Abstract
The sympathetic nervous system (SNS) plays an essential role in the regulation of metabolic and cardiovascular homeostasis. Low SNS activity has been suggested to be a risk factor for weight gain and obesity development. In contrast, SNS activation is characteristic of a number of metabolic and cardiovascular diseases that occur more frequently in obese individuals. Until recently, the relation between obesity and SNS behavior has been controversial because previous approaches for assessing SNS activity in humans have produced inconsistent findings. Beginning in the early 1990's, many studies using state of the art neurochemical and neurophysiological techniques have provided important insight. The purpose of the present review is to provide an overview of our current understanding of the regional specific alterations in SNS behavior in human obesity. We will discuss findings from our own laboratory which implicate visceral fat as an important depot linking obesity with skeletal muscle SNS activation. The influence of weight change on SNS behavior and the potential mechanisms and consequences of region specific SNS activation in obesity will also be considered.
1. Introduction
Obesity is a major public health problem. Approximately 65% of the U.S. population and 1 billion people worldwide are considered overweight or obese and at increased risk for metabolic and cardiovascular diseases (WHO, 1997). Obesity is much more than a cosmetic issue given its association with poor quality of life and reduced life expectancy (Peeters et al., 2003). In addition, obesity-related illnesses place a considerable burden on the economy by increasing rates of health care usage and associated costs. As much as 9.1% of all total health care expenditures and approximately 39.2 million lost work days each year have been attributed to obesity (Finkelstein et al., 2003).
The sympathetic nervous system (SNS) plays an essential role in the regulation of metabolic and cardiovascular homeostasis. Low SNS activity has been suggested to be a risk factor for future weight gain and obesity development (Ravussin and Tataranni, 1996). In contrast, SNS activation is characteristic of a number of metabolic and cardiovascular disorders, many of which occur more frequently in obese individuals. Obesity potentiates SNS activation in patients with hypertension (Grassi et al., 2000) and congestive heart failure (Grassi et al., 2003), and heightened SNS activity is associated with adverse clinical outcomes in these populations (Kaye et al., 1995, Schlaich et al., 2003).
The present review will focus on the evidence linking obesity with activation of the SNS and the particular insight provided by neurophysiological and neurochemical techniques. We will provide a brief overview of some of the major experimental techniques available to study region specific SNS behavior in humans, discuss the potential mechanisms and consequences of alterations in SNS behavior in obesity and discuss recent findings from our laboratory using overfeeding-induced weight gain as an experimental approach to better understand these issues. Finally, we will conclude our review with recommendations for future investigation.
2. Overview of Basic Anatomy and Physiology of the Sympathetic Nervous System
The motor outflow of the SNS originates almost exclusively in the intermediolateral cell column of the thoracic and upper lumber spinal cord (Loewy and Spyder, 1990). The relatively short axons of preganglionic neurons exit the spinal cord at the segment where their cell bodies reside and pass first in the ventral roots and subsequently in the white communicating rami to synapse with postganglionic neurons in the sympathetic ganglia. Acetylcholine is the predominate neurotransmitter released from sympathetic preganglionic nerves. Some preglanglionic axons bypass the sympathetic ganglion without synapsing and directly innervate the adrenal medulla via the splanchnic nerve. The postsynaptic or postganglionic neurons have long axons that extend to innervate target organs such as the blood vessels, kidney, heart, and other tissues and organs. The primary neurotransmitter of postganglionic sympathetic neurons is norepinephrine (NE), although several bioactive peptides, such neuropeptide Y, may be co-released.
In contrast to early views that activation of the SNS occurs in a global “all or none” fashion (Cannon, 1915), there is now considerable evidence that SNS outflow is regulated in a highly tissue specific manner (Morrison, 2001). Sympathetic nervous system outflow to one region may not reflect the magnitude or even the direction of SNS outflow to another. The cardiovascular and metabolic responses to SNS activation include vasoconstriction of blood vessels and increased oxygen consumption and substrate mobilization in tissues and organs.
3. Methods for Quantifying Sympathetic Nervous System Behavior
The methods for quantifying SNS activity in humans each possess distinct advantages and disadvantages with respect to sensitivity, reproducibility, invasiveness, and technical difficulty. In the sixty years following von Euler's identification of NE as the primary neurotransmitter released from sympathetic nerves (von Euler, 1948), countless investigators have sought to exploit this finding for the purpose of quantifying SNS activity. Urinary NE (and metabolite) excretion measurements are noninvasive and relatively easy to obtain but are limited as a measure of SNS activity is due to its lack of sensitivity and dependence on renal function (Kopin, 1985). In addition, urinary NE measurements may provide some insight into “chronic” SNS activity but are not particularly useful for studying responses to acute stress (Dimsdale and Ziegler, 1991). In contrast, plasma NE concentrations tend to be more useful as indirect measure of SNS activity during acute stress. Unfortunately, the general applicability of plasma NE concentrations suffers from some important limitations. First, the NE entering circulation comprises only a small fraction of that released from presynaptic sympathetic varicosities and plasma NE concentrations do not provide insight into regional behavior of the SNS (Esler et al., 1988). Second, plasma NE concentrations are dependent on the rate of clearance from the circulation and not simply the rate of NE release (Esler et al., 1988). Finally, plasma NE concentrations have limited reproducibility, particularly when compared with other direct measures of SNS activity (Grassi et al., 1997).
In light of these limitations, isotope dilution methods have been developed to assess regional NE spillover (i.e., the amount of NE secreted by presynaptic terminals that enters into the circulation of a particular tissue or organ). Regional NE spillover rate can powerful provide insight into the SNS behavior of virtually every tissue/organ accessible by catheterization (Esler et al., 1988, Esler et al., 1990). Adrenal epinephrine secretion can be assessed using similar methodology. Measurements of whole body NE spillover provide a measure of global SNS outflow (Esler et al., 1988), although it is important to emphasize that these measurements will be influenced by the net effect of SNS outflow to all the individual tissues/organs innervated by the SNS (i.e., regional NE spillovers). In the hands of appropriately trained individuals, these measurements exhibit excellent reproducibility and sensitivity. However, there are some limitations of using regional NE spillover as a measure of SNS activity. The technique is invasive and relies on a number of assumptions which vary in the degree to which they are upheld under different conditions (Esler et al., 1988). In addition, NE spillover measurements can be influenced by factors other than sympathetic nerve traffic such as presynaptic modulation (Gothert, 2003), metabolism of NE, and changes in blood flow (Esler et al., 1990). Thus, the main challenge in interpreting these measurements lies with discriminating any observed changes (or differences) in SNS activity due to alterations in sympathetic nerve traffic from those attributable to other factors. Importantly, the addition of measurements of the spillover of NE metabolites can provide important insight into the processes that lie close to or within sympathetic nerve endings (Eisenhofer et al., 1992). Similarly, measurements of regional blood flow can provide important insight in determining the degree to which differences in blood flow between comparison groups or conditions represents a confounding factor.
In contrast to the above mentioned methods for assessing SNS activity in humans, the microneurographic technique provides a direct measure of efferent postganglionic sympathetic nerve traffic (Wallin and Fagius, 1988, Vallbo et al., 2004). The technique involves the percutaneous insertion of a tungsten microelectrode into a skin or peripheral skeletal muscle nerve fiber (most commonly, the peroneal nerve). Microneurographic recordings are extremely sensitive and highly reproducible (Wallin et al., 1993) and both single- (Macefield et al., 2002) or multi-unit (Wallin and Fagius, 1988) recordings can be obtained. As with NE spillover measurements, microneurographic recordings can be obtained under quite resting conditions or in response to a variety of stressors. Although minimally invasive, the quality of the recordings depend on a host of factors including technical proficiency of the investigator, characteristics of the electrode, proximity of the microelectrode to the active nerve fiber, processing of the signal, and the cooperation of the subject. Importantly, microneurographic recordings are limited to postganglionic axons which lie close to the skin and are accessible by microelectrodes.
The value of the above mentioned methods for measuring SNS activity in humans can be enhanced by their combination. For example, combing microneurographic measurements with norepineprhine spillover measurements to skeletal muscle and/or plasma NE concentrations can provide insight into a broader aspect of sympathetic function; from action potential to neurotransmitter release and accumulation. Furthermore, the addition of target organ effect responses allow the consequences of sympathetic stimulation to be evaluated. The methods available for assessing effector responses to sympathetic stimulation are beyond the scope of this review. However, it is important to emphasize that none of the above mentioned methods for assessing SNS behavior provide insight into the target organ effector response to sympathetic stimulation.
4. Sympathetic Nervous System Behavior in Obesity: A Brief History
Historically, there have been two hypotheses regarding the nature of the predominate abnormality in SNS behavior in human obesity. Bray (Bray, 1991) used the acronym “MONA LISA” to describe his hypothesis that Most Obesities kNown Are Low In Sympathetic Activity. This view was based primarily on experimental evidence in rodents that exhibited low SNS activity and morbid obesity following lesions in the ventromedial hypothalamus. As such, low SNS activity was considered causal in the development of obesity. In contrast, Landsberg (Landsberg, 1986) viewed SNS activation targeting the heart, blood vessels and kidneys as a critical link to the well documented relation between obesity and hypertension (Hall, 2003, Davy and Hall, 2004).
Young and Macdonald (Young and Macdonald, 1992) reviewed over 40 human studies conducted prior to 1991 in an attempt to clarify whether obesity was associated with elevated or reduced SNS activity. The published literature supported both hypotheses as well as the possibility that no difference in SNS activity was evident in normal weight and obese individuals. However, several limitations of this literature were evident. The most significant limitation was that plasma NE concentrations were used as a measure of SNS activity (see above under “Methods to Assess Sympathetic Nervous System Behavior in Humans”). In addition, most of these studies failed to appropriately screen obese participants for the presence of overt metabolic and cardiovascular disease and none had considered the possibility that body fat distribution might contribute to individual differences in SNS behavior. As such, this literature should be considered cautiously and with these limitations in mind.
5. Region Specific and Whole Body Sympathetic Nervous System Behavior in Obesity
As emphasized above, SNS outflow is highly differentiated and elevated SNS ouflow to one organ may not be not be similar in magnitude or direction to SNS outflow targeting other organs. Therefore, any discussion of the SNS should consider the potential for regional specificity. In this regard, the availability of state-of-the-art neurochemical and neurophysiological techniques has improved our current understanding of the regional alterations in SNS outflow in obesity.
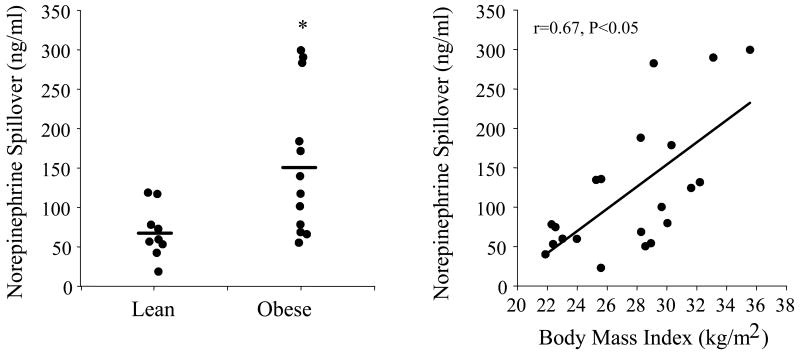
Cardiac SNS activity (i.e., cardiac NE spillover rate) is ∼50% lower in obese compared with nonobese normotensive humans (Vaz et al., 1997). However, cardiac SNS activity is twofold higher in hypertensive compared with normotensive obese and 25% higher compared with normal weight subjects. SNS activity to the kidney is also higher in obese compared with nonobese normotensive individuals (Vaz et al., 1997) (Figure 1), and there is a strong linear relation between body mass index (i.e., total body adiposity) and renal NE spillover rate (Vaz et al., 1997).
Figure 1.
Renal norepinephrine spillover in lean and obese humans (right panel) and relation between body mass index and renal norepinpehrine spillover in the pooled sample. *P<0.05 vs. lean. Used with permission. (from Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. 1997. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96:3423-3429).
Neither SNS activity to the splanchnic region nor adrenal epinephrine secretion appear to be noticeably altered in human obesity (Vaz et al., 1997), but NE spillover rate in white adipose tissue is reduced in obese compared with nonobese individuals (Coppack et al., 1998).
There is now considerable evidence based on direct recordings of post-ganglionic muscle sympathetic nerve activity (MSNA) supporting a link between body fat levels and SNS activity. The results of numerous studies indicate that obese humans demonstrate ∼50-100% higher levels of MSNA compared with their non-obese peers (Spraul et al., 1993, Scherrer et al., 1994, Spraul et al., 1994, Gudbjornsdottir et al., 1996, Grassi et al., 2000, Weyer et al., 2000, Alvarez et al., 2002). In contrast, Hugget et al. (Huggett et al., 2005) reported that MSNA was not elevated in overweight individuals. The reason(s) for this apparent discrepancy is not entirely clear but could be due, in part, to the selection of a group of overweight subjects with a primarily subcutaneous obese phenotype (see below under “6. Sympathetic Neural Activation in Obesity: Role of Abdominal Visceral Fat”).
Given the regional heterogeneity of SNS activation in obesity, it should not be surprising that the available evidence suggests that whole body SNS activity is not consistently altered in obesity (Vaz et al., 1997, Coppack et al., 1998, Rumantir et al., 1999).
6. Sympathetic Neural Activation in Obesity: Role of Abdominal Visceral Fat
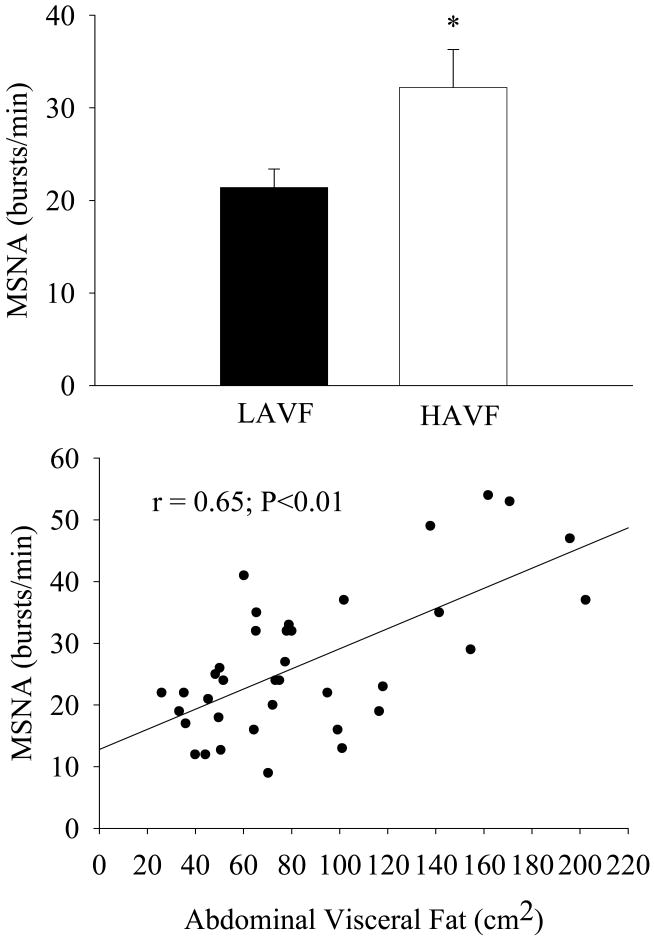
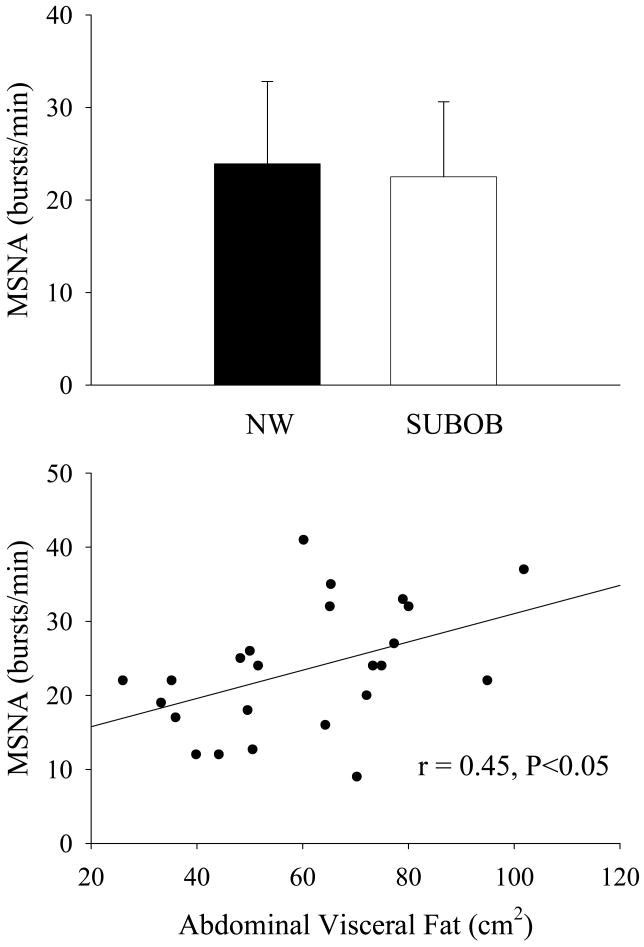
There is considerable interindividual variability in MSNA among subjects of similar age, gender and body mass index (Scherrer et al., 1994), but the reason(s) for this has/have been unclear until recently. We have observed ∼40% higher levels of MSNA in normotensive men with higher compared with lower abdominal visceral fat (via computed tomography) despite similar age, total fat mass, and abdominal subcutaneous fat (Alvarez et al., 2002) (Figure 2, top panel). In our study, MSNA was more closely associated with abdominal visceral fat (r=0.65, P<0.05, Figure 2, bottom panel) than with total fat mass (r=0.323, P<0.05) or abdominal subcutaneous fat (r=0.27, P=0.05). Importantly, the relation between skeletal MSNA and abdominal visceral fat was independent of total body fat (partial r=0.61, P<0.05). In contrast, MSNA was not increased in subcutaneous obese compared with non-obese men with similar levels of abdominal visceral fat (Alvarez et al., 2004) (Figure 3, top panel). However, abdominal visceral fat remained the only significant body composition or regional fat distribution related correlate in this pooled sample of men (r=0.45, P<0.05; Figure 3, bottom panel). Moreover, the relation between abdominal visceral fat and MSNA was also evident in normal weight (i.e., body mass index<25 kg/m2) humans (r=0.58, P<0.05) (Alvarez et al., 2004). Unfortunately, there is currently no information available regarding alterations in whole body SNS or SNS activity targeting other regions in visceral obesity.
Figure 2.
Basal muscle sympathetic nerve activity (MSNA) individuals with lower abdominal visceral fat (LAVF) and higher abdominal visceral fat (HAVF) (top panel) and relation between basal MSNA and abdominal visceral fat (bottom panel). *P<0.05 vs. baseline. (from Alvarez GE, Beske SD, Ballard TP, Davy KP. 2002. Sympathetic neural activation in visceral obesity. Circulation 106:2533-2536).
Figure 3.
Basal muscle sympathetic nerve activity (MSNA) in nonobese (NO) and subcutaneous obese (SUBOB) men (top panel) and relation between basal MSNA and abdominal visceral fat in the pooled sample (bottom panel). Used with permission. (from Alvarez GE, Ballard TP, Beske SD, Davy KP. 2004. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol 287:H414-418).
Gender- and age-related differences in body fat distribution may be important in understanding corresponding differences in MSNA between women and men, and between young and older adults (Ng et al., 1993). Jones and colleagues (Jones et al., 1996) reported that the gender difference in MSNA was no longer present after adjusting for differences in the waist-to-thigh ratio. Similarly, age-related differences in MSNA are reduced after adjusting for differences in waist circumference between young and older men (Jones et al., 1997a, Jones et al., 1997b). Whether the greater abdominal visceral fat accumulation that is characteristic of the metabolic syndrome, type 2 diabetes and hypertension also contributes to the higher MSNA levels observed in these disorders is not known.
7. Weight Change and Sympathetic Neural Behavior
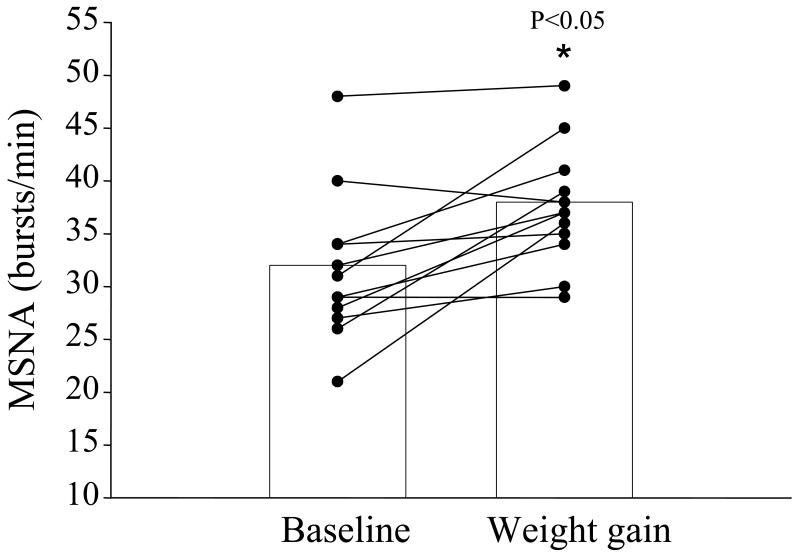
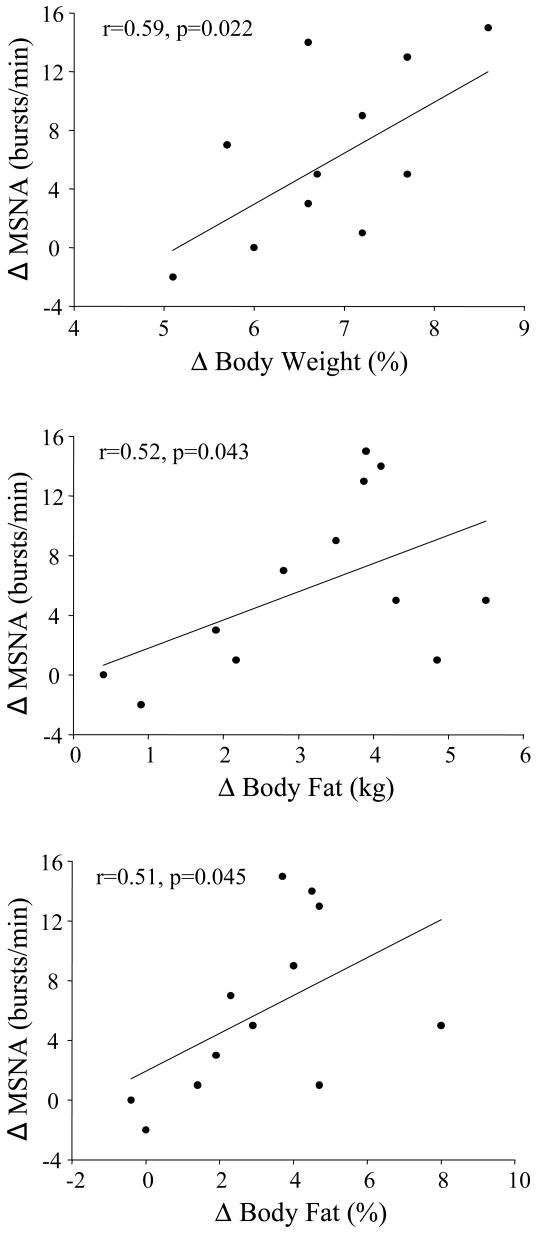
Cross-sectional studies have provided much needed insight on the relation between obesity and SNS activity in humans. However, the results of one study suggest that MSNA may not be elevated in overweight humans (Huggett et al., 2005). In addition, whether the higher MSNA observed in obese individuals is genetically determined and, perhaps, part of a phenotype that is evident prior to significant weight gain and obesity is not clear. We recently reported that modest overfeeding induced weight gain increased MSNA in healthy nonobese males (Figure 4) and that the magnitude of increase was related to the amount of body weight and fat gained (Figure 5). Importantly, these findings suggest that individuals who gain even modest amounts of weight demonstrate increases in SNS activity regardless of whether they become obese.
Figure 4.
Muscle sympathetic nerve activity (MSNA) at baseline and following weight gain in healthy nonobese men. *P<0.05 vs. baseline. Used with permission. (from Gentile CL, Orr JS, Davy BM, Davy KP. 2007. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol 292:R1834-1838).
Figure 5.
Top panel: Relation between the change in muscle sympathetic nerve activity (MSNA) with weight gain and percent change in body weight. Middle panel: Relation between the change in MSNA with weight gain and the change in body fat (kg). Bottom panel: Relation between the change in MSNA weight gain and change in percent body fat. *P<0.05 vs. baseline. Used with permission. (from Gentile CL, Orr JS, Davy BM, Davy KP. 2007. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol 292:R1834-1838).
Overfeeding induced weight gain may provide an insightful model to study the mechanisms mediating the relation between adiposity and SNS activation. As such, investigators could determine the causative role of a number of putative sympathoexcitatory molecules. For example, one could test the hypothesis that angiotensin II contributes to the SNS activation that occurs with weight gain (see below “8. Potential Mechanisms of Sympathetic Nervous System Activation in Obesity”). To address this, one could randomize subjects (normal weight or obese) to receive an angiotensin II receptor blocker or placebo and measure SNS activity (e.g., MSNA or renal NE spillover) before and following a period of weight gain. This approach is deemed more definitive than determining whether the reduction in MSNA with angiotensin II receptor blockade is greater in obese compared with nonobese because the former approach avoids potential differences in baseline SNS activity (in normal weight and obese), which can make interpretation of the responses to an intervention challenging. In addition, ensuring that a similar degree of blockade was achieved in lean and obese individuals would not be necessary if the weight gain model were utilized. Parenthetically, a similar but perhaps more acceptable approach would be to intervene during spontaneous weight regain following weight loss.
Weight loss is associated with a reduction in MSNA (Grassi et al., 1998, Trombetta et al., 2003, Straznicky et al., 2005) and whole body NE spillover (Schwartz et al., 1990). Unfortunately, there is currently no information available on whether weight loss lowers SNS activity to the kidney or other tissues/organs.
8. Potential Mechanisms of Sympathetic Nervous System Activation in Obesity
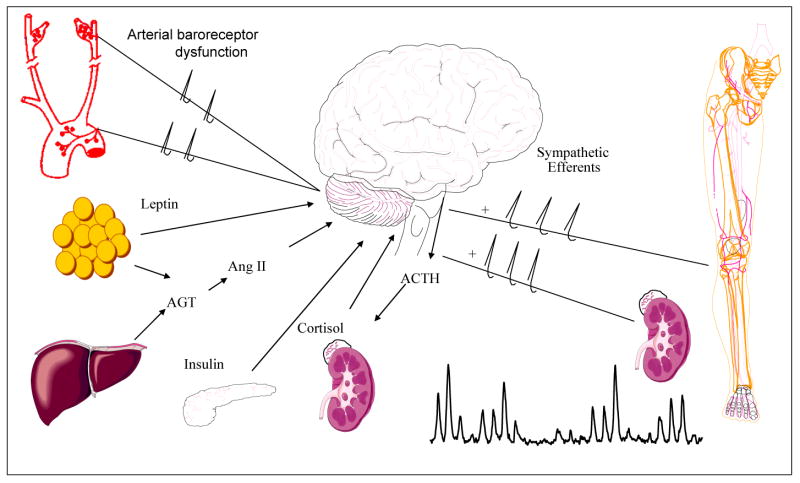
There are numerous potential mechanisms that could link obesity with SNS activation in humans (Figure 6). Chronic activation of supra-bulbar subcortical noradrenergic neurons has been suggested to contribute to peripheral SNS activation in heart failure (Lambert et al., 1995), essential hypertension (Ferrier et al., 1992), and aging (Esler et al., 2002). Thus, it is possible that this may also be a potential mechanism contributing to SNS activation in obesity. Unfortunately, the available evidence (Lambert et al., 1999) suggests this is not the case. However, whether brain NE spillover is increased in individuals with visceral obesity has not been determined to our knowledge.
Figure 6.
Potential mechanisms contributing to sympathetic nervous system activation in obesity (Davy and Hall, 2004). AGT=Angiotensinogen; Ang II=Angiotension II; ACTH=Adrenocorticotropin hormone. Used with permission. (from Davy KP, Hall JE. 2004. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol 286:R803-813).
Insulin circulates in proportion to body fat stores and acts on key brain regions to reduce food intake and increase energy expenditure (via sympathetically mediated thermogenesis) (Schwartz and Porte, 2005). Obesity, particularly visceral obesity, is frequently associated with hyperinsulinemia and, as such, insulin could contribution to elevated MSNA in these individuals. However, several lines of evidence suggest otherwise. First, fasting insulin concentrations have been related to MSNA in some (Scherrer et al., 1994, Monroe et al., 2000, Weyer et al., 2000) but not all studies (Alvarez et al., 2004). Second, intranasal delivery of insulin, which does not accumulate systemically, appears not to increase MSNA (Benedict et al., 2005). Third, acute insulin infusions increase MSNA in humans (Anderson et al., 1991), but the potential confound of baroreflex-mediated adjustments to systemic vasodilatation render these findings inconclusive. Finally, Scherrer et al. (Scherrer and Owlya, 1996) reported that MSNA was not increased in an insulinoma patient whose fasting insulin concentrations were dramatically elevated. In addition, surgical removal of the insulinoma did not reduce MSNA despite normalization of fasting insulin concentrations. Therefore, the role of endogenous hyperinsulinemia in producing tonically elevated MSNA in obesity remains unclear.
Leptin, the product of the OB gene, is another potential neurohumoral signal that could contribute to the elevated kidney or skeletal muscle SNS activity observed in obese humans. Leptin, which is secreted from adipocytes in proportion to fat mass, acts on hypothalamic neuronal targets to reduce energy intake and increase energy expenditure (Ahima and Flier, 2000). In rodents, leptin exerts influences on cardiovascular and renal function that are sympathetically-mediated (Haynes, 2000, Hall, 2003). In addition, acute infusions of leptin in rodents produces large increases in SNS outflow to brown adipose tissue, the kidney and lumbar vascular beds (Haynes, 2000). Chronically, leptin infusions produce significant sympathetically mediated blood pressure elevations in experimental animals (Hall, 2003). However, whether hyperleptinemia could be a potential mechanism contributing to SNS activation (and BP) elevations in obese humans is unclear. Leptin concentrations have been inconsistently related to MSNA (Snitker et al., 1997, Monroe et al., 2000, Alvarez et al., 2004). In addition, expression and secretion of leptin is lower in visceral compared with subcutaneous adipocytes (Russell et al., 1998) and circulating concentrations of the protein are similar in humans with higher compared with lower abdominal visceral fat but similar total and subcutaneous abdominal fat (unpublished observations). In addition, MSNA is similar in subcutaneous obese men and normal weight controls, despite three-fold higher leptin concentrations in the former (Alvarez et al., 2004) (Figure 4, bottom panel). Thus, the available evidence does not appear to support an obvious role for leptin signaling in mediating the SNS activation observed in human obesity.
Obesity is associated with activation of the renin-angiotensin-aldosterone system. The results of animal studies indicate that angiotensin II can act centrally to increase SNS activity (Reid, 1992). As such, it is possible that angiotensin II could increase SNS activity in a similar manner in obese humans. Muscle sympathetic nerve activity increases with angiotensin II infusion (Matsukawa et al., 1991) and decreases with angiotensin converting enzyme inhibition (Miyajima et al., 1999) in normotensive humans. Furthermore, multiple components of the renin-angiotensin system are expressed in adipose tissue and angiotensinogen, the major substrate for angiotensin II formation, is expressed to a greater extent in visceral than subcutaneous adipocytes (Engeli et al., 2000). Thus, increased angiotensin II could contribute to SNS activation in visceral obesity. Unfortunately, there is no direct experimental support for this possibility at the present time. However, the observation that angiotensin II receptor blockade reduces MSNA in obese hypertensive humans (Grassi et al., 2003) is consistent with, but does not definitively support, this concept.
The arterial baroreflex exerts a strong tonic inhibitory influence on SNS activity. Therefore, a reduction in this inhibitory influence could contribute to the higher SNS activity to muscle and kidney in obesity. However, in contrast to previous reports (Grassi et al., 2000), we (Alvarez et al., 2002) recently found that sympathetic baroreflex gain was not reduced in men with visceral obesity nor associated with any measure of total or regional adiposity. Furthermore, whether sympathetic baroreflex sensitivity determined from acute pharmacological perturbations of blood pressure has any relation with the long-term tonic influence of the arterial baroreflex on SNS activity is unclear. Nonetheless, the role of baroreflex function in the SNS activation that accompanies obesity needs to be explored further.
Visceral obesity has been hypothesized to be a neuroendocrine disorder characterized by dysregulation of hypothalamic-pituitary-adrenal axis and parallel activation of the SNS (Bjorntorp et al., 2000). Whether dysregulation of the HPA axis is critical to the activation of the SNS in the context of obesity is unclear. Acute infusion of adrenocorticotropin, a pro-opiomelanocortin hormone, increases MSNA in humans (Dodt et al., 1998) and one week of dexamethasone treatment produces greater reductions in MSNA in obese compared with nonobese individuals (Grassi et al., 2001). However, it is important to emphasize that the specific sites of action of dexamethasone are unclear.
Obesity is an important risk factor for obstructive sleep apnea (Young et al., 2002) and obstructive sleep apnea appears to be more closely associated with visceral obesity than total adiposity (Vgontzas et al., 2003). Narkiewicz et al. (Narkiewicz et al., 1998) reported that obesity, in the absence of obstructive sleep apnea, was not associated with elevated MSNA. Subsequently, Grassi et al. (Grassi et al., 2005) demonstrated, in a much larger sample, that MSNA was significantly increased in obese compared with nonobese subjects without sleep apnea. Therefore, while the presence of obstructive sleep apnea appears sufficient to produce, even augment, SNS activation in obese humans, its presence does not appear necessary for the full expression of the sympathoexcitatory phenotype observed in “uncomplicated” obesity.
9. Potential Consequences of Sympathetic Nervous System Activation in Obesity
The consequences of the region specific alterations in SNS activity in obese individuals have not been determined. However, there are several possibilities. First, the lower cardiac SNS activity observed in obese individuals (Vaz et al., 1997, Rumantir et al., 1999) may be cardioprotective, although the impact on total energy expenditure would be inconsequential. In addition, it is important to emphasize that the net impact of autonomic outflow on arrhythmogenesis in obesity remains unclear, in part, because cardiac vagal outflow is reduced in obesity (Arrone et al., 1995). Furthermore, the results of pharmacological studies suggest that cardiac SNS activity is higher, not lower, in obese compared with normal weight individuals (Arrone et al., 1995). The reasons for these discrepancies with the cardiac NE spillover studies (Vaz et al., 1997, Rumantir et al., 1999) are unclear, but upregulation of β-adrenergic receptors in response to low tonic cardiac SNS outflow is one possibility.
Second, the sympathetic nervous system innervates white adipose tissue and plays an important role in lipid mobilization (Bartness and Song, 2007). In humans, stimulation of the lateral femoral cutaneous nerve elicits lipolysis in the innervation area (Dodt et al., 1999). This lipolytic response is attenuated in obese individuals (Dodt et al., 2000). Whether the reduced SNS activity to white adipose tissue observed in obese subjects (Coppack et al., 1998) contributes to a corresponding lower basal rate of lipolysis (relative to fat mass) (Klein et al., 1988) is not known. In addition, SNS activity to white adipose tissue also has an inhibitory influence on fat cell proliferation (Bartness and Song, 2007). Therefore, it is also possible that the lower SNS activity to white adipose tissue in obesity may be associated with fat cell proliferation (Bartness and Song, 2007). However, it is important to emphasize that it is not known whether SNS activity to white adipose tissue is specifically reduced in different obesity phenotypes (e.g., visceral obesity).
Third, the higher MSNA observed in obese humans may be associated with enhanced sympathetic vasoconstrictor tone. In addition, upper body obesity appears to be associated with a different hemodynamic phenotype than lower body obesity. Individuals with an upper body fat distribution pattern demonstrate a lower cardiac output and higher systemic vascular resistance compared with individuals with a lower body fat distribution pattern (Jern, 1992). These observations are consistent with recent findings by Charkoudian et al. (Charkoudian et al., 2005) which suggest that there is balance between cardiac output and MSNA among healthy, normotensive individuals. Those individuals with a lower cardiac output exhibit higher levels of MSNA at rest. In turn, systemic vascular resistance is proportional to the level of MSNA. Thus, it is possible that the higher systemic vascular resistance observed in visceral obesity is due, at least in part, to higher MSNA and augmented sympathetic vasoconstrictor tone. Importantly, this may place individuals with visceral obesity at higher risk for developing hypertension.
Fourth, there is increasing evidence that the SNS plays an important role in the pathophysiology of obesity hypertension (Esler et al., 2001, Hall, 2003). However, SNS activity to the kidney (Vaz et al., 1997, Rumantir et al., 1999) and skeletal muscle (Spraul et al., 1993, Scherrer et al., 1994, Spraul et al., 1994, Gudbjornsdottir et al., 1996, Grassi et al., 2000, Weyer et al., 2000, Grassi et al., 2001, Alvarez et al., 2002) is elevated even in obese normotensive humans. Thus, SNS activity to these organs, particularly the kidney, is likely necessary but does not appear sufficient for the full expression of hypertension, at least by the standard clinical definition of 140/90 mmHg. However, it is possible that obesity may shift the population distribution of blood pressure to the right such that while many obese individuals have normal blood pressure (i.e.,< 140/90 mmHg) their arterial blood pressure is higher than would be expected at a lower body weight or level of adiposity. Importantly, the cardiovascular disease risks associated with elevated blood pressure appear to begin to increase at 115/75 mmHg (Lewington et al., 2002). Therefore, heightened SNS activity may raise arterial blood pressure in susceptible individuals (e.g., those with visceral obesity) into the prehypertensive range. Whether the duration of obesity, magnitude of SNS activation or other factors are important in determining which obese individuals ultimately develop hypertension remains unclear.
Finally, Landsberg (Landsberg, 1986) postulated that the increase in SNS activity that occurs with fat accumulation serves the homeostatic role of stimulating thermogenesis to prevent further weight gain. Although there is little information to support this idea in humans, both MSNA and energy expenditure are elevated in obese humans and MSNA is an important determinant of 24-hr energy expenditure in Caucasians but not Pima Indians (Spraul et al., 1993). In addition, low SNS activity may be a risk factor for future weight gain in Pima Indians (Ravussin and Tataranni, 1996). Interestingly, MSNA is lower than predicted based on total adiposity in individuals with a subcutaneous obesity phenotype (Alvarez et al., 2004). However, it is not known whether these individuals also demonstrate reduced energy expenditure and, as such, are predisposed to further weight gain.
10. Future Directions
There are many important avenues for future research but those considered to be most important included determining: 1) if low SNS activity to certain tissues/organs predisposes to weight gain; 2) if SNS activity to the kidney (or other regions) increase with weight gain; 3) the mechanisms responsible for SNS activation to skeletal muscle (or other regions) with weight gain (in obesity); and 4) the consequences of SNS activation to skeletal muscle (or other regions) with weight gain (in obesity).
Summary
In conclusion, the existing paradigms are not entirely consistent with our current understanding of SNS behavior in obese humans. Low sympathetic activity to some regions (e.g., skeletal muscle and adipose tissue) may be a risk factor for weight gain and obesity development. However, the contribution of the SNS is likely small and relevant in only certain susceptible individuals under conditions of positive energy balance. Nonetheless, there is considerable regional heterogeneity in the SNS activation associated with obesity. The renal and skeletal muscle circulations, but not the heart, appear to be primary targets for SNS activation. Abdominal visceral fat appears to be an important depot linking obesity and MSNA. However, the impact of this depot on SNS activity to the kidney or other regions is unknown. The available evidence does not consistently support a role of insulin or leptin in contributing to the SNS activation in human obesity. However, the limited evidence available suggests that the role of hypothalamic-pituitary-adrenal axis dysregulation or renin-angiotensin system activation may be worth pursuing further. The consequences of SNS activation in normotensive obese individuals are also unclear but may include blood pressure elevation to a level that is associated with elevated cardiovascular risk (i.e., above 115/75 mmHg). Both weight gain and loss are associated with predictable changes in skeletal muscle SNS activity but the impact on SNS to other tissues/regions has not been studied.
Acknowledgments
Dr. Davy's research was supported by National Institutes of Health Awards RO1 HL62283 and KO2 HL67227. Jeb Orr was supported by an American Heart Association predoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- Anderson E, Hoffman R, Balon T, Sinkey C, Mark A. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrone L, Mackintosh R, Rosenbaum M, Leibel R, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regul Integr Comp Physiol. 1995;269:R222–R225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- Benedict C, Dodt C, Hallschmid M, Lepiorz M, Fehm HL, Born J, Kern W. Immediate but not long-term intranasal administration of insulin raises blood pressure in human beings. Metabolism. 2005;54:1356–1361. doi: 10.1016/j.metabol.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Holm G, Rosmond R, Folkow B. Hypertension and the metabolic syndrome: closely related central origin? Blood Press. 2000;9:71–82. doi: 10.1080/08037050050151762. [DOI] [PubMed] [Google Scholar]
- Bray GA. Obesity, a disorder of nutrient partitioning: the MONA LISA hypothesis. J Nutr. 1991;121:1146–1162. doi: 10.1093/jn/121.8.1146. [DOI] [PubMed] [Google Scholar]
- Cannon W. Bodily Changes in Pain, Hunger, Fear, and Rage. New York: Appleton; 1915. pp. 22–39. [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack SW, Horowitz JF, Paramore DS, Cryer PE, Royal HD, Klein S. Whole body, adipose tissue, and forearm norepinephrine kinetics in lean and obese women. Am J Physiol Endo Metab. 1998;275:E830–834. doi: 10.1152/ajpendo.1998.275.5.E830. [DOI] [PubMed] [Google Scholar]
- Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- Dimsdale J, Ziegler M. What do plasma and urinary measures of catecholamines tell us about human response to stress? Circ. 1991;83(suppl II):II-36–II-42. [PubMed] [Google Scholar]
- Dodt C, Lonnroth P, Fehm HL, Elam M. Intraneural stimulation elicits an increase in subcutaneous interstitial glycerol levels in humans. J Physiol. 1999;521(Pt 2):545–552. doi: 10.1111/j.1469-7793.1999.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt C, Lonnroth P, Fehm HL, Elam M. The subcutaneous lipolytic response to regional neural stimulation is reduced in obese women. Diabetes. 2000;49:1875–1879. doi: 10.2337/diabetes.49.11.1875. [DOI] [PubMed] [Google Scholar]
- Dodt C, Wallin G, Fehm HL, Elam M. The stress hormone adrenocorticotropin enhances sympathetic outflow to the muscle vascular bed in humans. J Hypertens. 1998;16:195–201. doi: 10.1097/00004872-199816020-00010. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Esler MD, Meredith IT, Dart A, Cannon RO, 3rd, Quyyumi AA, Lambert G, Chin J, Jennings GL, Goldstein DS. Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation. 1992;85:1775–1785. doi: 10.1161/01.cir.85.5.1775. [DOI] [PubMed] [Google Scholar]
- Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. 2002;282:R909–916. doi: 10.1152/ajpregu.00335.2001. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- Ferrier C, Esler MD, Eisenhofer G, Wallin BG, Horne M, Cox HS, Lambert G, Jennings GL. Increased norepinephrine spillover into the jugular veins in essential hypertension. Hypertension. 1992;19:62–69. doi: 10.1161/01.hyp.19.1.62. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: how much, and who's paying? Health Aff (Millwood) Suppl. 2003:W3-219–226. doi: 10.1377/hlthaff.w3.219. [DOI] [PubMed] [Google Scholar]
- Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1834–1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- Gothert M. Modulation of noradrenaline release in human cardiovascular tissues. Pharmacol Toxicol. 2003;92:156–159. doi: 10.1034/j.1600-0773.2003.920403.x. [DOI] [PubMed] [Google Scholar]
- Grassi G, Bolla G, Seravalle G, Turri C, Lanfranchi A, Mancia G. Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci (Lond) 1997;92:285–289. doi: 10.1042/cs0920285. [DOI] [PubMed] [Google Scholar]
- Grassi G, Facchini A, Trevano FQ, Dell'Oro R, Arenare F, Tana F, Bolla G, Monzani A, Robuschi M, Mancia G. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–325. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo B, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell'Oro R, Trevano FQ, Bombelli M, Scopelliti F, Facchini A, Mancia G. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21:1761–1769. doi: 10.1097/00004872-200309000-00027. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell'Oro R, Turri C, Pasqualinotto L, Colombo M, Mancia G. Participation of the hypothalamus-hypophysis axis in the sympathetic activation of human obesity. Hypertension. 2001;38:1316–1320. doi: 10.1161/hy1201.096117. [DOI] [PubMed] [Google Scholar]
- Gudbjornsdottir S, Lonnroth P, Sverrisdottir Y, Wallin B, Elam M. Sympathetic nerve activity and insulin in obese normotensive and hypertensive men. Hypertension. 1996;27:276–280. doi: 10.1161/01.hyp.27.2.276. [DOI] [PubMed] [Google Scholar]
- Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep. 2000;2:311–318. doi: 10.1007/s11906-000-0015-1. [DOI] [PubMed] [Google Scholar]
- Huggett RJ, Scott EM, Gilbey SG, Bannister J, Mackintosh AF, Mary DA. Disparity of autonomic control in type 2 diabetes mellitus. Diabetologia. 2005;48:172–179. doi: 10.1007/s00125-004-1601-6. [DOI] [PubMed] [Google Scholar]
- Jern S. Hemodynamics of the male fat distribution pattern. Blood Pressure. 1992;1:21–29. [PubMed] [Google Scholar]
- Jones PP, Davy KP, Alexander S, Seals DR. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. Am J Physiol Endo Metab. 1997a;272:E976–E980. doi: 10.1152/ajpendo.1997.272.6.E976. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, Seals DR. Relations of total and abdominal adiposity to muscle sympathetic nerve activity in healthy older males. Int J Obes Relat Metab Disord. 1997b;21:1053–1057. doi: 10.1038/sj.ijo.0800515. [DOI] [PubMed] [Google Scholar]
- Jones PP, Snitker S, Skinner JS, Ravussin E. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol Endo Metab. 1996;270:E363–366. doi: 10.1152/ajpendo.1996.270.2.E363. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- Klein S, Young VR, Blackburn GL, Bistrian BR, Wolfe RR. The impact of body composition on the regulation of lipolysis during short-term fasting. J Am Coll Nutr. 1988;7:77–84. doi: 10.1080/07315724.1988.10720223. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37:333–364. [PubMed] [Google Scholar]
- Lambert GW, Kaye DM, Lefkovits J, Jennings GL, Turner AG, Cox HS, Esler MD. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation. 1995;92:1813–1818. doi: 10.1161/01.cir.92.7.1813. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Vaz M, Cox HS, Turner AG, Kaye DM, Jennings GL, Esler MD. Human obesity is associated with a chronic elevation in brain 5-hydroxytryptamine turnover. Clin Sci (Lond) 1999;96:191–197. doi: 10.1042/cs0960191. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Loewy A, Spyder K. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. [Google Scholar]
- Macefield VG, Elam M, Wallin BG. Firing properties of single postganglionic sympathetic neurones recorded in awake human subjects. Auton Neurosci. 2002;95:146–159. doi: 10.1016/s1566-0702(01)00389-7. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol. 1991;261:R690–696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- Miyajima E, Shigemasa T, Yamada Y, Tochikubo O, Ishii M. Angiotensin II blunts, while angiotensin converting enzyme inhibitor augments, reflex sympathetic inhibition in humans. Clin Exper Pharmacol Physiol. 1999;26:797–802. doi: 10.1046/j.1440-1681.1999.03122.x. [DOI] [PubMed] [Google Scholar]
- Monroe MB, Van Pelt RE, Schiller BC, Seals DR, Jones PP. Relation of leptin and insulin to adiposity-associated elevations in sympathetic activity with age in humans. Int J Obes Relat Metab Disord. 2000;24:1183–1187. doi: 10.1038/sj.ijo.0801364. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Tataranni PA. The role of altered sympathetic nervous system activity in the pathogenesis of obesity. Proc Nutr Soc. 1996;55:793–802. doi: 10.1079/pns19960079. [DOI] [PubMed] [Google Scholar]
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol Endo Metab. 1992;262:E763–778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, Brolin RE, Fried SK. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol Endo Metab. 1998;275:E507–515. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Owlya R. Sympathetic-nerve activity before and after resection of an insulinoma. New Engl J Med. 1996;335:1240–1242. doi: 10.1056/NEJM199610173351617. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Jaeger LF, Veith RC, Lakshminarayan S. The effect of diet or exercise on plasma norepinephrine kinetics in moderately obese young men. Int J Obes Relat Metab Disord. 1990;14:1–11. [PubMed] [Google Scholar]
- Snitker S, Pratley RE, Nicolson M, Tataranni PA, Ravussin E. Relationship between muscle sympathetic nerve activity and plasma leptin concentration. Obes Res. 1997;5:338–340. doi: 10.1002/j.1550-8528.1997.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Spraul M, Anderson EA, Bogardus C, Ravussin E. Muscle sympathetic nerve activity in response to glucose ingestion. Impact of plasma insulin and body fat. Diabetes. 1994;43:191–196. doi: 10.2337/diab.43.2.191. [DOI] [PubMed] [Google Scholar]
- Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–1735. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrao CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003;285:H974–982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- von Euler U. Identification of the sympathomimetic ergone in adrenergic nerves of cattle (sympathin N) with laevo-nor-adrenaline. Acta Physiol Scand. 1948;16:63–74. [Google Scholar]
- Wallin B, Kunimoto M, S J. Possible genetic influence on the strength of human muscle nerve sympathetic activity at rest. Hypertension. 1993;22:282–284. doi: 10.1161/01.hyp.22.3.282. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annual Review of Physiology. 1988;50:565–576. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension. 2000;36:531–537. doi: 10.1161/01.hyp.36.4.531. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Preventing and managing the global epidemic: Report of a World Health Organization Consultation on Obesity. Geneva: 1997. [PubMed] [Google Scholar]
- Young J, Macdonald I. Sypathoadrenal activity in human obesity: heterogeneity of findings since 1980. Int J Obes Relat Metab Disord. 1992;16:959–967. [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]