Abstract
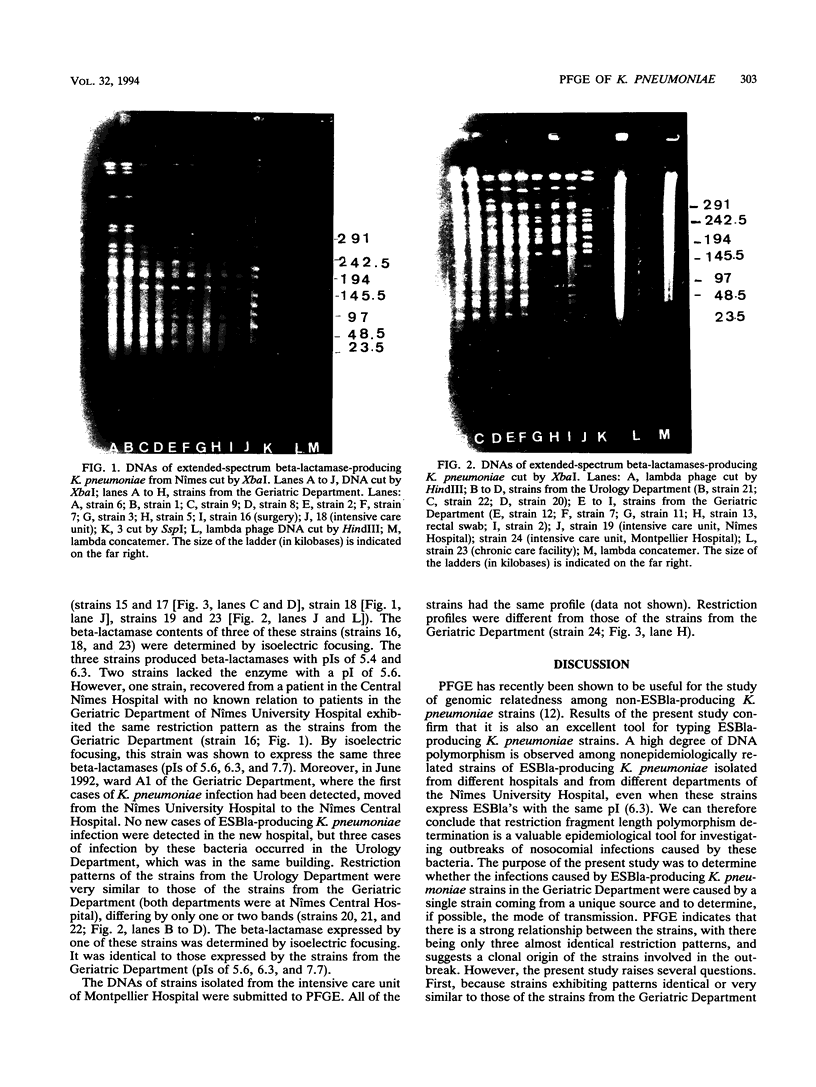
Twelve cases of infections caused by extended-spectrum beta-lactamase (ESBla)-producing Klebsiella pneumoniae were reported between August 1991 and March 1993 in the Geriatric Department of the Nimes University Hospital, where these bacterial had not been previously isolated. Restriction profiles of total genomic DNAs cleaved by XbaI and SpeI were compared by pulsed-field gel electrophoresis. The strains that were tested included the 12 isolates from K. pneumoniae-infected patients, strains recovered from rectal swabs of asymptomatic patients in the same ward, and strains isolated in other hospitals in Nîmes at the same time. The restriction profiles of the 12 isolates and those recovered from asymptomatic patients in the same ward were very similar. Over a period of more than 1 year, extended-spectrum beta-lactamases were not detected in K. pneumoniae isolates with restriction patterns different from that of the epidemic strain. It seems, therefore, that there was no transfer of a plasmid or a gene coding for ESBla to strains of K. pneumoniae that were different from the epidemic strain. At the same time, ESBla-producing K. pneumoniae isolates exhibiting restriction endonuclease profiles very different from that of the epidemic strain were isolated from other hospitals in Nîmes. None of these strains caused an outbreak. Pulsed-field gel electrophoresis, which allows precise characterization of strains beyond the species level, is a useful tool for studying the ESBla-producing K. pneumoniae strains involved in nosocomial outbreaks.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambaud M., Gerbaud G., Labau E., Marty N., Courvalin P. Possible in-vivo transfer of beta-lactamase TEM-3 from Klebsiella pneumoniae to Salmonella kedougou. J Antimicrob Chemother. 1991 Apr;27(4):427–436. doi: 10.1093/jac/27.4.427. [DOI] [PubMed] [Google Scholar]
- Arlet G., Sanson-le Pors M. J., Rouveau M., Fournier G., Marie O., Schlemmer B., Philippon A. Outbreak of nosocomial infections due to Klebsiella pneumoniae producing SHV-4 beta-lactamase. Eur J Clin Microbiol Infect Dis. 1990 Nov;9(11):797–803. doi: 10.1007/BF01967377. [DOI] [PubMed] [Google Scholar]
- Bingen E. H., Desjardins P., Arlet G., Bourgeois F., Mariani-Kurkdjian P., Lambert-Zechovsky N. Y., Denamur E., Philippon A., Elion J. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J Clin Microbiol. 1993 Feb;31(2):179–184. doi: 10.1128/jcm.31.2.179-184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buré A., Legrand P., Arlet G., Jarlier V., Paul G., Philippon A. Dissemination in five French hospitals of Klebsiella pneumoniae serotype K25 harbouring a new transferable enzymatic resistance to third generation cephalosporins and aztreonam. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):780–782. doi: 10.1007/BF01975048. [DOI] [PubMed] [Google Scholar]
- Chanal C. M., Sirot D. L., Petit A., Labia R., Morand A., Sirot J. L., Cluzel R. A. Multiplicity of TEM-derived beta-lactamases from Klebsiella pneumoniae strains isolated at the same hospital and relationships between the responsible plasmids. Antimicrob Agents Chemother. 1989 Nov;33(11):1915–1920. doi: 10.1128/aac.33.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertl R., Bandlow G. Epidemiological fingerprinting of Enterobacter cloacae by small-fragment restriction endonuclease analysis and pulsed-field gel electrophoresis of genomic restriction fragments. J Clin Microbiol. 1993 Jan;31(1):128–133. doi: 10.1128/jcm.31.1.128-133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L. Properties of plasmids responsible for production of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Jan;35(1):164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlier V., Nicolas M. H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988 Jul-Aug;10(4):867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- Maslow J. N., Brecher S. M., Adams K. S., Durbin A., Loring S., Arbeit R. D. Relationship between indole production and differentiation of Klebsiella species: indole-positive and -negative isolates of Klebsiella determined to be clonal. J Clin Microbiol. 1993 Aug;31(8):2000–2003. doi: 10.1128/jcm.31.8.2000-2003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A., Gerbaud G., Sirot D., Courvalin P., Sirot J. Molecular epidemiology of TEM-3 (CTX-1) beta-lactamase. Antimicrob Agents Chemother. 1990 Feb;34(2):219–224. doi: 10.1128/aac.34.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B., Willey S. H., Papanicolaou G. A., Medeiros A. A., Eliopoulos G. M., Moellering R. C., Jr, Jacoby G. A. Outbreak of ceftazidime resistance caused by extended-spectrum beta-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990 Nov;34(11):2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., De Champs C., Chanal C., Labia R., Darfeuille-Michaud A., Perroux R., Sirot J. Translocation of antibiotic resistance determinants including an extended-spectrum beta-lactamase between conjugative plasmids of Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 1991 Aug;35(8):1576–1581. doi: 10.1128/aac.35.8.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot J., Chanal C., Petit A., Sirot D., Labia R., Gerbaud G. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated beta-lactamases markedly active against third-generation cephalosporins: epidemiologic studies. Rev Infect Dis. 1988 Jul-Aug;10(4):850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]
- de Champs C., Sirot D., Chanal C., Poupart M. C., Dumas M. P., Sirot J. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991 Apr;27(4):441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]