based regulation of auto-reactive T cell responses and potentially lead to a decreased propensity to elicit neuroprotective T cell responses.
Acknowledgement
M.J.C. was supported in part by grants from NIH, UC Riverside and an unrestricted gift from Merck.
References
- [1].Lo D, Feng LL, Li L, Carson MJ, Crowley M, Pauza M, et al. Integrating innate and adaptive immunity in the whole animal. Immunol Rev. 1999;169:225–39. doi: 10.1111/j.1600-065x.1999.tb01318.x. [DOI] [PubMed] [Google Scholar]
- [2].Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10(5):351–3. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- [3].Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix C. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006 doi: 10.1111/j.1600-065X.2006.00441.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bechmann I. Failed central nervous system regeneration: a downside of immune privilege? Neuromol Med. 2005;7(3):217–28. doi: 10.1385/NMM:7:3:217. [DOI] [PubMed] [Google Scholar]
- [5].Melchior B, Puntambekar SS, Carson MJ. Microglia and the control of autoreactive T cell responses. Neurochem Int. 2006 doi: 10.1016/j.neuint.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11(5):417–40. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- [7].Bauer J, Rauschka H, Lassmann H. Inflammation in the nervous system: the human perspective. Glia. 2001;36(2):235–43. doi: 10.1002/glia.1112. [DOI] [PubMed] [Google Scholar]
- [8].Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol. 2006;6(5):404–16. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- [9].Tremlett H. What went wrong in the natalizumab trials comment? Lancet. 2006;367(9521):1484–5. doi: 10.1016/S0140-6736(06)68643-1. [DOI] [PubMed] [Google Scholar]
- [10].Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: has the disease outgrown its name? Ann Neurol. 2006;60(2):162–73. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- [11].Cree B. Emerging monoclonal antibody therapies for multiple sclerosis. Neurologist. 2006;12(4):171–8. doi: 10.1097/01.nrl.0000204859.15501.6b. [DOI] [PubMed] [Google Scholar]
- [12].Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202(1):145–56. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barger SW, Moerman AM, Mao X. Molecular mechanisms of cytokine-induced neuroprotection: NFkappaB and neuroplasticity. Curr Pharm Des. 2005;11(8):985–98. doi: 10.2174/1381612053381594. [DOI] [PubMed] [Google Scholar]
- [14].Yune TY, Lee SM, Kim SJ, Park HK, Oh YJ, Kim YC, et al. Manganese superoxide dismutase induced by TNF-beta is regulated transcriptionally by NF-kappaB after spinal cord injury in rats. J Neurotrauma. 2004;21(12):1778–94. doi: 10.1089/neu.2004.21.1778. [DOI] [PubMed] [Google Scholar]
- [15].Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24(33):7324–34. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49(6):681–97. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [17].Massa PT, Aleyasin H, Park DS, Mao X, Barger SW. NFkappaB in neurons? The uncertainty principle in neurobiology. J Neurochem. 2006;97(3):607–18. doi: 10.1111/j.1471-4159.2006.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12(5):518–25. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- [19].Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27(8):387–93. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- [20].Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Topphoff US, Vogt J, et al. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46(3):421–32. doi: 10.1016/j.neuron.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [21].Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–7. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- [22].Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- [23].Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89(5):1092–100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- [24].Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40(2):218–31. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31(2):95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [26].Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol. 1999;154(2):481–94. doi: 10.1016/S0002-9440(10)65294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Volterra A, Steinhauser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47(3):249–57. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- [28].Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int. 2004;45(23):259–64. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- [29].Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36(2):145–55. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- [30].Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J. Appl. Physiol. 2006;100(1):307–17. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2(5):409–23. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- [32].Gimenez MA, Sim J, Archambault AS, Klein RS, Russell JH. A tumor necrosis factor receptor 1-dependent conversation between central nervous system-specific T cells and the central nervous system is required for inflammatory infiltration of the spinal cord. Am J Pathol. 2006;168(4):1200–9. doi: 10.2353/ajpath.2006.050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Salzet M, Vieau D, Day R. Crosstalk between nervous and immune systems through the animal kingdom: focus on opioids. Trends Neurosci. 2000;23(11):550–5. doi: 10.1016/s0166-2236(00)01642-8. [DOI] [PubMed] [Google Scholar]
- [34].Sears HC, Kennedy CJ, Garrity PA. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130(15):3557–65. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- [35].Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238(2):274–88. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- [36].Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239(4837):290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- [37].Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88(16):7438–42. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, et al. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46(4):369–79. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- [39].Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15(11):7712–26. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pedersen EB, McNulty JA, Castro AJ, Fox LM, Zimmer J, Finsen B. Enriched immune-environment of blood-brain barrier deficient areas of normal adult rats. J Neuroimmunol. 1997;76(12):117–31. doi: 10.1016/s0165-5728(97)00038-6. [DOI] [PubMed] [Google Scholar]
- [41].McCluskey LP, Lampson LA. Local immune regulation in the central nervous system by substance P vs. glutamate. J Neuroimmunol. 2001;116(2):136–46. doi: 10.1016/s0165-5728(01)00295-8. [DOI] [PubMed] [Google Scholar]
- [42].Flaris NA, Densmore TL, Molleston MC, Hickey WF. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993;7(1):34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- [43].Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66(1):74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- [44].Priller J. Robert Feulgen Prize Lecture. Grenzganger: adult bone marrow cells populate the brain. Histochem Cell Biol. 2003;120(2):85–91. doi: 10.1007/s00418-003-0559-7. [DOI] [PubMed] [Google Scholar]
- [45].Matsumoto Y, Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987;17(1):71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- [46].Nathan C, Ding A. TREM-1: a new regulator of innate immunity in sepsis syndrome. Nat Med. 2001;7(5):530–2. doi: 10.1038/87846. [DOI] [PubMed] [Google Scholar]
- [47].Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, et al. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83(6):1309–20. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171(2):594–9. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- [49].Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A dap12-mediated pathway regulates expression of cc chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194(8):1111–22. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Paloneva J, Autti T, Raininko R, Partanen J, Salonen O, Puranen M, et al. CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology. 2001;56(11):1552–8. doi: 10.1212/wnl.56.11.1552. [DOI] [PubMed] [Google Scholar]
- [51].Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (SCID) mice. J Neurosci. 1999;19(11) doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Stalder AK, Carson MJ, Pagenstecher A, Asensio VC, Kincaid C, Benedict M, et al. Late-onset chronic inflammatory encephalopathy in immune-competent and severe combined immune-deficient (SCID) mice with astrocyte-targeted expression of tumor necrosis factor. Am J Pathol. 1998;153(3):767–83. doi: 10.1016/S0002-9440(10)65620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schwartz M, Moalem G, Leibowitz Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22(7):295–9. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- [54].Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- [55].Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189(5):865–70. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hohlfeld R. Immunologic factors in primary progressive multiple sclerosis. Mult Scler. 2004;10(Suppl 1):S16–21. doi: 10.1191/1352458504ms1026oa. discussion S21-2. [DOI] [PubMed] [Google Scholar]
- [57].Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci USA. 2005;102(52):19045–50. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Byram SC, Carson MJ, Deboy CA, Serpe CJ, Sanders VM, Jones KJ. CD4+T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24(18):4333–9. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18(15):5804–16. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mix E, Pahnke J, Ibrahim SM. Gene-expression profiling of experimental autoimmune encephalomyelitis. Neurochem Res. 2002;27(10):1157–63. doi: 10.1023/a:1020925425780. [DOI] [PubMed] [Google Scholar]
- [61].Tsunoda I, Kuang LQ, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: induction of relapsing-remitting and progressive forms of EAE in H2s mouse strains. Brain Pathol. 2000;10(3):402–18. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Korn T, Magnus T, Jung S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J. 2005;19(13):1878–80. doi: 10.1096/fj.05-3748fje. [DOI] [PubMed] [Google Scholar]
- [63].Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55(1):127–34. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [64].Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22(1):72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [65].Okada K, Yamashita U, Tsuji S. Modulation of Na(+)-dependent glutamate transporter of murine astrocytes by inflammatory mediators. J UOEH. 2005;27(2):161–70. doi: 10.7888/juoeh.27.161. [DOI] [PubMed] [Google Scholar]
- [66].Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53(7):688–95. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]