Summary
Anting, the plumage-dipping behavior to which ants (mostly formicines) are commonly subjected by birds (mostly passerines), is shown in tests with hand-raised Blue Jays (Cyanocitta cristata) and the ant Formica exsectoides to be instinctive: the birds displayed typical renditions of the behavior on the first occasion that they encountered ants. Evidence is presented supportive of the view that anting is a strategy by which birds render ants fit for ingestion. Formicine ants are ordinarily protected by their formic acid-containing spray. Being wiped into the bird’s plumage causes them to discharge that spray, without harm to the bird, to the point of almost total emptying of the glandular sac in which the secretion is stored. The ants are therefore essentially secretion-free by the time they are swallowed. Further evidence indicates that it is the ant’s possession of the acid sac that triggers the anting behavior in the bird. If F. exsectoides are surgically deprived of their acid sac, they are eaten by the birds without first being subjected to anting. Data are also presented indicating that the ant’s crop, which is especially capacious in formicines (its contents may amount to over 30% of the formicine’s mass), and which appears to survive the anting procedure intact, constitutes, at least when laden, a valuable component of the trophic package that the bird accesses by anting.
Keywords: Cyanocitta cristata, Formica exsectoides, chemical defense, coevolution, predation, formic acid
Introduction
The peculiar behavior known as “anting” (Fig. 1A, D-E), whereby birds grasp ants and wipe these into their plumage prior to ingesting them, has long been of interest to naturalists (Chisholm 1959; Simmons 1985; Whitaker 1957). The behavior is highly stereotyped and typically involves the bird seizing one or more ants in the bill, and then, while holding one or both wings outstretched and the tail bent forward between the legs, wiping the ants into the primary feathers of wings and tail. Anting has been reported for over 200 species of birds, mostly passerines (Chisholm 1944, 1959; Potter 1970, Simmons 1966, 1985; Whitaker 1957), and it appears to be carried out principally with ants of the subfamily Formicinae (Brackbill 1948; Groskin 1950; Ivor 1943; McAtee 1938; Nice 1945; Potter 1970; Staebler 1942; Whyte 1981) whose members eject a potently irritating secretion from their poison gland when attacked. In formicine ants the primary component of that secretion is formic acid, which may make up more than 50% of the fluid. The ants hold the secretion in a large storage sac of the gland, the reservoir (herein referred to as the acid sac), from which the fluid is ejected forcibly as a jet (Hölldobler & Wilson 1990; Stumper 1960). It has long been claimed that by anting birds cause the ants to discharge their secretion into the bird’s plumage.
Fig. 1.
(A) Hand-raised Blue Jay, anting (see text for details). (B-C) Blue Jays seizing individual F. exsectoides ants, preparatory to subjecting these to anting; note that the ants are being grasped by the thorax and that in (C) the bird has drawn the nictitating membrane over the eye (as many birds do, protectively, when bearing down to attack an insect). (D-E) Typical stances assumed by Blue Jays during anting (in D, the ant is barely discernable, held in the bird’s bill); in E, the bird is wiping the ant into the primary feathers of the right wing. (F) F. exsectoides remnants in feces of one of our Blue Jays.
Two primary hypotheses have been advanced to explain anting, one fumigatory, the other dietary. The fumigatory hypothesis postulates that by causing the ant’s secretion to be voided into its plumage, the bird is essentially giving itself a “shampooing”, whereby potentially harmful ectoparasites, including both arthropods and microbes, are brought under chemical control (Ali 1937; Ehrlich et al. 1986; Simmons 1966). That hypothesis, while seemingly widely held among ornithologists (Revis & Waller 2004), is not supported by evidence: avian ectoparasites appear not to be adversely affected by ant secretions or their components, under in vivo conditions (Judson and Bennett 1992; Revis & Waller 2004; Whitaker 1957).
The dietary hypothesis, in contrast, argues that by inducing the ants to emit their secretion, birds are essentially using their body as a “napkin”, to rid intended morsels of their noxious effluent and render them fit for ingestion (Adlersparre 1936; Chisholm 1944; Sauer 1957). This hypothesis, which we favor, has been bolstered by recent evidence. Thus, for instance, it has been shown that starlings are more likely to subject formicine ants to anting if the ants are offered to them on an empty stomach, that is, when the birds would be needing to cope with enteric exposure to the full strength of the secretion (Judson and Bennett 1992). We ourselves have shown that in Blue Jays anting may occur also when the birds are offered spraying insects other than ants, as for example bombardier beetles, and that the behavior, which causes the bombardiers to eject their spray, occurs as a preamble to ingestion of the beetles. But as we also found, there are other Jays (Scrub Jays, Aphelocoma coerolescens), that do not ant with bombardiers, but instead subject these to “sand wiping” prior to ingestion (literally to a vigorous wiping in the soil, which also causes the beetles to deplete their glands), demonstrating thereby that it is for purposes of “disarming” the prey, rather than self-dousing, that anting ordinarily occurs (Eisner et al. 2005).
Pursuant to these earlier findings, we here report data, derived from observations and experiments with hand-reared Blue Jays (Cyanocitta cristata) and a formicine ant (Formica exsectoides), which shed light on some aspects of the anting process, while offering support for the notion that anting is primarily, if not necessarily exclusively, a food-preparatory procedure. Specifically, we provide evidence of the following:
Anting is an inborn behavior. A first exposure to F. exsectoides, on the part of naïve, hand-raised Blue Jays, elicited bouts of anting indistinguishable from those performed by experienced birds.
Anting with F. exsectoides in Blue Jays is contingent upon the ant possessing an intact defensive apparatus. Experimental ants from which the acid sac has been excised, are generally eaten without first being subjected to anting.
Anting does indeed result in the preingestive emptying of the ant’s acid sac. Formica exsectoides that were retrieved from the birds after having been subjected to anting, but before the birds had proceeded to swallow them, proved to have depleted acid sacs.
Anting seemingly occurs without the rupture and loss of contents of the ant’s crop. Our data on this point, while not as extensive as we would have liked, are supportive of the view that anting in birds serves not only for “disarming” the formicine ant, but for accessing the ant’s crop contents, which can be substantial. In F. exsectoides we show the crop, when replete, to amount to about a third of the ant’s mass.
Here we present these results, together with a discussion of their implications.
Materials and methods
Blue Jays (Cyanocitta cristata)
Six Blue Jays were used in the study, all raised from the nestling stage by hand-feeding. Two were given to us years ago, before we had any idea that we would some day be gathering information on anting. It was these two birds that we knew not to have had any exposure to live insects prior to our having received them. The other four Jays were made available to us years later, and it was with these that we obtained the bulk of the data presented here. These birds, which were several years old at the time of testing, had had some laboratory experience with insects before we obtained them, and we could not be certain that they had never been exposed to ants. We housed these four birds in a large walk-in aviary, except during days of testing, when they were transferred individually to special glass-fronted cubical cages, 0.8 m to the side. They took readily to these enclosures, having previously been subjected to feeding tests in them.
We maintained all Jays on commercial bird food and water, to which they had access at all times, except during experimental sessions, when the food dish was removed.
Ants (Formica exsectoides)
Several thriving colonies of this ant, derived from nests found locally in the environs of Ithaca, NY, were set up individually in large glass aquaria and maintained on mealworms (larvae of Tenebrio molitor) and water (and an occasional supplement of other insects). Workers of this ant are ferociously aggressive and prone to bite and eject their formic acid-containing spray in response to even slight provocation.
Anatomically such workers conform to the typical formicine body plan, being in possession of both a large crop, and a large formic acid sac. The photos shown here (Fig. 2) of another formicine ant, Camponotus floridanus, that we studied in Florida (Eisner et al. 1993), are illustrative of this structural arrangement. If allowed to feed, and kept undisturbed so as not to be caused to spray, the formicine worker can be shown upon dissection to have an abdomen (or gaster as it is called in ants) accommodating little besides these two sacs (Fig. 2C and D). A predator intent on feeding on such an item, if it is to render the item palatable and gain access to the full measure of its contained nutrients, must therefore have a way both of ridding the item of its defenses, and of preserving the integrity of the crop.
Fig. 2.
Photos of Camponotus floridanus, a formicine ant similar in its major features to F. exsectoides. (A) Worker ant. (B) Head of ant, beside acid sac (A.S.) and crop (Cr), extricated from the abdomen; the ant was allowed to gorge on stained (red) honey solution, accounting for the color of the crop, in which acquired nutrient was held (pv = proventriculus; oe = esophagus). (C) Headless, legless workers, one with abdomen intact, the other with the abdomen torn open, to allow the acid sac and crop to bulge out. (D) Same as preceding, but with entire alimentary canal pulled out (mg = midgut, where much of the digestion and absorption occurs). Reference bar = 2 mm.
Anting: an inborn behavior
The two Blue Jays initially received, which we knew to have had no previous exposure to insects, were presented, at various times in their fist year of life, with a diversity of insects, including F. exsectoides. The latter were given singly or in groups to the separately-caged birds, and the results were noted and photographed.
Contingency of anting upon the ant’s possession of its acid sac
Two categories of F. exsectoides workers were offered to the four Jays tested: intact workers (the controls), in possession of their normal defensive apparatus, and operated workers (the experimentals), from which the acid sac was excised. Removal of the sac, which opens on the abdominal tip, was effected simply by grasping individual ants (immobilized by brief confinement to a freezer) by the thorax with forceps, while pulling on the abdominal tip with a second pair of forceps, until the tip with its attached sac pulled free (severance at the level of the rectum prevented the gut from being extricated as well). The operation could be carried out without noticeable loss of fluid from the sac. The sac itself is cuticle-lined and leak-proof, but some secretion, we thought, might have leaked imperceptibly from the gland opening during the excision procedure and bestowed a lingering acidic odor upon the ants. To cope with this eventuality, and insure that our sacless ants were free of residual odor at the time of testing, we did not offer these ants to the birds until at least two hours following the surgery. Since the ants remained fully viable for upward of six hours following the operation, such delayed use was of no negative consequence.
The testing involved presenting ants of either category, in groups of 5, to individual birds, in small cylindrical dishes (8 cm diameter, 4 cm height), rendered escape-proof by having their inner vertical surface coated with “Fluon AD1,” a commercial Teflon-based liquid which upon drying leaves a film too slippery for ants to traverse. An individual daily feeding session involved presenting 4 such dishes in succession, to an individual caged bird and keeping track of the fate of all 20 ants thus offered.
Each of the birds was tested in 16 such daily sessions, 8 with experimental sacless ants, and eight with intact controls. Sessions were on consecutive days, with breaks for weekends. Sequence of sessions for each bird was: 4 with experimentals; 4 with controls; 4 with experimentals; 4 with controls. The total number of ants tested with the birds was therefore 640 per each of the two ant categories.
Fate of ants was scored in accord to the following criteria: E, if they were eaten outright; AE, if they were eaten after being subjected to anting; AR, if they were rejected after being subjected to anting; R, if they were rejected after mere inspection (that is, seizure in the bill); and I, if they were ignored (not touched).
Anting was judged to have occurred if the bird, at least once, with its wings partly or completely spread, and the ant in its bill, dipped the ant into its plumage.
Precautions were taken to prevent the control ants from ejecting some of their secretion prematurely, before presentation to the birds. Transference of such ants from laboratory colony to feeding dish was effected by coaxing the ants into individual vials without seizing them. Dissection of ants killed by freezing after being treated in this fashion showed their acid sacs to be of undiminished content (visual comparison with sacs of nestmates frozen and dissected directly after being taken from the colony).
Depletion of the ant’s acid sac in consequence of anting
A quantitative determination was made of the mass of fluid lost from the acid sac of F. exsectoides as a result of anting. To this end measurements were made of the fluid content of the sacs of two groups of ants (all individually weighed beforehand), one exposed to Blue Jays and subjected to anting (the experimentals; N=22 ants), the other (the controls; N=21 ants), unexposed to the Jays.
Sac fluid content was determined by the same procedure for both experimentals and controls. Sacs were dissected from the individual ants, blotted off with filter paper, and weighed. They were then laid out on filter paper again, and re-weighed, after having been punctured so as to cause their secretory contents to be drained away. Subtraction of the second weight from the first, provided a measure of the mass of fluid in the sac.
The sacs of the control ants were obtained simply by taking individual ants from our laboratory colonies and dissecting these after killing them by freezing. Precaution was taken, as in other tests, to prevent the ants from being induced to spray in the course of their transferal to the freezer.
The experimental ants were ones that had been attacked by the birds and subjected to the plumage-dipping phase of the anting process, but had been retrieved before the birds had a chance to swallow them. To thwart a bird in the midst of the anting process, we simply waited for it to complete what we judged to be a normal amount of feather-dipping, and then startled it by tapping on the glass-paneled front of the cage or clapping our hands. While such distractive behavior did not always cause the bird to drop the ant, it did so on sufficient occasions.
The experimental ants were dead or fatally maimed when taken from the cage for dissection (they were, for purposes of matching their treatment with that of the controls, also placed in a freezer for some minutes prior to dissection). They proved not to be grossly deformed. Their abdomen was almost always intact, and extrication of the acid sac posed no problem. Observation and photography (Fig. 1 B, C) had told us that the birds tended, most often, to seize ants by the thorax, and that the abdomen is usually spared preingestive malaxation. The sac, in fact, in the experimental ants, appeared consistently to be imperforate.
From the values we had obtained for whole body mass of the ants, prior to dissection, we calculated (by subtraction of the mean mass of experimentals from the mean mass of controls) the loss in body mass incurred by the ants in consequence of anting.
It was while in the course of dissecting the ants that we made the incidental observation that the crop too, like the acid sac, was consistently undamaged in the experimentals and about as replete with fluid as in the controls. This is not to say that the crops were filled to capacity. They were only partly filled, a condition that appeared to have been the norm for worker crops in our ant colonies at the time. We did not, however, take weighings of the crops.
Nutrient carrying capacity of the ant’s crop
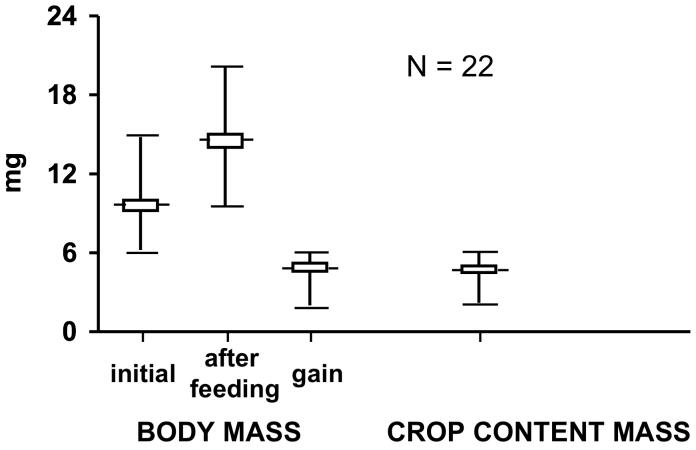
To obtain a value for the carrying capacity of the crop we took a group of F. exsectoides (N=22) from our colonies and weighed them, both before and after they were confined individually (for 3-5 min, a time which we had determined sufficed for the ants to feed to repletion) to small dishes with access to 0.5 M aqueous sucrose solution (presented as a large drop on a piece of aluminum foil). The difference in the two weighings provided a measure of the amount of fluid ingested. The ants were then killed by freezing and their crop was extricated whole, blotted off, and weighed (the crop was a bulging sac in the F. exsectoides thus fed, comparable in dimension to what it is in the gorged C. floridanus shown in Fig. 2C). No loss of crop contents occurred during this procedure (no fluid appeared to leak from the esophagus or proventriculus, and none seemed to seep across the impervious, cuticle-lined crop wall). The crop was then cut open, drained, and weighed a second time, after being once again blotted off. The difference in the two weighings provided a measure of the mass of crop content.
Results
Anting: an inborn behavior
We were sufficiently taken by the finding that our two naïve Blue Jays manifested normal anting on the occasion of their very first encounter with F. exsectoides, that we made provision to photograph the behavior when they performed it only days later, on a subsequent presentation of the ants. The photo here presented (Fig. 1A) speaks for itself in that it shows a typical rendition of anting being executed by one of these essentially inexperienced birds. It was noted, incidentally, that both birds, quite consistently, ate the ants that they subjected to anting.
Contingency of anting upon the ant’s possession of its acid sac
The results were essentially the same with all four birds, and are presented here (Fig. 3), lumped for the total number of ants offered (640 sacless experimentals; 640 intact controls). As is evident, incidence of anting was high with the control ants (61%, the sum of the AE and AR categories) and close to zero (3%) with the experimentals, indicating a strong correlation of anting with possession of the acid sac. It was true also that while the sacless experimentals were virtually all eaten outright (95.9%), the controls, if eaten, were for the most part (45.5%) first subjected to anting. Only relatively few of the controls (28.3%) were eaten outright. Overall, removal of the sac increased the acceptability of the ants: the sum total of control ants that were rejected or ignored (that is, the sum of the AR, R, and I categories) amounted to a greater percentage in the controls (27.4 %) than in the experimentals (1.1 %).
Fig. 3.
Fate* of ants, in feeding tests with Blue Jays, plotted as a function of the ants’ possession of the acid sac (experimentals = without sac; controls = with sac). Data are lumped for all four birds. Differences between experimentals and controls are significant (Chi Square > 37; p<<0.001) for all five fate categories. * E = eaten outright; AE = anted and eaten; AR = anted and rejected; R = rejected after contact inspection; I = ignored.
Examination of the feces (Fig. 1F) produced by the birds as a sequel to ingestion of these ants revealed no differences in the degree of breakup of the remains of ants of the two categories (visual estimate).
Depletion of the ant’s acid sac in consequence of anting
As is evident from Fig.4, ants weighed after having been subjected to anting (experimentals) were lighter than those not exposed to birds (controls), by an amount (ΔMant = 2.40 mg) not much greater (about 2X) than the mass of secretion (ΔMsac = 1.25 mg) calculated to have been expelled from the sacs of the ants in consequence of anting. We conclude that ants lose little more than sac fluid when subjected to anting, and that, since their sacs are left virtually empty after anting, they spray exhaustively when put through the feather-dipping preliminaries of anting by an attacking bird.
Fig. 4.
Loss of body mass (left), and of acid sac content mass (right), incurred by F. exsectoides in consequence of anting. Two groups of ants are compared: experimentals (N=22), comprising ants that were subjected to anting; and controls (N=21), made up of ants that were unexposed to birds. Values are given as mean ± SEM, and range. On left are given the values from which loss in body mass (ΔMant) was calculated; on right are the values used in calculation of the loss in sac contents (ΔMsac). The difference in ΔMant and ΔMsac is significant (p>0.01) but relatively small.
Nutrient carrying capacity of the ant’s crop
The weight gained by F. exsectoides in consequence of feeding on sucrose solution (Fig. 5) matched closely (p>0.1) the weight of the crop contents of the ants after such feeding. We conclude that the food imbibed by these ants was taken entirely into the crop (and that the ants we used in these tests had empty or nearly empty crops prior to feeding, in consequence, possibly, of their laboratory colonies having been somewhat underfed at the time).
Fig. 5.
Formica exsectoides body mass gain, incurred in consequence of feeding to repletion (3-5 min) on aqueous 0.5M sucrose solution (left), and the relation of this gain to crop content mass (right). Values are given as mean ± SEM plus range. The increase in ant mass does not differ significantly from crop content mass (p<0.1), indicating that the fluid imbibed by the ant is taken up in its entirety by the crop.
Discussion
The conclusions we derive from our study, admittedly, are shaped by our view that anting is essentially a prey-preparatory process, an avian strategy for dealing pre-ingestively, in a manner meant to be protective, with insects that spray, especially ants of the subfamily Formicinae. Ants of this subfamily are of worldwide distribution, as are insectivorous birds in their extraordinary variety, so that it makes sense that a mechanism should exist, of wide prevalence in birds, that enables these animals to prey on formicine ants. It also makes sense for such a mechanism to be programmed genetically for instinctive expression, in birds generally, as it evidently is in Blue Jays. We were by no means the first to demonstrate that the capacity to ant is inborn in birds. Others, including Sauer (1957), Querengässer (1973), and Weisbrod (1971), in tests with captive birds, showed these to ant quite normally when first presented with ants.
We demonstrate that the “decision” to ant on the part of our birds was contingent upon the ant’s possession of an intact spray apparatus. Only if deprived of their sac were our F. exsectoides treated consistently as edible. If in possession of the sac they tended to be eaten mostly if they were first subjected to anting. How the Jays “told the difference” between “armed” and “disarmed” ants remains unsettled, although it is probable that they did so based on chemosensory information (taste or smell): our normal ants can be expected to have ejected secretion when seized in the bird’s bill and could thereby have triggered the anting process. Formic acid could itself have been the “releaser” of the behavior and might well be the trigger of anting in formicines generally, as suggested by Poulson (1956), among others. But we know formic acid not to be the only elicitor of anting in nature. Bombardier beetles, for instance, trigger anting with their quinonoid effluent (Eisner et al. 2005), as may tenebrionids (Blaps), earwigs (Forficula), and millipedes (Trigoniulus), which also eject quinones (Monro et al. 1962, Peschke & Eisner 1987, Schildknecht & Weiss 1960) and which have been said to induce anting (Clunie 1976; references in Whitaker 1957). It would be interesting to know how sharply attuned anting is to chemical triggers. Noxious agents quite generally, one might think, including in particular substances one would class as irritants, such as are produced in fair variety by arthropods, could have the capacity to induce anting. Evidence to the effect that anting is loosely attuned to causative agents is apparent from the fact that the behavior has been noted occasionally to be elicited by entirely “irrelevant” materials (as, for example, raw onions, hair tonic, moth balls, prepared mustard, and burning matches; references in Whitaker 1957).
It seems demonstrated, if generalization from results with a single species is justified, that anting induces the depletion of the acid sac in formicine ants, and that the ants lose little beside sac contents in consequence of being subjected to anting. This was assumed to be the case by many students of anting, but had, to our knowledge, not been proven. Formicine ants are therefore indeed “disarmed” when subjected to anting. What is also interesting, because it introduces a new dimension to the interpretation of anting, is that the crop contents of the ant, unlike the sac contents, are not lost in the course of anting. While we lack rigorous data to prove the point, it seemed clear from our dissections of “anted ants” that the crop in these individuals was intact (no evidence of breakage or leakage) and of about the same size (equally full) as that of “unanted” controls. What this means is that the bird, in the course of disarming the ant, does not induce wastage of any of the ant’s usable innards, of which the crop contents may make up a major fraction. The crop, in fact, as we demonstrated by determination of its carrying capacity, can hold an equivalent of upward of 30% of the ant’s mass (or, relative to usable mass, actually more, given that most of the worker ant is taken up by its indigestible skeleton).
The crop of formicine ants is of very special significance. Ants do not construct honeycombs for the storage of their nutrient reserves. If they store food at all, in liquid form for communal use, it is primarily in their crop. The strategy is exploited to the fullest in the Formicinae, whose crop is outsize and adapted for prolonged and massive storage of liquid nutrient (Eisner 1957). The worker formicine, when foraging, has the capacity to take up food in quantity greater than it needs for itself. It filters the food upon intake (by means of a special infrabuccal chamber), so as to exclude particulate matter, and stores the liquid filtrate in the crop (Eisner & Happ 1962). There it is held until needed, either by its nestmates or itself. It provisions nestmates by regurgitative donation (trophallaxis) (Wilson & Eisner 1957) and provisions itself by pumping a fraction of its crop contents through the proventriculus into its midgut for absorption. The proventriculus is ordinarily tightly shut, and not permissive of fluid passage (Eisner 1957). Nutrient can thus be held in the crop for extended periods and tapped intermittently by both carrier and nestmate, as needed.
Of some significance was the finding that our birds, when anting, grasp the ants by the thorax rather than by the abdomen, explaining perhaps why the crop is not damaged in the course of anting.
Formicine workers, in the light of such consideration, and especially when they are well fed, take on the image of chemically protected “meals on wheels”, of ambulatory, spray-empowered, trophic pouches, which to an insectivore are clearly worth accessing. To the avian predator, anting appears to provide the means for securing such access. Feeding on ants could bring substantial rewards to a bird, in ant crop contents alone. An average-sized Blue Jay (85 g), ingesting 20 well fed ants the size of F. exsectoides, would take in on average somewhat less than 300 mg, of which about 100 mg (0.12 % of the bird’s mass) would be liquid nutrient from crops, presumably a highly utilizable commodity. The equivalent, for an 85 kg human, would be 100 g, or about two chicken eggs.
In the final analysis, however, we cannot claim, through our findings, to have laid to rest all other explanations of anting. The fumigatory hypothesis, for one, cannot be said to have been definitively refuted. Some bird ectoparasites, including microbes, might yet be found to be adversely affected by the feather dousing that occurs in consequence of anting. The passerine readiness to ant without ants, to spring into action and perform the anting maneuvers in response to chemical stimuli alone, may be an indication that anting, quite aside from its food preparative role, can have an anointing function. But need such anointing be strictly for self-medication, for curtailment of ectoparasites? We acknowledge that the possibility is far-farfetched, but could the dousing not serve also to augment the bird’s noxiousness, to decrease, at least temporarily, its vulnerability to predation? The resulting increase in fitness need not be insignificant. The hypothesis could easily be put to the test. Interestingly, protective chemical impregnation has been demonstrated for certain birds (species of Pitohui), but the defensive agent in their case is a steroidal alkaloid (homobatrachotoxin), acquired endogenously rather than from an outside source, and of proven activity against chewing lice (Dumbacher et al. 1992, 1999). Evidently there is interesting work yet to be done in this area of endeavor.
Acknowledgements
This study was supported in part by National Institutes of Health Grant AI02908. We thank Karen Hicks, Kenneth Dodge, and Pat Haberkern for much help, and Maria Eisner for preparing the illustrations and commenting on the manuscript. Comments on parts of the manuscript were also provided by Mark Deyrup, Helen Ghiradella, John Fitzpatrick, Mitch Masters, and E. O. Wilson. Janis Strope prepared the manuscript. The anatomical work on C. floridanus was done at the Archbold Biological Station, Lake Placid, Florida. This is paper 197 of the series Defense Mechanisms of Arthropods. No. 196 is Eisner et al., Chemoecology, in press.
References
- Adlersparre A. Zum Thema “Vögel und Ameisen”. Ornithol Monatsber. 1936;44:129–135. [Google Scholar]
- Ali S. Do birds employ ants to rid themselves of ectoparasites? Bombay Nat Hist Soc. 1936;38:628–631. [Google Scholar]
- Brackbill H. Anting by four species of birds. Auk. 1948;65:66–67. [Google Scholar]
- Chisholm AH. The problem of anting. Ibis. 1944;86:389–405. [Google Scholar]
- Chisholm AH. The history of anting. Emu. 1959;59:101–130. [Google Scholar]
- Clunie F. Jungle mynah “anting” with millipede. Notornis. 1976;23:77. [Google Scholar]
- Dumbacher JP, Beehler BM, Spande TF, Garraffo HM, Daly JW. Homobatrachotoxin in the genus Pitohui: Chemical defense in birds? Science. 1992;258:799–800. doi: 10.1126/science.1439786. [DOI] [PubMed] [Google Scholar]
- Dumbacher JP. The evolution of toxicity in Pitohuis: effects of homobatrachotoxin on chewing lice (Order Phthiraptera) Auk. 1999;116:957–963. [Google Scholar]
- Eisener T. A comparative morphological study of the proventriculus of ants (Hymenoptera: Formicidae) Bull Mus Comp Zool Harvard. 1957;116:439–490. [Google Scholar]
- Eisner T, Happ GM. The infrabuccal pocket of a formicine ant: a social filtration device. Psyche. 1962;69:107–116. [Google Scholar]
- Eisner T, Baldwin IT, Conner J. Circumvention of prey defense by a predator: ant lion vs. ant. Proc Nat Acad Sci USA. 1993;90:6716–6720. doi: 10.1073/pnas.90.14.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner T, Eisner M, Aneshansley DJ. Pre-ingestive treatment of bombardier beetles by jays: food preparation by “anting” and “sand-wiping”. Chemoecol. 2005;15:227–233. [Google Scholar]
- Ehrlich PR, Dobkin DS, Wheye D. The adaptive significance of anting. Auk. 1986;103:835. [Google Scholar]
- Groskin H. Additional observations and comments on anting in birds. Auk. 1950;67:201–209. [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Harvard Univ Press; USA, MA-Cambridge: 1990. [Google Scholar]
- Ivor HR. Further studies of anting in birds. Auk. 1943;60:51–55. [Google Scholar]
- Judson OP, Bennet TD. Anting as food peparation: formic acid is worse on an empty stomach. Behav Ecol Sociobiol. 1992;31:437–439. [Google Scholar]
- McAtee WL. Anting by birds. Auk. 1938;55:98–105. [Google Scholar]
- Monro A, Chadha M, Meinwald J, Eisner T. Defense mechanisms of arthropods. VI. Para-benzoquinones in the secretions of five species of millipedes. Ann Ent Soc Amer. 1962;55:261–262. doi: 10.1093/aesa/58.2.247. [DOI] [PubMed] [Google Scholar]
- Nice MM. Cowbirds anting. Auk. 1945;62:302–303. [Google Scholar]
- Peschke K, Eisner T. Defensive secretion of the tenebrionid beetle, Blaps mucronata: physical and chemical determinants of effectiveness. J Comp Physiol. 1987;161:377–388. doi: 10.1007/BF00603963. [DOI] [PubMed] [Google Scholar]
- Potter EF. Anting in wild birds, its frequency and probable purpose. Auk. 1970;87:692–713. [Google Scholar]
- Poulsen H. Experiments on anting by birds. Acta XI Congr Intern Orn. 1956;1954:608–610. [Google Scholar]
- Querengässer Über das Einemsen von Singvögeln und die Reifung dieses Verhaltens. J Ornithol. 1973;114:96–117. [Google Scholar]
- Revis HC, Waller DA. Bacterial and fungicidal activity of ant chemicals on feather parasites: An evaluation of anting behavior as a method of self-medication in songbirds. Auk. 2004;121:1262–1268. [Google Scholar]
- Sauer F. Ein Beitrag zur Frage des “Einemsens” von Vögeln. J Ornithol. 1957;98:313–317. [Google Scholar]
- Schildknecht H, Weiss KH. VI. Mitteilung über Insektenabwehrstoffe. Zur Kenntniss des Pygidialdrüsensekretes vom gemeinen Ohrwurm, Forficula auricularia. Zeitschr Naturforsch. 1960;15:755–757. [Google Scholar]
- Simmons KEL. A review of the anting behavior of passerine birds. British Birds. 1957;50:401–424. [Google Scholar]
- Simmons KEL. Anting and the problem of self-stimulation. J Zool London. 1966;149:145–162. [Google Scholar]
- Simmons KEL. Anting. In: Campbell B, Lack E, editors. A Dictionary of Birds. Buteo Books; USA, SD-Vermillion: 1985. p. 19. [Google Scholar]
- Staebler AE. A Robin anting. Wilson Bull. 1942;54:214–215. [Google Scholar]
- Stumper R. Die Giftsekretion der Ameisen. Naturwissenschaften. 1960;47:457–463. [Google Scholar]
- Weisbrod AR. Grooming behaviors of the blue jay. Living bird. 1971;10:271–284. [Google Scholar]
- Whitaker LM. A resume of anting, with particular reference to a captive orchard oriole. Wilson Bull. 1957;69:195–262. [Google Scholar]
- Whyte IJ. Anting in Blue-eared glossy starlings. Ostrich. 1981;52:185. [Google Scholar]
- Wilson EO, Eisner T. Quantitative studies of liquid food transmission in ants. Insectes Sociaux. 1957;4:157–166. [Google Scholar]