Abstract
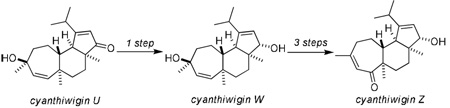
The conversion of cyanthiwigin U to cyanthiwigins W and Z is described.
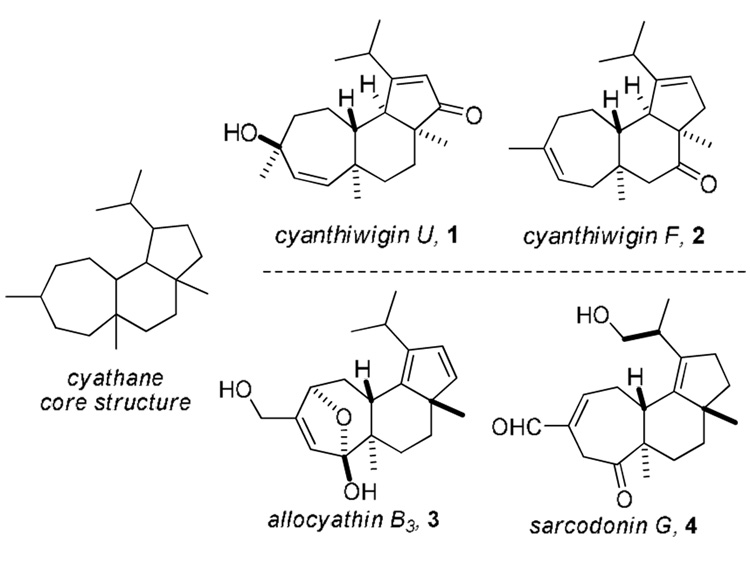
In 1992, two research groups independently described the isolation and structure elucidation of the first examples of the cyanthiwigins from two species of sea sponge.1,2 Their structural features clearly placed them in the cyathane class of diterpenoids although they could be differentiated from the majority by the syn-orientation of the angular methyl groups (Figure 1, cyanthiwigin U, 1 c.f. allocyathin B3).3
Figure 1.
Representative examples of cyathane diterpenes.
Members of the cyanthiwigin family, which has now grown to ~30 congeners4,5,6 have been reported to have noteworthy biological activities such as action against hepatitis B virus, human immunodeficiency virus, and Mycobacterium tuberculosis as well as anti-cancer properties. In light of their biological activities and low natural abundance, the cyanthiwigins are important targets for synthesis and to date total syntheses have been reported for (−)-cyanthiwigin U, 17 (+)-cyanthiwigin AC,8 and cyanthiwigin F, 2.9 In this Letter we report the total syntheses of cyanthiwigins W and Z.
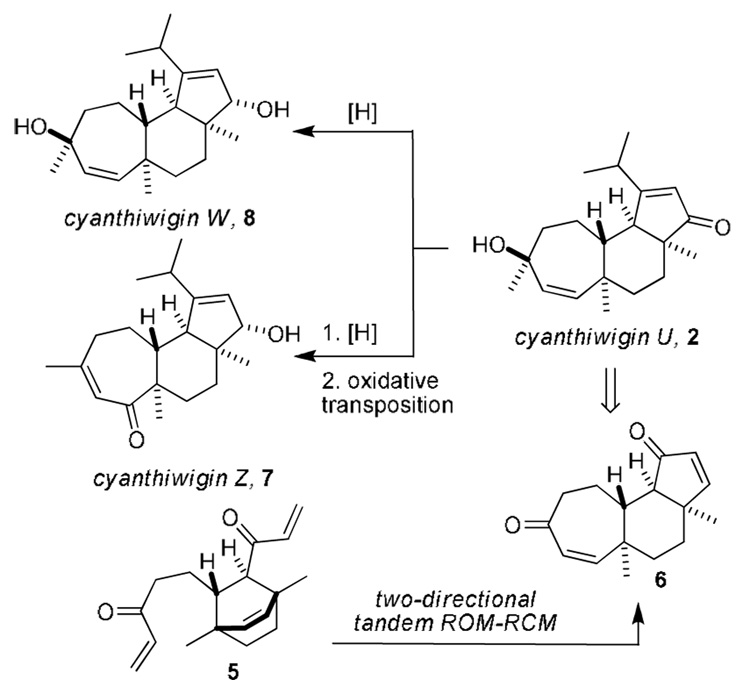
Our strategy for the synthesis of cyanthiwigin W and cyanthiwigin Z is based the same two-directional tandem ROM-RCM that we have previously described for the synthesis of cyanthiwigin U (5→6, Figure 2, details of the cyanthiwigin U synthesis have been reported previously7). With ready access to cyanthiwigin U, we expected that a diastereoselective 1,2-reduction of the cyclopentenone would lead to cyanthiwigin W, and the combination of a diastereoselective reduction and oxidative transposition of the tertiary allylic alcohol would provide cyanthiwigin Z.
Figure 2.
Overview of the plans for the synthesis of cyanthiwigin W and cyanthiwigin Z.
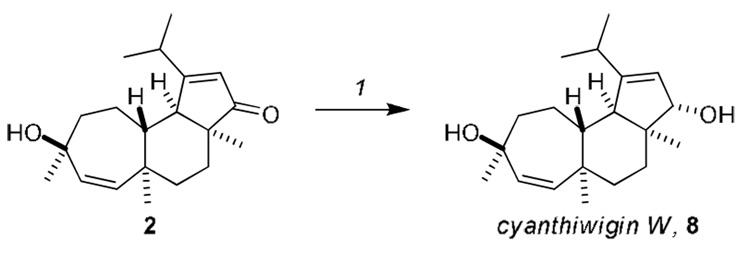
To our delight, subjecting cyanthiwigin U to standard Luche reduction10 conditions led to hydride delivery from the less hindered (albeit slightly concave) β-face in high yield to furnish cyanthiwigin W and 1-epi-cyanthiwigin W (d.r. = 9:1, Scheme 1). The epimers were readily separated on silica gel, and the cyanthiwigin W obtained by this route provided data that was in accord with that reported by Hamann and co-workers.4,11
Scheme 1.
Reagents and conditions: 1. NaBH4, CeCl3.7H2O, MeOH, 95%, d.r. = 9:1.
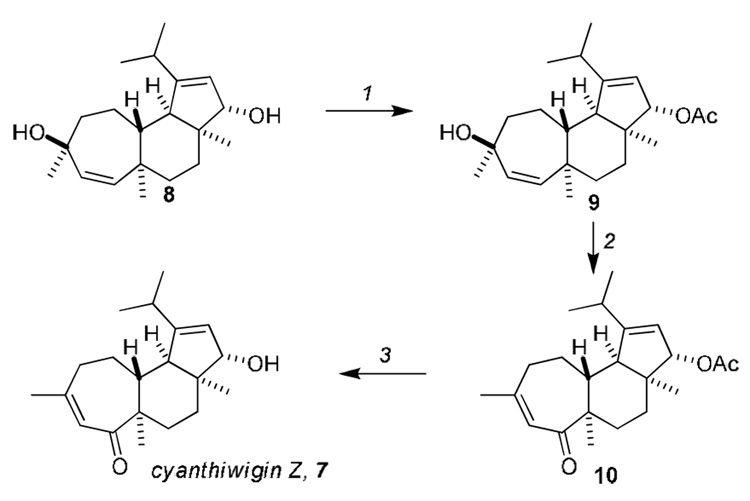
The conversion of cyanthiwigin W to cyanthiwigin Z commenced with selective acetylation of the secondary allylic alcohol with Ac2O/DMAP (8→9, Scheme 2). Subsequent Dauben oxidative transposition12 of the tertiary allylic alcohol with PCC led to enone 10, and this was followed by removal of the acetate with K2CO3 in MeOH to yield cyanthiwigin Z13 in 20% overall yield from cyanthiwigin W.
Scheme 2.
Reagents and conditions: 1.Ac2O, DMAP, CH2Cl2, 0 °C; 2. PCC, CH2Cl2, rt, 14h. 3. K2CO3, MeOH, rt, 3h, 20% (over 3 steps).
In conclusion, we have described the concise conversion of cyanthiwigin U to cyanthiwigins W and Z. Given the ready access to the core structures of the cyanthiwigins by either our route, or the Stoltz group’s strategy,9 these transformations provide an early indication of the encouraging prospects for the ready preparation of a variety of natural and unnatural cyanthiwigins in advance of biological studies.
Acknowledgments
Support for this research was provided by the National Cancer Institute (NCI CA110246). This work was facilitated by NMR facilities purchased partly with funds from an NSF Shared Instrumentation Grant (CHE-0131003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sennett SH, Pomponi SA, Wright AE. J. Nat. Prod. 1992;55:1421. doi: 10.1021/np50088a006. [DOI] [PubMed] [Google Scholar]
- 2.Green D, Goldberg I, Stein Z, Ilan M, Kashman Y. Nat. Prod. Lett. 1992;1:193. [Google Scholar]
- 3.For a review of synthetic studies on the cyathanes, see: Wright DL, Whitehead CR. Org. Prep. Proc. Int. 2000;32:307.
- 4.Peng J, Walsh K, Weedman V, Bergthold JD, Lynch J, Lieu KL, Braude IA, Kelly M, Hamann MT. Tetrahedron. 2002;58:7809. [Google Scholar]
- 5.Peng J, Avery MA, Hamann MT. Org. Lett. 2003;5:4575. doi: 10.1021/ol035592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng J, Kasanah N, Stanley CE, Chadwick J, Fronczek FR, Hamann MT. J. Nat. Prod. 2006;69:727. doi: 10.1021/np050197e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeiffer MWB, Phillips AJ. J. Am. Chem. Soc. 2005;127:5334. doi: 10.1021/ja0509836. [DOI] [PubMed] [Google Scholar]
- 8.Reddy TJ, Bordeau G, Trimble L. Org. Lett. 2006;8:5585. doi: 10.1021/ol062304h. [DOI] [PubMed] [Google Scholar]
- 9.Enquist JA, Jr, Stoltz BM. Nature. 2008;453:1228. doi: 10.1038/nature07046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemal AL, Luche J-L. J. Am. Chem. Soc. 1981;103:5454. [Google Scholar]
-
11.Cyanthiwigin W: [α]D +89 (c 0.05, MeOH); Lit. +97 (c 0.08, MeOH). Comparison of 1H and 13C NMR data:
Natural Synthetic§ 1H δ (mult, J) 13C δ 1H δ (mult, J) 13C δ 1β 4.73 (s) 77.9 4.73 (s) 77.9 2 5.27 (s) 126.5 5.27 (s) 126.6 3 157.6 157.6 4α 1.94 (d, 8.4) 55.9 1.94 (d, 10.0) 55.9 5β 1.48 (m) 50.5 1.47–1.49 (m) 50.5 6 39.6 39.6 7α 1.32 (m) 38.9 38.9 7β 1.62 (m) 1.59–1.73 (m) 8α 1.35 (m) 28.3 1.31–1.40 (m) 28.4 8β 1.68 (m) 1.59–1.73 (m) 9 48.6 48.6 10α 1.48 (m) 26.9 1.47–1.49 (m) 27.0 10β 1.86 (m) 1.82–1.91 (m) 11α 1.59 (m) 42.5 1.59–1.73 (m) 42.6 11β 1.83 (m) 1.82–1.91 (m) 12 72.0 72.0 13 5.38 (d, 12.8) 136.8 5.38 (d, 12.4) 136.8 14 5.12 (d, 12.8) 140.4 5.11 (d, 12.7) 140.4 15 1.23 (3H, s) 30.4 1.23 (3H, s) 30.4 16 0.95 (3H, s) 18.0 0.95 (3H, s) 18.1 17 0.85 (3H, s) 24.1 0.86 (3H, s) 24.1 18 2.46 (m) 30.8 2.43–2.50 (m) 30.8 19 1.10 (3H, d, 6.8) 21.3 1.10 (3H, d, 6.6) 21.3 20 1.04 (3H, d, 6.8) 22.8 1.04 (3H, d, 6.9) 22.8 §Definitive assignments were made by a combination of HSQC and HMBC experiments.
- 12.Dauben WG, Michno DM. J. Org. Chem. 1977;42:682. [Google Scholar]
-
13.Cyanthiwigin W: [α]D −151 (c 0.01, MeOH); Lit. −160 (c 0.03, MeOH). Comparison of 1H and 13C NMR data:
Natural Synthetic§ 1H δ (mult, J) 13C δ 1H δ (mult, J) 13C δ 1β 4.71 (s) 77.7 4.71 (s) 77.6 2 5.29 (s) 126.2 5.29 (s) 126.6 3 156.7 156.7 4 2.12 (d, 10.8) 53.6 2.13 (d, 10.2) 53.5 5 1.57 (m) 52.4 1.53–1.63 (m) 52.4 6 34.2 34.2 7α 1.42 (m) 46.6 1.40–1.48 (m) 46.6 7β 1.60 (m) 1.53–1.63 (m) 8α 1.47 (m) 28.0 1.40–1.48 (m) 28.1 8β 1.74 (m) 1.74–1.76 (m) 9 48.1 48.1 10α 1.57 (m) 27.9 1.53–1.63 (m) 28.0 10β 1.77 (m) 1.76–1.79 (m) 11α 2.15 (m) 37.8 2.15–2.19 (m) 37.8 11β 2.33 (m) 2.32–2.34 (m) 12 152.5 152.5 13 5.71 (s) 127.2 5.71 (s) 127.2 14α 208.8 208.4 14β 15 1.83 (3H, s) 25.7 1.84 (3H, s) 25.6 16 1.06 (3H, s) 15.3 1.07 (3H, s) 15.4 17 0.88 (3H, s) 23.7 0.88 (3H, s) 23.7 18 2.43 (m) 30.0 2.40–2.45 (m) 30.0 19 1.07 (3H, d, 6.8) 21.0 1.07 (3H, d, 6.9) 21.0 20 1.23 (3H, d, 6.8) 21.3 1.13 (3H, d, 6.6) 21.3 §Definitive assignments were made by a combination of HSQC and HMBC experiments.