Abstract
A total of 218 adults with an average age of seventy-eight years participated in a study of memory performance in community elders. A computer-generated random zip code list of adults ≥70 years of age was purchased and a four-phase telephone-screening plan was adopted. During the second year, the sampling plan had to be changed, with a convenience-sampling plan being adopted to recruit adequate numbers of African-American subjects. Fifty-seven percent of the African-American subjects (N = 55) and 68% of the White subjects (N = 83) were recruited from random sampling methods. As compared to the random sample, the convenience sample was significantly older (80 vs 76), had more depression (12 vs 9), had lower physical functioning (46 vs 65), and less vitality (48 vs 60). On meta-memory, the convenience sample scored higher than the random sample on achievement (3.84 vs 3.69), and lower on task (3.75 vs 3.85). The convenience sample scored significantly lower than the random sample on memory performance (15 vs 18), and memory self-efficacy (26 vs 33). More research is needed to document normative measures for cognitive function and to facilitate accurate comparisons between African-American elderly and other elderly.
Keywords: African American, Elderly, Memory Performance, Cognition, Memory Self-Efficacy
Introduction
African Americans, the largest minority group in the United States, are the victims of a disturbing history of exploitation by health researchers.1–2 Further, they have been and continue to be under-represented in government-funded research. Recent evidence from 28 ongoing randomized clinical trials of cardiovascular disease, diabetes mellitus, hypertension, and renal disease indicated that African Americans represented, an average of only 4.6% of the samples.3 Ironically, however, epidemiological data indicate that the rates of leading causes of morbidity and mortality in the United States are higher in African Americans than in White Americans.4–6 Therefore, the United States Public Health Service now mandates the inclusion of minorities in all research unless compelling evidence of unfeasibility can be presented.
When they have participated in research, African Americans have often received biased treatment.7–8 Many investigators have capitalized on this population's limited understanding of research and have recruited participants without obtaining adequate informed consent.9–10 The focus has been on acquiring “willing” participants, regardless of the backlash this abuse of trust would create for future African Americans' participation in research. As a result, African Americans have developed fear and skepticism about government-funded research,11–13 and their fear of involvement in studies has negatively influenced the perceptions of both the community and its health care professionals regarding the importance of research. Health care providers for African Americans hesitate to become involved in research with their client base and do not encourage clients' participation because of the negative perceptions about medical research held by many African Americans.14 Due to the historical absence of cultural sensitivity, the African-American community has erected nearly impenetrable barriers to research.15–16
There are more than 2.5 million African Americans aged 65 and older in the United States.17 Yet, African Americans are seriously under-represented in research on aging, especially cognitive aging. The Consortium to Establish a Registry of Alzheimer's Disease (CERAD) was the first organized attempt to recruit African Americans into cognitive aging research.18–19 Two years after its initiation, African Americans represented only 10% of the sample. This led CERAD to form a minority enrollment committee and to try to identify the barriers to enrolling African Americans in the registry. A new protocol was implemented, and over the next two years, the number of African-American participants in research doubled. Recently, other investigators have suggested the use of culturally sensitive strategies to build the trust of the African-American community in research and; therefore to enhance recruitment.
One problem in recruiting African Americans is that cognitive aging studies involve complex ethical and social implications, though researchers often analyze their findings without examining the specific issues of privacy, confidentiality, methodology, deception, informed consent, justice and equitable treatment, scientific freedom, ownership of data, values and epistemology, and risk/benefit ratio.20 For example, patients 60 years of age and older (N = 3954) who completed the Short Portable Mental Status Questionnaire during a physician's office visit and were diagnosed with moderate to severe cognitive impairment, were more likely to be African-American than Caucasian (85.8% vs 61.3%, respectively, P = .05).21 At hospital discharge, Proctor et al22 found that among African-American (N = 189) and Caucasian (N = 231) elderly with an average age of 77 and a diagnosis of either stroke, chronic obstructive pulmonary disease, or congestive heart failure, the African Americans had significantly (P≤.01) greater cognitive impairment than did their White counterparts, whether mild (43.8% vs 24.5%, respectively) or severe (15.6% vs 7.6%, respectively). Cognitive impairment was determined by the Blessed Dementia Scale.23 Shadlen and colleagues24 found that among African Americans (N = 38) and Whites (N = 415) with early onset Alzheimer's disease and an average age of 77 years, the African Americans had significantly lower mean MMSE scores (17.2 vs 20.2, respectively). This difference in cognitive function persisted after controlling for age, education, duration of AD symptoms, and impairment in activities of daily living. All the findings from these studies support the traditional view that African Americans have a higher rate of cognitive impairment than do their White counterparts; however, only White subjects have been studied in an environment of trust. Further, behavioral evaluations tend to bias findings against African Americans. Therefore, African Americans have been forced to adopt the coping behaviors of others, which is not conducive to accuracy in the findings.
Several researchers suggest establishing relationships with community health centers, educating the community and its health care providers about the benefits of research, using direct telephone calls to recruit minority participants, and addressing fears and skepticism about government-funded research.9,10,19,25–27 Thompson et al28 advocate conducting pilot work and pretesting instruments developed specifically for this population, as well as using indigenous interviewers with minority participants.29 Other investigators have suggested in-home visits with minority elderly as a strategy to deal with recruitment and retention barriers. Through a telephone survey, Norton et al30 compared the cognitive function of community-based older adult respondents and non-respondents and found that cognitive impairment, as determined by the Mini Mental State Exam (MMSE) score, was greater in the non-respondents. These researchers also found that a recruitment method involving face-to-face contact with the cognitively impaired participants increased the rate of participation from 63% to 93%.
This paper on reports the recruitment and retention of elderly African Americans, and their measurement results from a study of factors associated with meta-memory and memory performance. The aims of this study were 1) to determine whether meta-memory is associated with memory performance among the elderly; 2) to determine whether the relationship between memory performance and meta-memory varies depending on health, depression, and self-efficacy beliefs; and 3) to evaluate whether the relationship between memory performance and meta-memory varies by age, gender, race, or education level.
Methods
Subjects
To obtain subjects for the study, a computer generated random zip code list of adults over the age of 70 living in the Cleveland, Ohio metropolitan area was purchased, and a four-phase telephone-screening plan to determine eligibility for the study was implemented. This screening plan was used to eliminate anxiety and stress among individuals who might be ineligible for study inclusion because of early or mild cognitive impairment. On the first telephone call, eligibility was determined by asking four questions related to language, age, ethnicity, and living arrangement. Language eligibility was determined by the ability to speak English. Age eligibility was defined as 70 years of age and older. Ethnicity was categorized as either being African-American or Caucasian. Living arrangement eligibility was defined as not living in a nursing home. During the second telephone call, the individual was queried as to whether he or she was interested in participating in a study of memory and health. If interested, a flyer was mailed to the house describing the study. During the third telephone call, if interest and informed consent were affirmed, the Short Portable Mental Status Questionnaire (SPMSQ), a 10-item questionnaire developed to detect intellectual impairment in older adults living in the community or residing in institutions, was administered.31 The SPMSQ classifies cognitive impairment into four categories: intact, mild impairment, moderate impairment, and severe impairment.32 To be included in the study, individuals had to score ≥8. The SPMSQ was administered over the telephone by a trained nursing research assistant who followed a written script. While the SPMSQ is able to screen out seriously impaired subjects, it is not capable of screening the mildly impaired. Therefore, those scoring ≥8 on the SPMSQ were tested further with the Mini-Mental State Exam. The results were not disclosed at the time of the telephone call. The individual was thanked for his/her participation and mailed a $2 grocery coupon.
A total of 218 subjects (149 females and 69 males) completed the study (Table 1). The majority (63%, N = 138) were recruited by random telephone procedures; the remainder (37%, N = 80) were recruited by convenience and snowballing methods. Fifty-seven percent of the African-American subjects (N = 55) and 68% of White subjects (N = 83) were recruited using random sampling methods (Table 1). The participants ranged in education level from 3 years to 25 years (M = 12.07, SD = 3.40), and in age from 66 years to 97 years (M = 77.75, SD = 6.24).
Table 1.
Numbers of subjects by race and sampling frame
| (N=138) Random | (N=80) Convenience | |||
|---|---|---|---|---|
| X | % | X | % | |
| African Americans | 55 | (57%) | 41 | (43%) |
| Whites | 83 | (68%) | 39 | (32%) |
| Males | 53 | (76%) | 16 | (24%) |
| Females | 85 | (57%) | 64 | (43%) |
There were many dropouts during the four phases of recruitment. The total dropout rate from the random sample was 86% for African-American subjects and 75% for the White subjects. Although the dropout rates remained almost constant for the first and second calls for both African Americans and Whites, dropout numbers increased for African Americans and decreased for Whites after the second call.
After the first year, the representation of the African-American subjects was lower than anticipated, so a group of African-American (N = 31) dropouts were telephoned by an African-American graduate research assistant. Six common themes emerged from the telephone interviews. The dropouts: 1) lacked trust in the local university (institution); 2) did not understand the purpose of the study or how it related to them; 3) were afraid of strangers in their homes; 4) were generally uncomfortable with the procedure/protocol; 5) were uncomfortable about how the findings would be used; and 6) were embarrassed about appearing uneducated/unintelligent.
As a result of this information, a number of changes were made in the sampling procedure. First, an African-American graduate student was made a permanent member of the research team. Previously, two African-American research assistants had conducted in-home interviews, but had not made the initial telephone contact with potential subjects. Second, a relationship was established with the administrative manager of the Golden Age Centers to begin recruiting in subsidized high-rise apartments with African-American elderly residents. Third, the team established rapport with the social work staff in each of the high-rise apartments, in order to have a contact person at each site. Fourth, individuals were referred to the research team members by the contact person in each apartment building. These strategies were successful and the size of the African-American sample increased.
Those individuals who made one or two errors on the SPMSQ were included in the next phase of the study. During the fourth telephone call, the individual was informed that he or she qualified for a complete home interview; if the person was interested, the address was confirmed and a home interview was scheduled. The Mini-Mental State Exam (MMSE) was used to rule out those with cognitive impairments during the home interview. The MMSE contains 11 questions to be answered without a time limit. Scores can range from 0–30, with a score of 23 or less indicating cognitive impairment. A score between 18 and 22 usually indicates mild cognitive impairment, and a score between 0 and 17 indicates severe cognitive impairment. The MMSE was tested in the National Institute of Mental Health Epidemiological Catchment Area Program surveys conducted between 1980 and 1984 on a mega sample of adult participants in Baltimore, Md; Durham, NC; Los Angeles, Calif; New Haven, Conn; and St. Louis, Mo. However, among the elderly (over 60 years of age) and the poorly educated (less than eighth grade), the MMSE, when used as the sole criterion, may over-estimate the prevalence of cognitive impairment. Participants with borderline cognitive impairments and pre-clinical dementia may go undetected (r = .90). To be eligible for the study, subjects had completed the screening procedure and scored ≥23 on the MMSE.
Materials
Subjects who completed the screening procedure were then administered the study instruments. Memory self-efficacy was operationalized with the Memory Self-Efficacy Questionnaire (MSEQ), derived from Bandura's self-efficacy theory.33 The Rivermeade Behavioral Memory Test (RBMT) served as the principal memory measure.34 The RBMT measures everyday memory, and functions as a bridge between laboratory-based measures of memory, and assessments obtained by questionnaires and observations. Depression was operationalized with the Center for Epidemiological Studies Depression Scale (CES-D), a measure of depression designed for research.35–36 Health was measured with the Medical Outcomes Study Health Scale (SF-36), a measure of an individual's self-perceived health status.37 Meta-memory, the memory components of knowledge, beliefs, and affect, was measured with the Meta-memory in Adulthood (MIA) questionnaire.38 The MIA consists of seven subscales, which measure strategy, task, capacity, change, anxiety, achievement, and locus.
Results
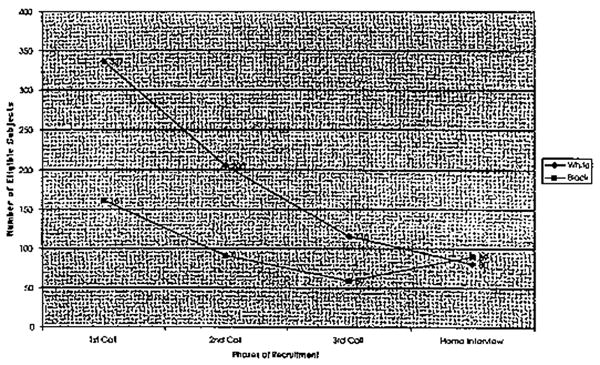
A total of 529 individuals were contacted by telephone. Of this random sample (N = 529), 31 (6%) individuals refused to participate during the first call (Figure 1). Of the 298 individuals contacted during the second telephone call, 76 refused to participate. Of the 255 individuals contacted during the third telephone call, 80 (31%) refused to participate. By the fourth telephone call, only one of the 170 individuals contacted refused.
Fig 1.
Changes of Subject Number.
Those individuals who agreed to participate during the third telephone call (N = 175) were administered the SPMSQ to determine cognitive eligibility. Of those administered the SPMSQ, 46% percent of the sample made 0 errors, 29% made 1 error, 17% made 2 errors, 5% made 3 errors, 3% made 4 errors, and <1% made 5 errors. Therefore, 92% of the individuals contacted (N = 161) during the third telephone call were eligible to participate in the memory testing. A total of 14 individuals were excluded based on their error rate (≥3 errors) on the SPMSQ.
All individuals were living in the community, in either private homes (N = 139), subsidized high-rise apartments (N = 39), or assisted living facilities (N = 40). Sixty-seven percent of the sample were widowed (N = 148), 27% were married (N = 58), and 6% (N = 12) were single. Sixty-five percent of the subjects were living alone, 25% were with a spouse, and 5% were with their children. The remaining 5% were divided between other living arrangements, such as living with a companion or a grandchild. Of the total sample, 56% (N = 122) were Caucasians and 44% (N = 96) were African-Americans. Of the African Americans, 43% (N = 41) were recruited through convenience methods; of the Caucasian sample, 32% (N = 39) were recruited by convenience methods (Table 1).
The convenience sample was older than the random sample (80 vs 76, P<.0001), had more depression (12 vs 9, P<.03), and reported lower physical functioning (46 vs 65), P<.0001, and less vitality (48 vs 60, P<.0007) (Table 2 and 3). Differences in cognitive function were marginally significant, with the convenience sample testing lower on post hoc comparisons (M = 27.05, SD = 2.23), F(1, 216) = 4.27, P<.04.
Table 2.
Demographic variables
| (N=138) Random | (N=80) Convenience | ||||
|---|---|---|---|---|---|
| X | SD | X | SD | P | |
| Age | 76.35 | 4.92 | 80.18 | 7.45 | .0001 |
| Education | 12.16 | 3.11 | 11.93 | 3.87 | NS |
| Depression | 8.78 | 7.98 | 11.63 | 10.95 | .03 |
| Cognition | 27.71 | 2.30 | 27.05 | 2.23 | .04 |
Table 3.
Health profile variables
| (N=138) Random | (N=80) Convenience | ||||
|---|---|---|---|---|---|
| X | SD | X | SD | P | |
| Physical functioning | 64.64 | 28.22 | 45.88 | 29.22 | .0001 |
| Role physical | 62.32 | 40.44 | 58.75 | 43.05 | NS |
| Bodily pain | 70.39 | 25.93 | 65.26 | 28.29 | NS |
| General health | 59.38 | 21.08 | 55.56 | 19.65 | NS |
| Vitality | 59.57 | 21.27 | 48.13 | 27.43 | .0007 |
| Social functioning | 81.36 | 26.79 | 83.15 | 26.10 | NS |
| Role emotional | 82.15 | 32.27 | 80.83 | 35.91 | NS |
| Mental health | 80.99 | 17.36 | 79.40 | 17.16 | NS |
Of the African-American elders, there were no differences in age, cognition, or depression scores between the random (N = 55) and convenience (N = 41) samples. There were no differences in memory performance or memory self-efficacy scores. Of the meta-memory variables, only three subscale scores differed significantly between the random and convenience samples: achievement, locus, and task. The convenience sample scored higher than the random on achievement (3.83 vs 3.68, P = .03) and on locus (3.59 vs 3.35, P = .01). On task, the random sample scored higher than the convenience (3.75 vs 3.59, P = .009).
There were significant health variable differences between the random and convenience samples of African-American elders, with the convenience sample scoring higher on mental health, (84.59 vs 75.27, P = .0), role function, (68.49 vs 91.85, P = .00009), role physical (73.78 vs 50.45, P = .006), and social functioning (90.61 vs 73.65, P = .001).
Memory variables
On meta-memory, the convenience sample scored significantly higher on achievement (3.84 vs 3.69), P<.006, and marginally, but significantly, lower on task (3.75 vs 3.85), F(1, 216 = 4.43, P<.04) (Table 4). The convenience sample scored significantly lower on memory performance (15 vs 18, P<.0001), and memory self-efficacy (26 vs 33, P<.01) (Table 5).
Table 4.
Meta-memory variables
| (N=138) Random | (N=80) Convenience | ||||
|---|---|---|---|---|---|
| X | SD | X | SD | P | |
| Achievement | 3.69 | .36 | 3.84 | .39 | .006 |
| Anxiety | 3.09 | .54 | 3.21 | .62 | NS |
| Capacity | 3.10 | .50 | 3.03 | .57 | NS |
| Change | 2.61 | .54 | 2.48 | .61 | NS |
| Locus | 3.40 | .50 | 3.47 | .53 | NS |
| Strategy | 3.37 | .59 | 3.32 | .61 | NS |
| Strategy internal | 3.30 | .65 | 3.21 | .64 | NS |
| Strategy external | 3.45 | .73 | 3.42 | .82 | NS |
| Task | 3.85 | .30 | 3.75 | .38 | .037 |
Table 5.
Memory performance and self-efficacy variables
| (N=138) Random | (N=80) Convenience | ||||
|---|---|---|---|---|---|
| X | SD | X | SD | P | |
| Memory performance | 17.69 | 3.70 | 15.16 | 5.01 | .0001 |
| Memory self-efficacy | 32.65 | 18.35 | 26.33 | 16.04 | .01 |
Discussion
A major limitation of this study is the representativeness of the sample. According to Kerlinger,39 a random sample must represent the population from which it was drawn and must have approximately the characteristics of the population relevant to the research in question. Even though the original plan was to recruit a random sample, it was necessary to add convenience methods, to recruit an adequate sample of African-American elderly. Therefore, a convenience sample was used to increase the sample size so that it was more representative of the inner-city population being studied (44% African-American and 56% Caucasian).
Our findings indicated that there were no differences in depression, memory performance, memory self-efficacy, and memory strategy use between the convenience and random samples of African-American elderly. However, there were significant differences on health and meta-memory variables. Caucasian elders had a stronger awareness of their memory's ability and value than did African Americans. However, the convenience sample scored higher on the achievement subscale of the meta-memory measure. Achievement is related to motivation, and is based on the perceived importance of having a good memory and performing well on memory tasks. The convenience sample had the most to lose from a failing memory since they were living primarily in subsidized high-rise apartment complexes, and wanted to maintain their independence for as long as possible. The desire to maintain independence may be an essential factor in the achievement of functional memory; therefore, the convenience sample's scores on achievement were not surprising.
For health variables, the convenience sample of African-American elderly scored significantly higher than the random sample on mental health, role function, role physical, and social functioning. The high-rise apartment complexes in which the convenience sample lived provided a ready source of social interaction on a daily basis. Social interaction promotes an awareness of one's self image and self-perception, and encourages mental stimulation. These African Americans exhibited a high degree of motivation and the desire to test and improve their memories. Once their fear of failure had dissipated, the individuals who participated in the study learned a great deal about their cognitive skills and potential ways of retaining and improving their memory through cognitive testing. All sampling plans should incorporate cultural sensitivity, because it promotes knowledge and growth for the discipline and for the population being studied.
Anderson12 has identified three areas of concern that must be addressed to increase African Americans' participation in research. First, due to a history of distrust of the medical care system, communication between African Americans and the system is inherently weak. Second, African Americans often have a past or present orientation to life, rather than a future orientation, which is typical of research. Finally, African Americans do not trust the National Institutes of Health (NIH) and are suspicious of the research process since they believe NIH does not ensure their safety.
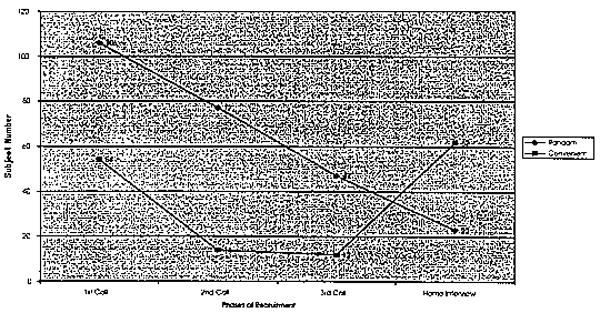
All these concerns may be alleviated or diminished through direct contact with potential subjects. This study found that when the interviewer made direct contact, the dropout rate was 0% for both African Americans and Whites. African Americans' doubts and skepticism dissipated once they met with the interviewers, and made their own appraisals. This is not possible with indirect contact. Potential subjects need a mechanism by which to establish acceptance of the study, and the researchers implementing the study. Wrobel and Shapiro40 have proposed that a face-to-face encounter in an individual's home increases minority retention. We would add that the interviewer should make an initial contact to develop trust before arriving at the home, so that the visit is not aborted. In this study, 51% of the eligible African-American participants refused during the third telephone call to schedule a home interview (Figure 2). By contrast, only 28% of the eligible White participants refused the home interview. Face-to-face contact or some other trust-building strategy or contact with the elderly African-American subjects might have prevented these losses. Morse et al41 validated this finding with their study of community research on AIDS. At some of the research sites, nurses with experience treating HIV-infected individuals identified barriers and executed strategies to enhance participants' recruitment, retention, and compliance. These sites had the highest rates of participant retention and compliance, suggesting that creative strategies can breach the social and ethical barriers to research among vulnerable populations.
Fig 2.
Sampling Strategy for African Americans.
In our study, many participants used subtle ways of refusing to take part in the study. Initially, they would agree to participate over the telephone, only to refuse by not completing the in-home interview, or by simply dropping out. Researchers attempted to minimize the distrust by increasing the number of follow-up telephone calls, but African Americans' fear of involvement persisted. In a hypertension study, Pavlik et al42 experienced the same behaviors among African Americans living in low-income zip-code areas. Of the people contacted, 97% (N = 2037) completed the initial telephone survey and 65% (N = 1324) of these individuals agreed to an onsite visit; nevertheless, only 26% (N = 344) of those who agreed to visit actually participated. Our findings, as well as those of Pavlik and colleagues, suggest that household screening, rather than indirect contact, facilitates recruitment and retention by reducing distrust among the population to be studied. Direct contact, such as face-to-face screening, sensitizes researchers to the culture of potential participants, while simultaneously increasing these prospects' trust.
Rogler,15–16 Sieber and Stanley20 note the impact of researchers' personal biases on the research process. Unfortunately, many researchers have treated African-American participants as second-class citizens or as intellectually handicapped individuals. Their findings often reflect the submission of African-American participants, rather than advances in knowledge. Personal biases may play an important role in causing researchers to make inaccurate interpretations, and unjust applications. Cultural sensitivity must become standard procedure when working with minority populations. This requires researchers to make both substantive and methodological adaptations. Greater cultural sensitivity in the original sampling plan might have prevented many of the difficulties experienced in the course of this study. Cultural sensitivity should also be incorporated into the normative measures for cognitive function, to facilitate accurate comparisons between African-American elderly and other elderly.
Acknowledgments
Support for this research was provided by NIH-NINR R15 NR0420. Our thanks to all of the graduate students from the Frances Payne Bolton School of Nursing who helped with data collection. Ezra C. Holston, P-NP, MSN, RN, New York University was supported by the American Nurses Association, Ethnic Minority Fellowship Program, funded by Substance Abuse and Mental Health Services Administration (@T06 SM151555).
References
- 1.Jones JH. Bad Blood: The Tuskeegee Syphilis Experiment. New York, NY: The Free Press; 1981. [Google Scholar]
- 2.Thomas SB, Quinn SC. The Tuskeegee syphilis study, 1932 to 1972: implications for HIV education and AIDS risk education programs in the Black community. Public Health Then and Now. 1991;81:1498–1505. doi: 10.2105/ajph.81.11.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders E. Recruitment of African-American patients for clinical trials—the ALLHAT challenges Antihypertensive and Iipid-lowering trial to prevent heart attack. J Natl Med Assoc. 1995;87:627–629. [PMC free article] [PubMed] [Google Scholar]
- 4.Kington RS, Smith JP. Socioeconomic status and racial and ethnic differences in functional status associated with chronic diseases. Am J Public Health. 1997;87:805–810. doi: 10.2105/ajph.87.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strawbridge WJ, Kaplan GA, Camacho T, Cohen RD. The dynamics of disability and functional change in an elderly cohort: results from the Alameda County study. J Am Geriatr Soc. 1992;40:799–806. doi: 10.1111/j.1532-5415.1992.tb01852.x. [DOI] [PubMed] [Google Scholar]
- 6.Whitfield KE, Seeman TE, Miles TP, et al. Health indices as predictors of cognition among older African Americans: MacArthur studies of successful aging. Ethn Dis. 1997;7:127–136. [PubMed] [Google Scholar]
- 7.Brawley OW, Tejeda H. Minority inclusion in clinical trials issues and potential strategies. J Natl Cancer Inst Monogr. 1995;17:55–57. [PubMed] [Google Scholar]
- 8.Hornblum AM. Acres of Skin: Human Experiments at Holmesburg Prison. New York, NY: Routledge; 1998. [Google Scholar]
- 9.Allen M. The dilemma for women of color in clinical trials. J Am Med Womens Assoc. 1994;49:105–109. [PubMed] [Google Scholar]
- 10.Millon-Underwood S, Sanders E, Davis M. Determinants of participation in state-of-the-art cancer prevention, early detection/screening, and treatment trials among African Americans. Cancer Nurs. 1993;16:25–33. [PubMed] [Google Scholar]
- 11.Robinson SB, Ashlely M, Haynes MA. Attitude of African Americans regarding prostate cancer clinical trials. J Community Health. 1996;21:77–87. doi: 10.1007/BF01682300. [DOI] [PubMed] [Google Scholar]
- 12.Anderson NB. Appealing to diverse audiences: reaching the African-American community. J Natl Med Assoc. 1995;87:647–649. [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas CR, Pinto HA, Roach M, III, Vaughn CB. Participation in clinical trials: is it state-of-the-art treatment for African Americans and other people of color? J Natl Med Assoc. 1994;86:177–182. [PMC free article] [PubMed] [Google Scholar]
- 14.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogler LH. Methodological sources of cultural insensitivity in mental health research. Am Psychol. 1999;54:424–433. doi: 10.1037//0003-066x.54.6.424. [DOI] [PubMed] [Google Scholar]
- 16.Rogler LH. The meaning of culturally sensitive research in mental health. Am J Psychiatry. 1989;146:296–303. doi: 10.1176/ajp.146.3.296. [DOI] [PubMed] [Google Scholar]
- 17.Miles TP, Bernard MA. Morbidity, disability, and health status of Black American elderly: a new look at the oldest-old. J Am Geriatr Soc. 1992;40:1047–1054. doi: 10.1111/j.1532-5415.1992.tb04485.x. [DOI] [PubMed] [Google Scholar]
- 18.Ballard EL, Nash F, Raiford K, Harrell LE. Recruitment of Black elderly for clinical research studies of dementia: the CERAD experience. Gerontologist. 1993;33:561–565. doi: 10.1093/geront/33.4.561. [DOI] [PubMed] [Google Scholar]
- 19.Welsh KA, Ballard E, Nash F, Raiford K, Harrell L. Issues affecting minority participation in research studies of Alzheimer's disease. Alzheimer Dis Assoc Disord. 1994;8:38–48. [PubMed] [Google Scholar]
- 20.Sieber JE, Stanley B. Ethical and professional dimensions of socially sensitive research. Am Psychol. 1988;43:49–55. doi: 10.1037//0003-066x.43.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med. 1995;122:422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Proctor EK, Morrow-Howell N, Chadiha L, Braverman AC, Darkwa O, Dore P. Physical and cognitive functioning among chronically ill African-American and White elderly in home care following hospital discharge. Med Care. 1997;35:782–791. doi: 10.1097/00005650-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Blessed G, Tomlinson BE, Roth M. Blessed-Roth dementia scale (DS) Psychopharmacol Bull. 1988;24:705–708. [PubMed] [Google Scholar]
- 24.Shadlen MF, Larson EB, Gibbons L, McCormick WC, Teri L. Alzheimer's disease symptom severity in Blacks and Whites. J Am Geriatr Soc. 1999;47:482–486. doi: 10.1111/j.1532-5415.1999.tb07244.x. [DOI] [PubMed] [Google Scholar]
- 25.Gautier MA, Clarke WP. Gaining and sustaining minority participation in longitudinal research projects. Alzheimer Dis Assoc Disord. 1999;13:S29–S33. doi: 10.1097/00002093-199904001-00008. [DOI] [PubMed] [Google Scholar]
- 26.Hall WD. Representation of Blacks, women, and the very elderly in 28 major randomized clinical trials. Ethn Dis. 1999;9:333–340. [PubMed] [Google Scholar]
- 27.Stoy DB, Curtis CR, Dameworth KS, et al. The successful recruitment of elderly Black subjects in a clinical trial: the CRISP experience. J Natl Med Assoc. 1995;87:280–287. [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson EE, Neighbors HW, Munday C, Jackson JS. Recruitment and retention of African-American patients for clinical research: an exploration of response rates in an urban psychiatric hospital. J Consult Clin Psychol. 1996;64:1–7. doi: 10.1037//0022-006x.64.5.861. [DOI] [PubMed] [Google Scholar]
- 29.Miller KW, Wilder LB, Stillman FA, Becker DM. The feasibility of a street-intercept survey method in an African-American community. Am J Public Health. 1997;87:655–658. doi: 10.2105/ajph.87.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norton MC, Breitner JC, Welsh KA, Wyse BW. Characteristics of non-responders in a community survey of the elderly. J Am Geriatr Soc. 1994;42:1252–1256. doi: 10.1111/j.1532-5415.1994.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 31.Dalton JE, Pederson SL, Blom BE, Holmes NR. Diagnostic errors using the Short Portable Mental Status Questionnaire with a mixed clinical population. J Gerontol A Biol Sci Med Sci. 1987;42:512–514. doi: 10.1093/geronj/42.5.512. [DOI] [PubMed] [Google Scholar]
- 32.Fillenbaum G, Heyman A, Williams K, Prosnitz B, Burchett B. Sensitivity and specificity of standardized screens of cognitive impairment and dementia among elderly Black and White community residents. J Clin Epidemiol. 1990;43:651–660. doi: 10.1016/0895-4356(90)90035-n. [DOI] [PubMed] [Google Scholar]
- 33.Berry JM, West R, Dennehey DM. Reliability and validity of the memory self-efficacy questionnaire. Dev Psychol. 1989;25:701–713. [Google Scholar]
- 34.Cockburn J, Smith PT. The Rivermeade Behavioral Memory Test Supplement 3: Elderly People. Bury St Edmunds, Suffolk: Thames Valley Test Company; 1989. [Google Scholar]
- 35.Radloff LS, Teri L. Use of the center for epidemiological studies-depression scale with older adults. Clin Gerontol. 1986;5:119–136. [Google Scholar]
- 36.Hertzog C, VanAlstine J, Usala PD, Hultsch DF, Dixon R. Measurement properties of the center for epidemiological studies depression scale (CES-D) in older populations. J Consult Clin Psychol. 1990;2:64–72. [Google Scholar]
- 37.War JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 38.Dixon RA, Hultsch DF, Hertzog C. The metamemory in adulthood (MIA) questionnaire. Psychopharmacol Bull. 1988;24:671–688. [PubMed] [Google Scholar]
- 39.Kerlinger FN. Foundations of Behavioral Research. 3rd. New York, NY: Holt, Rinehart and Winston; 1986. [Google Scholar]
- 40.Wrobel AJ, Shapiro NEK. Conducting research with urban elders: issues of recruitment, data collection, and home visits. Alzheimer Dis Assoc Disord. 1999;13:S34–S38. [PubMed] [Google Scholar]
- 41.Morse EV, Simon PM, Besch CL, Walker J. Issues of recruitment, retention, and compliance in community-based clinical trials with traditionally underserved populations. Appl Nurs Res. 1995;8:8–14. doi: 10.1016/s0897-1897(95)80240-1. [DOI] [PubMed] [Google Scholar]
- 42.Pavlik VN, Hyman DJ, Vallbona C, et al. Response rates to random digit dialing for recruiting participants to an onsite health study. Public Health Rep. 1996;111:444–450. [PMC free article] [PubMed] [Google Scholar]


