Abstract
Heparin, a widely used anticoagulant, is being rapidly displaced by low-molecular-weight heparins. Recently, certain lots of heparin have been associated with anaphylactoid-type reactions resulting from contamination with oversulfated chondroitin sulfate. This impurity has also contaminated low-molecular-weight heparins obtained by chemical and enzymatic depolymerization of heparin. The sensitivity of oversulfated chondroitin sulfate to five different depolymerization processes similar to ones used in preparing low-molecular-weight heparins is reported.
Heparin is a polydisperse mixture of linear acidic polysaccharides. An anticoagulant, heparin has been in clinical use over the past 75 years.1-3 Heparin is unique as one of the oldest drugs currently still in widespread clinical use, one of the first biopolymeric drugs, and one of the few carbohydrate drugs.3 Heparin is isolated by extraction from animal tissues, most commonly porcine intestines, and is a member of the glycosaminoglycan (GAG)a family. Low-molecular-weight heparins (LMWHs), prepared by the controlled partial depolymerization of heparin, have been introduced as anticoagulant/antithrombotic agents over the past 25 years.4 The introduction of LMWHs primarily resulted from an improved understanding of the molecular basis of the biochemistry associated with the coagulation cascade.5-7 LMWH is an agent that is more specific than heparin (which acts at many points in the cascade), and it was thought that it might provide more subtle regulation of coagulation and reduce the major hemorrhagic side effects associated with heparin. The success of LMWHs has been primarily ascribed to their enhanced subcutaneous bioavailability and improved pharmacokinetics.8 LMWHs are produced by the degradation of heparin by enzymatic9-12 and chemical β-elimination13-15 and oxidation.10,16-18 LMWHs can be analyzed by size-exclusion chromatography to determine molecular weight and polydispersity.19,20 Polyacrylamide gel and capillary electrophoresis and nuclear magnetic resonance (NMR) spectroscopy have also been used to analyze LMWHs.21-23
Recently, a new, rapid onset, acute side effect associated with heparin was observed, believed to be caused by an anaphylactoid response.23,24 This spike in adverse events was associated with an oversulfated chondroitin sulfate (OSCS) contaminant in certain lots of heparin.25 OSCS had been previously prepared from chondroitin sulfate (CS), another member of the GAG family having similar backbone structure with heparin, by chemical sulfonation, and was shown to have anticoagulant activity.26 Initial reports have suggested that this OSCS contaminant was also present in LMWHs.27
In this paper, five literature methods similar to those used to prepare LMWHs, Dalteparin sodium (nitrous acid),10 Tinzaparin sodium (heparin lyase),9,11,12 Centaxarin sodium (periodate-oxidation),17,18 Enoxaparin (Clexane) sodium (alkaline treatment),14 and Ardeparin sodium (H2O2)16 were used to degrade heparin, OSCS, and a mixture of heparin and OSCS. Polyacrylamide gel electrophoresis (PAGE) was used to detect the sensitivity of heparin and OSCS to each preparation method. This study should provide useful information for the control of the manufacture of LMWH and monitoring the purity of LMWHs.
OSCS was prepared from CS according to a modified procedure from Maruyama et al.26 (Supporting Information). Heparin, OSCS, and the mixture of them were degraded following the protocols used for LMWH preparation (Supporting Information).
1H and 13C NMR spectra were recorded for synthetic OSCS (Figure 1A,B). The spectra of synthetic OSCS matched the contaminant.25,26 In addition, COSY (not shown) and HMQC (Figure 1C) spectra were recorded for the sodium salt form of OSCS, and the proton and carbon signals in the repeating disaccharide of OSCS were assigned (Supporting Information Table S1).
Figure 1.
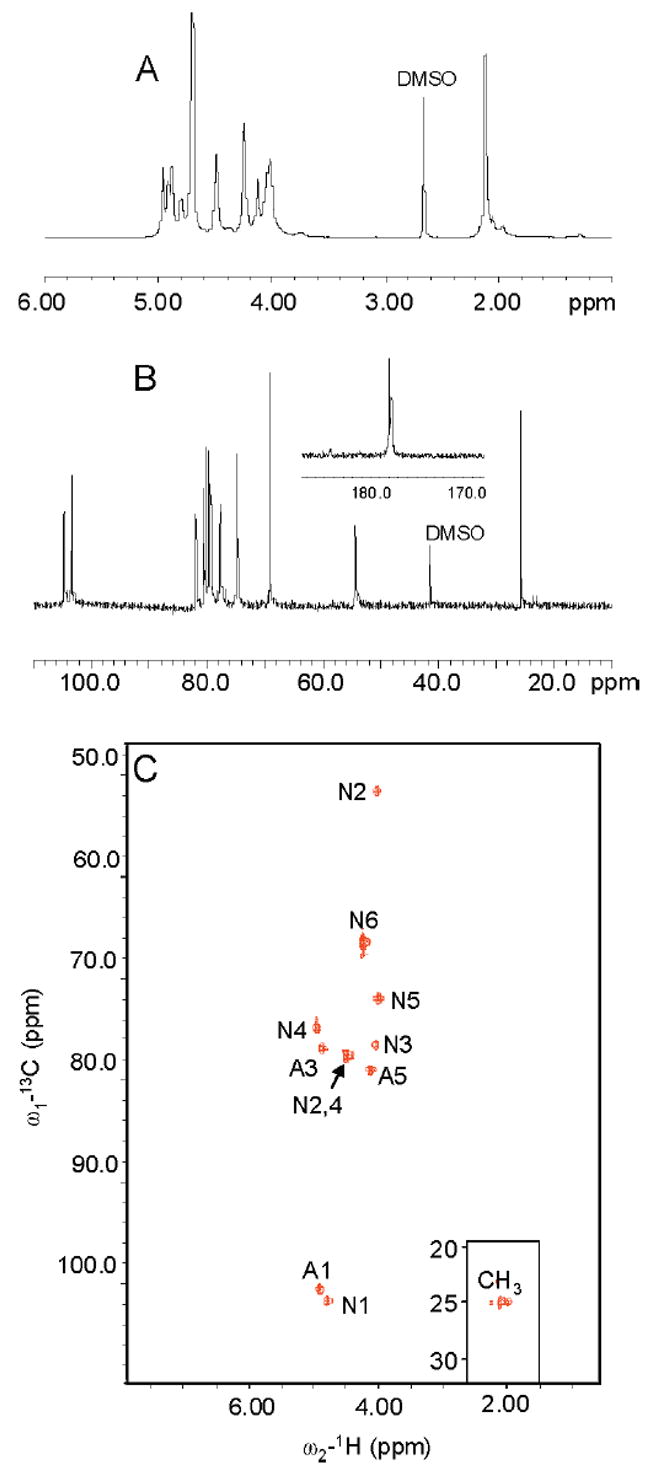
NMR spectra of OSCS. (A) 1H NMR spectrum of OSCS; (B) 13C NMR spectrum of OSCS; (C) HMQC spectrum of OSCS. N, GalNAc residues; A, GlcA residues.
PAGE analysis of heparin and OSCS samples, treated with nitrous acid, are presented in Figure 2A. Heparin was sensitive to nitrous acid (lane 3 to 4), while OSCS was not degraded (lane 5 to 6), and the 1:1 mixture of heparin and OSCS was partially degraded (lane 7 to 8). No difference in the 1H NMR spectra of OSCS sample, taken before and after nitrous acid treatment, was observed (Supporting Information Figure S1B). These results clearly demonstrate the reactivity of nitrous acid depolymerization toward heparin due to the presence of N-sulfo groups in heparin but the lack of reactivity toward OSCS because the glucosamine residues are substituted by N-acetyl groups.28
Figure 2.
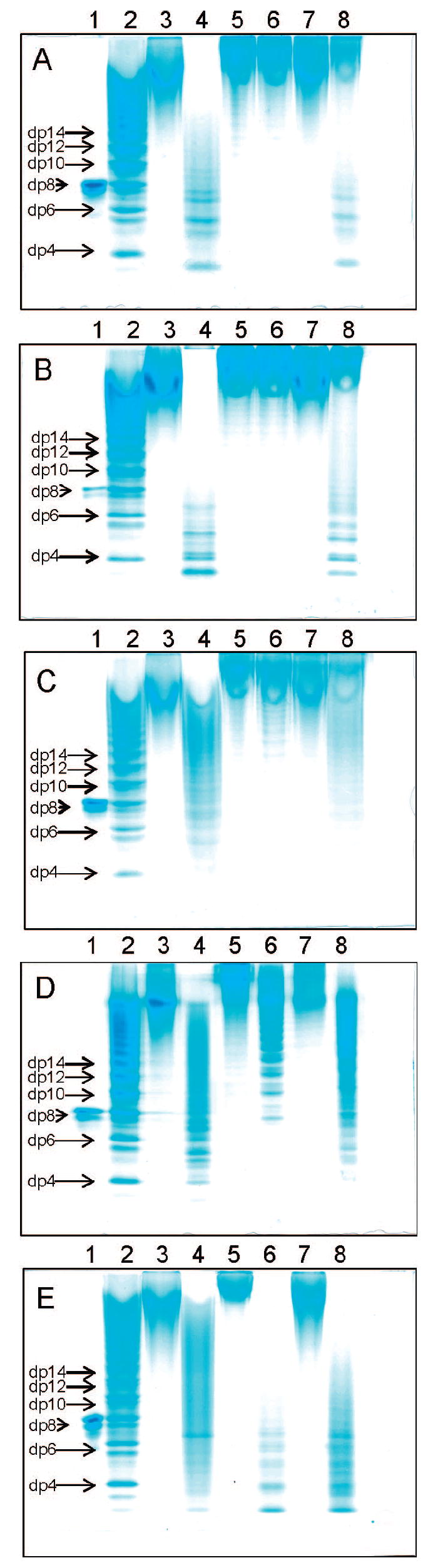
PAGE analysis of heparin, OSCS, LMWHs, and degraded OSCS. (A) PAGE analysis of samples from nitrous acid degradation. (B) PAGE analysis of samples from enzymatic degradation. (C) PAGE analysis of samples from periodate-oxidation. (D) PAGE analysis of samples from alkaline treatment. (E) PAGE analysis of samples from hydrogen peroxide degradation, in which 1–8 are: 1, octasaccharide, derived from heparin; 2, heparin oligosaccharide standards;22 3, heparin; 4, depolymerized heparin; 5, OSCS; 6, depolymerized OSCS, 7, 1:1 mixture of heparin: OSCS; 8, depolymerized 1:1 mixture of heparin:OSCS.
PAGE analysis of heparin, OSCS, and treated with heparin lyase I are presented in Figure 2B. Heparin was sensitive to heparin lyase I (lanes 3 to 4), while OSCS was resistant to heparin lyase I (lane 5 to 6). The 1:1 mixture of heparin and OSCS showed somewhat lower sensitivity than expected, consistent with the partial inhibition of heparin lyase I by OSCS.29 No degradation of OSCS was observed by 1H NMR spectroscopy following heparin lyase I treatment (Supporting Information Figure S1C).
PAGE analysis of heparin and OSCS following periodate treatment are presented in Figure 2C. Heparin was periodate sensitive (lanes 3 to 4), while OSCS was insensitive to periodate treatment. Periodate reacts with vicinal diols,17,18 such as C-2 and C-3 in iduronic or glucuronic acid residues. This degradation cannot be observed in OSCS because all of the hydroxyl groups in its glucuronic acid residues are substituted with sulfo groups. No degradation was observed on periodate-oxidation treatment by 1H NMR spectroscopy (Supporting Information Figure S1D).
PAGE analysis of heparin, OSCS, and samples subjected to alkaline treatment (base catalyzed β-eliminative cleavage of their benzyl esters) are presented in Figure 2D. Heparin was sensitive (lane 3 to 4) as was OSCS (lane 5 to 6), although the degree of depolymerization was lower for OSCS than for heparin. When a 1:1 mixture of heparin:OSCS was subjected to alkaline treatment (lane 7 to 8), continuous banding extended through the entire gel, reflecting a mixture of LMWH and β-eliminated OSCS. Base catalyzed β-elimination is a process that is favored when the acidic proton and the leaving group are located in a trans configuration. The iduronic acid residues in heparin possess a 4,5-trans configuration and are sensitive to β-elimination under basic conditions and afford a ΔUA residue at the nonreducing end of the product formed.13-15 ΔUA residues are observed in OSCS following base catalyzed β-elimination. The ultraviolet absorbance of a 4 mg/mL sample at 232 nm increased from 0.300 to 0.627 AU after base catalyzed β-elimination. The H-4 signal of the ΔUA residue in the nonreducing end of the resulting OSCS product is clearly present at 5.9 ppm in the 1H NMR spectrum (Supporting Information Figure S2). The ratio of the signals assigned to ΔUA H-4, glucuronic acid H-1, and N-acetyl CH3 group is 1:16.5:50 (Supporting Information Figure S3). On the basis of these integrations, a chain length of 16.5 disaccharide units can be calculated, corresponding to a reduction of the average molecular weight of OSCS to 12 kDa. The average molecular weight of untreated OSCS and alkaline treated OSCS were confirmed by gel permeation chromatography (GPC) as 18 and 13 kDa, respectively. The formation of product, containing ΔUA residues, suggests that the glucuronic acid residues in OSCS adopt a 4,5-trans-like orientation, different from the 4,5-cis configuration commonly observed in chondroitin sulfate. Thus, base catalyzed β-elimination can degrade OSCS, albeit to a much lower extent than base catalyzed β-elimination degrades heparin in the production of a LMWH. The complex mixture, obtained on the base eliminative cleavage of contaminated heparin, might make it more difficult to analyze contaminated LMWHs produced by this method.
PAGE analysis of heparin and OSCS, depolymerized using H2O2 oxidation, are presented in Figure 2E. Heparin was sensitive to H2O2 oxidation (lane 3 to 4) as was OSCS (lane 5 to 6). The H2O2 oxidative depolymerization of OSCS was more extensive than heparin. When a 1:1 mixture of heparin: OSCS was degraded (lane 7 to 8), continuous banding extended through the entire gel reflecting a mixture of LMWH and degraded OSCS. The NMR spectrum of H2O2 treated OSCS shows no assignable H-1 signals, clearly demonstrating the oxidative cleavage of most of the glycosidic linkages. The signal for the acetyl groups also shifted upfield due to the loss of sulfo groups. To further confirm these changes, the OSCS degraded by H2O2-oxidation was analyzed by LC-MS (Supporting Information Figures S4 and S5). At pH 2.0, this treatment completely converted OSCS to monosaccharide and smaller products. At pH 5.0 and 7.0, higher oligosaccharides were observed with the reducing-end as ring-opened, disulfated uronic acid or galactosamine residues having their C1 oxidized to a carboxyl group. The complex mixture produced by H2O2 treatment of OSCS may make it difficult to analyze contaminated LMWHs produced by this method.
Nitrous acid treatment, using a method similar to that used to prepare Dalteparin, failed to depolymerize OSCS. Heparin lyase I treatment, similar to the method used to prepare Tinzaparin, failed to depolymerize OSCS. Periodate-oxidation, a method similar to that used to prepare Centaxarin, also failed to depolymerize OSCS. Thus, LMWHs prepared from a contaminated batch of heparin using these processes would result in a product containing intact, resistant high-molecular-weight OSCS contaminant, which can be easily identified. In contrast, using a base catalyzed β-eliminative process, similar to the process used to prepare Enoxaparin, affords a complex mixture of LMWH and an OSCS having a reduced molecular weight that might mask the presence of contaminant in certain assays. Finally, H2O2 treatment, using a process similar to that used to prepare Ardeparin, more efficiently depolymerizes OSCS than heparin and affords a complex mixture consisting of LMWH and depolymerized OSCS, which would also be difficult to detect contaminated batches. On the basis of the PAGE analysis, H2O2 treated OSCS shows more complete depolymerization and base catalyzed β-eliminative OSCS shows a more subtle decrease in molecular weight than the corresponding LMWH, each posing unique problems for the size-based analysis and potentially for size-based purification of a contaminated products.
Acknowledgments
This work was supported by the National Institutes of Health HL62244 and GM38060.
Footnotes
Abbreviations: GAG, glycosaminoglycan; LMWH, low-molecularweight heparin; OSCS, oversulfated chondroitin sulfate; CS, chondroitin sulfate; COSY, correlation spectroscopy; HMQC, heteronuclear multiple quantum coherence; ΔUA, 4-deoxy-α-l-threo-hex-4-enopyranosyluronic acid; AU, absorbance units; GPC, gel permeation chromatography; LC-MS, liquid chromatography–mass spectrometry; dp, degree polymerization.
Supporting Information Available: Experimental procedures and analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Capila I, Linhardt RJ. Heparin–protein interactions. Angew Chem Int Ed. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Linhardt RJ. Heparin: an important drug enters its seventh decade. Chem Ind. 1991;2:45–50. [Google Scholar]
- 3.Linhardt RJ, Toida T. Carbohydrates in drug design. Marcel Dekker; New York: 1997. pp. 277–341. [Google Scholar]
- 4.Barrowcliffe TW. Low molecular weight heparin(s) Br J Hamaetol. 1995;90:1–7. doi: 10.1111/j.1365-2141.1995.tb03373.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl U, Lidholt K, Spillmann D, Kjellen L. More to “heparin” than anticoagulation. Thromb Res. 1994;75:1–32. doi: 10.1016/0049-3848(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg RD. Biochemistry and pharmacology of low molecular weight heparin. Semin Hematol. 1997;34:2–8. [PubMed] [Google Scholar]
- 7.Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490–6505. [PubMed] [Google Scholar]
- 8.Linhardt RJ. 2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem. 2003;46:2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 9.Langer RS, Linhardt RJ. Heparinase derived anticoagulants. 4,396,762. US Patent. 1981
- 10.Lormeau J-C, Maine Ml, Choay J, Petitou M. Oligosaccharides having anti-Xa activity and pharmaceutical compositions containing them. 4,401,662. US Patent. 1980
- 11.Nielsen JI. Process of using light absorption to control enzymatic depolymerization of heparin to produce low molecular weight heparin. 5,106,734. US Patent. 1987
- 12.Lormeau J-C, Petitou M, Choay J. Method for obtaining biologically active mucopolysaccharides of high purity, by controlled depolymerization of heparin. 5,019,649. US Patent. 1987
- 13.Lopes LL. Depolymerization method of heparin. 4,981,955. US Patent. 1990
- 14.Uzan A. Sulfated polysaccharides obtained from heparin, preparation process, pharmaceutical composition and use thereof. 5,849,721. US Patent. 1995
- 15.Piani SB, Tamagnone GCdR, Alpino RRB, Milani MRTdR, Fantuz MB. Heparin derivatives and process fro their preparation. 5,010,063. US Patent. 1989
- 16.Fussi F. Process for obtaining low molecular weight heparins endowed with elevated pharmacological properties and product so obtained. 4,281,108. US Patent. 1981
- 17.Cifonelli JA, King JA. Structural characteristics of heparan sulfates with varying sulfate contents. Biochemistry. 1977;16:2137–2141. doi: 10.1021/bi00629a014. [DOI] [PubMed] [Google Scholar]
- 18.Islam T, Butler M, Sikkander SA, Toida T, Linhardt RJ. Further evidence that periodate cleavage of heparin occurs primarily through the antithrombin binding site. Carbohydr Res. 2002;337:2239–2243. doi: 10.1016/s0008-6215(02)00229-x. [DOI] [PubMed] [Google Scholar]
- 19.Ahsan A, Jeske W, Hoppensteadt D, Lormeau JC, Wolf H, et al. Molecular profiling and weight determination of heparins and depolymerized heparins. J Pharm Sci. 1995;84:724–727. doi: 10.1002/jps.2600840612. [DOI] [PubMed] [Google Scholar]
- 20.Mulloy B, Gee C, Wheeler SF, Wait R, Gray E, et al. Molecular weight measurements of low molecular weight heparins by gel permeation chromatography. Thromb Haemostasis. 1997;77:668–674. [PubMed] [Google Scholar]
- 21.Desai UR, Wang H, Ampofo SA, Linhardt RJ. Oligosaccharide composition of heparin and low-molecular-weight heparins by capillary electrophoresis. Anal Biochem. 1993;213:120–127. doi: 10.1006/abio.1993.1394. [DOI] [PubMed] [Google Scholar]
- 22.Edens RE, al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, et al. Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 23.Linhardt RJ, Loganathan D, al-Hakim A, Wang HM, Walenga JM, et al. Oligosaccharide mapping of low molecular weight heparins: structure and activity differences. J Med Chem. 1990;33:1639–1645. doi: 10.1021/jm00168a017. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, et al. Contaminated Heparin Associated with Adverse Clinical Events and Activation of the Contact System. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruyama T, Toida T, Imanari T, Yu G, Linhardt RJ. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydr Res. 1998;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 27.Greb E. EMEA Report Assesses Contaminated-Heparin Risk. Electronic Newsletter of Pharmaceutical Technology. Jun 12, 2008. p. 523176. http://pharmtech.findpharma.com/pharmtech/Online+Only/EMEA-Report-Assesses-Contaminated-Heparin-Risk/ArticleStandard/Article/detail/
- 28.Bienkowski MJ, Conrad HE. Structural characterization of the oligosaccharides formed by depolymerization of heparin with nitrous acid. J Biol Chem. 1985;260:356–365. [PubMed] [Google Scholar]
- 29.Linhardt RJ, Fitzgerald GL, Cooney CL, Langer R. Mode of action of heparin lyase on heparin. Biochim Biophys Acta. 1982;702:197–203. doi: 10.1016/0167-4838(82)90503-9. [DOI] [PubMed] [Google Scholar]


