Abstract
Diffusion tensor imaging (DTI) was performed in 39 right-handed children to examine structural hemispheric differences and the impact of age, socioeconomic status and sex on these differences. Apparent diffusion coefficient (ADC) values were smaller in the left relative to the right temporal, prefrontal, anterior internal capsular, and the thalamic regions and fractional anisotropy (FA) values were larger in the left as compared to right internal capsule, thalamus, and cingulate. Significant region-by-sex interactions disclosed that the relation of DTI asymmetries to performance depended on sex including the relation of temporal lobes to reading comprehension and the relation of frontal lobes to solving applied mathematical problems.
Keywords: adolescents, arithmetic, asymmetry, brain, children, diffusion tensor imaging, reading
Historically, hemispheric specialization has been a central issue in neuropsychology and behavioral neurology as chronicled by Arthur Benton (Benton, 1970, 1977) in his scholarly critiques and reviews of this topic. Dr. Benton’s pioneering research on hemispheric functional asymmetries in perception (Fontenot & Benton, 1972) complimented his studies of focal brain lesions in patients (Hamsher, Levin, & Benton, 1979). Advances in brain imaging techniques have facilitated investigation of hemispheric specialization and structural asymmetries. Neuroimaging studies of hemispheric asymmetry have utilized a region of interest (ROI) approach to study differences in linear surface area or volumes, but now diffusion tensor imaging (DTI) (Alexander, Lee, Lazar, & Field, 2007) facilitates investigation of the microstructure of white matter tracts and their role in hemispheric asymmetries. With impetus provided by Dr. Benton’s research and historical papers on hemispheric specialization and his contributions to developmental neuropsychology, we utilized DTI methods to analyze the relation of white matter tract microstructure to reading and arithmetic skills in typically developing children and adolescents.
Diffusion Tensor Imaging
DTI is a promising tool to non-invasively examine and quantify changes in white matter pathways associated with normal development and under pathological states (Alexander et al., 2007; Smith et al., 2007). Interpretation of pathological changes requires adequate understanding of hemispheric asymmetries in the healthy brain, and, in pediatric populations, how development and sex may impact normative values.
DTI, which assesses myelination in vivo, is based on the characteristic of myelin sheaths and cell membranes of white matter tracts that restrict the movement of water molecules (Mori & Zhang, 2006). As a result, water molecules move faster along the major axis of nerve fibers rather than perpendicular to them. This characteristic, which is referred to as anisotropic diffusion and is measured by fractional anisotropy (FA), is determined by the thickness of the myelin sheath and of the axons. FA ranges from 0 to 1, where 0 represents maximal isotropic diffusion (e.g., free diffusion in all directions) and 1 which represents maximal anisotropic diffusion, i.e., parallel to the major axis of a white matter tract. Isotropic diffusion of water in multiple directions is measured by the apparent diffusion coefficient (ADC) and other measures of diffusivity. In general, measures of diffusivity such as ADC are inversely related to FA, though the relationship is complex and influenced by several potential factors. Increases in FA may result from changes in fiber organization, but may also relate to developmental myelination, increased fiber density or axonal diameter as well as the ratio of intracellular/extracellular space (Mori & Zhang, 2006). FA generally has greater variability throughout the brain in terms of regional differences than measures of diffusivity such as ADC (Schmithorst, Holland, & Dardzinski, 2007). Decreases in diffusivity such as ADC may relate more to increases in fiber density, but can also be affected by increased axonal diameter and myelination (Schmithorst et al., 2007). Thus, while both ADC and FA reflect changes in the microstructure of the white matter, the interaction between these two indices is complex and incompletely understood. Additionally, the relation of ADC and FA to white matter microstructure may be influenced by dynamic processes during childhood and adolescent development (Mori & Zhang, 2006).
Developmental Studies of Hemispheric Asymmetry
In the following sections, we use the term “leftward” to indicate that a DTI index is larger in the left hemisphere than the right hemisphere, whereas “rightward” refers to a larger value in the right than left hemisphere. As described in a later section, most DTI studies of hemispheric asymmetry have involved adult subjects. However, a DTI study in 8 to 12 year olds which used ROI methodology found higher left hemisphere FA in the anterior limb of the internal capsule and centrum semiovale (Snook, Paulson, Roy, Phillips, & Beaulieu, 2005). In comparison, a recent study (Bonekamp et al., 2007) using DTI tractography in typically developing children and adolescents demonstrated significant asymmetries for ADC in several regions including decreased ADC on the right in the body of the corpus callosum, and decreased ADC on the left in frontal white matter, genu of the corpus callosum, anterior white matter, and the superior fronto-occipital fasiculus. FA differences were higher in the left in the superior coronal radiata, cingulum, and the centrum semiovale.
Age-Related Changes in FA and Diffusivity
Developmental studies in infants and children using DTI have also disclosed age-related differences in FA and ADC, (Barnea-Goraly et al., 2005; Bonekamp et al., 2007; Giorgio et al., 2008; Mori & Zhang, 2006; Mukherjee et al., 2001; Schmithorst, Wilke, Dardzinski, & Holland, 2002; Schneider, Il’yasov, Hennig, & Martin, 2004; Schneiderman et al., 2007; Snook et al., 2005). Developmental changes in DTI parameters have been interpreted as evidence for age-related increases in myelination and thickening of the axon diameter (Sakuma et al., 1991), increased organization of white matter tracts (Alexander, Hasan, Lazar, Tsuruda, & Parker, 2001), reduced water content and increased cohesiveness and compactness of the fiber tracts (McGraw, Liang, & Provenzale, 2002), and reduced extra-axonal space (Beaulieu & Allen, 1994). In school-aged children age-related increases in FA have been reported in specific regions including the white matter of prefrontal cortex, the internal capsule, corpus callosum, centrum semiovale, and the connections by which visual information is transferred from visual association cortex to more anterior regions (Barnea-Goraly et al., 2005; Mukherjee et al., 2001; Schmithorst et al., 2002; Snook et al., 2005). Fiber tracts for which age-related increases in FA have been reported include the superior longitudinal fasiculus and the cingulum (Bonekamp et al., 2007), and more widespread areas throughout the brain (Snook, Plewes, & Beaulieu, 2007). Corresponding to age-related increases in FA, the ADC decreases with age as diffusion becomes increasingly anisotropic in specific areas such as the cerebral peduncles, frontal and temporal white matter, anterior and posterior limbs of the internal capsule, corpus callosum, temporo-occipital white matter, superior corona radiata, superior longitudinal fasciculus, cingulum, and centrum semiovale (Bonekamp et al., 2007; Snook et al., 2005) in addition to more diffuse changes (Snook et al., 2007). Changes in diffusion are generally considered to be greatest within the first four years of life (Mukherjee et al., 2001; Schneider et al., 2004) and are generally less apparent by young adulthood (Snook et al., 2005)
Hemispheric Asymmetries in Adults
Brain hemispheric asymmetries have been identified in both postmortem and imaging studies in adults, including structural and functional imaging. The assumption has been that these asymmetries relate to differences in cognitive and neurobehavioral functioning (Colibazzi et al., 2007; Thoma, Yeo, Gangestad, Lewine, & Davis, 2002). For example, in the frontal lobes, right frontal protrusions have been identified (Chui & Damasio, 1980; Kertesz, Polk, Black, & Howell, 1990; LeMay, 1976; Zilles et al., 1996) as has rightward cortical asymmetry on volumetric analysis using magnetic resonance imaging (MRI) (Carne, Vogrin, Litewka, & Cook, 2006). Positron emission tomography (PET) has revealed right greater than left cerebral glucose metabolism in the lateral aspects of the frontal lobes, but left greater than right metabolism in the medial frontal lobes (Willis et al., 2002).
In the temporal lobes, left greater than right hemisphere asymmetry has been identified in the volume of the planum temporale (posterior temporal lobe) (Anderson, Southern, & Powers, 1999; Barrick et al., 2005; Dorsaint-Pierre et al., 2006; Geschwind & Levitsky, 1968; Good et al., 2001; Shapleske, Rossell, Woodruff, & David, 1999; Watkins et al., 2001) and in temporal white matter (Pujol et al., 2002). PET has revealed right greater than left cerebral glucose metabolism in the lateral aspects of the temporal lobes (Willis et al., 2002).
In the thalamus, the direction of asymmetries has varied across studies and imaging techniques. PET has revealed left greater than right cerebral glucose metabolism in the posterior thalamus (Willis et al., 2002). Consistent with PET data, proton magnetic resonance spectroscopy revealed higher N-acetyl-aspartate (NAA) concentration, NAA/Choline, and NAA/Creatine in the left thalamus (Nagae-Poetscher et al., 2004). The left thalamus has also been reported to have higher values of norepinephrine (Geschwind & Galaburda, 1985), and studies involving analysis of cytoarchitecture of the thalamic nuclei have demonstrated asymmetric enlargement of the left posterior thalamic nucleus in right-handed individuals (Galaburda & Eidelberg, 1982). In contrast, studies of thalamic volume in healthy adults and controls for patient populations have demonstrated equivocal results, with some studies reporting right greater than left, and others finding left greater than right volumes, or no asymmetry, depending on the particular region of thalamus that was utilized (Arciniegas et al., 1999; Csernansky et al., 2004; Gilbert et al., 2001; Hazlett et al., 1999; Portas et al., 1998; Staal, Hulshoff Pol, Schnack, van der Schot, & Kahn, 1998; Szabo, Lancaster, Xiong, Cook, & Fox, 2003; Watkins et al., 2001). Moreover, mean diffusivity using diffusion weighted imaging (DWI) was greater in the right versus left thalamus, indicating potential differences in white matter organization (Fabiano, Horsfield, & Bakshi, 2005).
Finally, in the cingulate, PET has revealed left greater than right cerebral glucose metabolism in the superior cingulate (Willis et al., 2002). Volumetric and MRI texture analysis studies have also revealed asymmetries in the cingulate and paracingulate regions. DTI studies in adults have revealed greater anisotropy in the left hemisphere as compared to the right in the cingulum (Gong, Jiang, Zhu, Zang, He et al., 2005; Gong, Jiang, Zhu, Zang, Wang et al., 2005; Kubicki et al., 2003; Park et al., 2004), internal capsule (Peled, Gudbjartsson, Westin, Kikinis, & Jolesz, 1998), uncinate (Kubicki et al., 2002), superior longitudinal fasciculus (Makris et al., 2005), temporal and temporal-parietal areas (Cao, Whalen, Huang, Berger, & DeLano, 2003; Powell et al., 2006; Rodrigo et al., 2007), anterior limb of the external capsule, posterior limb of the internal capsule, thalamus, and cerebral peduncles (Ardekani, Kumar, Bartzokis, & Sinha, 2007). Using DTI tractography, the size and density of temporal lobe fibers has also been greater in the left than right hemispheres (Nucifora, Verma, Melhem, Gur, & Gur, 2005; Wakana et al., 2007).
DTI in Relation to Cognitive Development
Studies have also reported a relation between DTI indices such as FA and cognitive skills in children. Olesen et al. (2003) found in typically developing children that FA in left frontal subregions, the corpus callosum, and left temporal-occipital region was related to working memory and left temporal FA was related to reading ability. These relations between white matter maturation and cognitive ability were still present after co-varying for age, implying that individual differences in brain development were not entirely attributable to chronologic age. Similar to the results for working memory, age-related decreases in reaction time (RT) on a go-no go task were directly related to increased FA and lower diffusivity in frontostriatal fibers of school aged children and young adults (Liston et al., 2005). Taken together, developmental DTI findings indicate that age-related, increased anisotropic diffusion in task-relevant fiber systems is strongly associated with improved reading, working memory and response inhibition in children, adolescents, and young adults.
Sex Differences
Structural ROI and functional neuroimaging studies have revealed sex differences in hemispheric asymmetries (Clements et al., 2006; Shaywitz et al., 1995; Sullivan, Rose, Rohlfing, & Pfefferbaum, 2007). DTI in healthy children and adolescents has revealed significant sex differences in ADC in temporal white matter, with boys having larger values (greater diffusivity) than girls, and in the cingulum, where girls had larger ADC values than boys though no sex differences in FA (anisotropy) were found (Bonekamp et al., 2007). Females have been found to have proportionately larger gray matter volume in the dorsolateral prefrontal cortex and superior temporal gyrus (Schlaepfer et al., 1995). Females have also been found to have larger language areas including the planum temporale and inferior frontal gyri (Harasty, Double, Halliday, Kril, & McRitchie, 1997) that correspond to the increased neuronal density observed in language areas in females as compared to males (Witelson, Glezer, & Kigar, 1995). Hsu et al. (2008) has shown a variety of age by sex related FA changes, particularly in the frontal region. Silveri et al. (2006) have also shown sex differences in FA related to differences in the development of response inhibition. Of particular relevance to the current study, Shaywitz et al. (2007) have shown that while reading is generally lateralized by functional brain imaging to the left occipitotemporal region, females may have more bi-hemispheric representation (Shaywitz et al., 1995).
Current Study and Hypotheses
In a group of children and adolescents with orthopedic injury not involving the brain, DTI analyses were used to examine lateralized differences in pathway structure of the cerebral hemispheres and to test several hypotheses about the relation of such asymmetries to cognitive function. We predicted that DTI findings of white matter microstructure would confirm anatomical asymmetries reported in previous ROI studies. Furthermore, we predicted that some of these DTI-defined asymmetries would differ depending on age and sex. Lastly, these DTI-defined asymmetries were predicted to have functional significance for arithmetic and reading abilities.
METHODS
Subjects
DTI was acquired using a research protocol without sedation in a group of 39 right-handed (Oldfield, 1971) children and adolescents (10F, 29M) who sustained an orthopedic injury and had been recruited as the comparison group for a larger study investigating the effects of pediatric head injury. These children were prospectively recruited from Level 1 trauma centers and children’s hospitals in Houston, Dallas and Miami, and underwent DTI at about four months post-injury. All children were imaged on Philips Intera 1.5T scanners using identical acquisition parameters. Mean age of the group at the time of scanning was 12.58 years. Mechanism of injury included 31 low-speed (predominantly sports-related or fall) and 8 high speed (predominantly motor vehicle crashes) injuries. Descriptive statistics on the characteristics of these children are in Table 1 Exclusion criteria included pre-injury mental deficiency or psychosis. Children were also attending mainstream classes prior to their injuries. Conventional imaging sequences were also performed as part of the protocol and read by a pediatric board-certified neuroradiologist, and no child in the cohort reported here demonstrated any abnormalities on imaging.
Table 1.
Demographic and Injury Characteristics
| Mean | SD | Range | ||
|---|---|---|---|---|
| Age at testing (years) | 12.58 | 2.38 | 8.47–17.03 | |
| Post-injury interval (months) | 4.04 | 0.78 | 2.70–5.72 | |
| Social Composite Index (z-score) | 0.13 | 0.86 | −1.34–1.86 | |
| Distribution | ||||
| Gender | 29 Male, 10 Female | |||
| Race/Ethnicity | 13 Caucasian, 12 Hispanic, 11 African American, 2 Biracial, 1 Asian | |||
| Handedness | 39 Right-handed, 0 Left-handed | |||
| Mechanism of Injury | 20 Sports-related, 6 Fall, 1 Hit by falling object, 1 Hit by motor vehicle, 2 Motor vehicle crash, 5 Motorcycle crash, 1 Recreation vehicle crash, 2 Bicycle crash, 1 Other | |||
Thirty-three of the 39 children who underwent DTI provided data on four subtests of the Woodcock Johnson-III (Woodcock & Mather, 2001): Passage Comprehension, Letter-Word Identification, Applied Problems, and Calculations.
DTI Acquisition
Transverse multislice spin echo, single shot, echo planar imaging (EPI) sequences were used (10150.5 ms TR, 90 ms TE, 2.7 mm slices, 0 mm gap). A 256 mm FOV (RFOV=100%) was used with a measured voxel size of 2.69 × 2.69 × 2.7 mm and a reconstructed voxel size of 2.00 × 2.00 × 2.7 mm. Diffusion was measured along 15 directions (number of b-value = 2, low b-value = 0 and high b-value = 860 sec/mm2). To improve signal to noise ratio, high-b images were acquired twice and averaged. Each acquisition took approximately five minutes 45 seconds, and 55 slices were acquired.
DTI Analysis
DTI Fiber Tracking Analysis of Regions of Interest
The Philips diffusion affine registration tool (Netsch & van Muiswinkel, 2004) was used to remove shear and eddy current distortion and head motion prior to calculating FA maps with Philips fiber tracking 4.1V3 Beta 4 software. ROIs were drawn manually using the protocols described below, then the automated Philips three-dimensional fiber tracking tool was utilized to determine fiber tracks passing through ROIs. Mean FA and ADC of the fiber system, which was automatically generated by the software, was used as the quantitative measure for DTI variables. The algorithm for fiber tracking is based upon the Fiber Assignment by Continuous Tracking (FACT) method (Mori, Crain, Chacko, & van Zijl, 1999). For each of the ROIs listed below, we used standard parameters where tracking terminated if the FA in the voxels decreased below 0.2 or if the angle between adjacent voxels along the track was larger than 6.75 degrees. Philips software generated an automatic mean FA and ADC for each of the ROIs. The rationale for proposing the following ROIs was the ease of identification on DTI and reproducibility of the protocols, predicted asymmetry based on previous structural and functional imaging studies, and the role of some of these regions in cognitive processes such as language and arithmetic.
Internal capsule
Four measures based upon fiber projections from the internal capsule were utilized: 1) right anterior limb; 2) left anterior limb; 3) right posterior limb; and, 4) left posterior limb. ROIs were drawn on FA color maps in the axial plane using boundaries similar to those described by Mori et al. (1999).
Frontal Lobes
Measures of frontal white matter were performed on both the right and left hemispheres. These ROIs were drawn in the coronal plane, on a slice just anterior to the first slice where the genu of the corpus callosum was visible. Right and left sides were calculated separately, and all white matter within the boundaries was included.
Temporal Lobes
Measures of temporal white matter were performed in the right and left hemispheres in the sagittal plane, with the ROI drawn around the temporal white matter including the temporal stem to include the inferior longitudinal fasciculus.
Thalamus
ROIs were created on FA color maps in the axial plane. The thalamic ROI was limited to the anterior thalamic radiations originating from the medial aspect of the thalamus.
Cingulate
ROIs were created on FA color maps in the left and right parasagittal planes, independently using co-registered b=0 images to cross check boundaries as necessary. Three seed points were placed linearly along the cingulate fibers running in an anterior-posterior direction, and the multi-ROI function was used to select fibers that were common to the three ROIs.
Intra-rater and Inter-rater Agreement
To examine intra-rater agreement, each patient’s DTI variables were measured twice; intra-class correlation coefficients (ICC) exceeded 0.95 for all DTI indices. Inter-rater agreement was also assessed by measurement of the corpus callosum by two different raters in ten percent of the cases in both groups; ICCs again exceeded 0.95.
Woodcock Johnson III
Academic achievement in reading and arithmetic was performed within two weeks of imaging using the Woodcock Johnson Tests of Achievement-III (Woodcock & Mather, 2001). These widely used tests of academic achievement have been validated in the child TBI population. Letter/Word Identification measures reading decoding for single letters and words, a skill that predicts reading competence. Passage Comprehension, measures text comprehension by asking the child to point to a picture representing a phrase and at a more difficult level to provide a missing key word that makes sense in the passage. Calculations measures performance of simple mathematic computations presented in a traditional problem format. Applied Problems, requires that the child to listen to the problem, decide which operation to follow, and then perform a relatively simple calculation. Because the problems include extraneous information, the child must determine the appropriate mathematical operations to use and which numbers to include in the calculation. Standard scores are provided for each measure, with higher scores reflecting better performance. Internal consistency coefficients for these subtests range from 0.83 to 0.92. Because of the multiple comparisons to be made, and the problems with family-wise statistical error, all cognitive relationships were based solely on these four measures.
Statistical Analysis
An asymmetry index (AI) was created for both FA and ADC for each of the ROIs using the following formula: (left − right)/[(right + left)/2], similar to previous studies (Bonekamp et al., 2007). T-tests were used to examine differences in FA and ADC between the right and left hemispheres for males and females separately. General linear models were then used to further examine the relation of sex, age and socioeconomic status on the AI (for both ADC and FA) for each ROI. General linear models were also used to examine the relation of performance on the four WJ-III subtests to relevant ROI AIs. Specifically, we examined the relation of temporal AI (ADC only) to language subtests (i.e., Letter-Word Identification and Passage Comprehension) and the relation of an ROI with tracts ascending to the parietal areas (posterior limb of the internal capsule AI, FA only) to mathematics subtests (i.e., Calculation and Applied Problems). We also examined the relation between Frontal AI (ADC only) and Applied Problems, as cognitive components such as executive functioning necessary to perform well on this subtest are thought to be mediated by frontal areas.
RESULTS
Hemispheric Differences
Hemispheric asymmetries in ADC were significant in the temporal lobes (t(38)= −4.07, p=0.0002), prefrontal regions (t(38)=−2.48, p=0.018), anterior limb of the internal capsule (t(38)=−2.61, p=0.013), and the anterior radiations of the thalamus (t(38)=−2.19, p=0.035), with the right hemisphere ADC larger than the left in nearly all ROIs when the pooled data (males and females together) were used, indicating increased diffusivity in the right hemisphere as compared to the left. Figure 1 illustrates mean ADC values for right and left hemisphere ROIs. The ROIs with significant hemispheric differences are denoted in Figure 1.
Figure 1.
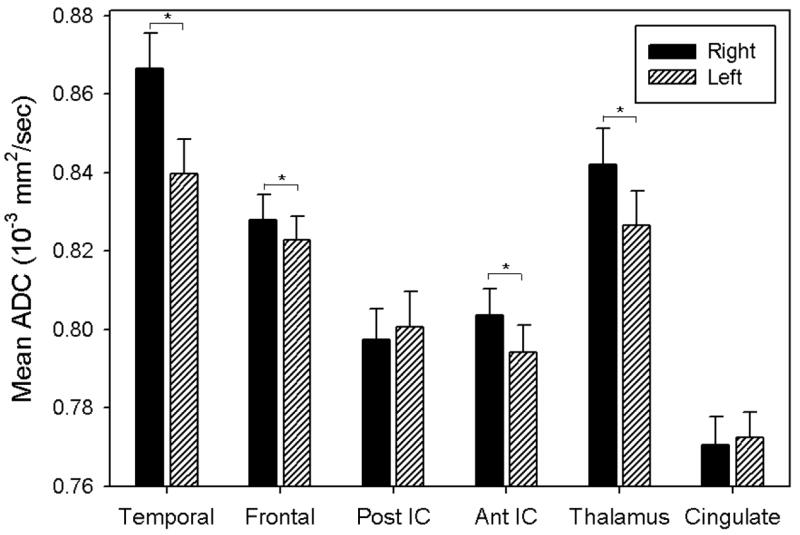
Histogram of mean ADC by ROI and hemisphere. Error bars represent standard error.
For FA, the right and left hemispheres differed for the posterior limb of the internal capsule (t(38)=4.96, p<0.0001), the anterior limb of the internal capsule (t(38)=2.21, p=0.033), the anterior radiations of the thalamus (t(38)=2.38, p=0.023) and the cingulate (t(38)=8.56, p<0.0001), with increased FA in the left hemisphere in nearly all instances. Of the ROIs shown in Figure 2, the cingulate had the greatest hemispheric asymmetry in FA. This asymmetry in FA for the cingulate is illustrated in Figure 3 for a representative subject. Means and standard deviations for all regions for both FA and ADC are presented in Table 2.
Figure 2.
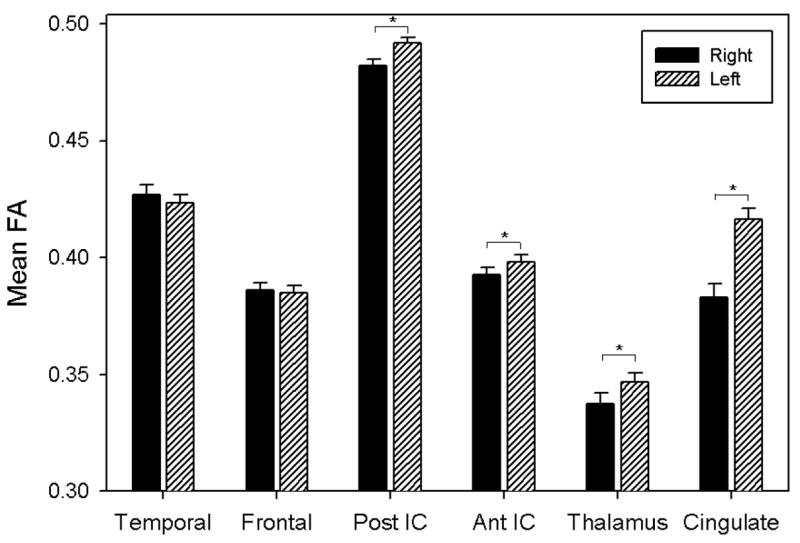
Histogram of mean FA for ROIs by hemisphere. Error bars represent standard error.
Figure 3.
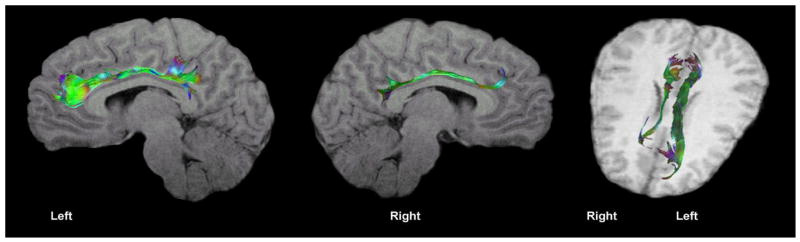
DTI showing left greater than right cingulate FA in a 13 year old right-handed male adolescent. Consistent with convention, green color indicates fibers traversing in an anterior-posterior direction, red color indicates right to left orientation of fibers, and blue indicates superior to inferior orientation.
Table 2.
Means and Standard Deviations for FA and ADC
| FA | ADC | |||
|---|---|---|---|---|
| Region of Interest | Mean | SD | Mean | SD |
| Right Frontal | 0.386 | 0.020 | 0.828 | 0.041 |
| Left Frontal | 0.385 | 0.019 | 0.823 | 0.038 |
| Right Temporal | 0.427 | 0.025 | 0.866 | 0.057 |
| Left Temporal | 0.424 | 0.021 | 0.840 | 0.056 |
| Right Cingulate | 0.383 | 0.037 | 0.771 | 0.044 |
| Left Cingulate | 0.416 | 0.030 | 0.772 | 0.040 |
| Right Thalamus | 0.337 | 0.028 | 0.842 | 0.057 |
| Left Thalamus | 0.347 | 0.025 | 0.826 | 0.055 |
| Right Anterior Internal Capsule | 0.393 | 0.018 | 0.804 | 0.043 |
| Left Anterior Internal Capsule | 0.398 | 0.018 | 0.794 | 0.045 |
| Right Posterior Internal Capsule | 0.482 | 0.017 | 0.797 | 0.049 |
| Left Posterior Internal Capsule | 0.492 | 0.016 | 0.801 | 0.057 |
Impact of Sex
Sex did not significantly affect the AI for ADC for any of the ROIs, though small (non-significant) differences between males and females are evident in some AI means as illustrated in Figure 4.
Figure 4.

ADC Asymmetry Index plotted by ROI and sex. Negative values reflect larger right than left hemisphere ADC values, whereas positive values indicate that ADC values were larger in the left hemisphere than the right hemisphere. Error bars represent standard error.
Figure 5 demonstrates differences in the FA AI between male and female children and adolescents. In analyses of the FA AI for each ROI, sex (F(1,36)=14.23, p<0.001) did relate to left-right asymmetry in the frontal lobes, reflecting right greater than left frontal FA in girls, but not in boys (Figure 5). Apart from this difference in frontal FA, no other region significantly differed in AI by sex. Insert Figure 5
Figure 5.
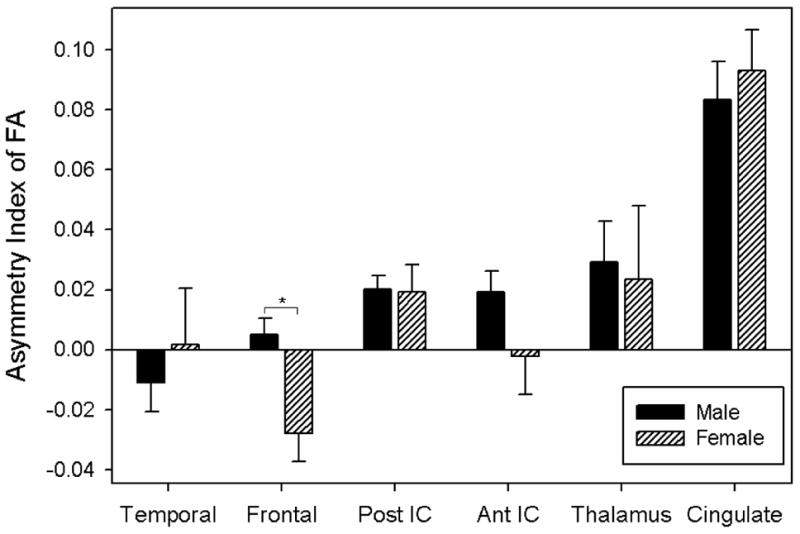
FA Asymmetry Index plotted by ROI and sex. Negative values reflect larger right than left hemisphere FA values, whereas positive values indicate that FA values were larger in the left hemisphere than the right hemisphere. Error bars represent standard error.
Impact of Age and Socioeconomic Status
There was no significant difference in age between males and females included in this cohort. Analyses of the AI for ADC in each ROI revealed a significant interaction between sex and socioeconomic status in relation to the temporal lobe ADC (F(1,35)=5.22, p=0.029) where socioeconomic status as measured by the Socioeconomic Composite Index (SCI) was inversely related to AI in females, but not in males. In girls, the mean temporal lobe ADC AI was negative; thus, lower SCI was related to a greater asymmetry if right hemisphere diffusivity was greater than the left or lesser asymmetry if the left hemisphere diffusivity was greater than the right hemisphere.
Age was also related to asymmetry in FA with AI decreasing with older age, (t(36)=−3.01, p=0.005). Figure 6 shows that younger age was related to a greater leftward difference in frontal FA, whereas the rightward asymmetry in frontal FA during adolescence was smaller in magnitude. Age was not related to asymmetry for ADC.
Figure 6.
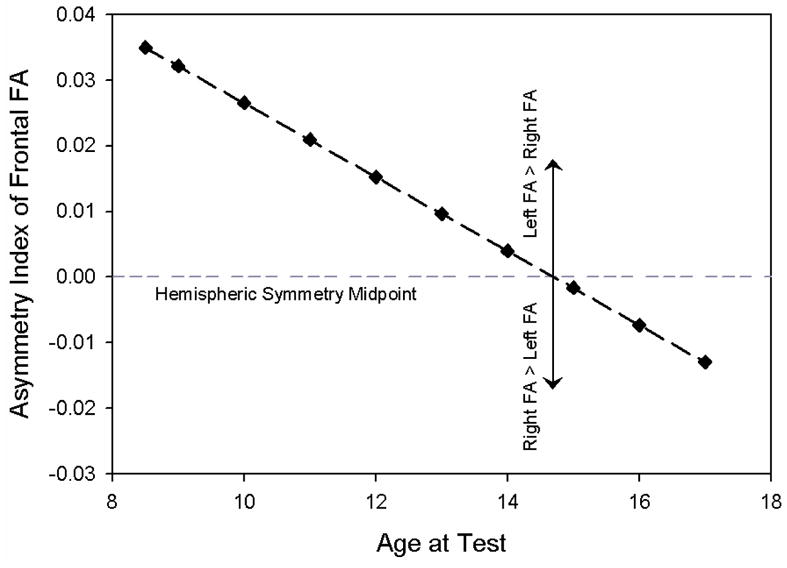
FA Asymmetry Index in the frontal lobes plotted by age showing greater leftward asymmetry in younger children and smaller rightward asymmetry in adolescents.
DTI Findings in Relation to Performance on the Woodcock Johnson III
Although no significant overall relation was found between the temporal lobe AI for ADC and the Passage Comprehension subtest of the WJ-III, a sex by region interaction was detected (F(1,36)=5.19, p=0.031) whereby an AI associated with decreased diffusivity or lower ADC (increased fiber size and/or density) in the left as compared to right temporal lobe was related to better reading comprehension in males (t(26)=−2.55, p=0.0172), but not in females (Figure 7). Age also impacted this relation between temporal lobe asymmetry and performance as seen in a region by age interaction (F(1,36)=5.09, p=0.033), wherein the relation between temporal lobe AI and reading efficiency weakened with age. However, there was not a sex by age interaction. Data were centered for children at 13 years of age and at an SCI z-score of 0.
Figure 7.

WJ-III Passage Comprehension standard score plotted for males and females by Asymmetry Index (AI) for temporal lobe ADC with negative AI values reflecting higher diffusivity in the right temporal lobe than the left temporal lobe. Higher right relative to left temporal diffusivity was related to better reading in males, but not in females.
We found a significant region by sex interaction in the relation between frontal lobe asymmetry in diffusivity and performance on the Applied Problems subtest (F(1,27)=9.12, p=0.006). As seen in Figure 8, an AI associated with increased diffusivity (high ADC) in the right as compared to left hemisphere in the frontal lobes was related to accuracy of solving complex arithmetic problems in females (t(27)=−2.80, p=0.009), but not in males. No age by sex interactions were noted. Data were again centered for children at 13 years of age and at an SCI z-score of 0.
Figure 8.

WJ-III Applied Problems standard score plotted by Asymmetry Index (AI) for frontal ADC with negative AI values reflecting higher diffusivity in the right frontal lobe than the left frontal lobe. Higher right relative to left frontal diffusivity was related to more accurate solution of problems in females, but not in males
No other significant relations were found between AI for any region and performance on the WJ-III achievement tests. Analyses of the WJ-III measures and AI indices also were calculated using standard scores and the results were not substantially different using raw scores with age included as a covariate.
DISCUSSION
In a previous DTI study of neurodevelopment, Snook et al. (2005) found lateralized asymmetries in only a few structures using an ROI method in typically-developing children aged 8 to 12 years old. Our study differs from Snook et al. in the use of DTI tractography and an acquisition protocol which included more diffusion-sensitizing gradient directions and better spatial resolution. Using DTI tractography similar to the methodology in the present study, Bonekamp et al. (2007) found significant asymmetry in ADC in several regions including left greater than right ADC in the frontal lobes, genu, and the superior fronto-occipital fasciculus, whereas ADC was higher in the right side of the corpus callosum body. For FA, Bonekamp et al (2007) found that values were higher in left than right hemisphere for superior coronal radiata, cingulum, and central semiovale whereas FA was higher for right frontal white matter than left. Asymmetry in ADC reflects relatively higher diffusivity in the hemisphere with the larger value, suggesting increased white matter fiber size and density and larger axon diameter in the opposite hemisphere with lower diffusivity. In comparison with the Bonekamp et al. findings, our ADC data indicate higher values in several right hemisphere ROIs than homologous regions in the left hemisphere with no significant left greater than right differences. Asymmetry in FA reflects higher anisotropic diffusion parallel to the axis of white matter tracts in the hemisphere with the larger value. Although our results and Bonekamp et al.’s report show left greater than right FA in several ROIs, we did not confirm their finding of a rightward frontal asymmetry in FA. These differences in findings for the two studies may be attributable to differences in the imaging protocol and subjects as Bonekamp et al. recruited typically developing children and adolescents, whereas we studied similarly aged youth who had sustained orthopedic injury.
In addition, we found AIs in the temporal lobes, anterior limb of the internal capsule and the anterior thalamic radiations for ADC and in the anterior and posterior limbs of the internal capsules and the anterior thalamic radiations for FA. Therefore the current findings extend the number of regions of documented white matter asymmetries for diffusivity and anisotropy in the maturing brain. Agreement between our data and studies of asymmetry using DTI in healthy adult populations suggests that these anatomical asymmetries begin early in brain development and become established by adolescence.
Developmental studies using DTI have shown that the measures of FA and diffusivity are related to age and interpreted as surrogate markers of myelination (Mori & Zhang, 2006), fiber organization, fiber density, axonal diameter and the ratio of extracellular to intracellular space. Such measures are also linked to cognitive development (Deary et al., 2006; Schmithorst, Wilke, Dardzinski, & Holland, 2005) where sex differences may be present (Hsu et al., 2008). Decreased diffusivity may be an indication of increased fiber size, density, or myelination (Schmithorst et al., 2007) as reflected by Luders et al. (2007) who observed increased posterior corpus callosum thickness to be positively associated with IQ. Temporal lobe fiber tracts course through the posterior corpus callosum, consistent with the possibility that regional temporal lobe DTI findings in males contributed to WJ-III passage comprehension and also likely involved other fiber tracts that were interhemispheric in nature.
In the present study, hemispheric asymmetry in diffusivity interacted with sex in relation to reading comprehension and arithmetic. For males, increased rightward asymmetry in temporal diffusivity was positively related to reading comprehension, whereas this pattern was not seen in females. We infer that relatively low left temporal diffusivity was related to increases in myelination and in both fiber size and density of white matter tracts reflecting maturation of this region. With generally higher verbal skills in girls relative to boys and diffusivity decreasing with typical brain development (Bonekamp et al., 2007), we interpret low left relative to right temporal diffusivity as an indication of brain maturation in boys, a condition that may enhance reading comprehension (Olesen et al., 2003). Consistent with a dissociation related to maturation of a specific brain region, academic skill, and sex, we also found that increased ADC reflecting greater diffusivity of the right frontal region relative to the left was positively related to solving arithmetic problems by females, but not by males. This finding, which implies low left frontal relative to right frontal diffusivity in girls is related to arithmetic skill, may reflect enhanced maturation of this region conducive to improved efficiency in quantitative skills. The gender-specific dissociation we found in regional white matter maturation and academic skill may reflect differences in speed of development between boys and girls. Accordingly, the the temporal lobe pattern in boys may reflect a relatively late functional commitment in reading, whereas the results in girls may be due to a later commitment in prefrontal representation of skills involved in solving arithmetic problems. In relation to Dr. Benton’s contributions to developmental neuropsychology, it would be of interest to compare the AIs in the present study to a sample of children with dyslexia and to a group of children with developmental dyscalculia. Based on our findings, the relation of temporal lobe AI in diffusivity to reading might be altered in dyslexic boys and the relation of frontal AI to arithmetic in girls would differ from the pattern shown in the present study.
In comparison with our findings in children, a leftward asymmetry of FA in adult women relative to men has also been reported (Szeszko et al., 2003). In that study, greater FA in the left hemisphere correlated with better verbal comprehension and verbal memory in women but not men, indicating a sex-specific relation between structure and ability, which is again consistent with our results of a sex-related interaction with performance on applied problems. However, in our study, leftward asymmetry in FA decreased with age as adolescents tended to have the opposite pattern with higher FA values in the right frontal region than the left. Other sex-specific relations have been demonstrated using magnetic resonance spectroscopy (MRS) which revealed a correlation between intelligence and N-acetyl-aspartate (NAA) concentrations in the frontal regions of women but not men (Jung et al., 2005). Our results are also consistent with previous findings of stronger relationship between intelligence and functional connectivity in the left hemisphere generally relative to the right hemisphere (Schmithorst & Holland, 2006).
However, in contrast to our findings, previous DTI studies in children have revealed lower anisotropy and higher diffusivity in the frontal lobes in females as compared to males, which were hypothesized to relate to increased crossing of white matter fiber tracts, resulting in lower FA values (Schmithorst et al., 2007). The frontal lobes are considered one of the latest regions to develop (Casey, Giedd, & Thomas, 2000; Huttenlocher & Dabholkar, 1997; Wilke, Sohn, Byars, & Holland, 2003), and this development may occur later in boys than in girls. A significant sex by age interaction was found in a study by Schmithorst et al. (2007) whereby boys displayed a positive correlation of FA with age and girls displayed a negative correlation of FA with age. Girls also exhibited a faster rate of decrease in right frontal diffusivity with age as compared to boys. In the present study, frontal FA was higher in the right relative to the left hemisphere of girls, and this asymmetry was greater than in boys. However, higher right frontal relative to left frontal ADC was related to arithmetic proficiency in girls, but not in boys. With a generally inverse relation between FA and ADC, relatively low left frontal ADC may reflect maturation of this region.
The cause of gender differences remains unclear, but may include genetic, hormonal, psychosocial, and environmental influences. The recent work by Hsu et al. (2008) even raises the question as to whether these differences actually relate to distinct sexual dimorphism in the developing brain as a marker of hormonal influences. In addition, whether these volumetric changes are caused by individual cells, cortical laminae, distinct cytoarchitectonic regions, specific nuclei, neurotransmitter systems or vascular territories are involved remains unresolved. Another issue is neuronal pruning, where changes in DTI white matter may reflect a selection process that relates to intact and viable pathways (D’Arceuil et al., 2007) as well as experience-dependent changes in brain structure (Aydin et al., 2007).
Absolute differences in asymmetry are small in this and previous studies, and the functional significance of many of these is undetermined. In a recent DTI study of healthy children and adolescents, Bonekamp et al. (2007) demonstrated mean asymmetry indices in the range of -4% to 9% for FA and -6% to 3% for ADC. The present study revealed AIs ranging from -16% to 28% for FA and -16% to 12% for ADC suggesting greater variability in our patients as compared with the community sample recruited by Bonekamp et al. Differences in volumetric studies are even smaller (<1%) (Carne et al., 2006). As DTI methods of analysis have been available for less than a decade, data are now just emerging to show how brain asymmetries develop and their functional significance.
Limitations of our study include its cross-sectional design. Longitudinal DTI could further elucidate the effects of white matter development in children and the relation to changes in cognition. The volume of white matter increases throughout childhood and extends to early adulthood (Gogtay et al., 2004; Paus et al., 2001). Assets of our study include the good reliability in the DTI analysis, both within and between examiners. In addition, we demonstrated a relation between DTI measures of white matter integrity and cognitive data. Behavioral assessment of the patients was done independently of the DTI analysis, thus mitigating examiner bias.
In summary, these findings add to the literature of age and sex-dependent hemispheric asymmetries in white matter microstructure. We also demonstrated sex and region-specific relations of asymmetry in diffusivity to academic achievement scores with distinct patterns for reading comprehension and arithmetic problems. Although, we examined only elements of mathematical ability and reading comprehension, it is plausible that these white matter microstructural differences have more widespread relationships with cognitive development than what could be explored in the current investigation. DTI methods provide an exciting window into normal and abnormal brain development that holds the promise of informing neuroscientists and clinicians about neural mechanisms mediating cognition.
Acknowledgments
This research was supported by grant NS-21889 awarded to Harvey S. Levin, Ph.D. by the National Institutes of Health. We gratefully acknowledge the assistance of Summer Lane and Lori Cook for their assistance in patient recruitment and Stacey K. Martin for her assistance in manuscript preparation. We would also like to acknowledge the generous support by Mission Connect of the TIRR Foundation.
References
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45(5):770–780. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Southern BD, Powers RE. Anatomic asymmetries of the posterior superior temporal lobes: a postmortem study. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(4):247–254. [PubMed] [Google Scholar]
- Arciniegas D, Rojas DC, Teale P, Sheeder J, Sandberg E, Reite M. The thalamus and the schizophrenia phenotype: failure to replicate reduced volume. Biol Psychiatry. 1999;45(10):1329–1335. doi: 10.1016/s0006-3223(97)00459-9. [DOI] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging. 2007;25(2):154–167. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Aydin K, Ucar A, Oguz KK, Okur OO, Agayev A, Unal Z, et al. Increased gray matter density in the parietal cortex of mathematicians: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2007;28(10):1859–1864. doi: 10.3174/ajnr.A0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Mackay CE, Prima S, Maes F, Vandermeulen D, Crow TJ, et al. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24(3):678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31(4):394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- Benton AL. Hemispheric cerebral dominance. Isr J Med Sci. 1970;6(2):294–303. [PubMed] [Google Scholar]
- Benton AL. Historical notes on hemispheric dominance. Arch Neurol. 1977;34(2):127–129. doi: 10.1001/archneur.1977.00500140081019. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Whalen S, Huang J, Berger KL, DeLano MC. Asymmetry of subinsular anisotropy by in vivo diffusion tensor imaging. Hum Brain Mapp. 2003;20(2):82–90. doi: 10.1002/hbm.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne RP, Vogrin S, Litewka L, Cook MJ. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci. 2006;13(1):60–72. doi: 10.1016/j.jocn.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chui HC, Damasio AR. Progressive dialysis encephalopathy (“dialysis dementia”) J Neurol. 1980;222(3):145–157. doi: 10.1007/BF00313113. [DOI] [PubMed] [Google Scholar]
- Clements AM, Rimrodt SL, Abel JR, Blankner JG, Mostofsky SH, Pekar JJ, et al. Sex differences in cerebral laterality of language and visuospatial processing. Brain Lang. 2006;98(2):150–158. doi: 10.1016/j.bandl.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Colibazzi T, Zhu H, Bansal R, Schultz RT, Wang Z, Peterson BS. Latent volumetric structure of the human brain: Exploratory factor analysis and structural equation modeling of gray matter volumes in healthy children and adults. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, et al. Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry. 2004;161(5):896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- D’Arceuil H, Liu C, Levitt P, Thompson B, Kosofsky B, de Crespigny A. Three-Dimensional High-Resolution Diffusion Tensor Imaging and Tractography of the Developing Rabbit Brain. Dev Neurosci. 2007 doi: 10.1159/000110503. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006;66(4):505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhune VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, et al. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129(Pt 5):1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Fabiano AJ, Horsfield MA, Bakshi R. Interhemispheric asymmetry of brain diffusivity in normal individuals: a diffusion-weighted MR imaging study. AJNR Am J Neuroradiol. 2005;26(5):1089–1094. [PMC free article] [PubMed] [Google Scholar]
- Fontenot DJ, Benton AL. Perception of direction in the right and left visual fields. Neuropsychologia. 1972;10(4):447–452. doi: 10.1016/0028-3932(72)90007-3. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Eidelberg D. Symmetry and asymmetry in the human posterior thalamus. II. Thalamic lesions in a case of developmental dyslexia. Arch Neurol. 1982;39(6):333–336. doi: 10.1001/archneur.1982.00510180011002. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161(837):186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, He Y, Xie S, et al. Side and handedness effects on the cingulum from diffusion tensor imaging. Neuroreport. 2005;16(15):1701–1705. doi: 10.1097/01.wnr.0000183327.98370.6a. [DOI] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S, et al. Asymmetry analysis of cingulum based on scale-invariant parameterization by diffusion tensor imaging. Hum Brain Mapp. 2005;24(2):92–98. doi: 10.1002/hbm.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Hamsher K, Levin HS, Benton AL. Facial recognition in patients with focal brain lesions. Arch Neurol. 1979;36(13):837–839. doi: 10.1001/archneur.1979.00500490051008. [DOI] [PubMed] [Google Scholar]
- Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54(2):171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Byne W, Wei TC, Spiegel-Cohen J, Geneve C, et al. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry. 1999;156(8):1190–1199. doi: 10.1176/ajp.156.8.1190. [DOI] [PubMed] [Google Scholar]
- Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, et al. Gender differences and age-related white matter changes of the human brain: A diffusion tensor imaging study. Neuroimage. 2008;39(2):566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, et al. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage. 2005;26(3):965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Polk M, Black SE, Howell J. Sex, handedness, and the morphometry of cerebral asymmetries on magnetic resonance imaging. Brain Res. 1990;530(1):40–48. doi: 10.1016/0006-8993(90)90655-u. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann N Y Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal Microstructure Modulates Efficient Recruitment of Cognitive Control. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37(4):1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol. 2002;179(6):1515–1522. doi: 10.2214/ajr.179.6.1791515. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Nagae-Poetscher LM, Bonekamp D, Barker PB, Brant LJ, Kaufmann WE, Horska A. Asymmetry and gender effect in functionally lateralized cortical regions: a proton MRS imaging study. J Magn Reson Imaging. 2004;19(1):27–33. doi: 10.1002/jmri.10429. [DOI] [PubMed] [Google Scholar]
- Netsch T, van Muiswinkel A. Quantitative evaluation of image-based distortion correction in diffusion tensor imaging. IEEE Trans Med Imaging. 2004;23(7):789–798. doi: 10.1109/TMI.2004.827479. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16(8):791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23(1):213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Peled S, Gudbjartsson H, Westin CF, Kikinis R, Jolesz FA. Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Res. 1998;780(1):27–33. doi: 10.1016/s0006-8993(97)00635-5. [DOI] [PubMed] [Google Scholar]
- Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, et al. Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry. 1998;43(9):649–659. doi: 10.1016/s0006-3223(97)00339-9. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez-Sala A, Deus J, Cardoner N, Sebastian-Galles N, Conesa G, et al. The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. Neuroimage. 2002;17(2):670–679. [PubMed] [Google Scholar]
- Rodrigo S, Naggara O, Oppenheim C, Golestani N, Poupon C, Cointepas Y, et al. Human subinsular asymmetry studied by diffusion tensor imaging and fiber tracking. AJNR Am J Neuroradiol. 2007;28(8):1526–1531. doi: 10.3174/ajnr.A0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, et al. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology. 1991;180(1):229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61(3):129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. Neuroimage. 2006;31(3):1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, et al. Diffusion tensor anisotropy in adolescents and adults. Neuropsychobiology. 2007;55(2):96–111. doi: 10.1159/000104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29(1):26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373(6515):607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, et al. Age-related changes in reading systems of dyslexic children. Ann Neurol. 2007;61(4):363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn Reson Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34(1):243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack H, van der Schot AC, Kahn RS. Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry. 1998;155(12):1784–1786. doi: 10.1176/ajp.155.12.1784. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A. Postural sway reduction in aging men and women: Relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo CA, Lancaster JL, Xiong J, Cook C, Fox P. MR imaging volumetry of subcortical structures and cerebellar hemispheres in normal persons. AJNR Am J Neuroradiol. 2003;24(4):644–647. [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Ashtari M, Snyder PJ, Lieberman JA, Bilder RM. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol Psychiatry. 2003;53(5):450–459. doi: 10.1016/s0006-3223(02)01529-9. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Yeo RA, Gangestad SW, Lewine JD, Davis JT. Fluctuating asymmetry and the human brain. Laterality. 2002;7(1):45–58. doi: 10.1080/13576500143000122. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11(9):868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20(1):202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Willis MW, Ketter TA, Kimbrell TA, George MS, Herscovitch P, Danielson AL, et al. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res. 2002;114(1):23–37. doi: 10.1016/s0925-4927(01)00126-3. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15(5 Pt 1):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, Mather N. Woodcock-Johnson Tests of Achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, et al. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev. 1996;20(4):593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]