This is part of a series of articles on clinical trials that will run in the CMAJ News section throughout 2009.
The cost of conducting a clinical trial for a drug is rising like mercury on a summer afternoon, a trend that researchers say is hampering the development of new medicines and is bad news for academia, pharmaceutical companies and consumers.
From the 1980s to the 1990s, the clinical trial costs of drug development increased 5 times faster than preclinical costs, according to the Tuft Center for the Study of Drug Development. In 2003, some health economists in the United States estimated the average cost of bringing a drug to market at US$802 million. Estimates of typical research and development costs today are in the US$1.3 billion-to-US$1.7 billion range, though some health researchers dispute these numbers (see “Drug development costs hard to swallow,” page 279).
Though there is much debate about the numbers, there is little debate that they are getting bigger, which has some researchers worried.
“Reducing the costs of trials is absolutely crucial for the public good,” says Dr. Claiborne Johnston, director of the University of California, San Francisco Neurovascular Disease and Stroke Center.
In a recent article, Johnston wrote that the average cost of developing a drug had, over the previous 20 years, risen at a rate that was 7.4% higher than inflation and that clinical trials were responsible for most of the increase (Ann Neurol 2007;62[6]:A6-7). Escalating costs are limiting innovation, wrote Johnston, and society bears the burden of high development costs through higher drug prices.
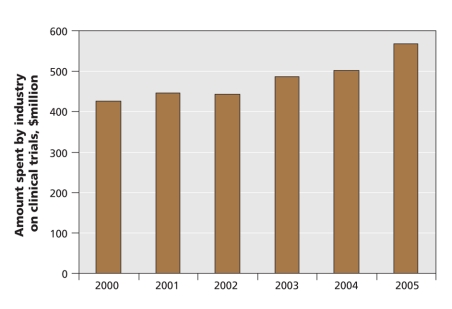
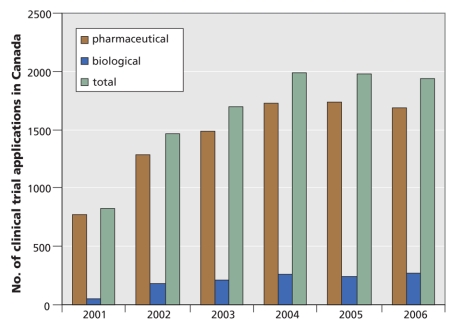
One reason for the increase is related to the drug industry's incredible success in recent years. From 2000 to 2005, Canadian drug companies increased their investment in clinical trials from $425.7 million to $567.1 million, according to the Patented Medicine Prices Review Board (Figure 1). The number of clinical trial applications for pharmaceutical and biological products in Canada increased from 825 in 2001 to 1938 in 2006, reports the Health Products and Food Branch of Health Canada (Figure 2).
Figure 1: Amount spent by the Canadian pharmaceutical industry on clinical trails. Source: Patented Medicine Prices Review Board.
Figure 2: Number of clinical trial applications in Canada for pharmaceutical and biological products. Source: Health Products and Food Branch, Health Canada.
All this research and development has yielded so many new drugs that it has become increasingly difficult for pharmaceutical companies to prove their products are better than those already on the market.
“Most new drugs are only incremental advances,” says Dr. Joel Lexchin, a professor at York University's School of Health Policy and Management, in Toronto, Ontario. “You need larger numbers of patients to demonstrate those very small new benefits.”
The rising costs show no sign of slowing, for many reasons. The need to accumulate more data to show modest benefits has resulted in drug companies investing more in recruitment for clinical trials. And the challenge of finding new products has led some companies to develop treatments for chronic conditions and degenerative diseases, and clinical trials for these types of drugs tend to be long, complex and expensive. Other cost drivers include increasingly complex clinical trial protocol development, greater emphasis on data and site monitoring, increased use of technologies (such as computed axial tomography and magnetic resonance imaging) and the delays caused by differing interpretations of compliance regulations (such as patient consent forms) by the many parties involved in multicentre trials.
“We are conducting bigger trials with more expensive interventions that require more frequent patient evaluations with hospital investigation tools,” says Dr. Ralph Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
The increasing cost of clinical research is affecting the drug industry in several ways. Many companies are conducting clinical trials in other countries, such as China and India, where costs can be as much as 60% lower. The industry has also become risk averse, some researchers say, and is not as willing to take chances on novel medicines.
“There are few new drugs out there. Even the blockbusters are now copy cats,” says Johnston. “A greater emphasis on copycats doesn't move society ahead that much.”
Research centres are also looking more closely at which types of clinical trials they will take on, fearing that certain projects will put them in a deficit. “The dilemma will be deciding what will be the scope of the research program and how trials will be proportioned,” says Meyer.
In Canada, the costs of delays in getting clinical trials off the ground is driving research dollars out of the country, says Ghislain Boudreau, director of medical affairs and clinical research at Pfizer Canada Inc. Getting the green light from Health Canada doesn't take much time, says Boudreau, but many delays are incurred because each province takes a different approach to ethics review and research contract negotiations.
“Whatever we gain at the beginning we are losing afterwards,” says Boudreau. “Other countries can begin recruiting very rapidly.”
To combat rising costs, some drug companies have off-loaded their research and development responsibilities to contract research organizations, which are believed to conduct trials more efficiently. Companies are also searching for inefficiencies in the clinical trail process, in hopes of streamlining it by focusing on key elements.
“There are opportunities for us to do a better job in managing the trail process,” says Johnston.
Efforts are also being made to harmonize clinical trial standards within and between countries. In Canada, stakeholders from academia, industry and government have begun discussions on harmonizing research contracts and the practices of review ethics boards. Then there is the International Conference on Harmonization, which aims to harmonize regulatory requirements in all major drug markets.
Reducing product failures at the later stages of the clinical trial process has also been stressed as a way to reduce costs. Companies have been looking at establishing effective surrogate end points — as opposed to clinical end points, which take longer and are more difficult to monitor — to weed out failures before moving to costly phase III trials. There are also talks of saving money by moving more rapidly to electronic data capture and by improving the recruitment process.
“Drug companies are talking about using genetic markers as a way of screening before entering people into studies to see who the product is most likely to be effective with and who is likely to have significant side effects,” says Lexchin, who adds that good clinical research is as important today as ever. “Clinical trials are still the basis for deciding how good and safe new drugs are, and that's true even more so now than in the past.” — Roger Collier, CMAJ
Figure. The boom in pharmaceutical development in recent decades has yielded so many new drugs that it has become increasingly difficult for drug companies to prove their products are better than those already on the market. To detect modest benefits, companies must run bigger and more expensive clinical trials. Image by: Reuters/Regis Duvignau