Abstract
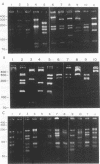
We applied a molecular typing approach for Helicobacter pylori that uses restriction fragment length polymorphism (RFLP) analyses of an 820-bp PCR-amplified portion of the ureC gene in H. pylori. The PCR products were digested with restriction enzyme HhaI, MboI, or MseI, and the fragments generated were analyzed by agarose electrophoresis. Among 25 independent clinical isolates, each showed a different pattern when a combination of the three RFLP patterns was used. Using this method, we studied isolates from the antrum or the body of the stomach of 14 patients before and after antibiotic therapy. Before treatment, successful isolation of H. pylori from the two sites of the stomach was possible for 12 of the 14 patients. For 10 of these 12 patients, each pair of isolates had identical RFLP profiles. For the other two patients (16.7%), however, isolates from the antrum and the body of the stomach had different RFLP profiles. Treatment was successful for 6 of the 14 patients; of the 8 patients with treatment failures, 5 had identical isolate pairs. In each case, the isolates found posttreatment were the same as the pretreatment isolates. For one of the patients who was colonized with two different isolates pretreatment, one of the isolates was identified at both sites after unsuccessful treatment. We also studied six long-term follow-up patients who had sequential biopsies at intervals of up to 5 months. Each follow-up isolate from each patient had the same RFLP profile as the initial isolate. This typing method provides a reliable and reproducible typing scheme for the study of H. pylori infections and indicates that infection with more than one H. pylori isolate is not rare.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyanz N., Bukanov N. O., Westblom T. U., Berg D. E. PCR-based RFLP analysis of DNA sequence diversity in the gastric pathogen Helicobacter pylori. Nucleic Acids Res. 1992 Dec 11;20(23):6221–6225. doi: 10.1093/nar/20.23.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beji A., Vincent P., Darchis I., Husson M. O., Cortot A., Leclerc H. Evidence of gastritis with several Helicobacter pylori strains. Lancet. 1989 Dec 9;2(8676):1402–1403. doi: 10.1016/s0140-6736(89)92020-5. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Burnie J. P., Lee W., Dent J. C., McNulty C. A. Immunoblot fingerprinting of Campylobacter pylori. J Med Microbiol. 1988 Oct;27(2):153–159. doi: 10.1099/00222615-27-2-153. [DOI] [PubMed] [Google Scholar]
- Clayton C. L., Kleanthous H., Morgan D. D., Puckey L., Tabaqchali S. Rapid fingerprinting of Helicobacter pylori by polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1993 Jun;31(6):1420–1425. doi: 10.1128/jcm.31.6.1420-1425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxall P. A., Hu L. T., Mobley H. L. Use of polymerase chain reaction-amplified Helicobacter pylori urease structural genes for differentiation of isolates. J Clin Microbiol. 1992 Mar;30(3):739–741. doi: 10.1128/jcm.30.3.739-741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pylori: its link to gastritis and peptic ulcer disease. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S87–S93. doi: 10.1093/clinids/12.supplement_1.s87. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Megraud F., Bonnet F., Garnier M., Lamouliatte H. Characterization of "Campylobacter pyloridis" by culture, enzymatic profile, and protein content. J Clin Microbiol. 1985 Dec;22(6):1007–1010. doi: 10.1128/jcm.22.6.1007-1010.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., Kureishi A., Wong S., Bryan L. E. Categorization of clinical isolates of Helicobacter pylori on the basis of restriction digest analyses of polymerase chain reaction-amplified ureC genes. J Clin Microbiol. 1993 May;31(5):1334–1335. doi: 10.1128/jcm.31.5.1334-1335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. J., Ali M. R., Nicholson G. I., Perez-Perez G. I., Blaser M. J. Long-term follow-up of voluntary ingestion of Helicobacter pylori. Ann Intern Med. 1991 Apr 15;114(8):662–663. doi: 10.7326/0003-4819-114-8-662. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Hunton C., Bickley J., Moreno M., Linton D. Ribosomal RNA gene restriction patterns of Helicobacter pylori: analysis and appraisal of Hae III digests as a molecular typing system. Epidemiol Infect. 1992 Aug;109(1):35–47. [PMC free article] [PubMed] [Google Scholar]
- Penfold S. S., Lastovica A. J., Elisha B. G. Demonstration of plasmids in Campylobacter pylori. J Infect Dis. 1988 Apr;157(4):850–851. doi: 10.1093/infdis/157.4.850. [DOI] [PubMed] [Google Scholar]
- Peterson W. L., Graham D. Y., Marshall B., Blaser M. J., Genta R. M., Klein P. D., Stratton C. W., Drnec J., Prokocimer P., Siepman N. Clarithromycin as monotherapy for eradication of Helicobacter pylori: a randomized, double-blind trial. Am J Gastroenterol. 1993 Nov;88(11):1860–1864. [PubMed] [Google Scholar]
- Prewett E. J., Bickley J., Owen R. J., Pounder R. E. DNA patterns of Helicobacter pylori isolated from gastric antrum, body, and duodenum. Gastroenterology. 1992 Mar;102(3):829–833. doi: 10.1016/0016-5085(92)90165-u. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]