Abstract
Background
The calcium-dependent proline-rich tyrosine kinase (Pyk2), a nonreceptor protein activated by tyrosine phosphorylation, links G protein-coupled receptors to vascular responses. We tested the hypothesis that enhanced vascular reactivity in DOCA-salt hypertensive mice are due to increased activation of Pyk2.
Methods and results
Aorta and small mesenteric arteries from DOCA-salt and uninephrectomized (UNI) male C57Bl/6 mice were used. Systolic blood pressure (mmHg) was higher in DOCA (126±3) vs. UNI (100±4) mice. Vascular responses to phenylephrine (1nM to 100μM) were greater both in aorta and small mesenteric arteries from DOCA-salt than UNI, but treatment with Tyrphostin A-9 (0.1μM, Pyk2 inhibitor) abolished the difference among the groups. Pyk2 levels, as well as phospho-Pyk2Tyr402, paxillin and phospho-paxillinTyr118 were increased in DOCA-salt aorta. Incubation of vessels with Tyrphostin A-9 restored phosphorylation of Pyk2 and paxillin.
Conclusion
Increased activation of Pyk2 contributes to increased vascular contractile-responses in DOCA-salt mice.
Keywords: Hypertension, paxillin, Pyk2 and DOCA-salt
INTRODUCTION
Proline-rich tyrosine kinase (Pyk2), also called cell adhesion kinase-β (CAKβ), calcium-dependent tyrosine kinase (CADTK) or related adhesion focal tyrosine kinase (RAFTK), is a member of a family of nonreceptor protein tyrosine kinases that associate with focal adhesions and are activated by a variety of extracellular stimuli (1, 2).
Activation of Pyk2 occurs in response to a variety of soluble and cell type-dependent stimuli, including hormones, growth factors and cytokines (3–5). Many of these stimuli, including noradrenaline (NA), angiotensin II (Ang-II) and endothelin-1 (ET-1), are able to induce a rapid and prolonged activation of Pyk2, via G protein-coupled receptor [GPCR (6, 7)].
Pyk2 phosphorylation occurs at multiple sites that are structurally and functionally distinct, and these events are thought to be tightly coupled in a sequential manner. In particular, tyrosine-402 (Tyr-402) has been identified as the key site for Pyk2 autophosphorylation and activation (8), with other tyrosine sites contributing to subsequent transphosphorylation and enhanced kinase activity (9, 10).
Pyk2 binds to proteins that interact with the cytoskeleton, such as paxillin (11) and Rho-GAP protein Graf (12), suggesting a role in regulation of the cytoskeleton, cellular morphology and function.
Recent studies have suggested that nonreceptor protein tyrosine kinases are involved in signaling cascades, such as extracellular regulated mitogen-activated protein (ERK1/2 MAP) kinase and phosphatidylinositol 3-kinase/Akt pathways, that regulate protein synthesis and cell function in vascular smooth muscle cells [VSMC (13–17)]. Furthermore, Ohtsu and colleagues (2005) showed that PKC/Pyk2 -dependent Rho/ROCK activation through PDZ-RhoGEF mediates Ang II-induced VSMC migration via JNK activation in VSMCs, providing a novel mechanistic role of the Rho/ROCK cascade that is involved in vascular remodeling (18).
Although it has been reported that Pyk2 phosphorylation leads to enhancement of contractile responses in Ang-II hypertensive animals(19, 20), a detailed investigation into the contribution of Pyk2 to other hypertension model has not been carried out.
Deoxycorticosterone acetate (DOCA)-salt hypertension is characterized by increased vascular reactivity to contractile stimuli (21–25) and by exhibiting increased levels of ET-1, or its precursor, prepro-ET-1 (26–28). Several reports showed that ET-1 is able to increase Pyk2 activity (29). Therefore, in the present study, we investigated if augmented agonist-induced contractile responses in vessels from DOCA-salt mice are due to increased activation of Pyk2.
MATERIAL AND METHODS
Animals
All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education. The animals were housed four per cage on 12-h light/dark cycle and fed a standard chow diet with water ad libitum.
Male C57BL/6 mice (10 weeks, The Jackson Laboratory, Maine, US) were used in this study. All mice were uninephrectomized under anesthesia with a mixture of ketamine (80 mg.kg−1) and xylazine (10 mg.kg−1). Uninephrectomized mice were given either no further treatment (UNI mice) or 1% NaCl plus 0.2% KCl in the drinking water and DOCA-salt silastic pellets (1g.kg−1) were implanted subcutaneously at the scapular region (DOCA-salt mice). The duration of treatment was 5 weeks.
Systolic blood pressure was measured in non-anaesthetized animals by tail cuff using a RTBP1001 blood pressure system (Kent Scientific Corporation Connecticut). At the end of 5 weeks of treatment, mice thoracic aorta or mesenteric arteries were submitted to the experimental procedures.
Vascular functional studies
After euthanasia, the mesentery and thoracic aorta were rapidly excised and placed in ice-cold physiological salt solution (PSS). First-order branches of mesenteric artery (≅ 2 mm in length with internal diameter ≅ 100 to 200μm) and thoracic aorta (4 mm in length) were carefully dissected and mounted as ring preparations on two stainless steel wires. The first-order mesenteric arteries were mounted in an isometric Mulvany-Halpern small-vessel myograph (model 610 M; Danish MyoTech, Aarhus, Denmark) whereas the aortic rings were mounted in standard organ chambers for isometric tension recording by a PowerLab 8/SP data acquisition system (ADInstruments Pty Ltd., Castle Hill, Australia). One wire was attached to a force transducer and the other to a micrometer. Both dissection and mounting of the vessels were carried out in cold (4°C) PSS. The segments were adjusted to maintain a passive force of 3.5mN for the first-order mesenteric arteries and 5mN for the aortic rings. Vessels were equilibrated for 60 minutes in PSS at 37°C, and continuously bubbled with 5% CO2 and 95% O2. Arterial integrity was assessed first by stimulation of vessels with 120 mM potassium chloride (KCl) and, after washing and a new stabilization time, by contracting the segments with phenylephrine (PE; 1μM). After wash and new stabilization period, the segments were incubated for 30 minutes with the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME 100μM) plus indomethacin (10μM), an inhibitor of prostanoid synthesis. Concentration-response curves to phenylephrine (PE, 1nM to 100μM) were performed in presence or absence of Tyrphostin A-9 [3,5-bis (1,1-Dimethylethyl)-4-hydroxyphenyl] methylene] propanedinitrile] (0.1μM), a Pyk2 inhibitor, both in aorta and first-order mesenteric arteries. Preliminary experiments were performed (data not shown) in order to establish the dose of Tyrphostin A-9 that would be used in the experiments.
Western Blot Analysis
Proteins (40 μg) extracted from aorta were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline solution with Tween (10%) for 1 hour at 24°C. Membranes were then incubated with antibodies (1:1000) overnight at 4°C. Antibodies were as follow: Pyk2 and phospho-Pyk2(Tyr402) (1:1000; Sigma Chemical Co.); paxillin and phospho-paxillin(Tyr118)+ (1:1000; Cell Signaling Technology Inc.). Immunoblots for nonphosphoproteins Pyk2 and paxillin were carried out in the same membranes used to evaluate their phosphorylated forms. After incubation with secondary antibodies, signals were revealed with chemiluminescence, visualized by autoradiography, and quantified densitometrically. Results are normalized to beta-actin protein and expressed as arbitrary units.
Drugs and Solutions
PSS of the following composition was used: 130 mM NaCl, 14.9 mM NaHCO3, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4·7H2O, 5.5 mM glucose, 1.56 mM CaCl2·2H2O and 0.026mM EDTA, (pH 7.4). Indomethacin, L-NAME and PE were purchased from Sigma Chemical Co. (St. Louis, MO). Tyrphostin A-9 was purchased from Calbiochem, (Gibbstown, NJ). All reagents used were of analytical grade. Stock solutions were prepared in deionized water, DMSO (tyrphostin A-9) and ethanol (indomethacin).
Data Analysis
Results are presented as mean±SEM (standard error of the mean). Contractions were recorded as changes in the displacement (mN) from baseline and are represented as mN for n experiments. Concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 4.0; GraphPad Software Inc., San Diego, CA, USA). Constraining curve-fit parameters were used to fit a sigmoid curve. Agonist potencies and maximum responses are expressed as pD2 (negative logarithm of the molar concentration of agonist producing 50% of the maximum response) and Emax (maximum effect elicited by the agonist), respectively. Statistically significant differences were calculated by one-way analysis of variance (ANOVA) or by Student’s t test. P<0.05 was considered significant.
RESULTS
Systolic blood pressure (mmHg) was higher in DOCA-salt mice (126±3; n=10), when compared with UNI mice (100±4; n=10).
Aorta from DOCA-treated animals displayed increased maximal force development to PE compared to UNI values [25.5±0.2 mN, vs. 15.1±0.1 mN, respectively; (Figure 1A)]. To verify the contribution of Pyk2 to the smooth muscle contraction, vessels were incubated with and without tyrphostin A-9, a Pyk2 inhibitor (Table 1). The difference in maximal force development to PE between the groups was abolished in the presence of tyrphostin A-9 (0.1μM), [10.7 ±1.4 mN DOCA vs. 8.7±0.8 mN UNI; (Figure 1B)]. Similar results were found in small mesenteric arteries. PE-induced contractile responses were greater in arteries from DOCA-treated animals (7.71±0.07 mN) compared to those in UNI [4.73±0.05 mN; (Figure 2A)]. Pyk2 inhibition with tyrphostin A-9 (0.1μM) abolished the differences in PE reactivity between small mesenteric arteries from the normotensive and hypertensive groups [4.49±0.1 mN DOCA vs. 4.08±0.03 mN UNI; (Figure 2B, Table 2)].
Figure 1. Increased PE-induced vasoconstriction in DOCA-salt aorta is prevented by Pyk2 blockade.
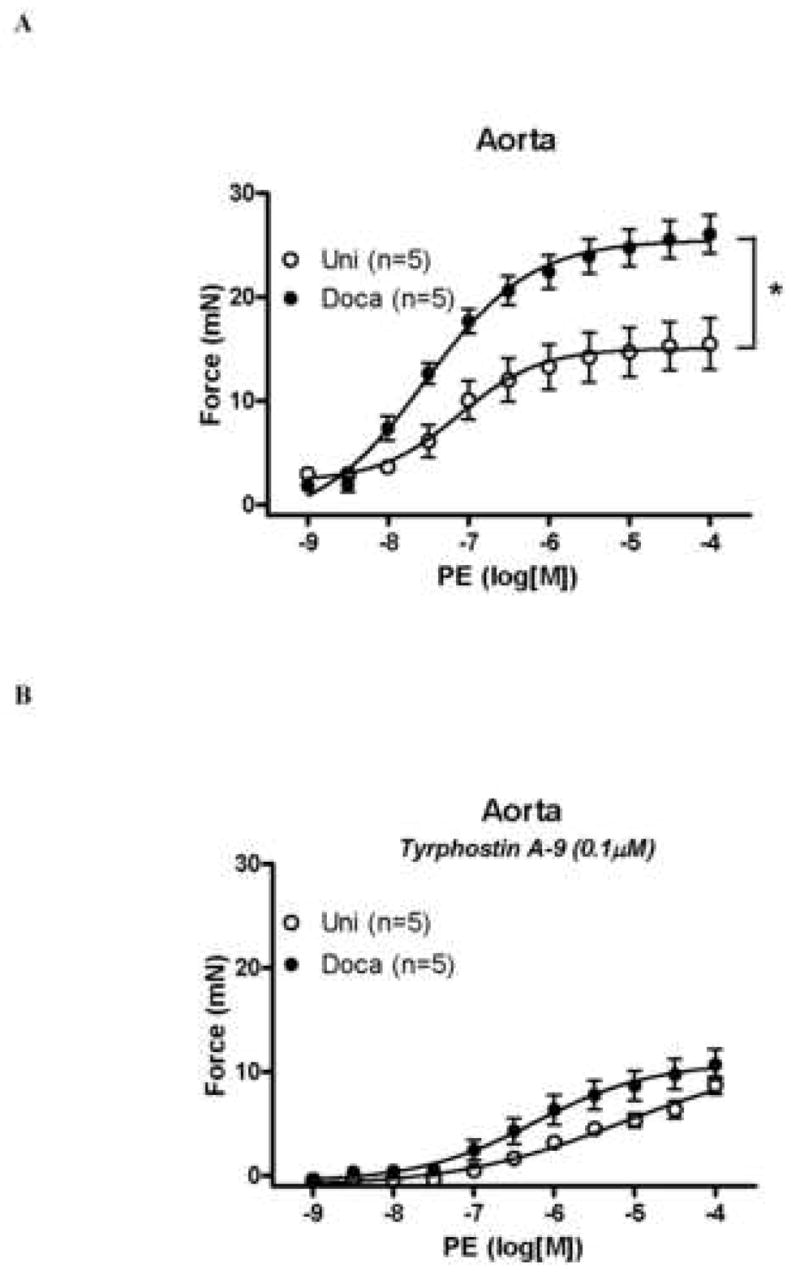
Concentration-response curve to PE in aorta A – from (○) UNI and (●) DOCA-salt mice, in the presence of L-NAME (100μM) and indomethacin (10μM) and B – in the presence of tyrphostin A-9 (0.1μM). Values are means ± SEM for n=5 experiments in each group. * p<0.05 vs. respective control.
Figure 2. Increased PE-induced vasoconstriction in DOCA-salt small mesenteric arteries is prevented by Pyk2 blockade.
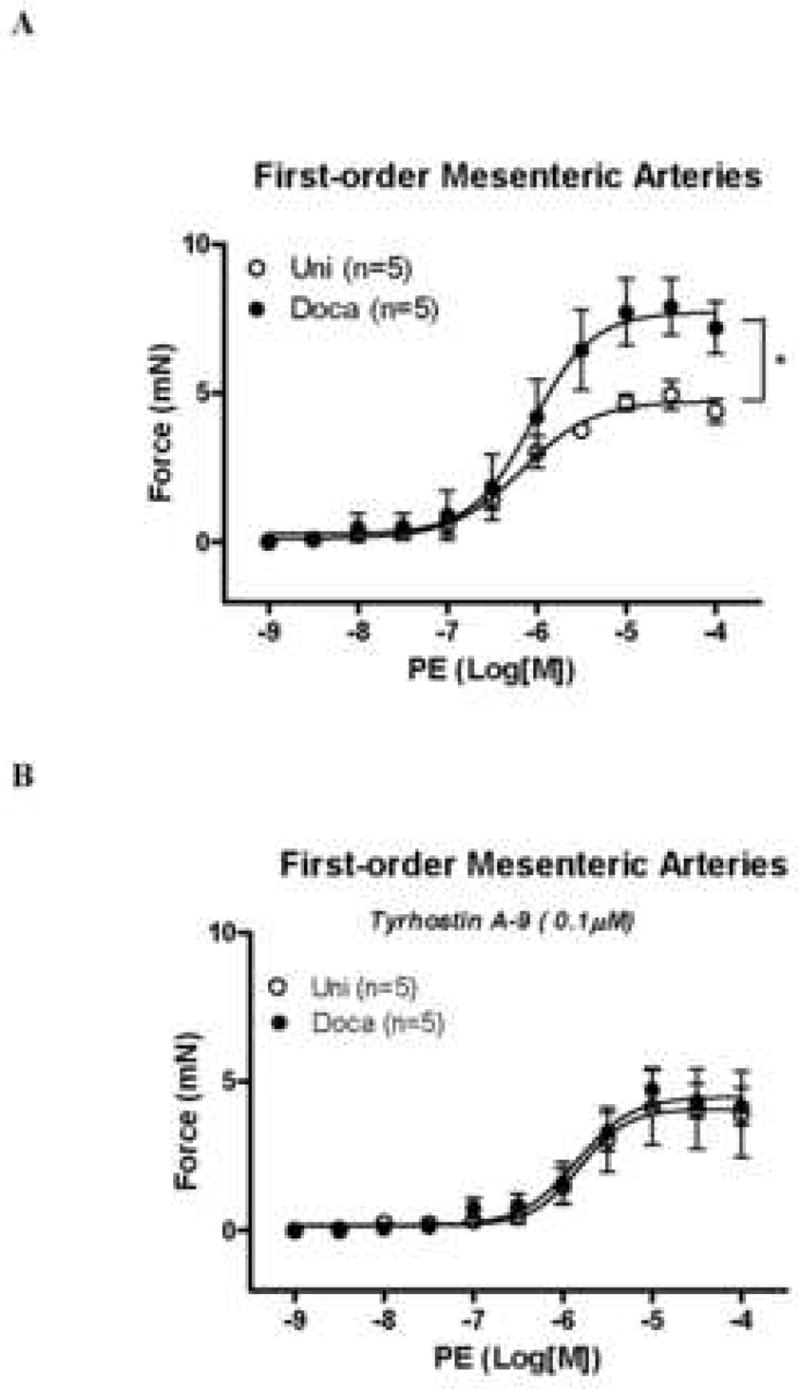
Concentration-response curve to PE in aorta A – from (○) UNI and (●) DOCA-salt mice, in the presence of L-NAME (100μM) and indomethacin (10μM) and B – in the presence of tyrphostin A-9 (0.1μM). Values are means ± SEM for n=5 experiments in each group. * p<0.05 vs. respective control.
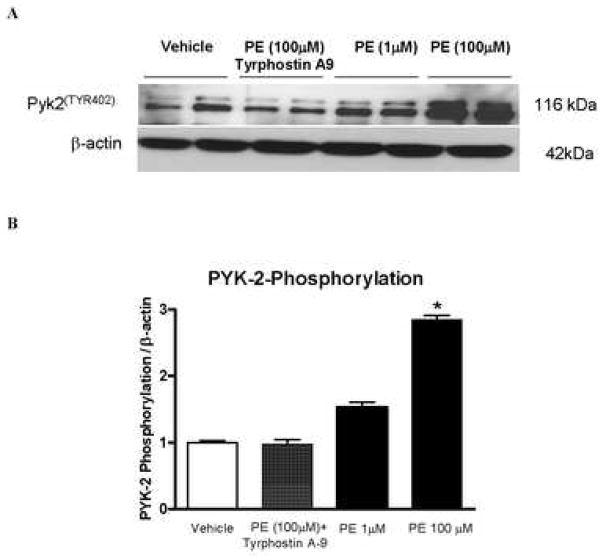
The next set of experiments was performed to confirm that PE induces Pyk2 activation. Indeed, stimulation of arteries with PE (1 and 100μM) during 5 minutes was able to significantly increase phosphorylated levels of Pyk2, in a concentration-dependent manner. Incubation with tyrphostin A-9 (30 minutes before PE stimulation) prevented increased phosphorylation of Pyk2 (Figure 3).
Figure 3. PE-induced Pyk2Tyr402 phosphorylation is prevented by Pyk2 inhibition.
A –Representative immunoblots to Pyk2Tyr402 phosphorylation and beta-actin in murine aorta. B – Corresponding bar graphs demonstrating that stimulation with PE (1 and 100μM – 5 minutes) induces increased Pyk2 Tyr402 phosphorylation. Incubation with tyrphostin A-9 (0.1mM – 30 minutes) prevented PE-induced Pyk2Tyr402 phosphorylation. Values are means ± SEM for n=5 experiments, after correction by beta-actin levels in each group. * p<0.05 vs. respective control.
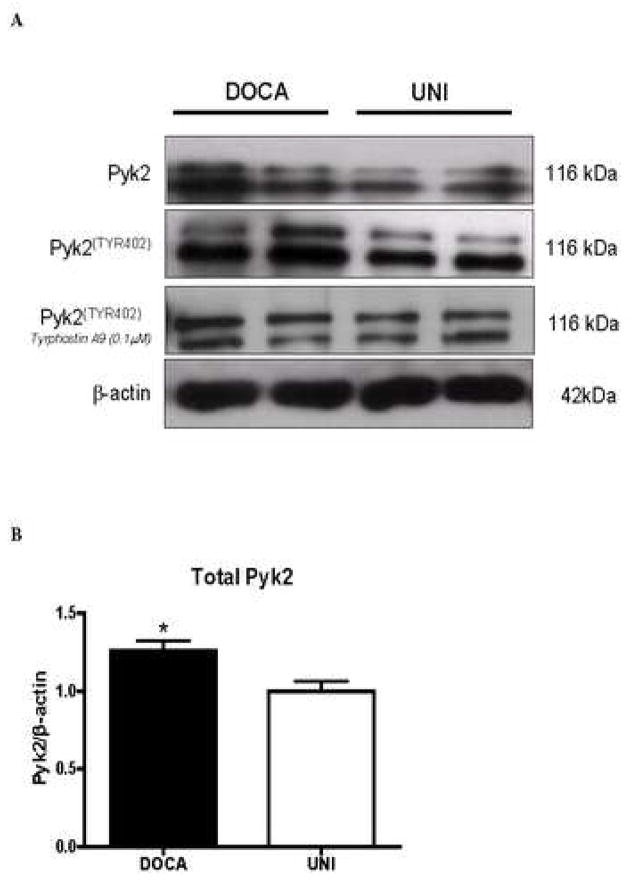
Under basal conditions, total and phosphorylated Pyk2 levels were increased in aortas from DOCA-salt animals, compared to UNI (Figure 4A-D). Incubation with tyrphostin A-9 (30 minutes) prevented increased basal phosphorylation of Pyk2 (Figure 4B).
Figure 4. Pyk2 and Pyk2Tyr402 phosphorylation levels are increased in aorta from DOCA-salt mice.
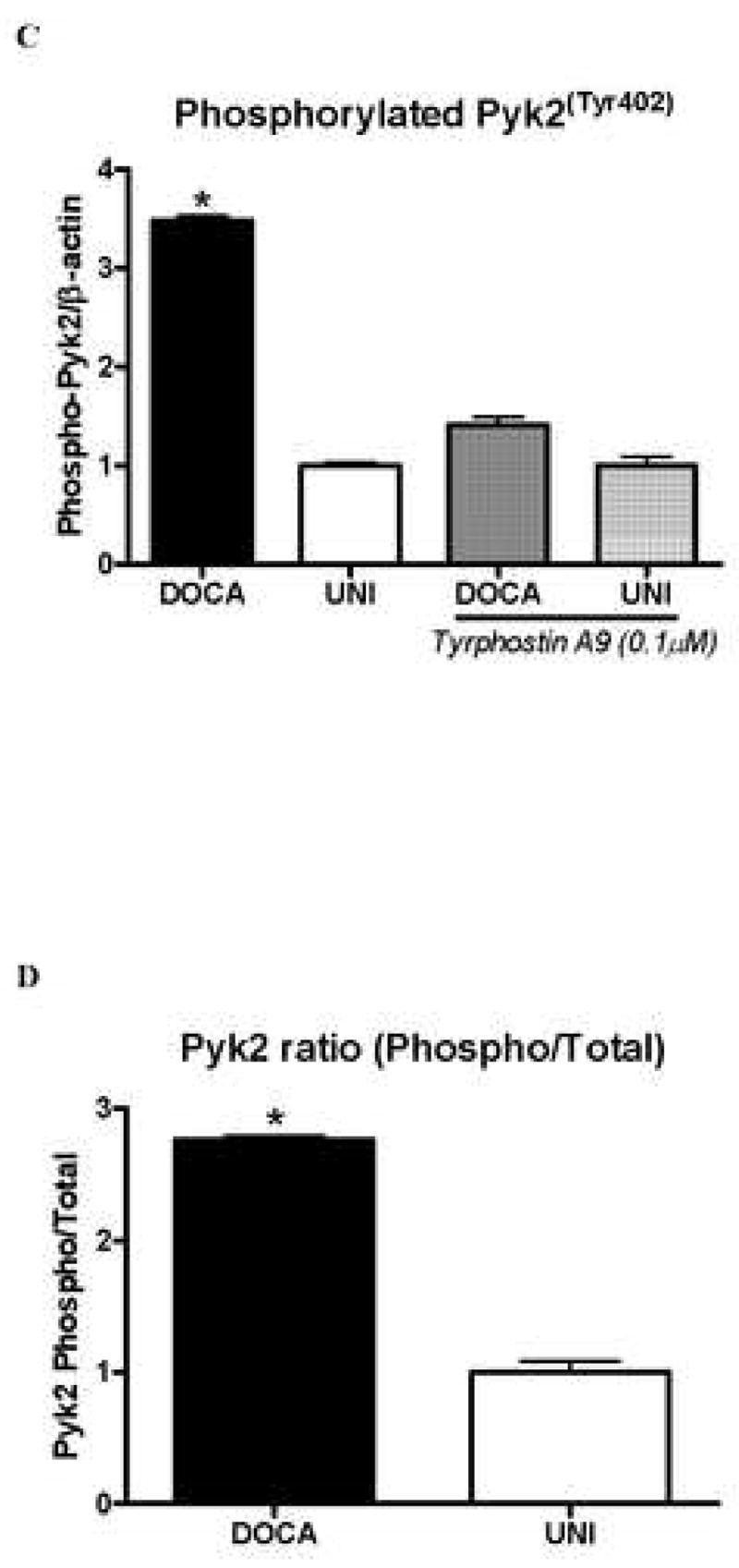
A – Representative immunoblots of Pyk2, phospho-Pyk2Tyr402 and beta-actin in aorta from UNI and DOCA-salt mice. Corresponding bar graphs demonstrating that under basal conditions, both. B – Pyk2 and C – Pyk2Tyr402 phosphorylation are increased in aorta from DOCA-salt mice. Incubation with tyrphostin A-9 prevented increased basal phosphorylation of Pyk2Tyr402 in aorta from DOCA-salt mice. D – Ratio between total and phosphorylated Pyk2 levels. Values are means ± SEM for n=5 experiments, after correction by beta-actin levels in each group. * p<0.05 vs. respective control.
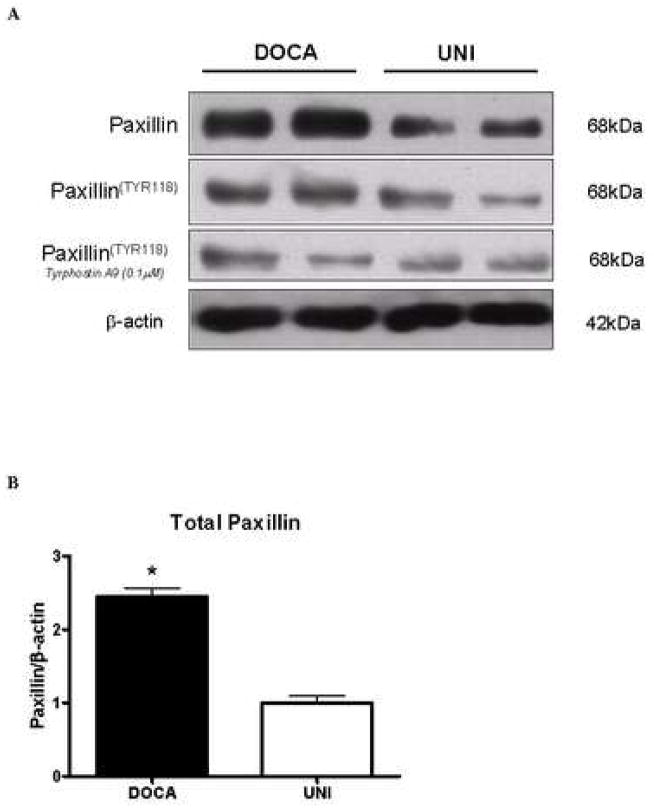
Pyk2 binds to proteins that interact with the cytoskeleton, such as paxillin (11). Therefore, we evaluated paxillin content in vessels from these animals. Paxillin levels, as well as phosphorylated phospho-paxillinTyr 118, were increased in aortas from DOCA-salt animals, compared with UNI mice (Figure 5A-D). Incubation with tyrphostin A-9 (30 minutes) prevented increased basal phosphorylation of paxillin (Figure 5B).
Figure 5. Activation of paxillin, a Pyk2 target, is increased in aorta from DOCA-salt mice.
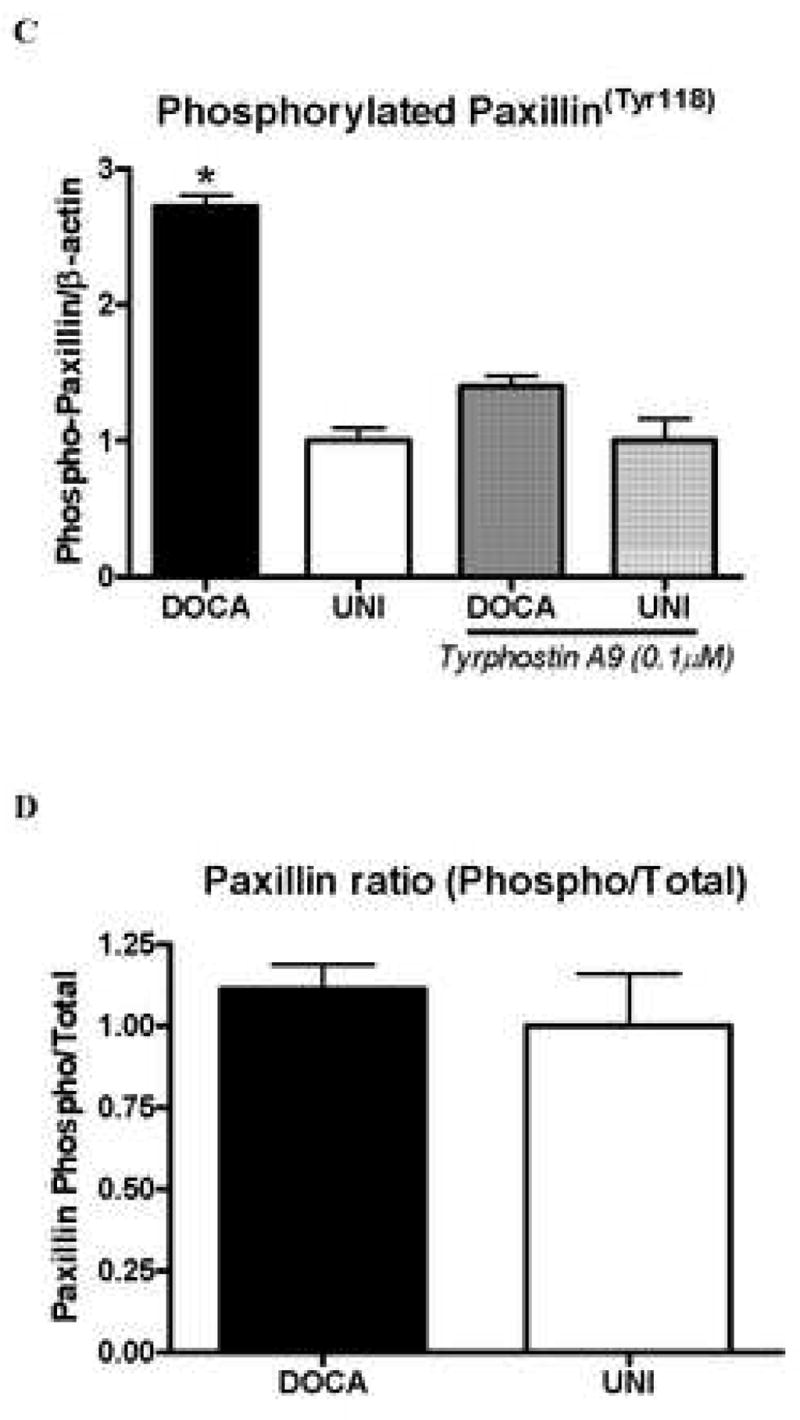
A – Representative immunoblots to paxillin, phospho-paxillinTyr118 and beta-actin in aorta from UNI and DOCA-salt mice. Corresponding bar graphs demonstrating that under basal conditions, both. B – paxillin and C – paxillinTyr118 phosphorylation are increased in aorta from DOCA-salt mice. Incubation with tyrphostin A-9 prevented increased basal phosphorylation of paxillinTyr118 in aorta from DOCA-salt mice D –Ratio between total and phosphorylated paxillin levels. Values are means ± SEM for n=5 experiments, after correction by beta-actin levels in each group. * p<0.05 vs. respective control.
DISCUSSION
DOCA-salt hypertension is characterized by increased vascular reactivity to contractile stimuli (21–25). To investigate if Pyk2 is implicated in the augmented PE-induced responses in DOCA-salt vessels, we used a pharmacologic approach to inhibit Pyk2 phosphorylation: tyrphostin A-9. This drug has been previously identified as a relatively selective inhibitor of this tyrosine kinase activity (30–34). Considering that tyrphostin A-9 may inhibit other tyrosine kinases, we also tested the effects of tyrphostin A-25, which has been shown to inhibit activity of a Pyk2 homologue, focal adhesion kinase (FAK), in some cell types (30). Tyrphostin A-25 had no effects on PE-induced response either in vessels from control or hypertensive animals (data not shown), suggesting a selective effect of tyrphostin A-9 on Pyk2. It is important to mention that knock-down of Pyk2 in vessels from DOCA-salt mice or induction of DOCA-salt hypertension in Pyk2 null mice represent more direct experimental approaches to show that Pyk2 contributes to enhanced vascular reactivity in mineralocorticoid hypertension.
To further confirm the Pyk2 inhibitory property of tyrphostin A-9, we showed that PE incubation induces the phosphorylation of Pyk2, in a concentration-dependent manner, and that incubation with tyrphostin A-9 prevents PE-induced Pyk2 phosphorylation. In this study, we observed that increased PE-induced response is prevented by incubation with tyrphostin A-9, both in aorta and small mesenteric arteries. These data support the hypothesis that Pyk2 activation is involved in the enhanced PE-induced contractile responses in DOCA-salt hypertension.
In a similar manner, aortas from DOCA-salt mice displayed increased levels of Pyk2, as well as augmented phosphorylation of Pyk2 under basal conditions. Furthermore, both the total and phosphorylated forms of the downstream target of Pyk2, paxillin, are augmented in DOCA-salt aorta. Tyrphostin A-9 also prevented increased Pyk2/paxillin phosphorylation in DOCA-salt aorta. We can speculate that in DOCA-salt hypertension, the Pyk2/paxillin pathway is up regulated, contributing to augmented responses to contractile stimuli.
Supporting these findings, Ohanin and colleagues (2005) showed that other agonists, such as NA and ET-1, can activate and induce association of Pyk2/paxillin with a cytoskeleton-enriched membrane fraction in the vascular tissue, The authors suggested that activation of paxillin is a common pathway used by GPCR agonists to regulate contraction (6, 35). Accordingly, paxillin itself is a substrate for the nonreceptor tyrosine kinase Pyk2 (36) and NA and ET-1 are able to increase phosphorylation of both Pyk2 and paxillin in a time course consistent with its involvement in the sustained phase of smooth muscle contraction (37–39).
In addition, the nonreceptor tyrosine kinase Pyk2 has an association with transcriptional activated cascades, suggesting the possibility of a role for Pyk2 in the regulation of cytoskeleton organization, and consequently of contractile responses (40, 41).
Pyk2 is described as an upstream regulator for the ERK1/2 signaling pathway (17). Considering that ERK1/2 phosphorylation is increased in aortas from DOCA-mice (data not shown), it is possible that Pyk2 upregulates ERK1/2 phosphorylation/activation and that increased ERK1/2 activity also contributes to the vascular functional changes associated with DOCA-salt hypertension. This hypothesis is currently being addressed in our laboratory. Interestingly, a recent report has shown that Pyk2 phosphorylation, as well as activation of ERK1/2 and Akt, is increased in a megakaryocyte lineage-specific focal adhesion kinase (FAK)-null mouse (FAK−/− megakaryocytes)(42).
Another important aspect to be observed is that DOCA-salt hypertension is characterized by increased levels of ET-1, or its precursor, prepro-ET-1 (26–28). Several reports showed that ET-1 is able to increase Pyk2 activity (29). The increased levels of ET-1 in DOCA-salt hypertension may be a rational explanation to the augmented activation of Pyk2 pathway in DOCA-salt rats. If this is the case, treatment of DOCA-salt animals with ET-1 antagonists should prevent increased activation of the Pyk2/paxillin pathway.
In summary, this study demonstrates for the first time that augmented response to constrictor stimuli in isolated vascular tissue from DOCA-salt treated mice are abolished by inhibition of Pyk2. These results suggest that increased activity of the Pyk2 pathway is an important mechanism contributing to increased vascular reactivity in hypertension.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH - HL71138 and HL74167) and Fundacao de Amparo a Pesquisa do Estado de Sao Paulo – FAPESP, Brazil.
ABBREVIATIONS
- Ang-II
Angiotensin II
- CADTK
Calcium-dependent tyrosine kinase
- CAKβ
Cell adhesion kinase-β
- DOCA-salt
Deoxycorticosterone acetate-salt induced hypertension
- Emax
Maximum effect elicited by the agonist
- ET-1
Endothelin-1
- ERK1/2
Extracellular regulated kinase 1/2
- GPCR
G protein-coupled receptor
- KCl
Potassium chloride
- L-NAME
Nω-nitro-L-arginine methyl ester
- NA
Noradrenaline
- pD2
Negative logarithm of the molar concentration of agonist producing 50% of the maximum response
- PE
Phenylephrine
- Pyk2
Proline-rich tyrosine kinase
- PSS
Physiological salt solution
- RAFTK
Related adhesion focal tyrosine kinase
- Tyrphostin A-9
[3,5-bis(1,1-Dimethylethyl)-4-hydroxyphenyl] methylene] propanedinitrile]
- UNI
Uninephrectomized mice
- VSMC
Vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, et al. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376(6543):737–45. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 2.Wu SS, Chiu TER. ANG II and LPA induce Pyk2 tyrosine phosphorylation in intestinal epithelial cells: role of Ca2+, PKC, and Rho kinase. Am J Physiol Cell Physiol. 2002;282(6):C1432–44. doi: 10.1152/ajpcell.00323.2001. [DOI] [PubMed] [Google Scholar]
- 3.Dikic I, Tokiwa G, Lev S, Courtneidge SAJS. A role for PYK2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383(6600):547–50. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 4.Litvak V, Tian D, Shaul YDSL. Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J Biol Chem. 2000;275(42):32736–46. doi: 10.1074/jbc.M004200200. [DOI] [PubMed] [Google Scholar]
- 5.Satoh A, Gukovskaya AS, Edderkaoui M, Daghighian M, Reeve J, Shimosegawa T, et al. Tumor necrosis factor-alpha mediates pancreatitis responses in acinar cells via protein kinase C and proline-rich tyrosine kinase 2. Gastroenterology. 2005;129(2):639–51. doi: 10.1016/j.gastro.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Ohanian V, Gatfield KJO. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension. 2005;6(1):93–9. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- 7.Touyz RMCB. Recent advances in angiotensin II signaling. Braz J Med Biol Res. 2002;35(9):1001–115. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- 8.Wu SS, Jácamo RO, Vong SKER. Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal. 2006;8(11):1932–40. doi: 10.1016/j.cellsig.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Dy RC, Cance WG, Graves LMHSE. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274(13):8917–24. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- 10.Shi CS, Sinnarajah S, Cho H, Kozasa T, Kehrl JH. G13alpha-mediated PYK2 activation. PYK2 is a mediator of G13alpha -induced serum response element-dependent transcription. J Biol Chem. 2000;275(32):24470–6. doi: 10.1074/jbc.M908449199. [DOI] [PubMed] [Google Scholar]
- 11.Salgia R, Avraham S, Pisick E, Li J, Raja S, Greenfield EA, et al. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J Biol Chem. 1996;271(49):31222–6. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- 12.Ohba T, Ishino M, Aoto HTS. Interaction of two proline-rich sequences of cell adhesion kinase beta with SH3 domains of p130Cas-related proteins and a GTPase-activating protein. Graf Biochem J. 1998;30(3):1249–54. doi: 10.1042/bj3301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocic P, Griffin TM, McRae CNPAL. Altered PYK2 phosphorylation by ANG II in hypertensive vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282(2):H457–65. doi: 10.1152/ajpheart.00546.2001. [DOI] [PubMed] [Google Scholar]
- 14.Rocic P, Lucchesi PA. Down-regulation by antisense oligonucleotides establishes a role for the proline-rich tyrosine kinase PYK2 in angiotensin ii-induced signaling in vascular smooth muscle. J Biol Chem. 2001;276(24):21902–6. doi: 10.1074/jbc.M101684200. [DOI] [PubMed] [Google Scholar]
- 15.Franchini KG, Torsoni A, Soares PHMJS. Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res. 2000;87(7):558–65. doi: 10.1161/01.res.87.7.558. [DOI] [PubMed] [Google Scholar]
- 16.Kuppuswamy D, Kerr C, Narishige T, Kasi VS, Menick DRGTC. Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. J Biol Chem. 1997;272:4500–8. doi: 10.1074/jbc.272.7.4500. [DOI] [PubMed] [Google Scholar]
- 17.Laser M, Willey CD, Jiang W, Cooper GT, Menick DR, Zile MR, et al. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem. 2000;275(45):35624–30. doi: 10.1074/jbc.M006124200. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, et al. Signal-Crosstalk Between Rho/ROCK and c-Jun NH2-Terminal Kinase Mediates Migration of Vascular Smooth Muscle Cells Stimulated by Angiotensin II ATVB. 2005;25:1831–6. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 19.Yin G, Yan CBCB. Angiotensin II signaling pathways mediated by tyrosine kinases. Int J Biochem Cell Biol. 2003;35(6):780–3. doi: 10.1016/s1357-2725(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 20.BCB. Angiotensin II signal transduction in vascular smooth muscle: pathways activated by specific tyrosine kinases. J Am Soc Nephrol. 1999;10(Suppl 11):S62–8. [PubMed] [Google Scholar]
- 21.Kim J, Lee YR, Lee CH, Choi WH, Lee CK, Kim J, et al. Mitogen-activated protein kinase contributes to elevated basal tone in aortic smooth muscle from hypertensive rats. Eur J Pharmacol. 2005;514(2–3):209–15. doi: 10.1016/j.ejphar.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Weber DSRCW. Enhanced relaxation to the rho-kinase inhibitor Y-27632 in mesenteric arteries from mineralocorticoid hypertensive rats. Pharmacology. 2001;63(3):129–33. doi: 10.1159/000056123. [DOI] [PubMed] [Google Scholar]
- 23.Chitaley K, Webb RC, Dorrance AMTMM. Decreased penile erection in DOCA-salt and stroke prone-spontaneously hypertensive rats. Int J Impot Res. 2001;13(5):s16–20. doi: 10.1038/sj.ijir.3900773. [DOI] [PubMed] [Google Scholar]
- 24.Wehrwein EA, Northcott CA, Loberg RDSWW. Rho/Rho kinase and phosphoinositide 3-kinase are parallel pathways in the development of spontaneous arterial tone in deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther. 2004;309(3):1011–9. doi: 10.1124/jpet.103.062265. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Fink GDJJD. Increased sympathetic venoconstriction and reactivity to norepinephrine in mesenteric veins in anesthetized DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293(1):160–8. doi: 10.1152/ajpheart.01414.2006. [DOI] [PubMed] [Google Scholar]
- 26.Cazaubon S, Chaverot N, Romero IA, Girault JA, Adamson P, Strosberg AD, et al. Growth factor activity of endothelin-1 in primary astrocytes mediated by adhesion-dependent and -independent pathways. J Neurosci. 1997;17(16):6203–212. doi: 10.1523/JNEUROSCI.17-16-06203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorokin A, Kozlowski P, Graves LAP. Protein-tyrosine kinase Pyk2 mediates endothelin-induced p38 MAPK activation in glomerular mesangial cells. J Biol Chem. 2001;276(24):21518–21. doi: 10.1074/jbc.M008869200. [DOI] [PubMed] [Google Scholar]
- 28.Daou GBAKS. Reactive oxygen species mediate Endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 signaling, as well as protein synthesis, in vascular smooth muscle cells. Free Radic Biol Med. 2004;37(2):208–15. doi: 10.1016/j.freeradbiomed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Rocic P, Govindarajan G, Sabri A, Lucchesi PA. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280(1):C90–9. doi: 10.1152/ajpcell.2001.280.1.C90. [DOI] [PubMed] [Google Scholar]
- 30.Bilder GE, Krawiec JA, McVety K, Gazit A, Gilon C, Lyall R, et al. Tyrphostins inhibit PDGF-induced DNA synthesis and associated early events in smooth muscle cells. Am J Physiol Cell Physiol. 1991;260(4 Pt 1):C721–C30. doi: 10.1152/ajpcell.1991.260.4.C721. [DOI] [PubMed] [Google Scholar]
- 31.Fuortes M, Melchior M, Han H, Lyon GJCN. Role of the tyrosine kinase Pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104(3):327–35. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakkakorpi PT, Nakamura I, Young M, Lipfert L, Rodan GALTD. Abnormal localisation and hyperclustering of (alpha)(V)(beta)(3) integrins and associated proteins in Src-deficient or tyrphostin A9-treated osteoclasts. J Cell Sci. 2001;114(1):149–60. doi: 10.1242/jcs.114.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Loeser RF, Forsyth CB, Samarel AMHJI. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278(27):24577–85. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aasheim HC, Delabie JEFF. Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood. 2005;5(7):2869–76. doi: 10.1182/blood-2004-08-2981. [DOI] [PubMed] [Google Scholar]
- 35.Avraham H, Park SY, Schinkmann KSA. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–33. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 36.Ward DT, Alder AC, Ohanian JVO. Noradrenaline-induced paxillin phosphorylation, ERK activation and MEK-regulated contraction in intact rat mesenteric arteries. J Vasc Res. 2002;39(1):1–11. doi: 10.1159/000048988. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki N, Miyauchi T, Tomobe Y, Matsumoto H, Goto K, Masaki T, et al. Plasma concentrations of endothelin-1 in spontaneously hypertensive rats and DOCA-salt hypertensive rats. Biochem Biophys Res Commun. 1990;167(3):941–7. doi: 10.1016/0006-291x(90)90614-s. [DOI] [PubMed] [Google Scholar]
- 38.Kohno M, Murakawa K, Horio T, Yokokawa K, Yasunari K, Fukui T, et al. Plasma immunoreactive endothelin-1 in experimental malignant hypertension. Hypertension. 1991;18(1):93–100. doi: 10.1161/01.hyp.18.1.93. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen PV, Parent A, Deng LY, Flückiger JP, Thibault GELS. Endothelin vascular receptors and responses in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1992;19(2 Suppl):II98–104. doi: 10.1161/01.hyp.19.2_suppl.ii98. [DOI] [PubMed] [Google Scholar]
- 40.Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, et al. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J Biol Chem. 2003;278(38):36661–8. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- 41.Du QS, Ren XR, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization, PYK2 autophosphorylation and focal adhesion targeting by FAK. J Cell Sci. 2001;114(Pt 16):2977–87. doi: 10.1242/jcs.114.16.2977. [DOI] [PubMed] [Google Scholar]
- 42.Hitchcock IS, Fox NE, Prévost N, Sear K, Shattil SJ, Kaushansky K. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111(2):596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]