Abstract
The goal of the study was to investigate the neural circuit recruited by adult readers during performance of a lexical decision task by assessing the relative timing of neurophysiological activity in the brain regions that comprise this circuit. The time course of regional activation associated with lexical decision was studied in 17 adult volunteers using magnetoencephalography. Following activity in mesial occipital cortices, activation progressed to lateral and ventral occipito-temporal regions (often encompassing the posterior portion of the middle temporal gyrus), followed by activity in the superior temporal gyri (STGp), motor/premotor cortices, and the inferior frontal gyrus. The latency of STGp activation relative to the latency of the motor response to the word stimuli did not support critical involvement of this area in lexical decision. Timing, word length, and word frequency effects found for activity in each area are discussed in relation to the purported roles of each region into the brain circuit for reading.
Models for the brain mechanism that supports reading postulate at least three relatively distinct subcomponents. Two posterior circuits, one involving ventral and lateral occipito-temporal cortices and the second involving posterior superior temporal and inferior parietal regions, appear to be complemented by an anterior circuit, encompassing inferior frontal regions. The ventral posterior circuit encompasses high-order visual association areas (occupying most of Brodmann’s area--BA--37) and is suitable for hosting neurophysiological processes responsible for graphemic processing and also possibly for the integration of orthographic, phonological and morphological information derived from print (Cohen, Dehaene, Naccache et al., 2000; Cohen, Lehericy, Chochon et al., 2002; Hagoort, Indefrey, Fiebach, Friederici, Muller, & von Cramon, 2002; Hart et al., 2000; Kuperberg et al., 2000; Mummery et al., 1998; Pugh et al., 1996; Rapcsak & Beeson, 2004; Warrington & Shallice, 1980). It is not clear at present if these processes include access to stored neural representations of familiar orthographic patterns. In cognitive-linguistic terms, the issue that remains unresolved is whether these processes operate on lexical or sublexical units. The region lying immediately anterior to the lateral portion of BA 37, and is part of the middle temporal gyrus (BA 21) may also be part of this circuit, and evidence is strongly suggestive for its involvement in lexical/semantic analysis of both spoken and printed language (Booth et al., 2002; Damasio & Damasio, 1983; Fiebach et al., 2002; Gaillard et al., 2001; Halgren et al., 2002; McCandliss, Cohen, & Dehaene, 2003; Pugh et al., 1996; Turkeltaub et al., 2002; Tyler, Marslen-Wilson, & Stamatakis, 2005). The second posterior circuit, includes at least one area, the posterior portion of the left superior temporal gyrus (BA 22) for which there is accumulated evidence that it is involved in the phonological processing of both spoken and printed stimuli (Beauvois & Derouesne, 1979; Binder et al., 2003; Caplan, Gow, & Makris, 1995; Joseph, Noble, & Eden, 2001; Joubert et al., 2004; Roux et al., 2004, Simos et al., 1999, 2000a). This circuit is said to include the supramarginal and angular gyri, but the precise role of these areas for phonological processing of printed stimuli is unclear. Finally, the anterior circuit which includes Broca’s area, appears to be involved in the articulatory recoding of both familiar and unfamiliar, pronounceable letter strings (e.g., Pugh et al., 1996; Fiez et al., 1999) and/or in the retrieval of semantic information from long-term memory (e.g., Gabrieli, Poldrack, & Desmond, 1998; Menard et al., 1996).
On the basis of a meta-analysis of data reported in 35 functional imaging studies (Jobard, Crivello, & Jurio-Mazoyer, 2003) it was suggested that neurophysiological processes associated with access to stored graphemic representations (either by familiar word stimuli or by pseudowords that contain word-like segments) take place in the left occipitotemporal region (BA 37). These studies have identified several regions associated with particular task contrasts and the manner in which task-specific regional activity may change in the course of development. Alternative interpretations of these data are, however, possible. For instance, differences in the degree of activation between words (stimuli that may have stored word-form representations) and pseudowords (stimuli that do not possess such representations) can take two mutually exclusive interpretations. Increased activity for words may be found in areas specialized for processing familiar orthographic stimuli. The same regions may show the opposite effect if increased degree of hemodynamic activity simply reflects increased demands for neurophysiological engagement in order to process less familiar orthographic stimuli (Jobard, Crivello, & Jurio-Mazoyer, 2003). It is also possible that pseudowords are often read aloud through access to multi-letter sublexical phonological representations they contain rather than through a letter-by-letter strategy, especially in deep orthographic systems, like English.
MEG could provide complementary information to that gained from fMRI BOLD and fMRI connectivity studies. Whereas fMRI BOLD studies have advanced knowledge of brain activation associated with specific processes isolated by a set of task contrasts and fMRI connectivity studies are leading to greater understanding of which regions of interest may be functionally connected (communicating) at the same time for a single, continuous task, MEG holds promise for illuminating the sequence of temporal activation of single or simultaneously activated regions of interest. Collectively, these different imaging modalities provide a larger picture of the brain basis for a complex function such as reading. Few studies have systematically addressed the temporal sequence of neurophysiological events leading to an overt behavioral response that signifies word recognition. For instance, Simos et al. (2002b) reported activation of both posterior circuits during a delayed word and pseudoword reading aloud task, but there were indications that posterior superior temporal gyrus (STGp) activity may not have been crucial for pronouncing exception words. Cortical activity in the posterior portion of MTG (BA 21) appeared to be more closely related to the pronunciation of words with reduced demands for phonological decoding. The delayed nature of the overt response required by the task and the requirement for active retrieval and production of the phonological representation of each printed stimulus did not permit a conclusive assessment of the role of STGp in word recognition. A similar picture emerged in two more recent studies (early activation of occipitotemporal cortices followed by activity in the left STGp between 200 and 600 ms after stimulus onset) but the data were again inconclusive with respect to the role of STGp in word recognition (Wilson, Leuthold, Lewis, Georgopoulos, & Pardo, 2005; Wydell, Vuorinen, Helenius, & Salmelin, 2003).
In the current study we recorded event-related magnetic fields to printed words in the context of a lexical decision task, reducing the need for phonological decoding of letter strings. Lexical decision is widely regarded as a task that assesses access to the nature of representations in the mental lexicon for words (e.g. phonological codes, orthographic codes, spreading semantic activation). Relatively rapid stimulus presentation and rate further encouraged participants, who were experienced adult readers, to adopt a rapid word recognition strategy based on visual/graphemic features. Word stimuli were all monosyllabic and varied systematically in frequency of occurrence and length in order to examine potential systematic changes in the degree and latency of regional activity as a function of lexical or visual characteristics, respectively. In addition, each acquisition session was repeated twice for each participant (with different but equivalent stimulus sets) ensuring that only reproducible sites of regional activation were taken into account in the analyses of the effects of stimulus type. This approach permitted assessment of individual variability in the anatomical layout of activation maps associated with word recognition.
Materials and methods
Participants
After signing a consent form, 17 right-handed adults, who were native English speakers (8 females and 9 males, ranging in age between 25 to 32 years with a mean age of 28 years), without history of neurological, psychiatric disorder, or learning disability, participated in the study.
Stimuli and task
Magnetoencephalography (MEG) scans were obtained during a go/no-go lexical decision task (e.g., Perea, Rosa, & Gomez, 2003). Single-syllable letter strings served as stimuli and were initially arranged in two lists (Long, consisting of 5–6 letter strings and Short, consisting of 3–4 letter strings). Word stimuli in each list were randomly mixed with an equal number of pseudowords matched for length and varied in frequency of appearance in print: there was a total of 160 Low Frequency words ranging between 1–20 appearances per million in the Kucera and Francis (1967) corpus, and an equal number of High Frequency words (30–570 appearances per million). Pseudowords were derived from real words (not used in the study) by substituting one or two letters. Table 1 lists key stimulus parameters. In order to assess the test-stability-generalizability of the results, each set of long and short words was randomly divided into two and administered to participants in two separate sessions (A and B). There was an equal number of high and low frequency words in each list and a matching number of pseudowords. The stimuli were printed black on a white background and projected centrally for 600 ms, one at a time (with a randomly varied SOA of 1–2 sec) through an LCD projector (Sharp Model XG-E690U) on a back-projection screen located approximately 60 cm in front of the participant. Long stimuli subtended up to 3° of visual angle horizontally and short stimuli up to 2.0°. Participants were instructed to press a non-magnetic response key with either their left or right index finger (counterbalanced between recording sessions for each participant) every time they read a real word. Both speed and accuracy was emphasized.
Table 1.
Stimulus characteristics
| Frequency of appearance | # letters | ||||
|---|---|---|---|---|---|
| Mean | SD | Range | |||
| Long | High Frequency | 112 | 50 | 30–400 | 5–6 |
| Low Frequency | 13 | 4 | 1–20 | 5–6 | |
| Short | High Frequency | 130 | 50 | 30–570 | 3–4 |
| Low Frequency | 13 | 5 | 3–4 | ||
Data collection and analysis
MEG recordings were made with a whole-head neuromagnetometer (Magnes 3600®, 4-D Neuroimaging, Inc., San Diego, CA) consisting of 248 axial gradiometer coils. The instrument is housed in a magnetically shielded room designed to reduce environmental magnetic noise that might interfere with biological signals. The signal was filtered online with a band pass between 0.1 and 50 Hz, digitized for 700 ms (254 Hz sampling rate) including a 150 ms prestimulus period, and subjected to an adaptive filtering procedure that is part of the 4-D Neuroimaging signal analysis package. The single trial event-related fields (ERFs) elicited by word stimuli were then averaged together after removing those during which an eye movement or blink had occurred (as indicated by a peak to peak amplitude in the electro-oculogram channel in excess of 50 μV). A minimum of 60 ERF epochs were collected to calculate each averaged waveform. Finally, the averaged epochs were digitally filtered with a low pass 20 Hz filter. In view of the fact that participants were instructed to respond manually only to word stimuli, neurophysiological data to pseudowords were not comparable to data associated with word stimuli and were not analyzed further.
The intracranial generators of the observed ERFs (henceforth referred to as “activity sources”) were modeled as single equivalent current dipoles (ECDs) and fitted at successive 4 ms intervals by using the nonlinear Levenberg-Marquardt algorithm. For a given point in time, the ECD fitting algorithm was applied to the magnetic flux measurements obtained from a group of 34–38 sensors, always including both magnetic flux extremes. The algorithm used in this study searched for the ECD that was most likely to have produced the observed magnetic field distribution at a given point in time. The ECD solutions were considered satisfactory if they were associated with a correlation coefficient of at least 0.9 between the observed and the “best” predicted magnetic field distribution. The ECD that accounted for the surface distribution of magnetic flux at each 4-ms time window identified the geometric center of the cortical patch producing the dipolar magnetic flux distribution at that time point. The derived activation maps consisted of strings of temporally contiguous activity sources that were typically localized in the same anatomical region. Identification of reproducible activity sources, across different conditions and sessions, was performed blindly with the aid of a clustering algorithm. For that purpose, the estimated activity sources from either two (in twelve participants) or six split data sets (in two participants) were merged and ranked by (i) the degree of latency overlap and (ii) spatial proximity using an automated algorithm developed by 4D Neuroimaging (for details see Papanicolaou et al., 2004). This method resulted in a limited set of well-delineated areas for each participant and hemisphere, which were then corregistered on anatomical MRI scans (T1-weight images: TR 13.6 ms; TE 4.8 ms; recording matrix 256× 256 pixels, 1 excitation, 240 mm field of view, and 1.4 mm slice thickness) obtained from every participant during a separate session.
Visual inspection of the resulting activation profiles showed that activity sources were found in three main areas in all participants in at least one hemisphere: motor and premotor cortex (Brodmann areas 4 and 6 or precentral and premotor areas [preC-preM]), the posterior portion of the superior temporal gyrus (BA 22 [STGp]), and the posterior portion of the middle temporal gyrus (BA 21 [MTGp]). In five participants activity was found in ventral occipitotemporal cortex instead of MTGp (BA 37 [VOT]). Activity foci in MTGp and VOT will henceforth be referred to as “posterior cluster”. Data were extracted and included in further analyses only for activity sources that appeared in a systematic fashion in a given area and hemisphere in at least 6/8 data sets (4 conditions × 2 sessions) for a given participant.
Analyses were performed for each session independently on four MEG-derived measures: The strength of neurophysiological activity corresponding to each ECD was indicated by two measures: estimated current moment (Q) of the net neuronal population response (in nanoAmpere-meters – nA-m), and global field power (RMS) of the measured magnetic flux used to calculate each activity source (in femtoTesla - fT). Current moment is a derived measure, the validity of which depends on the adequacy of the mathematical model used to estimate magnetic source parameters and also on the quality of the ECD solution at any given point in time. RMS on the other hand is a direct measure of the strength of magnetic flux produced by underlying electrical currents at a given point in time and is therefore independent of mathematical modeling considerations. Theoretically, RMS should provide the most stable index of the instantaneous strength of cortical activity provided that the relative position of the head with respect to the sensors remains constant across repeated measurements, as was the case in the present study. “Best” ECD Q (i.e., Q value associated with the ECD that had the best correlation coefficient) and peak RMS values across all consecutive activity sources forming each cluster served as estimates of the strength of electromagnetic activity for the duration of the corresponding cluster. Finally, the duration of regional, dipolar neurophysiological activity, as indicated by the number of consecutive ECDs localized in each reproducible cluster, was recorded. In our previous studies with healthy volunteers and patients, this method produced the most conclusive results as an index of the degree of task-specific regional cortical engagement (Breier et al., 1999, 2001; Maestu et al., 2002; Papanicolaou et al., 2004; Szymanski et al., 2001). Finally, the earliest time when activity sources were first noted in a particular region (onset latency expressed in milliseconds after stimulus onset) was examined in order to reconstruct the temporal progression of regional activity.
Given the importance that latency of activity has for the main objective of the study (determining the relative timing of regional activity during word recognition) a complementary to the ECD method of initial data analysis was used, Minimum Norm Estimate (MNE). This method affords greater spatial resolution and allows detection of simultaneous magnetic sources distributed along the entire cortical surface. The model assumes a continuous distribution of current along the cortical surface which has some minimum norm (Hämäläinen & Ilmoniemi, 1994). Estimated current sources were anatomically constrained by an MRI-derived surface model of each participant’s brain. This surface model was generated by a fully-automated cortical surface reconstruction procedure using FreeSurfer software (Dale, Fischl & Sereno, 1999) for producing a detailed geometric description (e.g. regular tessellation of the cortical surface consisting of equilateral triangles known as vertices) of the gray-white matter boundary of the neocortical mantle and the mesial temporal lobe. Each hemisphere consists of approximately 150,000 vertices (depending on each subject’s cortical surface area). For estimating current sources, the MNE software requires the Freesurfer-derived cortical surface reconstruction for defining the boundaries of a solution source space. A grid-spacing of 7 mm was used to construct icosahedrons to decimate the number of vertices from 150,000 to approximately 3,000 per hemisphere. Additionally, the MNE software was used to construct a single compartment boundary element model using triangular tessellations to model each vertex as a potential current dipole perpendicular to the cortical surface during the forward calculations. The inverse solution was subsequently reduced to obtaining an estimate of the scalar distribution of dipole strength across current sources within orientation-specific cortical patches of vertices (Dale & Sereno, 1993). Co-registration of each MEG dataset with its corresponding MRI dataset was performed using an automated co-registration routine within MNE which aligns digitization points in the MEG headshape file with the fiducial points demarcated on the outer skin surface reconstruction of the MRI.
The locations of reliable ECDs were used to identify ROIs where reliable magnetic activity was localized, the spatial extent of which was based on standard anatomical landmarks (posterior third of STG and MTG, VOT, and preC-preM region). Upon visual inspection of MNE activation maps at a minimum threshold sufficient to detect activity in all the ROIs listed above, an additional activation focus emerged in the inferior frontal gyrus (BA 44, 45). Activity in this region did not meet the rigorous criterion set for the ECD data (ECDs had to be found in at least 6/8 datasets in every subject), however, in view of the purported role of this region in word recognition (reviewed in (Jobard, Crivello, & Jurio-Mazoyer, 2003) it was included in analyses of the relative timing of regional activation. Estimated current waveforms were computed for each ROI by averaging current waveforms for each voxel comprising the corresponding ROI. In order to avoid the uncertainties inherent in using an arbitrary amplitude cut-off to determine activity onset in a particular region, the peak latency in the MNE ROI-averaged current waveform was used. As will be become apparent below, onset and peak latency values did not differ substantially from each other. This was probably due to the fact that the method used to derive onset values (requiring spatial overlap of ECDs in a given ROI in at least six out of eight data sets biased the data toward longer latencies, closer to the peak values. This likely explains why onset latency values for activity in ventral occipitotemporal cortices were somewhat longer than those typically reported in the context of reading tasks in previous studies by our group and others (Cohen et al., 2000; Simos et al., 2002b; Wydel et al., 2003).
Given that strength measures are, to some extent, intercorrelated, stimulus effects were examined using a multivariate approach (MANOVA) with four within-subjects variables, ROI (preC-preM, STGp, Posterior Cluster), Length (Long, Short words), Frequency (High, Low), and Hemisphere (left, right), and three measures (Q, RMS, and number of consecutive ECDs). This analysis was performed separately on data from each of the two Sessions in order to assess the stability of the results. Significant multivariate effects were further evaluated using univariate ANOVAs in order to determine the measure(s) most closely associated with a particular effect. Significant univariate interactions were followed up using simple main effects ANOVAs. Onset and peak latency data were submitted to separate ANOVAs with four within-subjects variables (Length: Long, Short words), Frequency (High, Low), Hemisphere (left, right), and Region (preC-preM, STGp, and Posterior Cluster for both measures; BA 44/45 was also included in the MNE peak latency data). In order to improve the stability of latency estimates for a given area and condition, data from both Sessions were averaged together. The Bonferroni correction method was used to maintain the type I error at the .05 level for each family of ANOVA tests. Statistical analyses were performed with SPSS 16.0.
Results
Reaction time data
The average hit rate (correct responses to words) was 94.5% (range across subjects and conditions: 93–95%, see Table 2). An ANOVA with Session, Length, and Frequency as within subjects factors failed to reveal significant main effects or interactions (p >.2 in all cases). Individual reaction time data were submitted to an ANOVA with length and frequency as within subjects factors revealing significant main effects for both (F[1,16] = 19.3, p <.0001 and F[1,16] = 51.3, p <.0001, respectively). Inspection of Table 2 indicates slower recognition RTs for long vs. short and low vs. high frequency words. Results were essentially identical across sessions.
Table 2.
Reaction times (ms) to word stimuli and response accuracy*
| Mean (SD) | # Hits (SD) | #False alarms | ||
|---|---|---|---|---|
| Long | High Frequency | 551 (45) | 93 (5) | 3 |
| Low Frequency | 566 (43) | 92 (4) | 3 | |
| Short | High Frequency | 523 (37) | 95 (6) | 4 |
| Low Frequency | 545 (37) | 93 (4) | 4 |
Average across sessions.
Latency and hemisphere effects
ECD data
Examples of ECD activation profiles for two representative participants (one with activity in MTGp and another with VOT activity) are shown in Figure 1. The four-way ANOVA on peak latency values revealed an ROI by Hemisphere interaction, F(2,32) = 13.70, p <.0001. No other main effects or interactions were significant (p >.3 in all cases). ROIs in each hemisphere were then ranked in ascending order of onset latency establishing the temporal progression of activity. Figure 2 shows the relative timing of activity across the six regions (three ROIs in each hemisphere) and the similarity in onset latency values between conditions. A one-way ANOVA with ROI/Hemisphere (with six levels) as the within-subjects factor revealed a significant linear trend, F(1,16) = 56.04, p <.0001 (p >.7 for all higher-order trends) indicating a regular progression of activity across the six regions. Finally, in order to determine pairs of regions that showed reliable onset latency asynchronies, a series of 15 one-way ANOVAs with two levels on the ROI/Hemisphere factor were computed (testing onset latency differences between a given region and each of the regions with later onsets). This procedure was performed on data collapsed across sessions and conditions. F-values were evaluated at a conservative alpha level of .003 to maintain probability of family-wise Type I error to approximately .05 given the total of 15 tests performed.
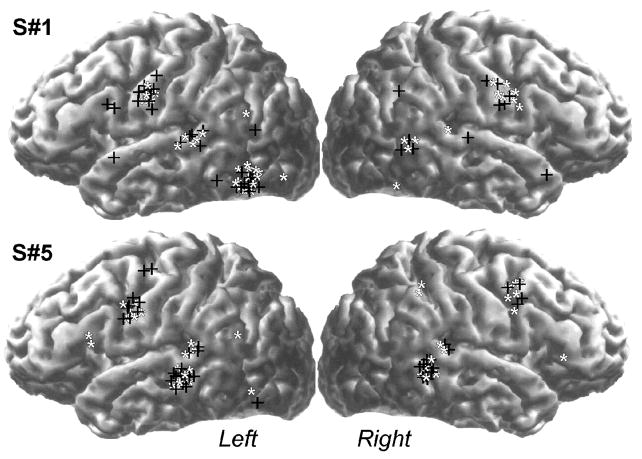
Figure 1.
Activation maps associated with processing of long-low frequency words for two participants demonstrating the two main profiles observed in the study. Participant 1 shows dipolar activity sources in ventral occipito-temporal cortices during the early stages of stimulus processing (onset = 200 ms), whereas the cluster of correspondingly early activity sources for participant 5 was located in the vicinity of the superior temporal sulcus. Magnetic sources are shown as stars and crosses for the first and second recording session, respectively.
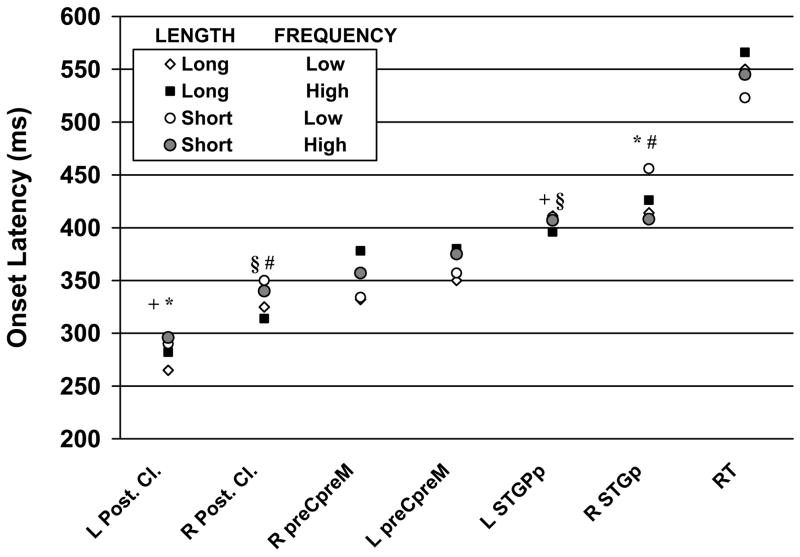
Figure 2.
Onset latency of activity indicating the earliest time (in milliseconds after stimulus onset) when magnetic activity was consistently detected in a particular region and stimulus condition across both sessions. Corresponding manual reaction times (RT) are included for reference. Sets of significantly (p <.001) different onset latencies are marked by the same symbol (+, §, *, or #). Abbreviations: Post. Cl.-ECD cluster in MTGp or ventral occipitotemporal cortex, preC-preM-Precentral-Premotor cortex, STGp-posterior portion of the superior temporal gyrus, in the right (R) and left (L) hemisphere.
As shown in Figure 2, magnetic activity was noted first in posterior temporal cortices (MTGp or VOT bilaterally) followed by bilateral perirolandic and premotor cortex activity. Although the delay between the onset of activity in the Posterior Cluster of areas and the earliest activity in area preC-preM was substantial (ranging from 44 to 96 ms across conditions) it failed to satisfy our rigorous alpha level (uncorrected p values ranged between .02 and .04). The delays in onset latency between the left Posterior Cluster of areas and STGp (both left and right) were significant (p <.0001), whereas the delay between right Posterior Cluster and STGp approached significance (p =.05-.01). The mean delay between the onset of activity in areas preC-preM and the onset of activity in STGp superior temporal gyrus were similar in magnitude (ranging from 40 to 80 ms) but none of these differences reached significance (uncorrected p values ranged between .3 and .04). On average, manual RTs were registered 180 ms after the onset of activity in preC-preM (200 ms, 190 ms, 170 ms, and 170 ms for long high frequency, long low frequency, short high frequency, and short low frequency words). This delay is expected if preC-preM activity is associated with the preparation and execution of a motor command for the button press. In a previous MEG study we reported a mean delay of approximately 150 ms between the onset of activity in premotor cortex and the onset of EMG activity in the contralateral hand (Castillo et al., 2004). There is also an estimated delay of 20–30 ms between the onset of distal muscle excitation and the onset of movement (Norman & Komi, 1979). Corresponding delays between the onset of activity in STGp and average RTs were 140 ms, 150 ms, 110 ms, and 130 ms.
MNE peak latency data were consistent with ECD findings. The four-way ANOVA on peak latency values revealed an ROI by Hemisphere interaction, F(3,48) = 140.78, p <.0001. No other main effects or interactions were significant (p >.5 in all cases). ROIs in each hemisphere were then ranked in ascending order of peak latency establishing the temporal progression of activity. Figure 3 shows the relative timing of activity across the eight regions (four ROIs in each hemisphere) as indicated by peak latency, and the similarity in peak latency values between conditions. As in the case of onset latency data the linear trend of region was significant, F(1,16) = 756.81, p <.0001 (p >.1 for all higher-order trends) indicating a regular progression of activity across the eight regions. Finally, in order to determine pairs of regions that showed reliable onset latency asynchronies, a series of 28 one-way ANOVAs with two levels on the ROI/Hemisphere factor were computed (testing onset latency differences between a given region and each of the regions with later onsets). This procedure was performed on data collapsed across sessions and conditions. F-values were evaluated at alpha = .001 to maintain probability of family-wise Type I error to approximately .05 given the total of 28 tests performed. As shown in Figure 3, peak latency differences between the posterior cluster areas and all subsequently peaking areas (including preC-preM and STGp) were significant. The activity peak in the inferior frontal ROI, bilaterally, took place with a significant delay from peak latency in all preceding areas, including preC-preM and STGp. Peak latency differences between preC-preM and STGp were not significant uncorrected p values ranged between .1 and .03).
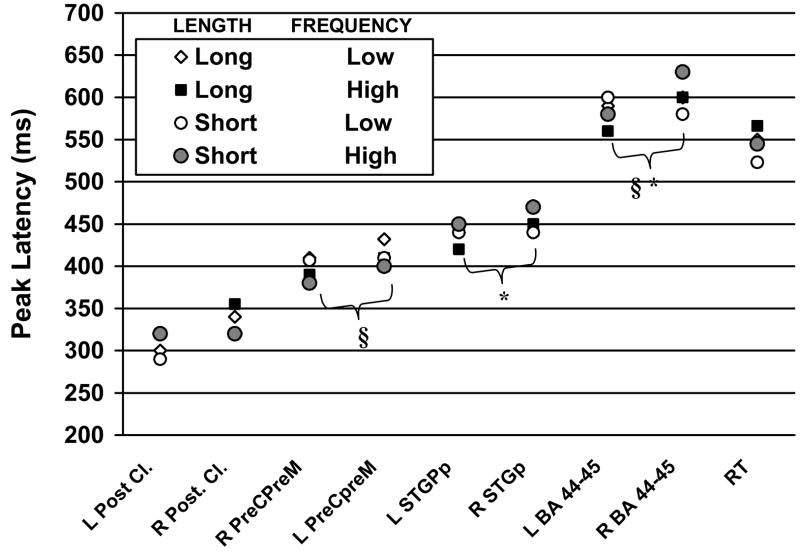
Figure 3.
Peak latency of activity in each ROI revealed by MNE analyses. Two more areas have been added to those reported in Figure 2 (ECD data): BA 44 and 45 in the inferior frontal gyrus bilaterally. Corresponding manual reaction times (RT) are included for reference. Pairs of significantly (p <.001) different onset latencies are marked by the same symbol (§, *). Peak latency in the posterior cluster of ROIs (VOT or MTGp) was took place significantly earlier than peak latency in all subsequent areas (symbols omitted from figure). Abbreviations: Post. Cl.-posterior cluster (MTGp or ventral occipitotemporal cortex), preC-preM-precentral-premotor cortex, STGp-posterior portion of the superior temporal gyrus, in the right (R) and left (L) hemisphere.
The average delay between the peak latency of activity in preC-preM and RT was 93 ms, 156 ms, 113 ms, and 145 ms for long high frequency, long low frequency, short high frequency, and short low frequency words. Corresponding delays between peak activity in STGp and average RT were 110 ms, 136 ms, 93 ms, and 95 ms. Finally, the peak activity in the inferior frontal ROI took place either slightly earlier (6 ms-long low frequency words) or after the corresponding averaged RT (range = 35–77 ms for the remaining conditions).
Length and frequency effects: ECD data
The four-way MANOVA interaction was significant for both sessions, Wilks Lamda =.408, F(6,60) = 5.66, p <.0001 for Session A, and Wilks Lamda =.12, F(6,60) = 14.16, p <.0001 for Session B. Given that main effects of ROI or simple-main effects of ROI within the levels of the other factors were not of interest, follow up analyses consisted of three-way univariate ANOVAs (Length by Frequency by Hemisphere) performed separately at each ROI on each of the three dependent variables (number of ECDs, Q, and RMS). Main effects, two and three way interactions are discussed further only if they reached statistical significance in the data sets for both sessions, in order to ensure that final results were stable over time (within participants).
Posterior temporal cluster
Across sessions there was a reliable Length by Hemisphere interaction for RMS (F[1,16] = 7.95, p <.01 for Session 1 and F[1,16] = 5.11, p <.04 for Session 2). Although a consistent tendency for long words to be associated with greater RMS measurements than short words was apparent for both sessions and in both hemispheres, only the long-short word difference in the right hemisphere reached significance after data from both sessions were collapsed together, F(1,16) = 12.32, p <.003 (p >.07 for data in the left hemisphere).
A Length by Frequency interaction was also found for Q measures. Simple main effects tests for Length at each frequency indicated a consistent tendency for greater Q values for long over short words which was restricted to low frequency stimuli. Again this tendency was apparent in the data sets from both sessions, but reached statistical significance when data were collapsed across sessions, F(1,16) = 38.22, p <.0001.
Finally, simple main effects tests contrasting frequency conditions at each word length indicated that low frequency, long words were associated with larger Q values than high frequency, long words in both sessions, F(1,16) = 5.14, p <.038 (Session A) and F(1,16) = 6.89, p <.018 (Session B). No significant effects were found on latency.
Superior temporal gyrus
A reliable Frequency by Hemisphere interaction was found for Q measures, F(1,16) = 26.13, p <.0001 (Session A) and F(1,16) = 11.11, p <.004 (Session B). Further tests indicated a consistent tendency for greater Q values in the low as compared to the high frequency condition. This tendency was restricted to dipolar magnetic data localized in the left superior temporal gyrus and reached statistical significance in the collapsed data across sessions, F(1,16) = 35.65, p <.0001. There was a smaller tendency in the same direction for right hemisphere activity which did not reach statistical significance (p >.052). Again, no consistent effects were found for the onset latency of activity.
preC-preM. Reliable Length by Frequency by Hemisphere interactions were found for both sessions for RMS, F(1,16) = 6.24, p <.024 (Session A) and F(1,16) = 8.30, p <.011 (Session B). Two-way ANOVAs indicated that the underlying Length by Frequency interaction was consistently significant only in the left hemisphere, F(1,16) = 10.34, p <.005 (Session A) and F(1,16) = 6.15, p <.025 (Session B). One way ANOVAs revealed reliable Length effects (long words eliciting greater RMS than short words) restricted to low frequency stimuli, F(1,16) = 4.56, p <.04 (Session A) and F(1,16) = 18.09, p <.001 (Session B).
Discussion
Timing information obtained with two complementary methods of MEG data analysis (ECD and MNE) indicated that neurophysiological activity in the posterior portion of the superior temporal gyrus (BA 22) associated with silent word reading takes place without a significant delay from activity in motor and premotor cortices (regardless of word frequency and length). The temporal delay between the onset of activity in the left BA 22 and the onset of the ensuing manual response does not appear to be sufficient to justify a key role of this region in the brain mechanism responsible for the required lexical decision. Data from a previous MEG study on the timing of cortical activity preceding a simple manual response (Castillo et al., 2004) showed a similar delay between the onset of activity in motor/premotor cortex and EMG activity in the contralateral hand (150 ms on average) as the delay between motor/premotor activity and mean RT in the present study. Presumably then, participants in the present study were prepared to make a response at the same time when significant activity in BA 22 was first detected, suggesting that under certain task requirements BA 22 may not be an indispensable component of the reading circuit. Previous MEG studies using naming tasks have reported activity in the left BA 22 at a sufficiently early latency to play a role in the construction of a phonological code (Salmelin, Schnitzler, Schmitz, & Freund, 2000; Simos et al., 2002b). Latency of activity in the left BA 22 was rather consistent across studies using different stimulus sets: ~400 ms in Salmelin et al. (2000), 467 ms in Simos et al. (2002b) and between 420–450 ms in the present study. In the context of pronunciation tasks, with average response times varying between 700 and 800 ms, activity in BA 22 peaked at approximately 300–400 ms prior to voice onset. In the context of the lexical decision task in the present study, however, with response latencies averaging 550 ms, BA 22 activity peaked a mere 100 ms prior to the mean manual RT. The same applies to the presumed role of inferior frontal cortex where hemodynamic activity is routinely observed in brain imaging studies of silent reading.
It has been suggested that lexical decisions on printed stimuli can be performed on the basis of visual/orthographic information alone (e.g., Seidenberg, 1985) especially for words characterized by high frequency of occurrence, an index of a high level of lexical and/or orthographic familiarity. Degree of familiarity is presumed to be inversely related to demands for phonological decoding: low frequency words are more likely to require phonological decoding than high frequency words at least for items characterized by a high degree of orthographic regularity as was the case for virtually all of the word stimuli used in the study (Turvey, Feldman, & Lukatela, 1984; Waters & Seidenberg, 1985). The fact that delays between activity in BA 22 (and BA 44/45 as well) and RT were similar regardless of word frequency implies that the presumed secondary role of these regions to word recognition during lexical decision persists in the face of stimulus variations which may affect lexicality and perhaps also the involvement of decoding processes. It should be noted that words and pseudowords in the present study were all single-syllable and matched for length (number of letters) and a no/no-go procedure was chosen in order to reduce response selection processes (Gordon, 1983) and enhance overall response speed (Perea, Rosa, & Gomez, 2003).
Which then are the component processes supported by cortical sites located within BA 22? There is ample evidence linking impairments in phonological processing of spoken and written language to acquired damage to the posterior superior temporal and the adjacent temporoparietal area in the left hemisphere (Beauvois & Derouesne, 1979; Caplan, Gow, & Makris, 1995). Results of hemodynamic brain imaging studies are also consistent with a role of posterior BA 22 sites in sub-word level phonological processing and analysis (Binder et al., 2000; Jacquemot et al., 2003; Majerus et al., 2005; Scott, Blank, Rosen, & Wise, 2000; Specht et al., 2003). Thus, in tasks that require phonological decoding, whether they involve oral or silent reading, activity in the left BA 22 appears to be associated with the engagement of neurophysiological processes responsible for the conversion of print to sound. These processes include, but are not limited to, storage of sublexical phonological representations, automatic retrieval and short-term maintenance of these representations in an active state that allows them to be maintained in consciousness (Hickok & Poeppel, 2000; Hughes et al., 2001; Mustovic et al., 2003; Wise et al., 2001). It has been proposed that the brain mechanism responsible for the aforementioned processes involves other areas as well, notably the adjacent supramarginal (Hautzel et al., 2002; Jonides et al., 1998) and angular gyri (Joubert et al., 2004). Most available evidence from lesion and electrical interference studies (Simos et al., 2000a, 2002b; Boatman, 2006) indicates, further, that BA 22 sites are indispensable components of the brain mechanism specialized for these processes. This view is consistent with developmental studies suggesting that the posterior-dorsal reading circuit, of which the left BA 22 is a major component, appears to be crucial during the early stages of learning to read (Simos et al., 2000b, 2000c, 2005), by hosting, primarily, neurophysiological processes that support phonological processing. In reading disabled students this area shows the most prominent changes in the degree and duration of activity in response to educational interventions during tasks that require phonological decoding (Simos et al., 2002a, 2007a).
It is important to note, however, that both imaging and lesion data do not clearly indicate exclusive involvement of the left STGp in phonological processing. There is also evidence that this region plays a crucial role for lexical/semantic processing of spoken language. Patients exhibiting the clinical profile of fluent aphasia, which typically results from damage to this region (Benson, 1985; Vignolo, 1988), almost invariably display notable deficits in a variety of experimental tasks tapping into lexical/semantic processing as well as in spontaneous speech and writing performance (Hagoort, 1993; Janse, 2006; Rodd, Davis, & Johnsrude, 2005). Moreover, these patients are often found to be more impaired in the elaboration (production, reading, and judgment) of grammatically irregular word forms, a function which presumably relies less on phonological processing/decoding and more on processing at the word level (Seidenberg, 1985; Ullman et al., 2005). There are also reports that at least in adult, experienced readers, the posterior portion of BA 22 is involved in lexical semantic processing during reading tasks. Brain imaging data using a variety of techniques support this conclusion. For instance, Okada and Hickok (2006) reported fMRI data implying that sites within the posterior portion of the superior temporal gyrus are involved in the storage and/or activation of lexical/semantic representations. Neurophysiological activity linked to word recognition in the same region has also been reported using direct epicortical recordings (optical imaging: Cannestra et al., 2000). Repetition priming at the whole-word phonological level may reduce activity in this region during performance of a lexical decision task (Haist et al., 2001). Several other hemodynamic studies have reported activity peaks in this region resulting from task comparisons which presumably isolate lexical (Fiebach et al., 2002; Fiez et al., 1999; Howard et al., 1992), or semantic processing of written words (Grossman et al., 2002; Heim et al., 2002; Perani et al., 1999). In the present study, the degree of activity in the left BA 22 was modulated by lexicality (stronger magnetic activity in response to low than high frequency words) implying a closer involvement of this region in processing unfamiliar written words, which presumably relies more heavily on phonological decoding. This processing mode is expected to be more prominent for regular low-frequency words (regularity was unfortunately not manipulated systematically in the present study). As explained in more detail above, the onset latency of activity in this region suggests, however, that BA 22 engagement was not an essential component of the brain circuit responsible for performing lexical decisions.
We don’t have sufficient data to determine whether activity in the left BA 22, in the context of the lexical decision task used in the present study, reflected processing at the lexical or sublexical level, or possibly both. If BA 22 were engaged in sublexical, phonologic processing, the timing data indicate that this type of process may not have been an essential component of the cognitive mechanism for recognizing words and may have occurred automatically as several lines of evidence suggests (Seidenberg, Petersen, Plaut, & MacDonald, 1996; Spoehr, 1978; Van Orden, 1987) but in the form of phonological recoding (e.g., Underwood & Thwaites, 1982). Similar findings have been reported by fMRI studies of silent word reading (Joubert et al., 2004). If, on the other hand, activity in the left BA 22 reflected processing at the lexical level, then its role, given the particular task demands, was probably redundant and other regions which showed activity earlier, namely the posterior middle temporal and occipitotemporal regions (see below). Regardless of the roles of BA 22 in reading, in general, the present findings imply that the intrinsic organization of the brain circuit for reading can be adjusted to particular task demands to produce the requisite cognitive decision and response, consistent with previous reports (Nakamura et al., 2006).
By using a complementary data analysis procedure that permits estimation of practically thousands of independent, simultaneously active sources at any given point in time after stimulus onset (MNE), in addition to concurring the finding concerning the relative timing of activity in STGp and response time, we also obtained evidence that neurophysiological activity in the inferior frontal gyrus (BA 44 & 45) peaks not only later than the peak of activity in STGp but also, for most conditions) after the manual response has taken place. This finding renders exceedingly unlikely the possibility that activity in this region plays a key role in the cognitive operations involved in word recognition, at least during speeded, silent reading. It should be pointed out that our finding does not preclude a key role of this region in tasks that pose different demands on the reading mechanism (requiring naming as opposed to a button press response) in adult experienced readers, and certainly does not diminish the purported role of this region during the acquisition of reading skill. At least in children, however, early engagement of the inferior frontal gyrus in the sequence of neurophysiological events involved in single word reading (aloud) is a sign of aberrant developmental of the reading mechanism, and is consistently found in students who experience severe reading difficulties (Simos et al., 2007a,b).
Another finding of the present study concerns individual variability in the precise location of reproducible activity during the early stages of the processing of printed words. One subgroup of 12 participants demonstrated activity in the posterior portion of the middle temporal gyrus near its border with BA 37. In the remaining participants activity with similar temporal and stimulus-related features was found in posterior-ventral occipitotemporal cortices. One possibility is that the precise anatomical location of the area dedicated to visual/orthographic processing may indeed differ across individuals, a fact that has simply been overlooked by previous hemodynamic studies. Alternatively, systematic individual variability may reflect the engagement of at least two functionally distinct regions in different participants. One region may correspond to the VWFA (in ventral occipitotemporal cortices: Cohen et al., 2000, 2002; Rapcsak & Beeson, 2004) and the other to a site within the superior temporal sulcus in the posterior portion of the middle temporal gyrus (area BA 21/37). The issue of inter-individual differences in activation maps during performance of language tasks has been systematically explored in few studies thus far (e.g., Seghier et al., 2004) and attributed to anatomical differences, variability in the layout of component neurophysiological processes, and differences in cognitive strategies (Nadeau et al., 1998; Ojemann et al., 1989; Rademacher et al., 1993; Steinmetz & Seitz, 1991). While there was systematic variability in the location of early activity in ventral occipitotemporal and posterior middle temporal gyri between participants, the method of data acquisition and analysis used in the present study ensured that only the most stable activity sources (across sessions and stimulus types) within individual datasets were considered.
In our data, activity in posterior middle temporal and occipitotemporal cortices took place sufficiently early during stimulus processing to play a key role in the lexical decision onto which the recorded manual response is presumably based. Further, activity in this area was modulated not only by visual word characteristics (word length) but also by word frequency, showing stronger magnetic responses to low than high frequency words. There is strong evidence that the ventral occipitotemporal region plays a crucial role in the brain mechanism responsible for storing (or automatically retrieving) orthographic information (knowledge regarding frequently occurring graphemic patterns; for a review see McCandliss, Cohen, & Dehaene, 2003). Damage to this region often results in a severe form of alexia characterized by ‘letter-by-letter reading’ (Binder and Mohr, 1992; Cohen et al., 2000; Leff, Scott, Rothwell, & Wise, 2001; Sakurai et al., 2000). Neuroimaging studies and studies using intracranial recordings have reported stronger/more extensive activation in this region to words and word-like nonwords than to consonant letter strings or nonsense characters (Cohen et al., 2000, 2002; Tarkiainen et al., 1999). A more direct link to lexical/semantic processing is suggested by findings of greater hemodynamic activation in response to low than to high frequency words (Kronbichler et al., 2004), and systematic lexical/semantic priming effects during the first 400 ms after stimulus onset on spectral components of the magnetic signal (McNab, Rippon, Hillebrand, Singh, & Swithenby, 2007). Our finding is consistent with the notion that ventral occipitotemporal cortices are involved in storing or gaining access to abstract orthographic representations. The latter may take the shape of “word forms” as suggested by a recent fMRI study that reported increased hemodynamic response of this region to low vs. high frequency words Kronbichler et al. (2004). Alternatively, the feature of orthographic familiarity displayed by high frequency words may also be operate at the sub-lexical level as suggested by findings that hemodynamic activity in this region is modulated by the degree of familiarity of letter combinations regardless of whether these sequences were part of words or not (Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006).
In some participants, activity clusters were consistently found in the posterior portion of the middle temporal gyrus near the border with BA 37. This region has been put forward by several studies as hosting neurophysiological processes involved in storing or accessing lexical/semantic information in printed stimuli (Pugh et al., 1996; Fiebach et al., 2002). Damage to this region has been linked to deficits in a variety of tasks tapping into lexica/semantic processing, such as semantic priming (Tyler, Marslen-Wilson, & Stamatakis, 2005). Moreover, the onset latency of magnetic activity in this region significantly correlated with pronunciation latency of orthographically exceptional words and this was clearly not the case for regular words and pseudowords (Simos et al., 2002b).
A final note regarding the length effect that was found in the present study for RT (slower RTs to longer words) is warranted here. More commonly length effects are found in the context of naming tasks (e.g., Weekes, 1997). For silent lexical decision tasks length effects are typically found during lateralized presentation of stimuli to the left visual field (Weekes, Capetillo-Cunliffe, Rayman, Iacoboni, & Zaidel, 1999), and for centrally presented low frequency words (Ferrand & New, 2003). Length effects have been reported, however, in the context of lexical decision tasks requiring a manual response (Grieco, Bettella, Marco Conti, Orioli, & Casco, 2007; for a recent review see New, Ferrand, Pallier, Brysbaert, 2006). Often length effects in the context of silent reading tasks are considered as indications of increased engagement of phonological decoding operations in reading (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001).
One possible explanation of the length effect found in the present study is that long words subtended large enough eccentricities so that a significant portion of each letter string was parafoveally presented within the left visual field, where length effects are supposed to be stronger (Ellis, 2004). However, the horizontal angular size of the stimuli used in this study (2–3°) implies that even the longest words fell entirely within the foveal region. Presentation parameters used in the study may have been responsible for the length effect. First, stimulus duration (600 ms) was relatively long compared to the majority of studies using exposure times shorter than 400 ms (incidentally longer exposure durations may allow refixations during reading which are more likely to occur with longer words, prolonging response times; Verilino-Perez, Colins, & Dore-Mazars, 2004). Second, stimuli were blocked for length creating a setting which may have encouraged consistent adoption of different strategies for long versus short stimuli. Strategic context effects have been previously reported in lexical decision tasks (e.g., Perea, Carreiras, & Grainger, 2004). For instance, longer exposure durations may permit decoding operations to take place. In principle, this tendency may have been enhanced by increased similarity between words and pseudowords in a particular list, which may be more of an issue with long than short words (New, Ferrand, Pallier, & Brysbaert, 2006).
Among the potential limitations of the present study is that the brain activation profiles presented here were obtained in the context of a task that may not fully represent the conditions of normal silent reading and in response to monosyllabic words. Lexical decision is but one of many reading or reading-related tasks that have been studied during a variety of brain imaging tasks. Also, lexical decision assesses some important reading-related processes--mainly access to the nature of representations in the mental lexicon for words (e.g. phonological codes, orthographic codes, spreading semantic activation). As such, it is a metalinguistic task requiring judgments of wordness. It is not necessarily the case that during on-line skilled reading of words in context that the brain or mind engages in exactly the same set of cognitive processes in identifying words. Thus the lexical decision task assesses some—and not all—aspects of processes related to reading. The nature of the task may have been responsible for the scarcity of inferior parietal activation which is often observed in brain imaging studies especially when overt pronunciation of printed stimuli is required (e.g., Nakamura et al., 2006). Moreover, in spite of the clear temporal pattern of regional activity observed, most of the activated regions are also active during non-reading tasks, casting doubt on a view of exclusive specificity for reading.
To conclude, the present findings suggest that the posterior portion of the superior temporal gyrus may not be critical part of the reading circuit when word identification does not involve pronunciation (i.e. in silent reading tasks such as lexical decision). Recognizing whether or not a word is in fact a real word thus appears not to require timely engagement of the left STGp and inferior frontal cortex under the conditions afforded by the lexical decision task used in this study. In view of the most commonly ascribed role of these regions in the cognitive function of reading by lesion and neuroimaging studies, this finding is consistent with the notion that phonological decoding and recoding operations are not indispensable components of word recognition at least in adult, skilled readers. Future studies varying decoding and response requirements (such as including a word naming condition and vary list composition in order to modulate decoding/recoding requirements) are needed to test these conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Panagiotis G. Simos, Department of Psychology, University of Crete, Greece
Kenneth Pugh, Haskins Laboratories.
Einar Mencl, Haskins Laboratories.
Stephen Frost, Haskins Laboratories.
Jack M. Fletcher, Department of Psychology, University of Houston
Shirin Sarkari, Vivian Lee Smith Center for Neurologic Research, Department of Neurosurgery, University of Texas Health Science Center Houston.
Andrew C. Papanicolaou, Vivian Lee Smith Center for Neurologic Research, Department of Neurosurgery, University of Texas Health Science Center Houston
References
- Balota DA, Chumbley JI. Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology: Human Perception & Performance. 1984;10:340–357. doi: 10.1037//0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- Beauvois MF, Derouesne J. Phonological alexia: three dissociations. Journal of Neurology, Neurosurgery and Psychiatry. 1979;42:1115–1124. doi: 10.1136/jnnp.42.12.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DF. Aphasia. In: Heilman K, Valenstein E, editors. Clinical neuropsychology. New York: Oxford University Press; 1985. [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115:1807–26. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–93. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–48. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman DF. Cortical auditory systems: speech and other complex sounds. Epilepsy & Behavior. 2006;8:494–503. doi: 10.1016/j.yebeh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002;16:251–61. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Papanicolaou AC, Zouridakis G, Wilmore LJ, Wheless JW, Constantinou JC, Maggio WW. Language dominance determined by magnetic source imaging: A comparison with the Wada Procedure. Neurology. 1999;22:938–945. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Wheless JW, Constantinou JEC, Papanicolaou AC. Hemispheric language dominance in children determined by magnetic source imaging. Journal of Child Neurology. 2001;16:124–130. doi: 10.1177/088307380101600211. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Black KL, Martin NA, Cloughesy T, Burton JS, Rubinstein E, Woods RP, Toga AW. Temporal and topographical characterization of language cortices using intraoperative optical intrinsic signals. Neuroimage. 2000;12:41–54. doi: 10.1006/nimg.2000.0597. [DOI] [PubMed] [Google Scholar]
- Caplan D, Gow D, Makris N. Analysis of lesions by MRI in stroke patients with acoustic–phonetic processing deficits. Neurology. 1995;45:293–298. doi: 10.1212/wnl.45.2.293. [DOI] [PubMed] [Google Scholar]
- Castillo EM, Simos PG, Wheless J, Baumgartner EJ, Breier JI, Billingsley RL, Sarkari S, Papanicolaou AC. Integrating sensory and motor mapping in a comprehensive MEG protocol. Clinical validity and replicability. Neuroimage. 2004;21:973–83. doi: 10.1016/j.neuroimage.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivard S, et al. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dale A, Sereno M. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio A. The anatomic basis of pure alexia. Neurology. 1983;33:1573–83. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Ellis AW. Length, formats, neighbours, hemispheres, and the processing of words presented laterally or at fixation. Brain & Language. 2004;88:355–66. doi: 10.1016/S0093-934X(03)00166-4. [DOI] [PubMed] [Google Scholar]
- Ferrand L, New B. Syllabic length effects in visual word recognition and naming. Acta Psychologica (Amst) 2003;113:167–83. doi: 10.1016/s0001-6918(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences (USA) 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, Xu B, Petrella JR, Balsamo L, Basso G. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;57:47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- Gordon B. Lexical access in lexical decision: Mechanisms of frequency sensitivity. Journal of Verbal Learning and Verbal Behavior. 1983;22:24–44. [Google Scholar]
- Grieco A, Bettella S, Conti M, Orioli M, Casco C. The effect of preview eccentricity in a lexical decision task. Visual Cognition. 2004;11:781–796. [Google Scholar]
- Hagoort P. Impairments of lexical-semantic processing in aphasia: Evidence from the processing of lexical ambiguities. Brain & Language. 1993;45:189–232. doi: 10.1006/brln.1993.1043. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The neural circuitry involved in the reading of German words and pseudowords: A PET study. Journal of Cognitive Neuroscience. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Haist F, Song AW, Wild K, Faber TL, Popp CA, Morris RD. Linking sight and sound: fMRI evidence of primary auditory cortex activation during visual word recognition. Brain & Language. 2001;76:340–350. doi: 10.1006/brln.2000.2433. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage. 2002;17:1101–16. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Medical Biological Engineering Computing. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Kraut MA, Kremen S, Soher B, Gordon B. Neural substrates of orthographic lexical access as demonstrated by functional brain imaging. Neuropsychiatry Neuropsychology & Behavioral Neurology. 2000;13:1–7. [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R. The cortical localization of the lexicons. Positron emission tomography evidence. Brain. 1992;115:1769–82. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Hughes HC, Darcey TM, Barkan HI, Williamson PD, Roberts DW, Aslin CH. Responses of human auditory association cortex to the omission of an expected acoustic event. Neuroimage. 2001;13:1073–1089. doi: 10.1006/nimg.2001.0766. [DOI] [PubMed] [Google Scholar]
- Jacquemot C, Pallier C, Le Bihan D, Dehaene S, Dupoux E. Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. Journal of Neuroscience. 2003;23:9541–9546. doi: 10.1523/JNEUROSCI.23-29-09541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse E. Lexical competition effects in aphasia: Deactivation of lexical candidates in spoken word processing. Brain & Language. 2006;97:1–11. doi: 10.1016/j.bandl.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, Tzourio-Mazoyer N. Impact of modality and linguistic complexity during reading and listening tasks. Neuroimage. 2007;34:784–800. doi: 10.1016/j.neuroimage.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Joseph J, Noble K, Eden G. The neurobiological basis of reading. Journal of Learning Disabilities. 2001;34:566–79. doi: 10.1177/002221940103400609. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux JM, Karama S, Lecours AR. Neural correlates of lexical and sublexical processes in reading. Brain & Language. 2004;89:9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-Day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: An fMRI study. Journal of Cognitive Neuroscience. 2000;12:321–341. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Leff AP, Scott SK, Rothwell JC, Wise RJ. The planning and guiding of reading saccades: a repetitive transcranial magnetic stimulation study. Cerebral Cortex. 2001;11:918–23. doi: 10.1093/cercor/11.10.918. [DOI] [PubMed] [Google Scholar]
- Maestú F, Ortiz T, Fernández A, Amo C, Martin P, Fernandez S. Spanish language mapping using MEG: A validation study. Neuroimage. 2002;17:1579–1586. doi: 10.1006/nimg.2002.1235. [DOI] [PubMed] [Google Scholar]
- Majerus S, Linden MV, Collette F, Laureys S, Poncelet M, Degueldre C, Delfiore G, Luxen A, Salmon E. Modulation of brain activity during phonological familiarization. Brain & Language. 2005;92:320–331. doi: 10.1016/j.bandl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McNab F, Rippon G, Hillebrand A, Singh KD, Swithenby SJ. Semantic and phonological task-set priming and stimulus processing investigated using magnetoencephalography (MEG) Neuropsychologia. 2007;45:1041–1054. doi: 10.1016/j.neuropsychologia.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Menard MT, Kosslyn SM, Thompson WL, Alpert NM, Rauch SL. Encoding words and pictures: A positron emission tomography study. Neuropsychologia. 1996;34:185–194. doi: 10.1016/0028-3932(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: divisible by what? Journal of Cognitive Neuroscience. 1998;10:766–77. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- Mustovic HK, Scheffler F, Di Salle F, Esposito JG, Hennig J, et al. Temporal integration of sequential auditory events: silent period in sound pattern activates human planum temporale. Neuroimage. 2003;20:429–434. doi: 10.1016/s1053-8119(03)00293-3. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, Williamson DJ, Crosson B, Gonzalez Rothi LJ, Heilman KM. Functional imaging: heterogeneity in task strategy and functional anatomy and the case for individual analysis. Neuropsychiatry, Neuropsychology & Behavioral Neurology. 1998;11:83–96. [PubMed] [Google Scholar]
- Nakamura K, Hara N, Kouider S, Takayama Y, Hanajim R, Sakai K, Ugawa Y. Task-guided selection of the dual neural pathways for reading. Neuron. 2006;52:557–564. doi: 10.1016/j.neuron.2006.09.030. [DOI] [PubMed] [Google Scholar]
- New B, Ferrand L, Pallier C, Brysbaert M. Reexamining the word length effect in visual word recognition: new evidence from the English Lexicon Project. Psychonomic Bulletin Reviews. 2006;13:45–52. doi: 10.3758/bf03193811. [DOI] [PubMed] [Google Scholar]
- Norman RW, Komi PV. Electromechanical delay in skeletal muscle under normal movement conditions. Acta Physiologica Scandinavica. 1979;106:241–8. doi: 10.1111/j.1748-1716.1979.tb06394.x. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Okada K, Hickok G. Identification of lexical–phonological networks in the superior temporal sulcus using functional magnetic resonance imaging. Neuroreport. 2006;17:1293–1296. doi: 10.1097/01.wnr.0000233091.82536.b2. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, Billingsley RL, Buchanan S, Wheless J, Maggio V, Maggio WW. Magnetoencephalography: a noninvasive alternative to the Wada procedure. Journal of Neurosurgery. 2004;100:867–76. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- Perea M, Carreiras M, Grainger V. Blocking by word frequency and neighbourhood density in visual word recognition: a task-specific response criteria account. Memory & Cognition. 2004;32:1090–102. doi: 10.3758/bf03196884. [DOI] [PubMed] [Google Scholar]
- Perea M, Rosa E, Gomez C. Influence of neighborhood size and exposure duration on visual-word recognition: evidence with the yes/no and the go/no-go lexical decision tasks. Perception & Psychophysics. 2003;65:273–86. doi: 10.3758/bf03194799. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–38. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Caviness VS, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cerebral Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62:2221–9. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rastle K, Kinoshita S, Lupker SJ, Coltheart M. Cross-task strategic effects. Memory & Cognition. 2003;6:867–76. doi: 10.3758/bf03196441. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex. 2005;15:1261–9. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Roux FE, Lubrano V, Lauwers-Cances V, Tremoulet M, Mascott CR, Demonet JF. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain. 2004;127:1796–810. doi: 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Takeuchi S, Takada T, Horiuchi E, Nakase H, Sakuta M. Alexia caused by a fusiform or posterior inferior temporal lesion. Journal of Neurological Sciences. 2000;178:42–51. doi: 10.1016/s0022-510x(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund HJ. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Schilling HH, Rayner K, Chumbley JI. Comparing naming, lexical decision, and eye fixation times: Word frequency effects and individual differences. Memory & Cognition. 1998;26:1270–1281. doi: 10.3758/bf03201199. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni J-M, Zimine I, Mayer E, Michel CM, Khateb A. Variability of fMRI activation during a phonological and semantic language task in healthy subject. Human Brain Mapping. 23:140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS. The time course of phonological code activation in two writing systems. Cognition. 1985;19:1–30. doi: 10.1016/0010-0277(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, Petersen A, Plaut DC, MacDonald MC. Pseudohomophone effects and models of word recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:48–62. doi: 10.1037//0278-7393.22.1.48. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Wheless JW, Constantinou JE, Gormley WB, et al. Localization of language-specific cortex by using magnetic source imaging and electrical stimulation mapping. Journal of Neurosurgery. 1999;91:787–96. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, Papanicolaou AC. Brain mechanisms for reading: the role of the superior temporal gyrus in word and pseudoword naming. Neuroreport. 2000a;11:2443–7. doi: 10.1097/00001756-200008030-00021. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: A Magnetic Source Imaging approach. Cerebral Cortex. 2000b;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Fletcher JM, Foorman BR, Bergman E, Fishbeck K, Papanicolaou AC. Brain activation profiles in dyslexic children during nonword reading: A magnetic source imaging study. Neuroscience Letters. 2000c;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Davis RN, Fitzgerald M, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002a;58:1203–13. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC. Brain mechanisms for reading words and pseudowords: An integrated approach. Cerebral Cortex. 2002b;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley-Marshall RL, Francis DJ, Castillo EM, Denton C, Papanicolaou AC. Early development of neurophysiological processes involved in normal reading and reading disability. Neuropsychology. 2005;19:787–98. doi: 10.1037/0894-4105.19.6.787. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton C, Papanicolaou AC. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychology. 2007a;21:485–96. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley-Marshall R, Denton CA, Papanicolaou AC. Intensive instruction affects brain magnetic activity associated with oral word reading in children with persistent reading disabilities. Journal of Learning Disabilities. 2007b;40:37–48. doi: 10.1177/00222194070400010301. [DOI] [PubMed] [Google Scholar]
- Specht K, Holtel C, Zahn R, Herzog H, Krause BJ, Motthagy FM, et al. Lexical decision of nonwords and pseudowords in humans: A positron emission tomography study. Neuroscience Letters. 2003;345:177–181. doi: 10.1016/s0304-3940(03)00494-4. [DOI] [PubMed] [Google Scholar]
- Spoehr KT. Phonological encoding in visual word recognition. Journal of Verbal Learning and Verbal Behavior. 1978;17:127–141. [Google Scholar]
- Steinmetz H, Seitz RJ. Functional anatomy of language processing: neuroimaging and the problem of individual variability. Neuropsychologia. 1991;29:1149–61. doi: 10.1016/0028-3932(91)90030-c. [DOI] [PubMed] [Google Scholar]
- Szymanski MD, Perry DW, Gage NM, Rowley HA, Walker J, Berger MS, Roberts TP. Magnetic source imaging of late evoked field responses to vowels: toward an assessment of hemispheric dominance for language. Journal of Neurosurgery. 2001;94:445–53. doi: 10.3171/jns.2001.94.3.0445. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–32. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turvey MT, Feldman LB, Lukatela G. The Serbo-Croatian orthographyconstraints the reader to a phonologically analytic strategy. In: Henderson L, editor. Orthographies and reading. London: Erlbaum; 1984. pp. 81–89. [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Stamatakis EA. Differentiating lexical form, meaning, and structure in the neural language system. Proceedings of the National Academy of Science. 2005;102:8375–8380. doi: 10.1073/pnas.0408213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT, Pancheva R, Love T, Yee E, Swinney D, Hickok G. Neural correlates of lexicon and grammar: Evidence from the production, reading, and judgment of inflection in aphasia. Brain and Language. 2005;93:185–238. doi: 10.1016/j.bandl.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Underwood G, Thwaites S. Automatic phonological coding of unattended printed words. Memory & Cognition. 1982;10:434–442. doi: 10.3758/bf03197645. [DOI] [PubMed] [Google Scholar]
- Van Orden GC. A ROWS is a ROSE: spelling, sound, and reading. Memory & Cognition. 1987;15:181–198. doi: 10.3758/bf03197716. [DOI] [PubMed] [Google Scholar]
- Verilino-Perez D, Colins T, Dore-Mazars K. Decision and metris of refixations in reading isolated words. Vision Research. 2004;44:2009–2017. doi: 10.1016/j.visres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the Visual Word Form Area? Neuroimage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vignolo L. The anatomical and pathological basis of aphasia. In: Rose FC, Whurr R, Wyke MA, editors. Aphasia. London: Whurr; 1988. [Google Scholar]
- Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Waters GS, Seidenberg MS. Spelling-sound effects in reading: Time course and decision criteria. Memory & Cognition. 1985;13:557–572. doi: 10.3758/bf03198326. [DOI] [PubMed] [Google Scholar]
- Weekes BS. Differential effects of number of letters on word and nonword naming latency. The Quarterly Journal of Experimental Psychology. 1997;50A:439–456. [Google Scholar]
- Weekes NY, Capetillo-Cunliffe L, Rayman J, Iacoboni M, Zaidel E. Individual differences in the hemispheric specialization of dual route variables. Brain & Language. 1999;67:110–33. doi: 10.1006/brln.1998.2045. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Leuthold AC, Lewis SM, Georgopoulos AP, Pardo PJ. The time and space of lexicality: a neuromagnetic view. Experimental Brain Research. 2005;162:1–13. doi: 10.1007/s00221-004-2099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Wydell TN, Vuorinen T, Helenius P, Salmelin R. Neural correlates of letter-string length and lexicality during reading in a regular orthography. Journal of Cognitive Neuroscience. 2003;15:1052–62. doi: 10.1162/089892903770007434. [DOI] [PubMed] [Google Scholar]