Abstract
Given that cancer and related disorders affect a wide spectrum of the world's population, and in most cases are progressive in nature, it is essential that future care must overcome the present limitations of existing therapies in the absence of toxic side effects. Mammalian forkhead transcription factors of the O class (FoxOs) may fill this niche since these proteins are increasingly considered to represent unique cellular targets directed against human cancer in light of their pro-apoptotic effects and ability to lead to cell cycle arrest. Yet, FoxOs also can significantly affect normal cell survival and longevity, requiring new treatments for neoplastic growth to modulate novel pathways that integrate cell proliferation, metabolism, inflammation and survival. In this respect, members of the FoxO family are extremely compelling to consider since these transcription factors have emerged as versatile proteins that can control angiogenesis, stem cell proliferation, cell adhesion and autoimmune disease. Further elucidation of FoxO protein function during neoplastic growth should continue to lay the foundation for the successful translation of these transcription factors into novel and robust clinical therapies for cancer.
Keywords: angiogenesis, cancer, immune system, oxidative stress, stem cells
Introduction
Mammalian forkhead transcription factors of the O class (FoxOs) that include FoxO1, FoxO3, FoxO4 and FoxO6 have emerged as critical regulators of cellular growth and proliferation and are therefore considered to be potential targets for therapeutic strategies directed against cancer. FoxO proteins are found throughout the body and are expressed in tissues of the reproductive system of males and females, skeletal muscle, the cardiovascular system, lung, liver, pancreas, spleen, thymus and the nervous system.1-6 The prior nomenclature for these proteins, such as forkhead in rhabdomyosarcoma (FKHR), the Drosophila gene fork head (fkh), and Forkhead RElated ACtivator (FREAC)-1 and -2, has been replaced. The current nomenclature for human Fox proteins places all letters in uppercase, otherwise only the initial letter is listed as uppercase for the mouse, and for all other chordates the initial and subclass letters are in uppercase.7 Initially, the FoxOs were first reported in fusion genes in human soft-tissue tumors and leukemias. FOXO1, termed forkhead in rhabdomyosarcoma (FKHR), and FOXO3a, also known as FKHRL1 (forkhead in rhabdomyosarcoma like protein 1), and their genes were identified through chromosomal translocations in alveolar rhabdomyosarcoma tumors.8 The acute leukemia fusion gene located in chromosome X (AFX), also known as the FOXO4 gene, was described as a gene that fused to MLL transcription factor as a result of the t(X; 11) chromosomal translocation in acute lymphoblastic leukemia.9 A fusion between FOXO2 and MLL also occurs in some cases of acute myeloid leukemia that also is believed to be identical to FOXO3a.10
FoxO Proteins as Transcription Factors
At least 100 forkhead genes and 19 human subgroups that range from FOXA to FOXS are now known to exist since the initial discovery of the fly Drosophila melanogaster gene forkhead.11 Forkhead proteins function as transcription factors to either inhibit or activate target gene expression and therefore, these proteins must bind to DNA through the forkhead domain that relies upon fourteen protein-DNA contacts. The forkhead domain in Fox proteins consists of three α-helices, three β-sheets, and two loops that are referred to as the wings,12 but not all winged helix domains are considered to be Fox proteins.13 On X-ray crystallography12 or nuclear magnetic resonance,14 the forkhead domain is described as a “winged helix” as a result of a butterfly-like appearance. High sequence homology is present in the α-helices and β-sheets with variations described in either absent β-sheets and loops or additional α-helices. Although both the first and second loops make contact with DNA, it is the second loop that can influence the stability of DNA binding. In addition, post-translational modification of FoxO proteins, such as phosphorylation or acetylation that block FoxO activity, alter the binding of the C-terminal basic region to DNA to prevent transcriptional activity.15 However, other mechanisms may influence DNA binding of forkhead proteins, such as variations in the N-terminal region of the DNA recognition helix, changes in electrostatic distribution, and the ability of forkhead proteins to be shuttled to the cell nucleus.5,16
FoxO Pro-apoptotic Pathways and Cell Cycle Regulation Block Neoplastic Progression
Genes linked to apoptosis may not necessarily lead to cell death since current studies have suggested additional roles for genes normally associated with apoptosis that can involve cellular replication and transcription. Yet, cellular apoptosis can lead to several degrees of pathology in diseases such as neurodegenerative disease, diabetes mellitus (DM) and cardiovascular injury.17 More importantly, regulation of apoptotic pathways appears to serve a critical juncture for the control of tumor growth and unregulated cell proliferation.5,18 Apoptotic cell death is considered to be a dynamic process that involves both early and late events. Membrane phosphatidylserine (PS) externalization is an early event during cell apoptosis that assists microglia to target cells for phagocytosis.19,20 This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress,21-23 since blockade of PSR function in microglia prevents the activation of microglia.24,25 As an example, externalization of membrane PS residues occur in cells during periods of oxidative stress that involve anoxia,26 reactive oxygen species (ROS) exposure,27 and with agents that produce ROS, such as 6-hydroxydopamine.28 In contrast to cells with PS exposure, the cleavage of genomic DNA into fragments is considered to be a later event during apoptotic injury.17,29 Endonucleases responsible for DNA degradation have been identified and include the acidic, cation independent endonuclease (DNase II), cyclophilins, and the 97 kDa magnesium—dependent endonuclease. In the nervous system, endonucleases include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease.17,29
Interestingly, the induction of apoptosis in cells through FoxO proteins may require pathways aligned with oxidative stress (Figs. 1 and 3). Oxidative stress occurs during the release of ROS that consist of oxygen free radicals and other chemical entities. Oxygen free radicals and mitochondrial DNA mutations have become associated with tissue injury, aging and accumulated toxicity for an organism.17 ROS include superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide and peroxynitrite.29 Most reactive species are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase, glutathione peroxidase, catalase and small molecules, such as vitamins C, E, D3 and nicotinamide, the amide form of niacin or vitamin B3.20,30,31 During periods of oxidative stress, FoxO transcription factors can lead to cell injury and apoptosis,32 since forkhead transcription factors such as FoxO1 and FoxO3a must be present for oxidative stress to result in apoptotic cell injury.33 Under other conditions of oxidative stress, FoxO3a in conjunction with c-Jun N-terminal kinase (JNK) have been shown to modulate an apoptotic ligand activating a Fas-mediated death pathway in cultured motoneurons,34 to lead to apoptosis through tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) and BH3-only proteins Noxa and Bim in neuroblastoma cells,35 and to promote pro-apoptotic activity of p53.36 Additional work shows that loss of FoxO expression during oxidative stress is protective to cells. For example, protein inhibition or gene knockdown of FoxO1 or FoxO3a can lead to reduction in ischemic infarct size in the brain,37 mediate protection of metabotropic glutamate receptors during vascular injury,38 enhance pancreatic β-cell or neuronal survival through NAD+ precursors during oxidative stress,39 and provide trophic factor protection with erythropoietin (EPO)40 and neurotrophins.41
Figure 1.
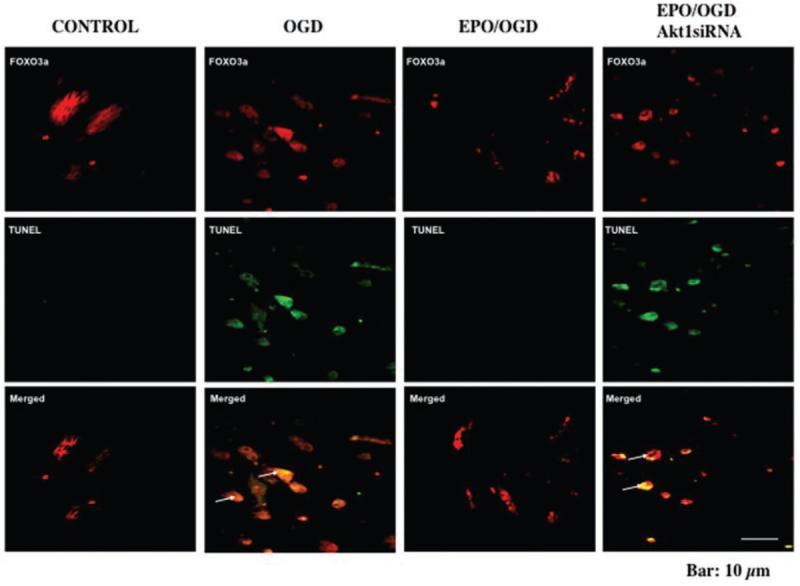
FoxO3a leads to apoptotic cell injury that can be prevented by erythropoietin (EPO) though Akt dependent pathways. Immunofluorescent double staining for FoxO3a and TUNEL was performed at 6 hours after oxidative stress with oxygen-glucose deprivation (OGD). EPO (10 ng/ml) during OGD prevents nuclear DNA degradation and FoxO3a nuclear translocation in the same rat brain endothelial cells (ECs) with no overlap of staining in merged images. In contrast, white arrows in merged images show both nuclear FoxO3a and TUNEL staining (yellow) in ECs with OGD alone or with combined EPO/OGD and Akt1 siRNA gene silencing, illustrating that EPO requires Akt1 to prevent FoxO3a nuclear translocation that leads to apoptotic DNA degradation. Control equals untreated ECs.
Figure 3.
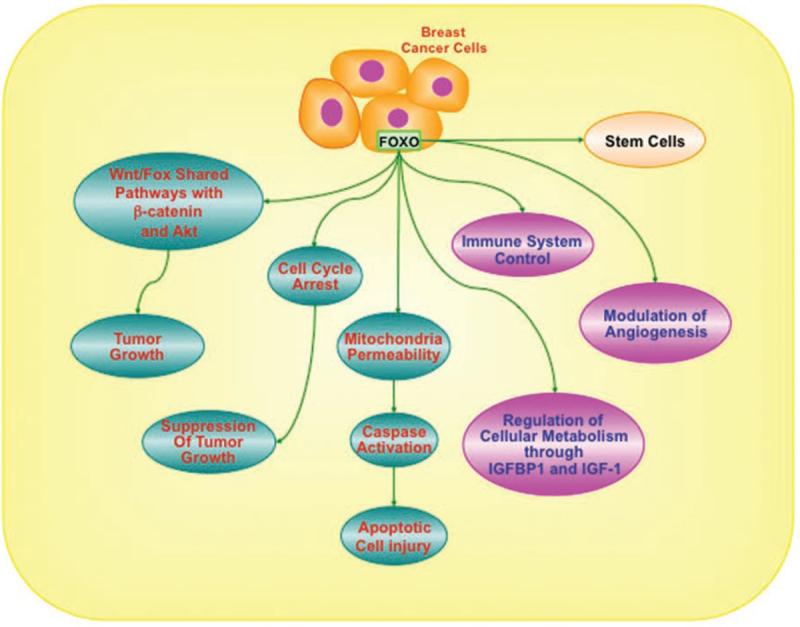
As an example in breast cancer cells, FoxO proteins influence of number of cell pathways that impact upon the immune system, cellular metabolism, angiogenesis, stem cell development, cell cycle regulation and apoptosis. FoxO proteins form an important component for processes that involve apoptosis following increased mitochondrial membrane permeability with subsequent caspase activation. FoxO proteins suppress tumor growth through cellular mechanisms that require cell cycle arrest, angiogenesis and immune system regulation. FoxO proteins also are intimately involved with pathways that control cellular metabolism that include insulin-like growth factor binding protein-1 (IGFBP1) and insulin-like growth factor-1 (IGF-1). In relation to the integration with novel signal transduction pathways, FoxO proteins interface with Wnt and Akt to modulate cellular proliferation and survival with common mechanisms that can impact upon pathways such as those associated with β-catenin.
FoxO proteins also appear to be ideal to regulate tumor growth not only through pro-apoptotic pathways, but also through the blockade of cell cycle progression (Fig. 3). For example, FoxO3a and FoxO4 can promote cell cycle arrest in mouse myoblastic cell lines through modulation of growth-arrest and DNA-damage-response protein 45.5,42 Treatment of chronic myelogenous leukemia cell lines with the Bcr-Abl tyrosine kinase inhibitor imatinib requires FoxO3a activation to antagonize cell proliferation and promote apoptotic cell death through increased TRAIL production43 (Fig. 2). In addition, the transcription factor E2F-1 that controls the induction of the cell cycle has been reported in cell lines to increase the endogenous expression of FoxO1 and FoxO3a to lead to cell cycle arrest.44 In contrast, the loss of FoxO3a activity in association with c-myc, p27 and nuclear factor-κB (NFκB) can result in cell cycle induction and malignant transformation of mouse cells in the presence of oncogene activation5,8 (Fig. 2). Other work suggests that FoxO proteins utilize the p53 upstream regulator p19(Arf) through myc to block cell cycle induction and lymphoma progression.45
Figure 2.
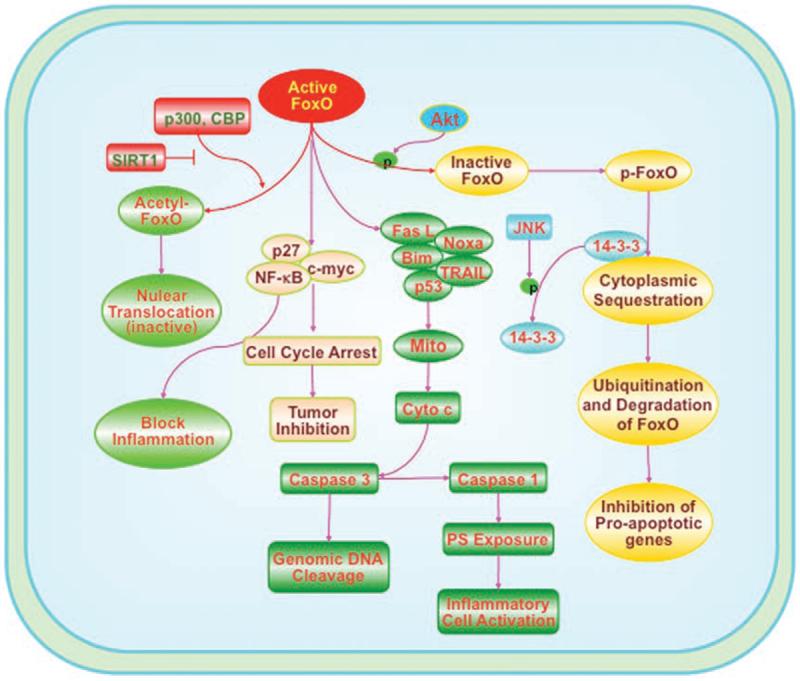
FoxO proteins employ multiple signal transduction pathways that affect cell proliferation, survival and immune system function. Post-translational modification of FoxO proteins involves pathways associated with phosphorylation, acetylation and ubiquitylation. The serine-threonine kinase protein kinase B (Akt) can prevent cellular apoptosis through the phosphorylation of FoxO proteins. Post-translational phosphorylation (p) of FoxO proteins will maintain FoxO transcription factors in the cytoplasm by association with 14-3-3 proteins and prevent the transcription of pro-apoptotic target genes. During FoxO protein activation, FoxOs can prevent inflammatory cell activation through the inhibition of nuclear factor-κB (NFκB). FoxO proteins can lead to apoptotic death pathways that involve mitochondrial (Mito) release of cytochrome c (Cyto c) and caspase activation through a Fas-mediated ligand (Fas L) death pathway, tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL), BH3-only proteins Noxa and Bim, or p53. Cell cycle inhibition that blocks tumor growth through FoxO protein activation can rely upon c-myc, p27 and NFκB. FoxO proteins may influence inflammatory cell activation through phosphatidylserine (PS) externalization on cells.
FoxO Proteins, Post-translational Control and Integration with Novel Cellular Signaling
Post-translational modification of FoxO proteins involves pathways associated with phosphorylation, acetylation and ubiquitylation8,46-48 (Fig. 2). In regards to the inhibition of FoxO protein activity, the serine-threonine kinase protein kinase B (Akt) is a primary mediator of phosphorylation of FoxO1, FoxO3a and FoxO4.8,49 Activation of Akt is usually cytoprotective, such as during hyperglycemia,50 hypoxia,51 β-amyloid (Aβ) toxicity,52 cardiomyopathy53 and oxidative stress.19,25,54 Akt can prevent cellular apoptosis through the phosphorylation of FoxO proteins.32 Post-translational phosphorylation of FoxO proteins will maintain FoxO transcription factors in the cytoplasm by association with 14-3-3 proteins and prevent the transcription of pro-apoptotic target genes.40,55 An exception in regards to the subcellular trafficking of FoxO proteins involves FoxO6. This FoxO protein usually resides in the nucleus of cells and is phosphorylated by Akt in the nucleus. FoxO6 does not contain a conserved C-terminal Akt motif which limits nuclear shuttling of this protein, but FoxO6 transcriptional activity can be blocked by growth factors independent of shuttling to the cytosol through a FoxO6 N-terminal Akt site.56
Modulation of Akt activity also oversees apoptotic pathways of caspases that may offer an alternative mechanism to regulate FoxO proteins. Caspases are a family of cysteine proteases that are synthesized as inactive zymogens that are proteolytically cleaved into subunits at the onset of apoptosis.57-59 The caspases 1 and 3 have been linked to the apoptotic pathways of genomic DNA cleavage, cellular membrane PS exposure, and activation of inflammatory cells24,60,61 (Fig. 2). Caspase pathways may be tied to the forkhead transcription factor FoxO3a since increased activity of FoxO3a can result in cytochrome c release and caspase-induced apoptotic death.35,38-40 Pathways that can inhibit caspase 3 activity appear to offer a unique regulatory mechanism. For example, caspase 3 cleavage of Fox3a can lead to pro-apoptotic amino-terminal (Nt) fragments that can lead to cell death. However, during caspase 3 inhibition, inactive phosphorylated FoxO3a remains intact and does not lead to apoptotic cell injury during oxidative stress.38-40
Post-translational modification of FoxO proteins also relies upon pathways associated with ubiquitylation and acetylation.62,63 Akt phosphorylation of FoxO proteins not only retains these transcription factors in the cytoplasm, but also leads to ubiquitination and degradation through the 26S proteasome.46,63 In the absence of Akt, IκB kinase (IKK) also can directly phosphorylate and block the activity of FoxO proteins, such as FoxO3a.5,8 This leads to the proteolysis of FoxO3a via the Ub-dependent proteasome pathway.8,46-48 The serum- and glucocorticoid-inducible protein kinase (Sgk), a member of a family of kinases termed AGC (protein kinase A/ protein kinase G/protein kinase C) kinases which includes Akt, also can phosphorylate and retain FoxO3a in the cytoplasm.64 Knowledge that Sgk and Akt can phosphorylate FoxO3a at different sites may offer new opportunities to more effectively prevent apoptotic cell injury that may be mediated by FoxO3a activity. Yet, phosphorylation of FoxO proteins does not always lead to negative regulation. The protein kinase mammalian sterile 20-like kinase-1 also can phosphorylate FoxO proteins directly and lead to their activation.65 The ability of sterile 20-like kinase-1 to activate FoxO proteins may be linked to JNK, since sterile 20-like kinase-1 can increase JNK activation.66 FoxO proteins also are acetylated by histone acetyltransferases that include p300, the CREB-binding protein (CBP), and the CBP-associated factor and are deacetylated by histone deacetylases, such as SIRT1, a NAD+-dependent deacetylase and the mammalian ortholog of the silent information regulator 2 (Sir2) protein5 (Fig. 2). Acetylation of FoxO proteins provides another avenue for the control of these proteins. Once acetylated such as by CBP, FoxO proteins may translocate to the cell nucleus but have diminished activity since acetylation of lysine residues on FoxO proteins has been shown to limit the ability of FoxO proteins to bind to DNA.67 In addition, acetylation can increase phosphorylation of FoxO proteins by Akt.67
Interestingly, FoxO proteins are associated with other novel signal transduction pathways tied to cell death. One pathway in particular involves proteins derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes. The Wnt proteins are secreted cysteine-rich glycosylated proteins that can control cell proliferation, differentiation, survival and tumorigenesis.68,69 More than eighty target genes of Wnt signaling pathways have been demonstrated in human, mouse, Drosophila, Xenopus and zebrafish. These genes are present in several cellular populations, such as neurons, cardiomyocytes, endothelial cells, cancer cells and pre-adipocytes.70 At least nineteen of twenty-four Wnt genes that express Wnt proteins have been identified in the human.68,69,71
One Wnt pathway controls target gene transcription through β-catenin, generally referred to as the canonical pathway.68,69 It is the β-catenin pathway that appears to tie FoxO proteins and Wnt signaling together.18 For example, in relation to Alzheimer's disease, A βis toxic to cells26,52 and is associated with the phosphorylation of FoxO1 and FoxO3a that can be blocked with ROS scavengers.72 A common denominator in the pathways linked to Aβ toxicity involves Wnt signaling through β-catenin. β-catenin may increase FoxO transcriptional activity and competitively limit β-catenin interaction with members of the lymphoid enhancer factor/T cell factor family73 and β-catenin also has been demonstrated to be necessary for protection against Aβ toxicity in neuronal cells.26
Additional shared signal transduction pathways between Wnt and FoxO proteins involve Akt. Processes that involve cellular proliferation, injury and immune system modulation with Akt and FoxO proteins also have parallel cellular pathways with Wnt. For example, Wnt relies upon Akt for the proliferation and differentiation of cardiomyocytes.74 In addition, reduction in tissue injury during pressure overload cardiac hypertrophy and the cytoprotective benefits of cardiac ischemic preconditioning also appear to depend upon Akt.68,69 Furthermore, Wnt overexpression can independently increase the phosphorylation and the activation of Akt to promote cellular protection and control microglial activation.26
Yet, other members of the forkhead family in addition to FoxOs also rely upon Wnt signaling in several scenarios that involve regulated as well as unchecked cell proliferation.68,69,75 For example, FoxD3 is activated by the Wnt pathway to control neural plate development76 and Foxl1 activates the Wnt/β-catenin pathway to increase extracellular proteoglycans, promote gastrointestinal cell proliferation, and possibly foster carcinogenesis.77 The Wnt pathway also utilizes forkhead members to modulate endocrine activity and can activate Foxn1 for regulatory control of thymic function.78 In other examples of cell development, Wnt signaling has been shown to rely upon Foxf1 and Foxf2 during intestinal maturation in murine models.79 In addition, Foxa2 in mice may be a significant component in early anterior-posterior axis polarization.80
In regards to tumorigenesis, deregulation of Wnt alone promotes activation of β-catenin that has been associated with the proliferation of medulloblastoma tumors.81 In addition, reduced expression of inhibitors of the Wnt pathway, such as axin, may foster lung cancer cell invasion.82 Multiple other studies also point to the activation of the Wnt pathway during gastric cancer. For example, Wnt5a expression has been correlated with advanced gastric cancer stages and a poor prognosis83 while experimental activation of the β-catenin pathway leads to the development of gastric tumors.84 In conjunction with forkhead proteins, loss of Foxl1 that can regulate the Wnt pathway and prevent β-catenin nuclear accumulation is believed to be a significant etiology for gastrointestinal tumorigenesis.77
FoxO Proteins, Stem Cells and Angiogenesis
The ability of FoxO proteins to block tumor progression most likely is tightly coupled to the modulation of stem cell proliferation and new vessel growth. The initial identification of FoxO proteins in soft-tissue tumors and leukemias, neoplasms now believed to harbor cancer stem cells for tumor self-renewal,81 suggests that FoxO proteins may be closely associated with the oversight of stem cell proliferation and differentiation (Fig. 3). For example, either simultaneous deletion of Foxo1, Foxo3a and Foxo4 or single deletion of Foxo3a in mice prevents the repopulation of hematopoietic stem cells and leads to apoptosis in these stem cell populations.85,86 Furthermore, vascular cytoprotective agents, such as the growth factor EPO,55,87,88 also may be required to modulate FoxO protein activity (Fig. 1) such as during erythroid progenitor cell development,42,89 suggesting that current clinical use of agents such as EPO during anemia or cancer may have less defined treatment implications for patients than originally anticipated.55,89 In cell culture and animal studies, EPO is cytoprotective in vascular cells and can stimulate postnatal neovascularization by increasing endothelial progenitor cell mobilization from the bone marrow.42,89,90 Interestingly, the ability of EPO to foster eythroid progenitor cell development is dependent upon the inhibition of FoxO3a activity,55,89 but also may require regulation of specific gene expression through an EPO-FoxO3a association to promote erythropoiesis in cultured cells.91 In relation to the reproductive potential of an organism, deletion of the FoxO3a gene results in the depletion of oocytes and subsequent infertility.92 Other work using a mouse model of FoxO3a overexpression in oocytes further suggests that FoxO3a retards oocyte growth and follicular development and leads to anovulation and luteinization of unruptured follicles.93 These studies may suggest a role for FoxO proteins, and specifically FoxO3a, in relation to not only the development of cancer stem cell niches, but also in regards to oocyte and follicular cell maturation. For example, in a small percentage of women who suffer from premature ovarian failure mutations in FOXO3a and FOXO1a have been observed.94
In addition to the modulation of stem cell development, FoxO proteins play a significant role to govern new vessel growth that can impact upon tumor cell growth and dispersion (Fig. 3). New capillary formation from pre-existing vessels into an avascular area is a process known as angiogenesis that is present during embryogenesis, during menstruation and during pathological processes that involve wound healing, chronic inflammation and tumor growth.69,89 FoxO proteins are intimately involved in endothelial cell development and angiogenesis. For example, Foxo3a−/− and Foxo4−/− mice develop without incidence and are indistinguishable from control littermates. Yet, mice that are singly deficient in Foxo1 die by embryonic day eleven and lack development of the vascular system.95 Other work illustrates that endothelial cell colonies in Foxo1-deficient mice fail to respond to vascular endothelial growth factor in a manner similar to wild-type endothelial cells,96 suggesting that FoxOs are necessary not only for the development of vascular cells, but also for the biological response to cellular mediators.
FoxO Proteins and Immune System Surveillance
In general, forkhead transcription factors have a vital role in maintaining immune system function and may influence the progression of tumor growth. For example, the forkhead family member FoxP3 can control the development and function of thymic-derived CD4(+) CD25(+) regulatory T cells (Treg) that impart autoimmunity. Loss of FoxP3 can result in autoimmune disorders.97 In addition, recent work identifies the expression of FoxP3 in tumor cells, such as melanoma,98 as well as in Tregs which may significantly affect patient mortality since the increased presence of Tregs in cancer patients combined with FoxP3 expression in tumors may impair antitumor autoimmune responses and lead to high mortality.99
In regards to FoxO proteins, these forkhead transcription factors also may impact upon neoplastic progression since they lead to the induction of apoptotic pathways and may influence early apoptotic membrane PS externalization (Fig. 3). The ability to regulate early apoptotic membrane PS exposure24 and inflammatory cell activity19 can ultimately impact upon cell survival since activated immune cells can lead to the phagocytic removal of tumor cells.21,29 Inflammatory cells, such as macrophages or microglia, require the activation of intracellular cytoprotective pathways to proliferate and remove injured cells.22,100 At times, this can be a beneficial process and form a barrier for the removal of foreign microorganisms and promote tissue repair during cell injury.30,42 However, inflammatory cells also may lead to cellular damage through the generation of ROS and through the production of cytokines.42 Interestingly, in mice deficient for Foxo3a, lymphoproliferation, organ inflammation of the salivary glands, lung and kidney, and increased activity of helper T cells results, supporting an important role for FoxO3a in preventing T cell hyperactivity.101 FoxO3a also appears to be necessary for neutrophil activity, since Foxo3a null mice are resistant to models of neutrophilic inflammation that involve immune complex-mediated inflammatory arthritis.102
In clinical studies, patients with rheumatoid arthritis and osteoarthritis show phosphorylation of FOXO3a in T lymphocytes as well as FOXO1 and FOXO4 in synovial macrophages, suggesting that loss of functional FOXO family members may lead to inflammatory cell activation in these disorders.103 FOXO1 gene transcript levels also are downregulated in peripheral blood mononuclear cells of patients with systemic lupus erythematosus and rheumatoid arthritis,104 illustrating a potential etiology through the loss of functional FOXO proteins for these disorders and possibly providing a biomarker of disease activity. Other work has demonstrated that FOXO1 protein regulates L-selectin expression that can regulate human T lymphocyte trafficking.105 More importantly, studies suggest a relationship between the regulation of immune system activity and the induction of apoptotic pathways that are dependent upon FoxO proteins. Prevention of inflammatory activation and apoptosis in the nervous system such as in systemic lupus erythematosus in animal models may require the upregulation of different Fox proteins, such as FoxJ1 and FoxO3a, that can block NFκB activation and interferon-gamma secretion.106 FoxO proteins also may work in concert with Fas signaling to clear activated T cells following a decrease in cytokine stimulation in patients with autoimmune lymphoproliferative syndromes,107 suggesting that activation of specific FoxO proteins may be beneficial for autoimmune disorders but may impair treatments designed to target tumor cells through immune mediated pathways.
FoxO Proteins and Cellular Metabolism
Clinical and experimental studies highlight the role of FoxO proteins during cellular metabolism that may affect the course of patients with cancer. Early work with FoxO proteins has shown that metabolic signaling with these transcription factors is conserved among multiple species including Caenorhabditis elegans, Drosophila melanogaster and mammals. FoxO proteins are homologous to the transcription factor DAuer Formation-16 (DAF-16) in the worm Caenorhabditis elegans that can determine metabolic insulin signaling and lead to lifespan extension,108,109 suggesting a significant role for FoxO proteins in relation to mammalian cell function.5,8 In fact, FoxO proteins can stimulate the insulin-like growth factor binding protein-1 (IGFBP1) promoter by binding to the insulin-responsive sequence (IRS)110 (Fig. 3). Both insulin and insulin-like growth factor-1 (IGF-1) can suppress this activity through activation of Akt.110,111
In clinical studies, analysis of the genetic variance in FOXO1a and FOXO3a on metabolic profiles, age-related diseases, fertility, fecundity and mortality have observed higher HbA1c levels and increased mortality risk associated with specific haplotypes of FOXO1a.112 These clinical observations may coincide with the demonstration in human endothelial progenitor cells that elevated glucose levels can reduce post-translational phosphorylation of FOXO1, FOXO3a and FOXO4 and allow for the nuclear translocation of these proteins to initiate an apoptotic program in endothelial progenitor cells.113 In experimental models, FoxO proteins may prevent the toxic effects of high serum glucose levels. Interferon-gamma driven expression of tryptophan catabolism by cytotoxic T lymphocyte antigen 4 may activate Foxo3a to protect dendritic cells from injury in nonobese diabetic mice.114 Additional studies have demonstrated that adipose tissue-specific expression of Foxo1 in mice improved glucose tolerance and sensitivity to insulin during an elevated fat diet.115 FoxO proteins also may protect against diminished mitochondrial energy levels known to occur during insulin resistance such as in the elderly populations.30,116,117 In caloric restricted mice that have decreased energy reserves, Foxo1, Foxo3a and Foxo4 mRNA levels were noted to progressively increase over a two year course.3 These observations complement studies in Drosophila and mammalian cells that demonstrate an increase in insulin signaling to regulate cellular metabolism during the upregulation of FoxO1 expression.118
However, the ability for FoxO proteins to maintain proper physiologic controls over cellular metabolism may be limited and occur only during specific circumstances. For example, mice with a constitutively active Foxo1 transgene have increased microsomal triglyceride transfer protein and elevated plasma triglyceride levels.119 Studies in cardiomyocytes also suggest detrimental results with enhanced FoxO activity. Increased transcriptional activity of FoxO1, such as by the Sirt1 activator resveratrol, can diminish insulin mediated glucose uptake and result in insulin resistance.120 In addition, overexpression of Foxo1 in skeletal muscles of mice can lead to reduced skeletal muscle mass and poor glycemic control,121 illustrating that activation of FoxO proteins also may impair cellular energy reserves. Additional investigations that block the expression of Foxo1 in normal and cachectic mice122 or reduce FoxO3 expression123 show the reverse with an increase in skeletal muscle mass or resistance to muscle atrophy. These results become especially relevant in patients with cancer and cachexia, since FoxO protein expression may further muscle wasting for these individuals. Given these concerns, one potential agent to consider for the maintenance of cellular metabolism in cancer patients is nicotinamide,20,57 an agent that also can inhibit FoxO protein activity.39 In patients with DM, oral nicotinamide protects β-cell function, prevents clinical disease in islet-cell antibody-positive first-degree relatives of type-1 DM, and can reduce HbA1c levels.20,57,116 Nicotinamide, which is closely linked to cell longevity pathways,124,125 may derive its protective capacity through two separate mechanisms of post-translational modification of FoxO3a. Nicotinamide not only can maintain phos-phorylation of FoxO3a and inhibit its activity, but also can preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis that can yield pro-apoptotic amino-terminal fragments.39
FoxO Proteins and Therapeutic Strategies for Cancer
Obviously, one of the most important clinical strategies for FoxO proteins involves treatments designed to control human cancer progression in light of the pro-apoptotic effects of FoxO proteins and their ability to block cell cycle progression. For example, studies with prostate cancer have shown that the tumor suppressor phosphatase and tensin homolog deleted on chromosome ten (PTEN) is mutated in approximately eighty percent of tumors with the loss of FOXO1 and FOXO3a activity. In cell cultures, overexpression of FoxO1 and FoxO3a in prostrate tumor cell lines also leads to apoptosis, suggesting that FoxO1 and FoxO3a are necessary for limiting prostate cell tumor growth.6 In addition, it has been shown that inhibition of FoxO3a activity can result in enhanced prostate tumor cell growth126 while agents that increase FoxO3a activity in both androgen sensitive and androgen insensitive prostate cell lines prevent prostate cancer cell progression.127 Furthermore, therapeutic strategies that rely upon the overexpression of a non-phosphorylatable form of FoxO3a that cannot be inactivated can sensitize prostate cancer cells to androgen-withdrawal-induced apoptosis.128 Yet, it should be noted that in prostate cell lines FoxO3a can be a positive regulator of androgen receptor expression and therefore may play a complex role in prostate cancer cell proliferation and growth inhibition.129 Other factors that control FoxO protein function also may play a role during prostate tumor progression. In prostate cancer cells, cyclin-dependent kinase 1 (CDK1) can become overexpressed and subsequently phosphylate FOXO1 to block its transcriptional activity and contribute to prostate tumorigenesis.130 In a similar manner, it has been shown that astrocyte-elevated gene-1 (AEG-1) can be upregulated in clinical prostate cancer,131 possibly lead to activation of Akt that suppresses FOXO3a132 and apoptosis in prostate tumor cells.
Initial investigations of FOXO3a in clinical breast cancer suggested that activation of FOXO3a was associated with lymph nodal metastasis and a poor prognosis.133 In contrast to these observations, other studies reported that FOXO3a was inactivated by IKK and that inactivation of FOXO3a was associated with a poor prognosis in breast cancer,134 suggesting that FOXO3a sub-cellular localization and pathways that enhance its activity could be used not only as prognostic assays but also as therapeutic targets. Other work in breast cancer cells demonstrate the tumor repressive ability of FoxOs by illustrating that increased activity of FoxO3a in association with JNK in breast cancer cell lines135 or in association with cyclin-dependent kinase inhibitor p27 in isolated human breast cancer cells can suppress breast cancer progression.136 In addition, FoxO proteins may be able to modulate estrogen function and indirectly block breast cancer growth. Overexpression of FoxO3a in breast cancer cell lines can decrease the expression of estrogen receptor regulated genes and inhibits 17beta-estradiol (E2)-dependent breast cancer growth.137
In addition to the ability to inhibit prostate and breast tumor growth, FoxO proteins may represent a viable option to control tumor progression in other tissues. FoxO proteins can function as redundant repressors of tumor growth. For example, somatic deletion in mice of Foxo1, Foxo3a and Foxo4 results in the growth of thymic lymphomas and hemangiomas.138 Other work illustrates that FoxO3a activation in colon carcinoma cell lines prevents tumor proliferation through Myc target genes that involve the Mad/Mxd family of transcriptional repressors.139 In addition, the loss of FoxO3a activity may participate in oncogenic transformation in B-chronic lymphocytic leukemia140 and in the progression of chronic myelogenous leukemia cell lines.43 Furthermore, studies suggest that some proteins, such as the Kaposi's sarcoma-associated herpesvirus latent protein LANA2, may specifically block the transcriptional activity of FoxO3a to lead to tumor growth.141 In cell models of endometrial cancer, pre-sensitization of cells to block Akt activation and foster transcription activity of FoxO1 enhances the effect of chemotherapy to limit tumor growth.142
Conclusions
The potential translation of FoxO proteins and their signal transduction pathways into viable therapeutic strategies against cancer offer exciting prospects for the future. FoxO proteins control several vital cellular pathways in relation to cell proliferation, metabolism, inflammation and survival. As a result, the ability of FoxO proteins to control cell cycle progression and promote apoptosis highlights the potential of FoxOs to become at the very least an important component for the development of new strategies against tumorigenesis. For example, use of triple mutant FoxO1 or FoxO3a expression in which three phosphorylation sites have been altered to prevent inactivation of this protein has been proposed as a potential therapeutic agent against melanoma tumors143 and endometrial cancer.144 Interestingly, new work suggests that the utilization and combination of multiple biomarkers may improve risk assessment for patients.145 Studies such as this offer additional support for the use of FoxO proteins as biomarkers of cancer progression. As an example, down-regulation of the phosphatidylinositol 3 kinase and Akt pathways have been associated with increased transcript levels for FOXO1a and FOXO3a in clinical prostate cancer samples and may indicate the onset of pre-cancerous changes or the progression of on-going tumor growth.146 Although loss of Akt activity in prostate cancer cells can result in enhanced FoxO3a activity and subsequent apoptosis of tumor cells,131 it is conceivable that early stages of cancer may lead to reduced Akt activity with insufficient levels of active forkhead transcription factors to limit tumor progression. In addition, the early and persistent expression of phosphorylated FOXO1a in gastric tumors may not only indicate the onset of cancer, but also suggest an improved prognosis for patients.147
Despite the presently known attributes of FoxO proteins to potentially treat a number of cancers, FoxO transcription factors also may have a “dark side” that can limit clinical utility. Further investigations are required since FoxO protein inhibition of cell cycle progression may not consistently lead to apoptotic cell death. Some investigations suggest that during oxidative stress, FoxO3a activation in association with SIRT1 can lead to cell cycle arrest, but not result in apoptotic cell injury.148 Furthermore, during hypoxic stress, forkhead transcription factors, such as FOXO3a, may potentiate anti-apoptotic pathways in breast cancer cells to further tumor growth.149 FoxO proteins also have been linked to potential chemotherapy drug resistance.150 Increased expression of MDR1 (P-glycoprotein) has been associated with chemotherapy drug resistance in breast cancer cells and recent work shows that FoxO1 can stimulate the transcriptional activity of MDR1 that may promote increased tolerance of tumor cells.151 In addition, the common pathways shared between Wnt and forkhead proteins may have another side that impacts upon the ability to control tumor growth.68,75 FoxO proteins may assist with β-catenin activation in the Wnt pathway and lead to tumor cell proliferation.69 In the presence of Wnt deregulation and increased β-catenin activity, tumorigenesis may ensue, such as with the proliferation of medulloblastoma tumors.81 It is clear that future basic and clinical investigations are necessary to continue to elucidate the immense potential of FoxO proteins as well as to understand the potential limits of these transcription factors.
Acknowledgements
This research was supported by the following grants (K.M): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/NIA.
Abbreviations
- Aβ
β-amyloid
- Akt
protein kinase B
- AFX
acute leukemia fusion gene located in chromosome X
- AGC
protein kinase A/protein kinase G/protein kinase C
- CBP
CREB-binding protein
- DAF-16
dauer formation-16
- DM
diabetes mellitus
- EPO
erythropoietin
- FKHR
forkhead in rhabdomyosarcoma
- FKHRL1
forkhead in rhabdomyosarcoma like protein 1
- IKK
IκB kinase
- IGF-1
insulin-like growth factor-1
- IGFBP1
insulin-like growth factor binding protein-1
- IRS
insulin-responsive sequence
- JNK
Jun N-terminal kinase
- NFκB
nuclear factor-κB
- PS
phosphatidylserine
- PTEN
tumor suppressor phosphatase and tensin homolog deleted on chromosome ten
- PSR
phosphatidylserine receptor
- ROS
reactive oxygen species
- Wg
drosophila wingless
References
- 1.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 2.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–34. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3 and 4 (FKHR, FKHRL1 and AFX) in the rat skeletal muscles. Microscopy research and technique. 2002;59:331–4. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 4.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6:134–40. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–37. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 7.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/fork-head transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- 8.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parry P, Wei Y, Evans G. Cloning and characterization of the t(X;11) breakpoint from a leukemic cell line identify a new member of the forkhead gene family. Genes Chromosomes Cancer. 1994;11:79–84. doi: 10.1002/gcc.2870110203. [DOI] [PubMed] [Google Scholar]
- 10.Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard OA. AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood. 1997;90:3714–9. [PubMed] [Google Scholar]
- 11.Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–58. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 12.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–20. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 13.Larson ET, Eilers B, Menon S, Reiter D, Ortmann A, Young MJ, Lawrence CM. A winged-helix protein from Sulfolobus turreted icosahedral virus points toward stabilizing disulfide bonds in the intracellular proteins of a hyperthermophilic virus. Virology. 2007;368:249–61. doi: 10.1016/j.virol.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Jin C, Marsden I, Chen X, Liao X. Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR. Biochemistry. 1998;37:6179–87. doi: 10.1021/bi980031v. [DOI] [PubMed] [Google Scholar]
- 15.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. 2007;35:6984–94. doi: 10.1093/nar/gkm703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397:233–46. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert opinion on therapeutic targets. 2008;12:905–16. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64:557–69. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–32. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 21.Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–75. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–18. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 26.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003;23:561–78. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 29.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation and injury. Histol Histopathol. 2007;22:1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008;6:281–4. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2008;68:1–9. doi: 10.55782/ane-2008-1666. [DOI] [PubMed] [Google Scholar]
- 32.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125–42. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2007;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–47. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 36.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006;103:9051–6. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 38.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006;3:107–17. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–43. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 40.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–50. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007;98:1949–58. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769:244–52. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007;21:2775–87. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 48.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 49.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 52.Chong ZZ, Li F, Maiese K. Erythropoietin requires NFkappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–9. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 54.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3 and 9. Exp Cell Res. 2004;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Heide LP, Jacobs FM, Burbach JP, Hoekman MF, Smidt MP. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem J. 2005;391:623–9. doi: 10.1042/BJ20050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13:883–95. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005;2:425–46. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 60.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003;23:320–30. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 61.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6:277–87. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 62.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100:11285–90. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–6. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 64.Leong ML, Maiyar AC, Kim B, O'Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278:5871–82. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 65.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 66.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal. 2008;20:892–906. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci USA. 2005;102:11278–83. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21:103–24. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218–32. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–40. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, Finkel T, Kusiak JW. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol. 2005;169:331–9. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with {beta}-Catenin Inhibits {beta}-Catenin/T Cell Factor Activity. J Biol Chem. 2008;283:9224–30. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 74.Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–51. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 75.Emami KH, Corey E. When prostate cancer meets bone: control by wnts. Cancer Lett. 2007;253:170–9. doi: 10.1016/j.canlet.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 76.Pohl BS, Knochel W. Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech Dev. 2001;103:93–106. doi: 10.1016/s0925-4773(01)00334-3. [DOI] [PubMed] [Google Scholar]
- 77.Perreault N, Sackett SD, Katz JP, Furth EE, Kaestner KH. Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev. 2005;19:311–5. doi: 10.1101/gad.1260605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3:1102–8. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- 79.Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–43. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 80.Kimura-Yoshida C, Tian E, Nakano H, Amazaki S, Shimokawa K, Rossant J, Aizawa S, Matsuo I. Crucial roles of Foxa2 in mouse anterior-posterior axis polarization via regulation of anterior visceral endoderm-specific genes. Proc Natl Acad Sci USA. 2007;104:5919–24. doi: 10.1073/pnas.0607779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sauvageot CM, Kesari S, Stiles CD. Molecular pathogenesis of adult brain tumors and the role of stem cells. Neurol Clin. 2007;25:891–924. doi: 10.1016/j.ncl.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 82.Xu HT, Wei Q, Liu Y, Yang LH, Dai SD, Han Y, Yu JH, Liu N, Wang EH. Overexpression of Axin Downregulates TCF-4 and Inhibits the Development of Lung Cancer. Ann Surg Oncol. 2007;14:3251–9. doi: 10.1245/s10434-007-9555-9. [DOI] [PubMed] [Google Scholar]
- 83.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–48. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 84.Tomita H, Yamada Y, Oyama T, Hata K, Hirose Y, Hara A, Kunisada T, Sugiyama Y, Adachi Y, Linhart H, Mori H. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res. 2007;67:4079–87. doi: 10.1158/0008-5472.CAN-06-4025. [DOI] [PubMed] [Google Scholar]
- 85.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a Is Essential for Maintenance of the Hematopoietic Stem Cell Pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 88.Cariou A, Claessens YE, Pene F, Marx JS, Spaulding C, Hababou C, Casadevall N, Mira JP, Carli P, Hermine O. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76:397–404. doi: 10.1016/j.resuscitation.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 89.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145–55. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, Kolbus A, Yamamoto K, Steinlein P, Verhaak RG, Mak TW, Beug H, Lowenberg B, von Lindern M. Differential regulation of Foxo3a target genes in erythropoiesis. Mol Cell Biol. 2007;27:3839–54. doi: 10.1128/MCB.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N. FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem. 2002;277:26729–32. doi: 10.1074/jbc.C200256200. [DOI] [PubMed] [Google Scholar]
- 93.Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- 94.Watkins WJ, Umbers AJ, Woad KJ, Harris SE, Winship IM, Gersak K, Shelling AN. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertility and sterility. 2006;86:1518–21. doi: 10.1016/j.fertnstert.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 95.Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–80. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–9. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 97.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Regulatory T cells and human disease. Clin Dev Immunol. 2007;2007:89195. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, Gedye C, Moss D, Ng SP, MacGregor D, Davis ID, Cebon J, Chen W. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–9. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 99.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–71. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–72. [PMC free article] [PubMed] [Google Scholar]
- 101.Lin L, Hron JD, Peng SL. Regulation of NFkappaB, Th activation and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–13. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 102.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–71. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 103.Ludikhuize J, de Launay D, Groot D, Smeets TJ, Vinkenoog M, Sanders ME, Tas SW, Tak PP, Reedquist KA. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum. 2007;56:2180–91. doi: 10.1002/art.22653. [DOI] [PubMed] [Google Scholar]
- 104.Kuo CC, Lin SC. Altered FOXO1 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Mol Med. 2007;13:561–6. doi: 10.2119/2007-00021.Kuo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–9. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 106.Sela U, Dayan M, Hershkoviz R, Cahalon L, Lider O, Mozes E. The negative regulators Foxj1 and Foxo3a are upregulated by a peptide that inhibits systemic lupus erythematosus-associated T cell responses. Eur J Immunol. 2006;36:2971–80. doi: 10.1002/eji.200636137. [DOI] [PubMed] [Google Scholar]
- 107.Bosque A, Aguilo JI, Alava MA, Paz-Artal E, Naval J, Allende LM, Anel A. The induction of Bim expression in human T-cell blasts is dependent on nonapoptotic Fas/CD95 signaling. Blood. 2007;109:1627–35. doi: 10.1182/blood-2006-05-022319. [DOI] [PubMed] [Google Scholar]
- 108.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–22. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 109.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–9. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 110.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–92. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 111.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–5. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 112.Kim JR, Jung HS, Bae SW, Kim JH, Park BL, Choi YH, Cho HY, Cheong HS, Shin HD. Polymorphisms in FOXO gene family and association analysis with BMI. Obesity (Silver Spring, Md. 2006;14:188–93. doi: 10.1038/oby.2006.24. [DOI] [PubMed] [Google Scholar]
- 113.Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, Lauro D, Fukamizu A, Lauro R, Federici M. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231–7. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 114.Fallarino F, Bianchi R, Orabona C, Vacca C, Belladonna ML, Fioretti MC, Serreze DV, Grohmann U, Puccetti P. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200:1051–62. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, Iskandar K, Suga K, Lombes M, Hayashi Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–76. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 116.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729–38. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–46. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kamagate A, Dong HH. Foxo1 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7:3162–70. doi: 10.4161/cc.7.20.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104:20517–22. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, downregulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–23. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 122.Liu CM, Yang Z, Liu CW, Wang R, Tien P, Dale R, Sun LQ. Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Ther. 2007;14:945–52. doi: 10.1038/sj.cgt.7701091. [DOI] [PubMed] [Google Scholar]
- 123.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–5. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, Vanberkum MF, Arking R, Freeman DC, Maiese K, Tzivion G. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–9. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008;5:159–70. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lynch RL, Konicek BW, McNulty AM, Hanna KR, Lewis JE, Neubauer BL, Graff JR. The progression of LNCaP human prostate cancer cells to androgen independence involves decreased FOXO3a expression and reduced p27KIP1 promoter transactivation. Mol Cancer Res. 2005;3:163–9. doi: 10.1158/1541-7786.MCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 127.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–50. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 128.Cornforth AN, Davis JS, Khanifar E, Nastiuk KL, Krolewski JJ. FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells. Oncogene. 2008;27:4422–33. doi: 10.1038/onc.2008.80. [DOI] [PubMed] [Google Scholar]
- 129.Yang L, Xie S, Jamaluddin MS, Altuwaijri S, Ni J, Kim E, Chen YT, Hu YC, Wang L, Chuang KH, Wu CT, Chang C. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2005;280:33558–65. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 130.Liu P, Kao TP, Huang H. CDK1 promotes cell proliferation and survival via phosphorylation and inhibition of FOXO1 transcription factor. Oncogene. 2008;27:4733–44. doi: 10.1038/onc.2008.104. [DOI] [PubMed] [Google Scholar]
- 131.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, Dahiya R. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–55. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 132.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–7. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jin GS, Kondo E, Miyake T, Shibata M, Takashima T, Liu YX, Hayashi K, Akagi T, Yoshino T. Expression and intracellular localization of FKHRL1 in mammary gland neoplasms. Acta medica Okayama. 2004;58:197–205. doi: 10.18926/AMO/32088. [DOI] [PubMed] [Google Scholar]
- 134.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 135.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC, Lam EW. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 136.Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007;67:9018–23. doi: 10.1158/0008-5472.CAN-07-1691. [DOI] [PubMed] [Google Scholar]
- 137.Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC, Lin SH, Hu MC. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, Downward J, Schulze A. Induction of Mxi1-SR{alpha} by FOXO3a Contributes to Repression of Myc-Dependent Gene Expression. Mol Cell Biol. 2007;27:4917–30. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ticchioni M, Essafi M, Jeandel PY, Davi F, Cassuto JP, Deckert M, Bernard A. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26:7081–91. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 141.Munoz-Fontela C, Marcos-Villar L, Gallego P, Arroyo J, Da Costa M, Pomeranz KM, Lam EW, Rivas C. Latent protein LANA2 from Kaposi's sarcoma-associated herpesvirus interacts with 14-3-3 proteins and inhibits FOXO3a transcription factor. J Virol. 2007;81:1511–6. doi: 10.1128/JVI.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoekstra AV, Ward EC, Hardt JL, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1. Gynecol Oncol. 2008;108:609–18. doi: 10.1016/j.ygyno.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 143.Gomez-Gutierrez JG, Souza V, Hao HY, Montes de Oca-Luna R, Dong YB, Zhou HS, McMasters KM. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer biology & therapy. 2006;5:875–83. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 144.Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149:1942–50. doi: 10.1210/en.2007-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 146.Hellwinkel OJ, Rogmann JP, Asong LE, Luebke AM, Eichelberg C, Ahyai S, Isbarn H, Graefen M, Huland H, Schlomm T. A comprehensive analysis of transcript signatures of the phosphatidylinositol-3 kinase/protein kinase B signal-transduction pathway in prostate cancer. BJU Int. 2008;101:1454–60. doi: 10.1111/j.1464-410X.2008.07540.x. [DOI] [PubMed] [Google Scholar]
- 147.Kim JH, Kim MK, Lee HE, Cho SJ, Cho YJ, Lee BL, Lee HS, Nam SY, Lee JS, Kim WH. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Mod Pathol. 2007;20:835–42. doi: 10.1038/modpathol.3800789. [DOI] [PubMed] [Google Scholar]
- 148.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 149.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–53. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 150.Gomes AR, Brosens JJ, Lam EW. Resist or die: Foxo transcription factors determine the cellular response to chemotherapy. Cell Cycle. 2008;7:3133–6. doi: 10.4161/cc.7.20.6920. [DOI] [PubMed] [Google Scholar]
- 151.Han CY, Cho KB, Choi HS, Han HK, Kang KW. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinogenesis. 2008;29:1837–44. doi: 10.1093/carcin/bgn092. [DOI] [PubMed] [Google Scholar]


