Abstract
Drug-associated memories are a hallmark of addiction and a contributing factor in the continued use and relapse to drugs of abuse. Repeated association of drugs of abuse with conditioned stimuli leads to long-lasting behavioral responses that reflect reward-controlled learning and participate in the establishment of addiction. A greater understanding of the mechanisms underlying the formation and retrieval of drug-associated memories may shed light on potential therapeutic approaches to effectively intervene with drug use-associated memory. There is evidence to support the involvement of serotonin (5-HT) neurotransmission in learning and memory formation through the families of the 5-HT1 receptor (5-HT1R) and 5-HT2R which have also been shown to play a modulatory role in the behavioral effects induced by many psychostimulants. While there is a paucity of studies examining the effects of selective 5-HT1AR ligands, the available dataset suggests that 5-HT1BR agonists may inhibit retrieval of cocaine-associated memories. The 5-HT2AR and 5-HT2CR appear to be integral in the strong conditioned associations made between cocaine and environmental cues with 5-HT2AR antagonists and 5-HT2CR agonists possessing potency in blocking retrieval of cocaine-associated memories following cocaine self-administration procedures. The complex anatomical connectivity between 5-HT neurons and other neuronal phenotypes in limbic-corticostriatal brain structures, the heterogeneity of 5-HT receptors (5-HTXR) and the conflicting results of behavioral experiments which employ non-specific 5-HTXR ligands contribute to the complexity of interpreting the involvement of 5-HT systems in addictive-related memory processes. This review briefly traces the history of 5-HT involvement in retrieval of drug-cue associations and future targets of serotonergic manipulation that may reduce the impact that drug cues have on addictive behavior and relapse.
Keywords: Serotonin receptors, cocaine, self-administration, memory retrieval, extinction, conditioned stimuli
Introduction
The abuse of alcohol, nicotine and illicit drugs comprises a global dilemma, marked by considerable morbidity and mortality [320]. Initial drug use typically occurs as a component of exploration, novelty or pleasure-seeking during adolescence [159,180], with a subpopulation of drug users exhibiting vulnerability to the transition from abuse to “addiction” [330]. Addiction is defined here as a chronic disorder characterized by persistent drug-seeking, which has been modeled extensively in animals in self-administration studies (Fig. 1) [169,335]. The incentive-motivational state focused on drug use seen in addiction appears to be fuelled by impaired neurobiology and reorganization of brain circuits. Affected brain circuits include motivational systems in the hypothalamus and brainstem and their reciprocal links with higher order limbic-corticostriatal structures involved in the complex cognitive, emotional and associative abilities of the brain [307]. Cortical regions, such as the prefrontal cortex and their associated circuits appear to be particularly sensitive to plasticity incurred due to repeated pairing of environmental stimuli (e.g., drug paraphernalia, people and places associated with drug use, etc). with exposure to abused drugs [14,116]. These associations between environmental cues and the drug-taking experience become well consolidated into memory [118,182] and imaging studies have verified that re-exposure to drug-related stimuli activate attention and memory circuits [69,116,236,326]. Drug-associated memories can trigger conditioned emotional responses in addicts [238] and “craving” (desire for drug) [22,109,121,162] which is often cited by individuals to explain their relapse [127,269].
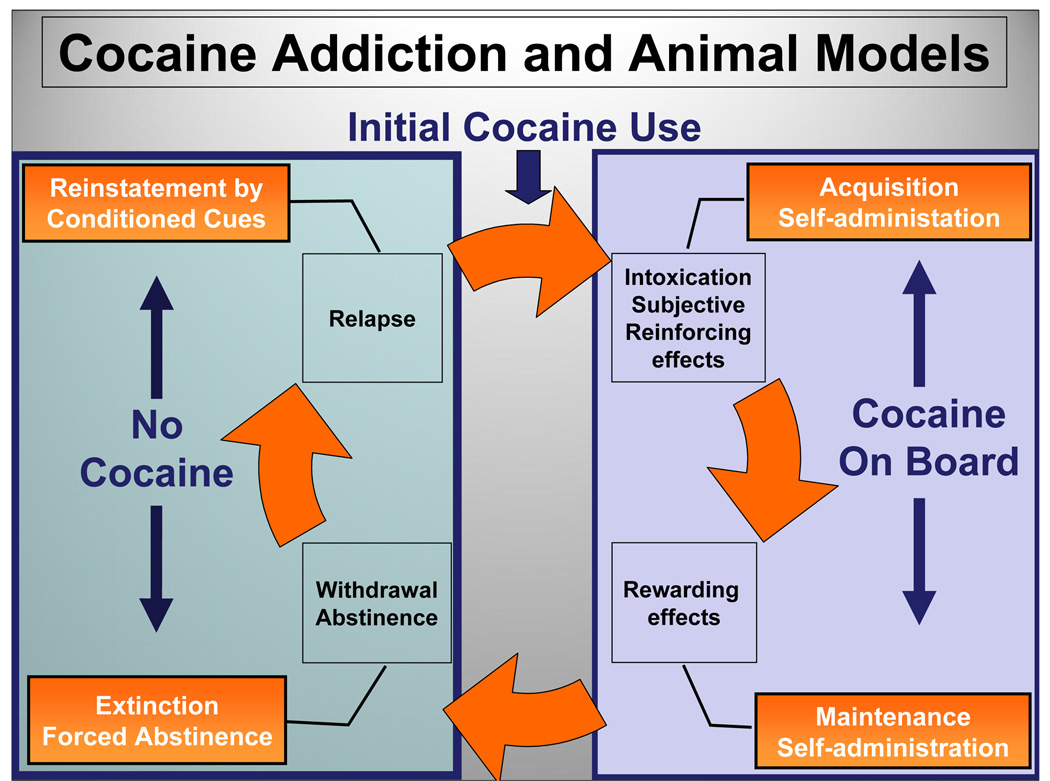
Fig. 1. Cocaine Addiction and Animal Models.
Addiction to cocaine or other abused substances is a chronic relapsing disorder characterized by persistent and compulsive drug-seeking and -craving that endures even after years of abstinence (adapted from [170,171]). The right side of the figure illustrates the component of the addictive cycle which is associated with the use of cocaine (“Cocaine on Board”). With initial use of cocaine, the intoxication (euphoria) and other subjective effects reinforce the behavioral output needed to continue drug use (reinforcing effects); these reinforcing effects increase the probability that drug-taking behavior will reoccur. The animal model of self-administration is useful to analyze the acquisition and consolidation of the learning of drug-taking behavior. A pleasurable subjective experience forms the basis of the rewarding effects of the drug, which play an essential role in its ability to serve as a reinforcer. The maintenance phase of self-administration is described by stable performance of the operant for delivery of cocaine and provides a window to investigate manipulations that alter expression of the behavior. The left side of the figure illustrates the component of the addictive cycle which is associated with the cessation of cocaine use (“No Cocaine”). The decision to cease using cocaine may be made voluntarily by the addict or under circumstances of duress, for example, under court-mandated treatment. Upon withdrawal from long-term use of this psychostimulant, abusers exhibit psychiatric symptoms characterized by both psychological (e.g., depression, anxiety, anhedonia, craving) and physiological symptoms (e.g., fatigue, hyperphagia, anergia). Once the ramifications of detoxification and withdrawal are handled, the addict enters a period of abstinence from cocaine use which may last from days to years. In self-administration models, behavioral components and neural mechanisms underlying abstinence may be investigated after a period of extinction or forced abstinence. However, abstinence is challenged by the potential for relapse to renewed use of cocaine. The reinstatement model of self-administration is useful for analysis of this phase of the addictive cycle as modeled by an animal homologue. Three major factors that may initiate drug-seeking behavior and relapse to drug use include exposure to drug-associated environmental stimuli, a “priming” or non-contingent dose of the drug itself or stress.
The process of association between the drug and experiential aspects of drug abuse is driven by Pavlovian (classical) learning [238] and there is a growing recognition and interest in understanding the biological and behavioral mechanisms underlying the encoding of drug-related memories. This interest is sensibly rooted in the fact that recovery is punctuated with remissions and relapses [205,237,300], and efficacious means to extend abstinence are needed. If addiction is a “disease of learning and memory” as recently proposed [138,326], then therapeutic gains may be achievable with a more complete understanding of the neurobiology of drug-related memories. The potential success of such approaches is suggested by observations that pharmacological manipulations can impair responsivity to drug-associated conditioned cues in humans [250,263,299] and animals [61,181,292].
Learning occurs during the three general phases of acquisition (initial learning), consolidation (molecular stabilization of long-term memory) and retrieval (recall of initial learning) [32,80,142,178]. The formation of memory is dependent upon dynamic changes in synaptic plasticity, an important neurochemical process through which protein modifications and/or synthesis result in altered synaptic strength (for comprehensive review, [141,148,176,202]). Two cellular models of the molecular mechanisms that underlie synaptic changes during learning and memory are long-term potentiation (LTP) and long-term depression (LTD) (for reviews, [175,196,199]). Activity-dependent modifications of N-methyl-D-aspartate receptors (NMDAR) and α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (AMPAR) in the postsynaptic neuron are well described mechanisms of these forms of neuroplasticity (for reviews, [59,77,179]). The most extensively studied mammalian form of synaptic plasticity is NMDAR–dependent LTP in hippocampal neurons [18]. In this situation, strong synaptic stimuli lead to a transient activation of NMDAR in dendritic spines and increased calcium released from endoplasmic reticulum resulting in the activation of multiple signaling cascades. Two key protein kinases within this pathway are calcium/calmodulin-dependent kinase type II (CaMKII) and cAMP-dependent protein kinase A (PKA) [21,90,193,333], both of which catalyze the phosphorylation and synaptic insertion of AMPARs into the PSD [52,65,129,192,195,251,295]. A strengthening of synaptic plasticity is subsequently achieved by stimulation of gene transcription and protein synthesis via the activation of several other downstream effectors including protein kinase C (PKC), Ras-guanosine triphosphatase activating protein (Ras-GTPase), extracellular signal-regulated protein kinase (ERK), p38 mitogen-activated protein kinase (p38 MAPK) and numerous PSD proteins [24,89,113,122,163,173,308,332,336]. The molecular mechanisms underlying LTP and hence synaptic plasticity are now viewed to be mechanistically important in the plasticity underlying the maladaptive reward learning which occurs following stimulant administration [146,153,319,334].
Glutamatergic systems critical to synaptic plasticity are under important modulatory control afforded by other neurotransmitter systems including monoaminergic neurotransmission [93,191,265,293]. Dopamine (DA) through actions at the D1R, in particular, facilitates LTP via alterations in AMPAR trafficking [108,143,334] and continues to be an important candidate in reward learning and long-term plasticity in addiction [59,139,146,153,155]. Serotonin also clearly modulates glutamatergic-encoded neuroplasticity [297,324], an observation which was made at the dawn of Kandel’s studies of synaptic plasticity underlying the simple classical conditioning of the gill-withdrawal reflex in Aplysia [33]. Receptors linked to adenylyl cyclase (AC) signaling are important mediators of this plasticity observed in Aplysia [11,60,125]. Of the 16 subtypes of 5-HT receptors (5-HTXRs), several couple to AC (5-HT1R, 5-HT4R, 5-HT6R and 5-HT7R), the 5-HT2R family couples to inositol phosphate signaling whereas the 5-HT3R is a ligand gated ion channel [20,96,134,164,235,262].
Serotonin signaling through several of these receptors may contribute to neural plasticity that underlies the encoding of drug associated memories. As a component of the complex processes that underlie the neurobiology of addiction, a greater understanding of serotonergic mechanisms in this aspect of addictive processes (Fig. 1) may open new doors to enhance recovery. Therapeutic gains have been reported in the treatment of alcoholism, smoking and illicit drug addictions with selective serotonin reuptake inhibitors (SSRIs) under certain conditions (e.g., [2,87,137,185,270,289,309,325]). Serotonergic manipulations may also prove therapeutic in psychostimulant addiction, which poses a particularly difficult public health problem due to the limited treatment options and extenuating ramifications for the individual user and society [133,165,220,296]. In the present review, we focus on the body of knowledge developed to suggest that 5-HT plays a role in stimulant-associated memories and the underlying synaptic plasticity. As the most complete information is available for the Erythroxylum coca alkaloid cocaine, we focus on this abused psychostimulant. A greater understanding of serotonergic mechanisms underlying the conditioned association between the cocaine exposure and environmental stimuli will provide new ideas for innovative approaches to treatment in addiction.
Cocaine
Cocaine use remains a significant public health problem across the globe [320]. Upwards of 15% of cocaine users are ultimately diagnosed with addiction [303,330], and recovery success is limited with a high degree of recidivism [91,303]. While behavioral and cognitive therapies can provide alternative treatment approaches, no proven effective and accessible medications for the treatment of this detrimental form of abuse and addiction have been validated despite an intense focus on their development [28,144,145,174,230,257,301].
Cocaine potently binds to the 5-HT transporter (SERT) to inhibit reuptake of 5-HT and increase efflux into the synapse [166] in addition to its extensively characterized ability to inhibit catecholamine reuptake [5,101,264]. Serotonergic manipulations (e.g., 5-HT precursors, neurotoxins, 5-HT agonists and antagonists) interfere with expression of the behavioral effects of cocaine (for review, [34,131,222,223,331]) while the neurochemical consequences of repeated cocaine exposure include altered expression of 5-HT transporters and receptor subtypes in both animals [10,67,240] and humans [189,245]. To date, much of the research which has examined the effects of serotonergic ligands on the behavioral effects of cocaine in animals has centered on the 5-HT1R and 5-HT2R subtypes [34,222], thus the role of these receptors in cocaine-associated memories will be the main focal objective for discussion in this review.
The 5-HT1R family forms the largest class of 5-HTXR subtypes and is comprised of the 5-HT1AR, 5-HT1BR, 5-HT1DR, 5-HT1ER and 5-HT1FR subtypes [177]. This receptor family couples to Gi/o proteins to preferentially inhibit cyclic adenosine monophosphate (cAMP) formation, although linkage to other signaling effectors has also been demonstrated (e.g., phosphatidylinositol pathway and PKC [20,262]). The 5-HT1AR and 5-HT1BR have received considerable attention in preclinical studies. For example, the stimulation of the 5-HT1AR [employing 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) and/or buspirone] reduced cocaine-evoked hyperactivity [48,258] (although see, [222]) and lever responding for cocaine in the rat self-administration model (see, Models for the Study of Cocaine-Associated Memories) [132,222,248]. Conversely, 5-HT1BR agonists enhanced cocaine-induced hyperactivity [260] and the reinforcing effects of cocaine inferred from an increase in lever responding for cocaine as measured in the self-administration paradigm [243,244,259]. However, studies with 5-HT1BR knockout (KO) mice found an increased propensity to self-administer cocaine, an effect which may indicate a decrease in the reinforcing effects of the psychostimulant [53,268]. A possible rationale for such differences comes from the increasing evidence that KO mice undergo important compensatory changes in other 5-HTXR subtypes and/or neurotransmitter systems during development [111,294] which may be implicated in observed behavioral effects in KO mouse models.
The 5-HT2R family (5-HT2AR, 5-HT2BR and 5-HT2CR) is linked preferentially to Gq/11 and activation of these receptors increases the hydrolysis of inositol phosphates and elevates cytosolic calcium [134,164,186,262]. Due to the limited distribution of the 5-HT2BR subtype in brain and lack of availability of selective 5-HT2BR ligands [83], the 5-HT2AR and 5-HT2CR are the most extensively researched subtypes of this family in addiction and memory formation processes [34,209]. Pretreatment with selective 5-HT2AR or 5-HT2CR ligands presents a relatively consistent pattern of effects on cocaine-induced behaviors in rodents (for review, [34]). An acute systemic injection of selective 5-HT2AR antagonists or 5-HT2CR agonists blocked, while 5-HT2AR agonists or 5-HT2CR antagonists enhanced, the hypermotive effects of cocaine at doses of the ligands that did not modify basal behavior [98,200,203]. Additionally, rates of responding for cocaine in a self-administration task is suppressed by a selective 5-HT2CR agonist and enhanced by a selective 5-HT2CR antagonist [98,99,120] suggesting an important role for the 5-HT2CR in control of the overt reinforcing effects of cocaine. In contrast, selective 5-HT2AR antagonists have no effects on rates of responding for cocaine in a self-administration task [97,98,233]. Based upon this partial dataset, once the 5-HT1R and 5-HT2R subtypes can be distinguished pharmacologically from one another, their influences include an excitatory role for the 5-HT1BR and 5-HT2AR and an inhibitory role for the 5-HT1AR and 5-HT2CR in the control of cocaine-induced behaviors.
Models for the Study of Cocaine-Associated Memories
The impact of cocaine on 5-HT systems coupled with the influence of 5-HT in the molecular cascade of events in the encoding of memories (see, Serotonin Mechanisms in Memory Formation) [148] supports a role for 5-HT neurotransmission in the processes through which cocaine-associated memories evolve [140,153,172,267]. These assessments of drug-associated memories have employed several different animal models [88,103,112,190,215,276,278,316]. The assay with the greatest construct validity, but also the most complex to dissect with regard to memory processes, is the drug self-administration procedure [15,88,110,124,266]. Cocaine self-administration models in animals have been adapted to explore drug-associated memories and the long-term consequences of cocaine intake in the laboratory. In one of its common variant in rodents, intravenous catheters are surgically implanted and subjects are allowed to complete an operant response (e.g., lever press, nose poke) to deliver a bolus injection of cocaine [110,147,291]. During the acquisition of a cocaine self-administration (Fig. 2), the learning paradigm promotes the establishment of a linkage between the cocaine-taking contextual (diffuse) cues, experimenter-delivered salient (discrete) environmental cues paired with the cocaine delivery, and the reinforcing and interoceptive effects of cocaine. In the language of classical conditioning, the effects of cocaine can be considered as an unconditioned stimulus (US). Repeated pairing of the US with the environmental context (e.g., the physical characteristics of the environment in which the drug is consumed including the presence of the houselights, levers, etc.) and discrete cues simultaneously delivered with cocaine (e.g., stimulus lights, tones, sound of drug delivery apparatus), a learned association between the conditioned stimuli (CS) and US (CS-US) is acquired. Thus, the CS complex is comprised of contextual and discrete cues. Following the training phase, the animals are subjected to an imposed withdrawal period (forced abstinence; [107,119,242]) or undergo extinction to the contingencies of the paradigm [99,152,286,288,291,292]. Following acquisition and either forced abstinence or extinction, retrieval of the conditioned association is assessed upon re-exposure to the CS complex and is implied by the behavioral output (e.g., lever press) in the absence of actual cocaine delivery (“drug-seeking”) [75,291]. The magnitude of operant responses on the previously drug-paired operandum can then be quantified as a measure of drug-seeking behavior supported by the CS complex [288]. Termed “cue-evoked reinstatement”, the behavioral output upon re-exposure to cocaine-associated cues shares aspects of construct and predictive validity to the human experience of relapse in the face of environmental triggers such as handling drug paraphernalia [58,68,238,327].
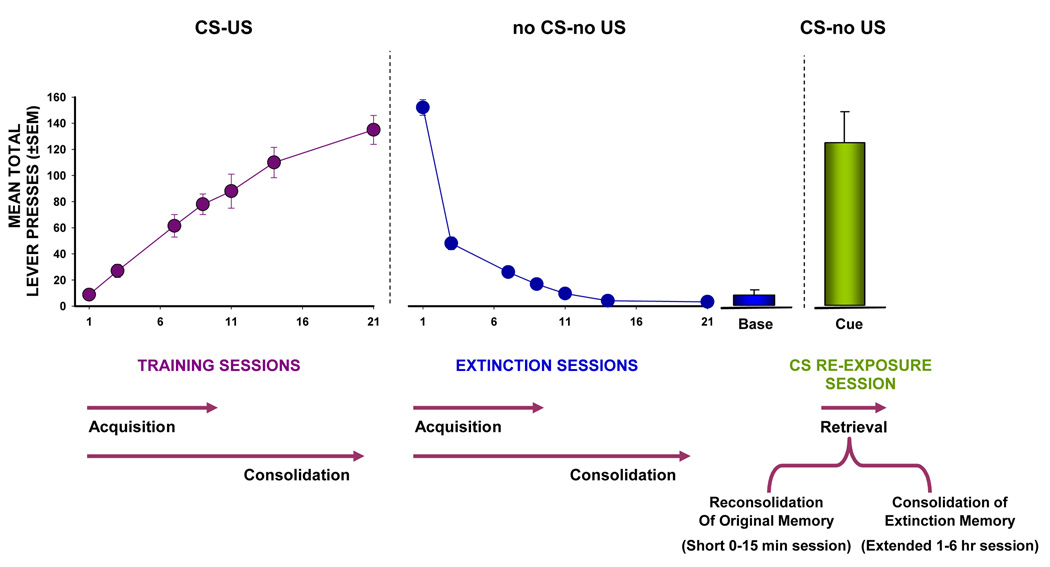
Fig. 2. Memory Processes Underlying Cocaine-Associated Memories in the Self-Administration Paradigm.
A typical cocaine self-administration paradigm involves completion of acquisition, extinction, and reinstatement phases. For illustrative purposes, the data are represented as mean (±SEM) lever presses per session during each stage of cocaine self-administration. The learned linkage between the cocaine-taking contextual (diffuse) cues, the salient (discrete) environmental cues [conditioned stimulus (CS) complex] previously paired with cocaine delivery (e.g., tone, stimulus, light), and the effects of cocaine (US) occurs during the acquisition phase (CS-US association). In most studies of reinstatement of drug-seeking, extinction of the behavior is established in the absence of the drug (no US) and the CS complex (no CS) resulting in the weakening of the association between the behavior (lever press, nose poke) and delivery of the primary reinforcer (cocaine). The previously established CS-US association is not eliminated but rather suppressed by new learning during extinction (no CS-no US association). Both acquisition of original (CS-US) and extinction (no CS-no US) learning are subject to consolidation processes requiring protein syntheses in which the STM of these associations are converted into LTM. Once lever press responding has achieved a low, stable baseline level (“Base”), the effectiveness of cue-induced reinstatement can be assessed (“CS”). The magnitude of operant responses on the previously drug-paired operandum upon re-exposure to the CS complex in the absence of actual cocaine delivery can then be quantified as a measure of drug-seeking behavior supported by the CS complex. The cue re-exposure session upon re-introduction of the CS complex may lead to retrieval and subsequent reconsolidation of the original self-administration memory (CS-US) or retrieval and consolidation of the newly formed extinction memory (no CS-no US) depending on the session duration.
The cue-elicited reinstatement paradigm employed as a component of self-administration studies normally involves extensive extinction training. This procedure typically involves repeated presentation of the diffuse contextual cues, but not the discrete CS cues, in the absence of the US (cocaine infusions) [66,94,287,288,291] to weaken the association between the behavior (e.g., lever press, nose poke) and the delivery of the primary reinforcer (drug) but maintain the incentive motivational value of the discrete cues [88,152,246,286,288,291,292]. Occasionally, the ability of the diffuse cues to elicit reinstatement has been examined, wherein extinction occurs in a different context (Context B) to that of the self-administration and cue-elicited reinstatement phases (Context A; [23,66,99,154,201]). In these paradigms, extinction is not a case of “forgetting,” but rather an active learning process that occludes, but does not “erase,” previously learned behaviors [26,86,247,261,306]. Thus, the previously established CS-US association is not eliminated but rather suppressed by new learning, which has been described as a “CS-no US” association [25,246]. Upon re-exposure to the discrete CS complex following extinction training, retrieval of the original memory (CS-US) or retrieval of the newly formed extinction memory (CS-no US) may result [44,160,224]. Reduction of lever responding in the presence of the CS complex via pharmacological manipulation is viewed as a disruption of the retrieval of cocaine-associated memories for cocaine-paired stimuli.
The remainder of the present assessment will elucidate the probable role for 5-HT actions at its receptors in the mechanisms underlying the retrieval of cocaine-associated memories (drug-seeking behavior) and suggest how 5-HT manipulations might prove useful in therapeutic approaches to suppress relapse in human cocaine addicts.
Serotonin Mechanisms in Memory Formation
The involvement of 5-HT in memory processing is not surprising given the fact that serotonergic pathways provide significant innervation to cortical and subcortical structures known to be involved in cognition from their parent neurons of origin in theraphe nuclei [7]. However, results from human and animal studies have yielded inconsistent findings concerning the directionality of its role. This situation reflects the complex nature of serotonergic processing of information in brain, the lack of available selective molecular and pharmacological tools as well as the need to evaluate the question at multiple levels of memory systems [211,318]. Some protocols have evaluated the impact of 5-HT1R and 5-HT2R ligands administered either before the learning session or after the learning session to tap into the acquisition and consolidation phases of memory, respectively [207,208], while only a handful of studies have specifically examined the role of these 5-HTXR in memory retrieval tasks. As return to cocaine use is often linked with retrieval of drug-associated memories (see Introduction), we will only review data employing tasks that tap into this aspect of memory-related behavior as most analogous to models employed to study cocaine-associated memories (see Models for the Study of Cocaine-Associated Memories).
There is experimental evidence that 5-HT1AR and 5-HT1BR signaling are involved in acquisition and retrieval of learning while the specific involvement of this receptor in consolidation of learning has not been extensively addressed. Pre-training administration of a 5-HT1AR agonist [e.g., flesinoxan, buspirone or 8-OH-DPAT] enhanced acquisition of new learning [35,63,208] in behavioral tasks associated with appetitive motivation, such as the delayed-matching-to-position or delayed nonmatch-to-sample tasks, whereas administration of the agonist impaired retention of new learning in spatial memory tasks [16,167]. In addition, administration of 8-OH-DPAT following the acquisition session (2hr) hindered consolidation of the new learning [167]. Interestingly, pretreatment with a 5-HT1AR agonist impaired retrieval of spatial and contextual cues in passive avoidance paradigms and the Morris water maze [6,49,51,150,188,188,218,221,253,284,311], an effect linked to stimulation of the postsynaptic, as opposed to the presynaptic, 5-HT1AR [49,241]. Contradictorily, one study reported an improvement in memory retrieval after pretreatment with a 5-HT1AR antagonist in the object recognition task [252], however, this observation has not been replicated in other paradigms of memory retrieval [210,212]. The 5-HT1AR may act in consort with glutamate systems to regulate memory processes as a 5-HT1AR antagonist reversed impairment of retrieval evoked by the NMDAR antagonist MK 801 in object recognition and radial arm maze tasks [19,50,252]. Pretraining administration of a 5-HT1BR antagonist enhanced acquisition of learning in an autoshaping task [213] while post-training administration of a preferential 5-HT1BR agonist impaired consolidation of learning in this same task [212]. Thus, while difficult to tease apart based upon the incomplete dataset, there is experimental evidence to suggest that the 5-HT1AR and 5-HT1BR play differential roles in phases of memory.
The 5-HT2R signaling pathway [186,262] is a serotonergic target of interest in understanding the acquisition and consolidation of learned behaviors and, to a lesser extent, the retrieval of memories engendered in associative-learning memory models (e.g., autoshaping tasks [206,207,256]). To date, only non-selective ligands have been employed to investigate the role of the 5-HT2AR and 5-HT2CR subtype in memory retrieval paradigms with predictably conflicting results (see Cocaine). The 5-HT2A/2CR antagonists (e.g., such as methysergide and mesulergine) investigated to date have been reported to interfere with memory retrieval in tasks involving recall of visual cues in a delayed non-match to sample task [197], although opposite effects have been reported in auditory and radial arm maze memory tasks [13,274,275].
The interpretation of these discrepant data must take into account the nature and degree of difficulty of the paradigms employed, the selectivity of the ligands and doses used (e.g., 8-OH-DPAT possesses affinity for both the 5-HT1AR and 5-HT7R [126]), the neural circuit involved (e.g., hippocampus versus prefrontal cortex), the synaptic localization (e.g., whether pre- versus postsynaptic 5-HTXR are manipulated), the developmental stage and age of animals at time of testing (e.g., adolescent versus adult), the extent of training (e.g., one trial versus multiple trials) and the timing of the administration of the ligands (i.e., injection before or after training session or retrieval test, etc.). These factors taken in conjunction with a comparative assessment of selective 5-HTXR ligands and doses across several behavioral tasks are required to clarify the role of 5-HT1R and 5-HT2R in memory retrieval processes. However, from a general consideration of the present collection of studies, the stimulation of postsynaptic 5-HT1AR and blockade of 5-HT2A/2CR may interfere with memory retrieval processes, and thus serve as potential candidates in altering recall of cocaine-associated memories.
Serotonin and Cocaine-Associated Memories
The involvement of individual 5-HTXR and transduction systems in mediating cocaine-associated memories requires further exploration with a goal to uncover which serotonergic manipulations might promise translation to the clinic as effective adjuncts to therapy in cocaine addiction. The clinical human psychopharmacology research with selective 5-HTXR ligands is truly limited due to the paucity of selective pharmacological compounds available for use in humans [74,117,272,280]. Of the pharmacological manipulations assessed in humans to date, increases in serotonergic tone via administration of the 5-HT transporter substrate fenfluramine were shown to reduce cocaine craving in human cocaine-dependent subjects [39–41,255]. Preclinical studies have also illustrated that the re-exposure to cocaine-associated cues [29,47] or even to other reward-associated cues [214] results in significant alterations of 5-HT efflux in the prefrontal cortex suggesting that retrieval of cocaine-associated memories involves cortical 5-HT function. Paradoxically, pharmacological manipulations that either decrease (e.g., administration of the neurotoxin 5,7-dihydroxytryptamine) or increase extracellular 5-HT (e.g., administration of a SSRI) prior to exposure to the CS complex in a reinstatement session reduced behavioral responding elicited by cues previously associated with cocaine in rats [9,38,312,313]. In the remainder of this article, we will review the handful of published observations in support of the role of 5-HT substrates in the processes involved in the retrieval of cocaine-associated memories.
Cocaine-Associated Memories and 5-HT1R
The 5-HT1AR agonist impairs the memory retrieval of spatial and contextual cues (see Serotonin Mechanisms in Memory Formation) thus the prediction is that 5-HT1AR agonist would also block retrieval of cocaine-associated memories. To date, a selective 5-HT1AR agonist at doses that act at either pre- or postsynaptic 5-HT1AR has not been assessed in reinstatement sessions. However, following acquisition of cocaine self-administration and subsequent extinction of the operant response, acute administration of the 5-HT1AR antagonist WAY 100635 30 min prior to test did not alter cue-induced reinstatement [37]. Thus with only a limited amount of data the role of 5-HT1AR in retrieval of cocaine-associated memories remains unclear.
There is a paucity of studies determining the role of the 5-HT1BR in memory retrieval as noted, however, 5-HT1BR agonists are reported to potentiate the reinforcing effects of cocaine. Thus, one might expect a 5-HT1BR agonist to enhance retrieval of cocaine-associated memories. However, acute administration of the 5-HT1B/1AR agonist RU24969 15 min prior to test reduced retrieval of cocaine-associated memories following extinction [1]. The effects of RU24969 were deemed to be mediated by 5-HT1BR stimulation as administration of the selective 5-HT1BR antagonist GR127935 reversed the effects of RU24969 on cocaine-associated memories; the antagonist alone had no effect [1]. The differential control afforded by 5-HT1BR agonists over the behavioral consequences of exposure to cocaine-associated cues versus the reinforcing effects of cocaine may be consistent with dissociable neural mechanisms suggested to underlie the reinforcing effects of a psychoactive drug versus conditioned effects of the drug [54,92,204]. Similar to the effects observed with cocaine-associated cues, the 5-HT1BR agonist also reduced the level of responding elicited with sucrose-associated cues [1]. As the administration of this ligand at the chosen doses did not affect spontaneous locomotor behavior (and assuming that RU24969 did not alter perceptual processes significantly), the authors concluded that RU24969 elicited a general decrease in motivation for appetitive stimuli (see below for further discussion) [1]. While further studies are required to delineate the exact role of 5-HT1AR and 5-HT1BR in retrieval of cocaine-associated memories, the limited dataset presented so far indicate that manipulation of either receptor subtype shows little promise in selectively suppressing the retrieval of cocaine-associated memories.
Cocaine- Associated Memories and 5-HT2AR/5-HT2CR
The 5-HT2R family is an important serotonergic target of interest in understanding the cellular and behavioral effects of cocaine (see Cocaine [34,222,223]). The results of studies that employed non-selective 5-HT2A/2CR antagonists in a handful of memory retrieval studies published to date are conflicting (see Serotonin Mechanisms in Memory Formation). However, a consistent pattern of effects of 5-HT2AR and 5-HT2CR ligands over cocaine-related behaviors as observed (see Cocaine [34]). Based on this research, selective 5-HT2AR antagonists and selective 5-HT2CR agonists might be expected to block the retrieval of cocaine-associated memories.
Early studies that employed less selective 5-HT2A/2CR antagonists did not observe changes in retrieval of cocaine-associated memories following extinction training [37,282]. More recent studies indicate that pretreatment with selective 5-HT2AR antagonists potently blocked retrieval of cocaine-associated memories following extinction of operant responding in the absence of discrete cues [97,233]. The administration of M100907, the most selective 5-HT2AR antagonist available to date (volinanserin; [228,233]), effectively reduced retrieval of cocaine-associated memories observed under conditions that minimize the potential for confounds associated with the operant pretraining and the stress of food deprivation [55,123,323]. This effect does not appear to be related to general behavioral disruption as the same doses of the antagonist did not alter locomotor activity nor retrieval of sucrose-associated memories upon re-exposure to cues previously linked to sucrose self-administration [233]. Pretreatment with either a selective (RO 60-0175) or non-selective (mCPP, MK-212) 5-HT2CR agonist suppressed rates of lever responding on re-exposure to cues previously associated with cocaine self-administration after extinction training [36,99,231; Nic Dhonnchadha and Cunningham, unpublished data]. These suppressive effects were reversed by co-administration of 5-HT2CR antagonists, which alone failed to alter retrieval of cocaine-associated memories, indicating that the inhibition of lever responding upon re-exposure to cues linked to cocaine self-administration are mediated by the 5-HT2CR [36,99,231]. Additionally, the same doses of RO 60-0175 did not alter retrieval of cue-evoked memories in the sucrose self-administration paradigm [36]. In summary, these results point to ligands with selective 5-HT2AR antagonist or 5-HT2CR agonist properties as viable candidates to promote the disruption of retrieval of cocaine-associated memories.
Many studies examining the effects of serotonergic agonists in cue-elicited drug-seeking behavior employed antagonists to primarily verify the selectivity of the agonist. Only one dose of the antagonist is employed in most instances with the dose typically based upon previous experiments indicating a blockade/reversal of an effect of the chosen agonist (e.g., 5-HT1A/1BR) [36]. If one were to conduct a complete dose-response curve for the effects of the antagonist alone, a completely different story might be uncovered, e.g., inverse agonist effects. This is an important (as yet unanswered) question with respect to the involvement of the 5-HT2AR and 5-HT2CR in drug-seeking behavior, as a myriad of articles report inverse agonist activity in vitro and in vivo for the compounds employed as antagonists of these receptor subtypes [207,229,322]. Additionally, there is a growing literature about the tonic vs. phasic nature of 5-HT2AR and 5-HT2CR control in the brain. The 5-HT2AR does not appear to exert tonic influence upon DA neuronal firing or DA release (at least in the regions thus far studied), but stimulation of the 5-HT2AR with an exogenous agonist reveals an enhancement of DA neuronal output which is physiologically significant. The 5-HT2CR, on the other hand, appears to provide both tonic and phasic modulation of DA neurotransmission which is region dependent [34]. With the exception of the 5-HT2AR antagonist M100907 [233], further studies encompassing a wider range of doses of selective 5-HT1AR, 5-HT1BR and 5-HT2CR antagonists (as well as agonists and inverse agonists) are required to determine the involvement of these receptor subtypes in the retrieval of cocaine-related memories.
The ability of a 5-HT2AR antagonist or 5-HT2CR agonist to reduce the impact of drug-, but not food-associated, stimuli [36,233] may be related to the fact that neuronal substrates respond differently to drugs versus natural rewards and their associated stimuli. Cocaine has been described as “hijacking” the natural learning substrates [157,158,328] and resulting in maladaptive responses to cues previously associated with cocaine self-administration. Repeated exposure to cocaine or other drugs of abuse leads to persistent neural adaptations, such as changes in neuronal morphology as well as protein and gene expression in higher order limbic-corticostriatal structures involved in the complex cognitive, emotional and associative abilities of the brain [307]. These adaptations are thought to render this circuit hypersensitive to drugs and drug-associated stimuli and to play a prominent role in attributing incentive salience to stimuli, that is, the manner in which stimuli are perceived as attractive, causing drugs and drug-associated stimuli to become excessively ‘wanted’ [78,232,267,322]. Further studies examining the effects of selective serotonergic ligands in animal models of memory formation may aid in deciphering the impact of cocaine administration on normal processing stages [206].
The timing of injections of 5-HT ligands relative to experimental assessment of acquisition, consolidation and retrieval is very important. In the case of most published studies, ligand administration preceded the test for memory retrieval. In this case, several important controls must be considered to allow linkage of observed effects of treatment to disruption of memory recall. To assess the possibilities of performance deficits due to behavioral disruption consequent to the doses of a ligand, simple motility and exploratory patterns are frequently assessed. To measure the specificity of the ligand to affect memories for contextual and discrete cues associated with cocaine versus memories for contextual and discrete cues associated with other appetitive reinforcers, the 5-HTXR ligand manipulation may also be analyzed in a food or sucrose self-administration protocol and reward-seeking assessed in response to cues previously associated with food or sucrose self-administration, respectively. These control experiments for the observed effects of a certain ligand on retrieval of cocaine-associated memory are not ideal and need to be refined. For example, in addition to these performance and reward specificity considerations, a serotonergic pretreatment before memory retrieval tests could result in perceptual deficits that impair recognition of either the contextual or discrete cues of the CS complex. The examination of these ligands in parallel paradigms that do not involve appetitive-linked responses (e.g., spatial cue tasks) may help clarify this issue and refine the ability to interpret observations about retrieval of drug-associated memory in reinstatement self-administration models.
Possible Molecular Mechanisms Underlying the Role of 5-HT in Cocaine-associated Memories
Despite the undeniable support for 5-HTXR participation in learning and memory processes and evidence to indicate participation in cocaine-associated memories, the potential manner in which 5-HT contributes to the molecular basis for plasticity has been largely absent from discussion of memory formation in mammals. To date, our knowledge is derived from the elegant studies which examined memory storage underlying the gill-withdrawal reflex of Aplysia [148]. These studies indicate that memory formation underlying this reflex in Aplysia involves presynaptic mechanisms of LTP. In Aplysia, 5-HT release subsequent to tail stimulation leads to the activation of AC and a resultant rise in cAMP levels. In turn, cAMP activates PKA, closure of K+ channels and increased intracellular calcium release, mechanisms believed to contribute to classical conditioning of the gill-withdrawal reflex [11,60,125]. The exact 5-HTXR involved in the activation of AC involved in memory storage underlying the gill-withdrawal reflex of Aplysia by 5-HT remains unclear, but may involve 5-HT1R, 5-HT4R, 5-HT6R or 5-HT7R [62,82] similar to that observed at CA3 pyramidal neurons, cerebellar synapses and the amygdala [136,196,234].
The ability of postsynaptic 5-HT1AR stimulation to interfere with memory retrieval may involve the inhibition of AC activity and consequent impairment of the activity of PKA. Additionally 5-HT1AR stimulation can inhibit CaMKII activity [221] and the corresponding phosphorylation and increased functionality of AMPAR [42,283] which is necessary for LTP [12].
A role is suggested for the 5-HT2R family in establishing LTP in the cortex [56,84,85,302] possibly via the alteration of the phosphorylation state of AMPAR. Thus, 5-HT2A/2CR ligands may regulate the plasticity of memory retrieval, as ligand-receptor binding would result in increased calcium release from intracellular stores and activation of signaling cascades, resulting in the phosphorylation and synaptic insertion of AMPARs into the PSD [297]. Alternatively, 5-HT2A/2CR-induced impairment of memory retrieval may be achieved by inhibiting activation of a PKC-dependent tyrosine kinase signal cascade and a concomitant phosphoryaltion and upregulation of NMDAR as has been recently demonstrated in the basolateral amygdala (BLA) [56,194].
While data on the role of 5-HT in regulating key signaling cascades in many neuronal processes has been documented (e.g., ERK, p38MAPK, PKA/PKC; [31,135,187,315]), the manner in which these signaling cascades that involve these molecular events contribute to the influence of 5-HT systems over processes underlying the storage and retrieval of cocaine-associated memory formation requires further investigation and is an area ripe for exploration.
Serotonergic Manipulations of Cocaine-Associated Memories
The knowledge of neural mechanisms underlying addiction-associated memories is advancing rapidly and the serotonergic signaling pathway signifies a target for exploration of memory processes involved in learning that occurs in the self-administration paradigm. Importantly, the self-administration paradigm must be dissected and explored in the context of other relevant memory tasks. A more detailed understanding of the role of 5-HTXR manipulations at each stage as well as the molecular and cellular changes involved in memory formation during the various stages of formation of cocaine-associated memories is discussed here (see Fig. 2).
The self-administration assay provides an interesting, but complex, behavioral paradigm for the study of cocaine-associated memories. Chronic cocaine self-administration involves multiple exposures to the drug, the drug-taking context (e.g., self-administration chamber) and all of the discrete stimuli (e.g., stimulus lights, tone) associated with the drug-taking experience for periods of days to weeks. The strength of the associations made between cocaine and the contextual and discrete cues in the self-administration assay are dependent upon several phases of memory processing. To date, there has been limited focus on the processes underlying the specific memory phases of cocaine self-administration learning (i.e., acquisition, extinction, memory retrieval).
The first stage of acquisition (learning) involves the initial linkage of the new experience of cocaine with the contextual and discrete environmental cues. A short-term memory (STM) of this newly acquired experience is formed. This transient and labile memory trace becomes stabilized through a process of consolidation, dependent upon new protein synthesis through which STM traces are transformed into stable long-term memories (LTM) [70,81,81,114,149,202,225,271,281]. This process is associated with long-lasting modifications of synaptic plasticity that are mediated by structural remodeling of existing synapses and addition of new synaptic contacts [46,128]. The self-administration paradigm is perfectly amenable to pharmacological manipulation at the time of learning (during acquisition and consolidation) with the assessment of the effects of such manipulations on cocaine-associated memories accessed during retrieval sessions.
The consolidation of the CS–US associations that are in the process of being formed, for example, might be modified by a specific serotonergic intervention during each cocaine self-administration session when consolidation of STM to LTM is believed to occur. Candidate regions within the brain that may be involved include the BLA, the dorsal hippocampus and subdivisions of the subiculum as recent studies indicate these brain areas as important nuclei involved in the acquisition and consolidation of cocaine-associated memories that maintain cocaine-seeking behavior [17,95,105,106,198,329]. Additional research indicates that new protein synthesis is necessary for the learning and consolidation of cocaine self-administration [216]. An essential role for the cAMP-PKA-CREB pathway has been established for the consolidation process [3,149,254,310]. The knowledge that 5-HT can regulate this pathway (seeSerotonergic Mechanisms in Memory Formation; [8,317]) encourages the design of specific experiments to decipher the role of the serotonergic system in the consolidation and LTM formation of cocaine-associated memories. A possible confound in these experiments is the potential alteration in the reinforcing effects of cocaine induced by the 5-HTXR manipulation. A potential means to dissociate the effects of serotonergic ligands on the reinforcing effects of cocaine versus alterations in learning would be to assess the effects of these ligands during the acquisition versus maintenance stages of self-administration. In most cases, the ability of serotonergic ligands to alter the reinforcing effects of cocaine are measured during the maintenance phase, when levels of responding are stable. During the acquisition phase when the animals are learning the association between operant responding, cocaine delivery and CS presentation, both pre- and post-trial administration of the ligands could be used to temporally dissociate encoding (or acquisition) of information versus the consolidation of this information [30,207–209].
An experimental strategy to establish the serotonergic circuitry underlying phases of memory for cocaine-associated memories would involve intracranial microinjection of selective 5-HT1BR, 5-HT2AR or 5-HT2CR ligands into candidate brain nuclei. Pretraining administration of specific 5-HTXR ligands into the discrete brain region would allow analysis of involvement in the acquisition of the CS-US pairing upon testing in a subsequent retrieval session. Post-training manipulation of 5-HTXR at variable intervals (1 – 6 hours) after each training session would specifically answer the query as to whether a specific 5-HTXR manipulation can disrupt the consolidation of the CS-US memory in the cocaine self-administration paradigm.
A typical experimental paradigm involves completion of the acquisition phase to achieve stable performance in a cocaine self-administration followed by extinction training. As yet another (now novel) learning process, extinction training may reverse neuroadaptations normally observed during cocaine withdrawal and/or evoke new adaptations due to the novel extinction learning [79,285,290,304]. For example, while levels of tyrosine hydroxylase (TH), the rate-limiting enzyme for DA synthesis, were decreased in the nucleus accumbens (an area implicated in conditioned reward [156,277]) during withdrawal from cocaine self-administration, extinction training reversed this deficit, restoring/normalizing TH levels to those observed in untreated controls [285]. These results demonstrate that extinction-experienced animals differ from a neurobiological standpoint when compared with animals in cocaine withdrawal alone and also suggests that strategies that incorporate extinction training offer treatment strategies that could produce substantial long-term benefits.
Extinction sessions (or exposure-based therapies) are successfully employed to weaken pathogenic memories that are responsible for various anxiety disorders [72,100], however, extinction procedures have had limited therapeutic success in reducing relapse among drug-dependent patients [57,64,73,102,130,151,239]. There is a pervasive thought that if optimal parameters for cue exposure could be discovered (e.g., the right cues chosen, the appropriate number of sessions conducted), treatment strategies that incorporate extinction training behavior in conjunction with pharmacological treatment might increase treatment efficacy [64]. Similar to CS-US training that occurs during acquisition, extinction training incorporates acquisition and consolidation processes. Administration of 5-HTXR ligands that facilitate learning and memory before each extinction session may enhance extinction learning while 5-HTXR ligand administration after each extinction session might enhance consolidation, leading to improved effectiveness and/or shortening of the duration of extinction learning [71].
Consolidated memories appear to become labile again after “reactivation,” that is, soon after the memory is retrieved by reminder stimuli that apparently reactivate the original memory trace [219], as is the case in the cue-reinstatement model. In the case of cocaine self-administration [182], re-exposure to the CS (“reactivation”) might trigger the retrieval of CS–US associations and induce two competing processes known as “reconsolidation” and “extinction,” which are dependent on the duration and offset of the CS re-exposure event [76,81,247,249]. The so-called reconsolidation upon brief exposure to the conditioned stimuli requires de novo protein synthesis for the maintenance of the memory [226,227]. Reconsolidation is believed to ensure that the fragile memory returns to a stable state [225,279] and may provide a window in which to manipulate long-lasting drug-associated memories.
While much debate exists as to the similarities and/or differences between the original consolidation process and the reconsolidation phase, the predominant thinking is that the two processes engage distinct brain areas and molecular pathways [3,4,183]. The involvement of specific intracellular pathways in the molecular mechanisms underlying reconsolidation of cocaine-associated memories have been partially described and include activation of PKA, ERK, CREB and zif268 [27,161,168,184,217] and release of 5-HT [45]. The recent study by Canal and colleagues [45] reported that intra-amygdala injections of the protein synthesis inhibitor anisomycin resulted in the release of 5-HT near the site of injection. The authors suggest that 5-HT (along with other neurotransmitters) may be involved in the mechanisms through which anisomycin inhibits de novo protein synthesis and disrupts reconsolidation of a previously formed memory [45,115,273].
During the reconsolidation process, memory traces become labile and can be altered by various pharmacological manipulations [321]. To date, evidence from various studies suggests that administration of non-specific protein synthesis inhibitors (e.g., anisomycin), if applied immediately after memory retrieval, blocks subsequent expression of a previously acquired conditioned response [81,225]. As protein synthesis inhibitors do not easily cross the blood-brain barrier and can be toxic, they are unlikely to be used in studies in humans. Thus, future experiments with more benign compounds, such as selective 5-HTXR ligands, administered to animals acutely following a CS-retrieval session to reduce later memory expression (possibly through inhibiting reconsolidation mechanisms) are necessary to further delineate the role of serotonergic systems in reconsolidation of cocaine-associated memories. If 5-HT release is an early trigger in the cascade of events that control new protein synthesis required for memory reconsolidation processes [45], then inhibition of 5-HT release following the re-activation session might be expected to increase protein synthesis and facilitate the reconsolidation of the predominant memory that was retrieved during the session (see below). If the session is of long duration then facilitation and stabilization of the extinction memory would be expected.
Prolonged re-exposure to the CS in the absence of the US triggers the formation of a new memory trace that encodes the dissociation between the CS and the US (CS–no US; extinction memory), therefore competing with the original memory (CS–US) [305]. Thus, a drug that enhances the retrieval of the extinction memory (i.e., the consolidation of the CS–no US association) may also reduce the impact of cocaine-associated memories. Research indicates that the competing processes of reconsolidation and extinction require different molecular components [314] and inhibition of protein synthesis at this stage blocks the formation of this new extinction memory, leaving expression of the original memory unchanged [305]. To date, most studies which examined the effects of serotonergic ligands on retrieval of cocaine-associated stimuli employed an extinction phase within the self-administration protocol. Specific experiments have not yet addressed the question as to whether the 5-HTXR ligand altered retrieval of the original memory (CS-US) or the novel extinction memory (CS-no US). Studies to determine whether the impairment in cocaine-associated memories in subsequent reactivation tests involves disruption of reconsolidation or a potentiation of extinction would require regulating the re-exposure sessions and perhaps a comparison to a “forced abstinence” period. Re-exposure sessions of short duration (15 min) would ensure reconsolidation processes whereas longer sessions (1–3 hrs) would involve consolidation of CS-no US memories [261].
Knowledge of the mechanisms through which 5-HTXR manipulations may disrupt cocaine-associated memories after a period of forced abstinence versus extinction is also critical for understanding of cocaine-associated memories. Humans rarely undergo extinction per se, but rather, cease using drugs for other reasons, [88] and as noted, extinction procedures to date have been of limited therapeutic success in reducing relapse among drug-dependent patients [57,64,73,102,130,151,239]. It has also been suggested that animal models of cocaine-seeking assessed after forced abstinence may have stronger face validity for cue-induced relapse in humans relative to cocaine-seeking measured after explicit extinction training [104]. A relatively unexplored area is the effect of repeated ligand administration on the impact of cocaine-associated cues in the retrieval test. This is an important area of research as a medication with efficacy as an abstinence enhancer would be administered to cocaine-dependent patients beginning at termination of cocaine use and continuing for a period of time. For example, in a 12-week, double-blind placebo-controlled trial [220], the SSRI citalopram (Celexa®) in conjunction with contingency management significantly increased treatment retention and reduced craving and cocaine-positive urines in outpatient studies of cocaine-using subjects.
Understanding the role of 5-HT in the neural systems underlying consolidation, reconsolidation, reactivation and extinction and their special properties vis à vis drug-associated cues will open the door to selectively minimizing the strong stimulus properties of cocaine-associated memories [92,182,184]. Armed with this knowledge, we can then hope to develop serotonergic pharmacotherapies to specifically target processes underlying cocaine-associated memories.
Conclusion
One of the most challenging endeavors in neuroscience is to identify the molecular mechanisms that underlie drug-associated memory formation. While the role of specific serotonergic receptors in learning and memory processes underlying cue-evoked drug-seeking behavior is still under-examined, this is an area ripe for further investigation. The acquisition of cocaine-associated memories involves storage and encoding of cue-reward associations, and the expression of cue-induced drug-seeking behavior involves the retrieval of memories of such cue-reward associations (Fig. 2). While to date the attenuation of the incentive motivational effects of cocaine-associated cues has been proposed as the mechanism by which serotonergic regulation modulates drug-seeking behavior [36,38,98,99,231,233], serotonergic ligands may inhibit cue-induced drug-seeking behavior by interfering with aspects of memory encoding and retrieval [104,184,298], either by blocking retrieval of the original CS-US memory or facilitating the retrieval of the CS-no US association. These processes may involve modifications of the functional status of the targets within the serotonergic system (e.g., transporter, 5-HTXR or linked downstream signaling partners [43,149,209]) which contribute to the ability of serotonergic ligands to disrupt retention of a conditioned association between cocaine and environmental stimuli. Further research efforts to explore the manner in which 5-HT manipulations, in particular those involving 5-HT1BR, 5-HT2AR and 5-HT2CR, regulate consolidation, retrieval or reconsolidation of memory at molecular, cellular and behavioral levels, may clarify these potential mechanisms in reducing cue-elicited drug-seeking behavior and open new avenues to therapeutically enhance abstinence.
Acknowledgements
The work in this review was supported by grants from the National Institute on Drug Abuse DA 00260, DA 06511 and K05 DA 020087. This work was also partially supported by a Jeane B. Kempner Scholarship.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HTXR
5-HT receptor family
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- AC
adenylate cyclase
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors
- BLA
basolateral amygdala
- cAMP
cyclic adenosine monophosphate
- CaMKII
calcium/calmodulin-dependent kinase type II
- CR
conditioned response
- CREB
cAMP response element binding protein
- CS
conditioned stimulus
- DXR
dopamine receptor
- DA
dopamine
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- ERK
extracellular signal-regulated kinase
- FR
fixed ratio
- GPCR
G-protein coupled receptor
- IP
inositol triphosphate
- LTD
long-term depression
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate receptors
- p38 MAPK
p38 mitogen-activated protein kinase
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PLC
phospholipase C
- PSD
post-synaptic density
- Ras-GTPase
Ras-guanosine triphosphatase activating protein
- SSRI
selective serotonin reuptake inhibitor
- SERT
5-HT transporter
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase
- US
unconditioned stimulus
- US
unconditioned stimulus
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Acosta JI, Boynton FA, Kirschner KF, Neisewander JL. Stimulation of 5-HT1B receptors decreases cocaine- and sucrose-seeking behavior. Pharmacol Biochem Behav. 2005;80(2):297–307. doi: 10.1016/j.pbb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Daoud N, Lynch WJ, Penberthy JK, Breland AB, Marzani-Nissen GR, Johnson BA. Treating smoking dependence in depressed alcoholics. Alcohol Res Health. 2006;29(3):213–220. [PMC free article] [PubMed] [Google Scholar]
- 3.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28(1):51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63(9):999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 6.Ardenghi P, Barros D, Izquierdo LA, Bevilaqua L, Schroder N, Quevedo J, Rodrigues C, Madruga M, Medina JH, Izquierdo I. Late and prolonged post-training memory modulation in entorhinal and parietal cortex by drugs acting on the cAMP/protein kinase A signalling pathway. Behav Pharmacol. 1997;8(8):745–751. doi: 10.1097/00008877-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 8.Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER. A novel function for serotonin-mediated short-term facilitation in aplysia: conversion of a transient, cell-wide homosynaptic hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc Natl Acad Sci U S A. 2000;97(21):11581–11586. doi: 10.1073/pnas.97.21.11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155(1):18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- 10.Banks ML, Czoty PW, Gage HD, Bounds MC, Garg PK, Garg S, Nader MA. Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology. 2008;33(2):219–225. doi: 10.1038/sj.npp.1301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao JX, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1998;18(1):458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 13.Beatty WW, Rush JR. Spatial working memory in rats: effects of monoaminergic antagonists. Pharmacol Biochem Behav. 1983;18(1):7–12. doi: 10.1016/0091-3057(83)90242-3. [DOI] [PubMed] [Google Scholar]
- 14.Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry. 2001;6(3):205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- 15.Bergman J, Paronis CA. Measuring the reinforcing strength of abused drugs. Mol Interv. 2006;6(5):273–283. doi: 10.1124/mi.6.5.9. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand F, Lehmann O, Lazarus C, Jeltsch H, Cassel JC. Intraseptal infusions of 8-OH-DPAT in the rat impairs water-maze performances: effects on memory or anxiety? Neurosci Lett. 2000;279(1):45–48. doi: 10.1016/s0304-3940(99)00948-9. [DOI] [PubMed] [Google Scholar]
- 17.Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behav Brain Res. 2004;151(1–2):225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57(2):113–119. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Boast C, Bartolomeo AC, Morris H, Moyer JA. 5HT antagonists attenuate MK801-impaired radial arm maze performance in rats. Neurobiol Learn Mem. 1999;71(3):259–271. doi: 10.1006/nlme.1998.3886. [DOI] [PubMed] [Google Scholar]
- 20.Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326(2):553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 21.Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33(Pt 6):1354–1356. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- 22.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 23.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27(46):12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27(39):10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 26.Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. J Exp Psychol Anim Behav Process. 1994;20(1):51–65. [PubMed] [Google Scholar]
- 27.Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40(4):695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- 28.Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 2007;191(3):705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- 29.Bradberry CW, Rubino SR. Phasic alterations in dopamine and serotonin release in striatum and prefrontal cortex in response to cocaine predictive cues in behaving rhesus macaques. Neuropsychopharmacology. 2004;29(4):676–685. doi: 10.1038/sj.npp.1300386. [DOI] [PubMed] [Google Scholar]
- 30.Breen RA, McGaugh JL. Facilitation of maze learning with posttrial injections of picrotoxin. J Comp Physiol Psychol. 1961;54:498–501. doi: 10.1037/h0046436. [DOI] [PubMed] [Google Scholar]
- 31.Brown P, Gerfen CR. Plasticity within striatal direct pathway neurons after neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK 1/2 map kinase pathway. J Comp Neurol. 2006;498(3):415–430. doi: 10.1002/cne.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. Neuroscientist. 2007;13(5):492–505. doi: 10.1177/1073858407302725. [DOI] [PubMed] [Google Scholar]
- 33.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 34.Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6(18):1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- 35.Buhot MC, Wolff M, Benhassine N, Costet P, Hen R, Segu L. Spatial learning in the 5-HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn Mem. 2003;10(6):466–477. doi: 10.1101/lm.60203. [DOI] [PubMed] [Google Scholar]
- 36.Burbassi S, Cervo L. Stimulation of serotonin(2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;196(1):15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- 37.Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29(4):660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- 38.Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1–2):146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- 39.Buydens-Branchey, Branchey M, Fergeson P, Hudson J, McKernin C. Craving for cocaine in addicted users. Role of serotonergic mechanisms. Am J Addict. 1997;6(1):65–73. [PubMed] [Google Scholar]
- 40.Buydens-Branchey, Branchey M, Hudson J, Rothman M, Fergeson P, McKernin C. Effect of fenfluramine challenge on cocaine craving in addicted male users. Am J Addict. 1998;7(2):142–155. [PubMed] [Google Scholar]
- 41.Buydens-Branchey, Branchey M, Hudson J, Rothman M, Fergeson P, McKernin C. Serotonergic function in cocaine addicts: prolactin responses to sequential D,L-fenfluramine challenges. Biol Psychiatry. 1999;45(10):1300–1306. doi: 10.1016/s0006-3223(98)00268-6. [DOI] [PubMed] [Google Scholar]
- 42.Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277(39):36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- 43.Cammarota M, Bevilaqua LR, Barros DM, Vianna MR, Izquierdo LA, Medina JH, Izquierdo I. Retrieval and the extinction of memory. Cell Mol Neurobiol. 2005;25(3–4):465–474. doi: 10.1007/s10571-005-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cammarota M, Bevilaqua LR, Kerr D, Medina JH, Izquierdo I. Inhibition of mRNA and protein synthesis in the CA1 region of the dorsal hippocampus blocks reinstallment of an extinguished conditioned fear response. J Neurosci. 2003;23(3):737–741. doi: 10.1523/JNEUROSCI.23-03-00737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci U S A. 2007;104(30):12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carew TJ, Sutton MA. Molecular stepping stones in memory consolidation. Nat Neurosci. 2001;4(8):769–771. doi: 10.1038/90458. [DOI] [PubMed] [Google Scholar]
- 47.Carey RJ, Damianopoulos EN. Conditioned cocaine induced hyperactivity: an association with increased medial prefrontal cortex serotonin. Behav Brain Res. 1994;62(2):177–185. doi: 10.1016/0166-4328(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 48.Carey RJ, DePalma G, Damianopoulos E, Shanahan A, Muller CP, Huston JP. Evidence that the 5-HT1A autoreceptor is an important pharmacological target for the modulation of cocaine behavioral stimulant effects. Brain Res. 2005;1034(1–2):162–171. doi: 10.1016/j.brainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Carli M, Lazarova M, Tatarczynska E, Samanin R. Stimulation of 5-HT1A receptors in the dorsal hippocampus impairs acquisition and performance of a spatial task in a water maze. Brain Res. 1992;595(1):50–56. doi: 10.1016/0006-8993(92)91451-j. [DOI] [PubMed] [Google Scholar]
- 50.Carli M, Luschi R, Samanin R. (S)-WAY 100135, a 5-HT1A receptor antagonist, prevents the impairment of spatial learning caused by intrahippocampal scopolamine. Eur J Pharmacol. 1995;283(1–3):133–139. doi: 10.1016/0014-2999(95)00310-h. [DOI] [PubMed] [Google Scholar]
- 51.Carli M, Tranchina S, Samanin R. 8-Hydroxy-2-(di-n-propylamino)tetralin, a 5-HT1A receptor agonist, impairs performance in a passive avoidance task. Eur J Pharmacol. 1992;211(2):227–234. doi: 10.1016/0014-2999(92)90533-a. [DOI] [PubMed] [Google Scholar]
- 52.Carroll RC, Beattie EC, von ZM, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2(5):315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 53.Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol Biochem Behav. 2000;67(3):559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- 54.Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28(6):1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- 55.Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 56.Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience. 2003;119(1):53–63. doi: 10.1016/s0306-4522(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 57.Childress AR, McLellan AT, O'Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr Clin North Am. 1986;9(3):413–425. [PubMed] [Google Scholar]
- 58.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 60.Clark GA, Hawkins RD, Kandel ER. Activity-dependent enhancement of presynaptic facilitation provides a cellular mechanism for the temporal specificity of classical conditioning in Aplysia. Learn Mem. 1994;1(4):243–257. [PubMed] [Google Scholar]
- 61.Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30(1):145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- 62.Cohen JE, Onyike CU, McElroy VL, Lin AH, Abrams TW. Pharmacological characterization of an adenylyl cyclase-coupled 5-HT receptor in aplysia: comparison with mammalian 5-HT receptors. J Neurophysiol. 2003;89(3):1440–1455. doi: 10.1152/jn.01004.2002. [DOI] [PubMed] [Google Scholar]
- 63.Cole BJ, Jones GH, Turner JD. 5-HT1A receptor agonists improve the performance of normal and scopolamine-impaired rats in an operant delayed matching to position task. Psychopharmacology (Berl) 1994;116(2):135–142. doi: 10.1007/BF02245055. [DOI] [PubMed] [Google Scholar]
- 64.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 65.Cooke SF, Bliss TV. Long-term potentiation and cognitive drug discovery. Curr Opin Investig Drugs. 2005;6(1):25–34. [PubMed] [Google Scholar]
- 66.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116(1):169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 67.Cunningham KA, Paris JM, Goeders NE. Serotonin neurotransmission in cocaine sensitization. Ann NY Acad Sci. 1992;654:117–127. doi: 10.1111/j.1749-6632.1992.tb25960.x. [DOI] [PubMed] [Google Scholar]
- 68.Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21(3):111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 69.Daglish MR, Nutt DJ. Brain imaging studies in human addicts. Eur Neuropsychopharmacol. 2003;13(6):453–458. doi: 10.1016/j.euroneuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96(3):518–559. [PubMed] [Google Scholar]
- 71.Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006;19(5):571–581. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- 72.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 73.Dawe S, Powell J, Richards D, Gossop M, Marks I, Strang J, Gray JA. Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction. 1993;88(9):1233–1245. doi: 10.1111/j.1360-0443.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 74.De La Garza R, Newton TF, Kalechstein AD. Risperidone diminishes cocaine-induced craving. Psychopharmacology. 2005;178(2 – 3):347–350. doi: 10.1007/s00213-004-2010-8. [DOI] [PubMed] [Google Scholar]
- 75.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 76.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36(3):527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 77.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8(2):101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 78.Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 79.Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13(5–6):397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 80.Dudai Y. Molecular bases of long-term memories: a question of persistence. Curr Opin Neurobiol. 2002;12(2):211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 81.Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44(1):93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Dumitriu B, Cohen JE, Wan Q, Negroiu AM, Abrams TW. Serotonin receptor antagonists discriminate between PKA- and PKC-mediated plasticity in aplysia sensory neurons. J Neurophysiol. 2006;95(4):2713–2720. doi: 10.1152/jn.00642.2005. [DOI] [PubMed] [Google Scholar]
- 83.Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76(2):323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- 84.Edagawa Y, Saito H, Abe K. Serotonin inhibits the induction of long-term potentiation in rat primary visual cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(6):983–997. doi: 10.1016/s0278-5846(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 85.Edagawa Y, Saito H, Abe K. The serotonin 5-HT2 receptor-phospholipase C system inhibits the induction of long-term potentiation in the rat visual cortex. Eur J Neurosci. 2000;12(4):1391–1396. doi: 10.1046/j.1460-9568.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 86.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301(5636):1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 87.El-Mallakh RS, Abraham HD. MDMA (Ecstasy) Ann Clin Psychiatry. 2007;19(1):45–52. doi: 10.1080/10401230601163592. [DOI] [PubMed] [Google Scholar]
- 88.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esteban JA. AMPA receptor trafficking: a road map for synaptic plasticity. Mol Interv. 2003;3(7):375–385. doi: 10.1124/mi.3.7.375. [DOI] [PubMed] [Google Scholar]
- 90.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6(2):136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 91.European Monitoring Centre for Drugs and Drug Addiction. Cocaine and crack cocaine: a growing public health issue. EMCDDA. 2007
- 92.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fagen ZM, Mansvelder HD, Keath JR, McGehee DS. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann N Y Acad Sci. 2003;1003(1):185–195. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- 94.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174(1):1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 95.Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88(4):435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feuerstein TJ. Presynaptic receptors for dopamine, histamine, and serotonin. Handb Exp Pharmacol. 2008;(184):289–338. doi: 10.1007/978-3-540-74805-2_10. [DOI] [PubMed] [Google Scholar]
- 97.Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep. 2005;57(1):35–46. [PubMed] [Google Scholar]
- 98.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27(4):576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 99.Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- 100.Foa EB. Social anxiety disorder treatments: psychosocial therapies. J Clin Psychiatry. 2006;67 Suppl 12:27–30. [PubMed] [Google Scholar]
- 101.Fowler JS, Volkow ND, Wang GJ, Gatley SJ, Logan J. [(11)]Cocaine: PET studies of cocaine pharmacokinetics, dopamine transporter availability and dopamine transporter occupancy. Nucl Med Biol. 2001;28(5):561–572. doi: 10.1016/s0969-8051(01)00211-6. [DOI] [PubMed] [Google Scholar]
- 102.Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat. 1999;16(1):81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 103.Frenois F, Le MC, Cador M. The motivational component of withdrawal in opiate addiction: role of associative learning and aversive memory in opiate addiction from a behavioral, anatomical and functional perspective. Rev Neurosci. 2005;16(3):255–276. doi: 10.1515/revneuro.2005.16.3.255. [DOI] [PubMed] [Google Scholar]
- 104.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26(2):487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 106.Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23(10):2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- 107.Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology. 1998;135(2):151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 108.Gao C, Wolf ME. Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. J Neurosci. 2007;27(52):14275–14285. doi: 10.1523/JNEUROSCI.2925-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 110.Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9(4):285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- 111.Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155(1):1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 112.Giorgi O, Piras G, Corda MG. The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci Biobehav Rev. 2007;31(1):148–163. doi: 10.1016/j.neubiorev.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7(1):77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 114.Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory--a molecular framework. Nature. 1986;322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 115.Gold PE. The many faces of amnesia. Learn Mem. 2006;13(5):506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- 116.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, ia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol. 2000;20(3):305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 118.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295(3):1183–1191. [PubMed] [Google Scholar]
- 121.Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 122.Gutlerner JL, Penick EC, Snyder EM, Kauer JA. Novel protein kinase A-dependent long-term depression of excitatory synapses. Neuron. 2002;36(5):921–931. doi: 10.1016/s0896-6273(02)01051-6. [DOI] [PubMed] [Google Scholar]
- 123.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs : effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67(1):27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 124.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 126.Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487(1–3):125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 127.Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. J Subst Abuse Treat. 2006;31(4):355–364. doi: 10.1016/j.jsat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 128.Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2007 doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28(2):527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 130.Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend. 1994;34:87–97. doi: 10.1016/0376-8716(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 131.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27(46):12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Homberg JR, Arends B, Wardeh G, Raaso HS, Schoffelmeer AN, de Vries TJ. Individual differences in the effects of serotonergic anxiolytic drugs on the motivation to self-administer cocaine. Neuroscience. 2004;128(1):121–130. doi: 10.1016/j.neuroscience.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 133.Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75(1):196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 134.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71(4):533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 135.Hsiung SC, Tamir H, Franke TF, Liu KP. Roles of extracellular signal-regulated kinase and Akt signaling in coordinating nuclear transcription factor-kappaB-dependent cell survival after serotonin 1A receptor activation. J Neurochem. 2005;95(6):1653–1666. doi: 10.1111/j.1471-4159.2005.03496.x. [DOI] [PubMed] [Google Scholar]
- 136.Huang YY, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci. 2007;27(12):3111–3119. doi: 10.1523/JNEUROSCI.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;(1):CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 138.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 139.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 140.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 141.Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29(9):496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 142.Izquierdo I, Cammarota M, Medina JH, Bevilaqua LR. Pharmacological findings on the biochemical bases of memory processes: a general view. Neural Plast. 2004;11(3–4):159–189. doi: 10.1155/NP.2004.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69(6):375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 144.Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19(10):873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- 145.Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16(2):71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- 146.Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 147.Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6(6):339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- 148.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 149.Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2004;24(4–5):475–522. doi: 10.1007/s10540-005-2742-7. [DOI] [PubMed] [Google Scholar]
- 150.Kant GJ, Meininger GR, Maughan KR, Wright WL, Robinson TN, III, Neely TM. Effects of the serotonin receptor agonists 8-OH-DPAT and TFMPP on learning as assessed using a novel water maze. Pharmacol Biochem Behav. 1996;53(2):385–390. doi: 10.1016/0091-3057(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 151.Kasvikis Y, Bradley B, Powell J, Marks I, Gray JA. Postwithdrawal exposure treatment to prevent relapse in opiate addicts: a pilot study. Int J Addict. 1991;26(11):1187–1195. doi: 10.3109/10826089109062154. [DOI] [PubMed] [Google Scholar]
- 152.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168(1–2):21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 153.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 154.Kelamangalath L, Swant J, Stramiello M, Wagner JJ. The effects of extinction training in reducing the reinstatement of drug-seeking behavior: involvement of NMDA receptors. Behav Brain Res. 2007;185(2):119–128. doi: 10.1016/j.bbr.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 156.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 157.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86(1–2):11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 159.Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- 160.Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18(8):777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- 161.Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5(4):348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 162.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 163.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-Containing NMDA Receptors in Ras-ERK Signaling and AMPA Receptor Trafficking. Neuron. 2005;46(5):745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 164.Kitson SL. 5-hydroxytryptamine (5-HT) receptor ligands. Curr Pharm Des. 2007;13(25):2621–2637. doi: 10.2174/138161207781663000. [DOI] [PubMed] [Google Scholar]
- 165.Knapp WP, Soares BG, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007;(3):CD003023. doi: 10.1002/14651858.CD003023.pub2. [DOI] [PubMed] [Google Scholar]
- 166.Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199(3):649–661. [PubMed] [Google Scholar]
- 167.Koenig J, Cosquer B, Cassel JC. Activation of septal 5-HT1A receptors alters spatial memory encoding, interferes with consolidation, but does not affect retrieval in rats subjected to a water-maze task. Hippocampus. 2008;18(1):99–118. doi: 10.1002/hipo.20368. [DOI] [PubMed] [Google Scholar]
- 168.Koh MT, Bernstein IL. Inhibition of protein kinase A activity during conditioned taste aversion retrieval: interference with extinction or reconsolidation of a memory? Neuroreport. 2003;14(3):405–407. doi: 10.1097/00001756-200303030-00021. [DOI] [PubMed] [Google Scholar]
- 169.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 Suppl 1:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 170.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 171.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 172.Kourrich S, Rothwell PE, Klug JR. Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43(4):563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 174.Krasnova IN, Li SM, Wood WH, McCoy MT, Prabhu VV, Becker KG, Katz JL, Cadet JL. Transcriptional responses to reinforcing effects of cocaine in the rat hippocampus and cortex. Genes Brain Behav. 2008;7(2):193–202. doi: 10.1111/j.1601-183X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 175.Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8(9):687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 176.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5(1):45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 177.Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3(1):1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- 178.Laroche S. Cellular and molecular approaches to memory storage. Therapie. 2000;55(4):461–466. [PubMed] [Google Scholar]
- 179.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8(6):413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 180.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23(7):993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 181.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94(1–2):137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 182.Lee JL, Di CP, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47(6):795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 183.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304(5672):839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 184.Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26(22):5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74(8):1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leysen JE. 5-HT2 receptors. Curr Drug Target CNS Neurol Disord. 2004;3(1):11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- 187.Li M, Liu Y, Dutt P, Fanburg BL, Toksoz D. Inhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L463–L471. doi: 10.1152/ajplung.00133.2007. [DOI] [PubMed] [Google Scholar]
- 188.Liang KC. Pre- or post-training injection of buspirone impaired retention in the inhibitory avoidance task: involvement of amygdala 5-HT1A receptors. Eur J Neurosci. 1999;11(5):1491–1500. doi: 10.1046/j.1460-9568.1999.00561.x. [DOI] [PubMed] [Google Scholar]
- 189.Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155(2):207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- 190.Liu S, Cunningham KA. Serotonin2C receptors (5-HT2C R) control expression of cocaine-induced conditioned hyperactivity. Drug Alcohol Depend. 2006;81(3):275–282. doi: 10.1016/j.drugalcdep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 191.Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann N Y Acad Sci. 2003;1003:226–240. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- 192.Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- 193.MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18(1–2):71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 194.MacDonald JF, Kotecha SA, Lu WY, Jackson MF. Convergence of PKC-dependent kinase signal cascades on NMDA receptors. Curr Drug Targets. 2001;2(3):299–312. doi: 10.2174/1389450013348452. [DOI] [PubMed] [Google Scholar]
- 195.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 196.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 197.Markowska AL, Wenk GL. Serotonin influences the behavioral recovery of rats following nucleus basalis lesions. Pharmacol Biochem Behav. 1991;38(4):731–737. doi: 10.1016/0091-3057(91)90234-s. [DOI] [PubMed] [Google Scholar]
- 198.Martin-Fardon R, Ciccocioppo R, Aujla H, Weiss F. The dorsal subiculum mediates the acquisition of conditioned reinstatement of cocaine-seeking. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301589. In press. [DOI] [PubMed] [Google Scholar]
- 199.Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30(4):176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 200.McCreary AC, Cunningham KA. Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology. 1999;20(6):556–564. doi: 10.1016/S0893-133X(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 201.McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology (Berl) 1997;131(1):86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- 202.McGaugh JL. Memory--a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 203.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297(1):357–363. [PubMed] [Google Scholar]
- 204.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87(2):139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 205.Mendelson JH, Mello NK. Drug therapy: Management of cocaine abuse and dependence. New England Journal of Medicine. 1996;334(15):965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- 206.Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23(8):1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 207.Meneses A. Involvement of 5-HT(2A/2B/2C) receptors on memory formation: simple agonism, antagonism, or inverse agonism? Cell Mol Neurobiol. 2002;22(5–6):675–688. doi: 10.1023/a:1021800822997. [DOI] [PubMed] [Google Scholar]
- 208.Meneses A. A pharmacological analysis of an associative learning task: 5-HT1 to 5-HT7 receptor subtypes function on a pavlovian/instrumental autoshaped memory. Learn Mem. 2003;10(5):363–372. doi: 10.1101/lm.60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav Brain Res. 2007;184(1):81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 210.Meneses A, Hong E. 5-HT1A receptors modulate the consolidation of learning in normal and cognitively impaired rats. Neurobiol Learn Mem. 1999;71(2):207–218. doi: 10.1006/nlme.1998.3866. [DOI] [PubMed] [Google Scholar]
- 211.Meneses A, Manuel-Apolinar L, Rocha L, Castillo E, Castillo C. Expression of the 5-HT receptors in rat brain during memory consolidation. Behav Brain Res. 2004;152(2):425–436. doi: 10.1016/j.bbr.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 212.Meneses A, Perez-Garcia G. 5-HT(1A) receptors and memory. Neurosci Biobehav Rev. 2007;31(5):705–727. doi: 10.1016/j.neubiorev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 213.Meneses A, Terron JA, Hong E. Effects of the 5-HT receptor antagonists GR127935 (5-HT1B/1D) and MDL100907 (5-HT2A) in the consolidation of learning. Behav Brain Res. 1997;89(1–2):217–223. doi: 10.1016/s0166-4328(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 214.Merali Z, McIntosh J, Anisman H. Anticipatory cues differentially provoke in vivo peptidergic and monoaminergic release at the medial prefrontal cortex. Neuropsychopharmacology. 2004;29(8):1409–1418. doi: 10.1038/sj.npp.1300441. [DOI] [PubMed] [Google Scholar]
- 215.Michel A, Tambour S, Tirelli E. The magnitude and the extinction duration of the cocaine-induced conditioned locomotion-activated response are related to the number of cocaine injections paired with the testing context in C57BL/6J mice. Behav Brain Res. 2003;145(1–2):113–123. doi: 10.1016/s0166-4328(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 216.Mierzejewski P, Siemiatkowski M, Radwanska K, Szyndler J, Bienkowski P, Stefanski R, Kaczmarek L, Kostowski W. Cycloheximide impairs acquisition but not extinction of cocaine self-administration. Neuropharmacology. 2006;51(2):367–373. doi: 10.1016/j.neuropharm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 217.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47(6):873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 218.Misane I, Ogren SO. Multiple 5-HT receptors in passive avoidance: comparative studies of p-chloroamphetamine and 8-OH-DPAT. Neuropsychopharmacology. 2000;22(2):168–190. doi: 10.1016/S0893-133X(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 219.Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160(827):554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 220.Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33(3):367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 221.Moyano S, Del RJ, Frechilla D. Role of hippocampal CaMKII in serotonin 5-HT(1A) receptor-mediated learning deficit in rats. Neuropsychopharmacology. 2004;29(12):2216–2224. doi: 10.1038/sj.npp.1300504. [DOI] [PubMed] [Google Scholar]
- 222.Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81(3):133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 223.Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol Sci. 2006;27(2):105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 224.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36(4):567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 225.Nader K. Memory traces unbound. Trends Neurosci. 2003;26(2):65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 226.Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000;1(3):216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 227.Nader K, Wang SH. Fading in. Learn Mem. 2006;13(5):530–535. doi: 10.1101/lm.350906. [DOI] [PubMed] [Google Scholar]
- 228.National Institutes of Health Clinical Center (CC) An Investigation of the Antidepressant Efficacy of the 5-HT2A Antagonist, M100907. Combination with Citalopram in Treatment Resistant Depression. Clinicaltrials gov 2005. 2005 http://clinicaltrials gov/ct/show/NCT00070694 3-21-2006.
- 229.Navailles S, Moison D, Ryczko D, Spampinato U. Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J Neurochem. 2006;99(4):1311–1319. doi: 10.1111/j.1471-4159.2006.04188.x. [DOI] [PubMed] [Google Scholar]
- 230.Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- 231.Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18(8):791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- 232.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10(3):201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 233.Nic Dhonnchadha BÁ, Fox RG, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior. Psychopharmacology. 2008 doi: 10.1037/a0014592. (submitted manuscript) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6(11):863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 235.Noda M, Higashida H, Aoki S, Wada K. Multiple signal transduction pathways mediated by 5-HT receptors. Mol Neurobiol. 2004;29(1):31–39. doi: 10.1385/MN:29:1:31. [DOI] [PubMed] [Google Scholar]
- 236.Nutt D, Lingford-Hughes A, Daglish M. Future directions in substance dependence research. J Neural Transm Suppl. 2003;64:95–103. doi: 10.1007/978-3-7091-6020-6_6. [DOI] [PubMed] [Google Scholar]
- 237.O'Brien CP. Progress in the science of addiction. Am J Psychiatry. 1997;154(9):1195–1197. doi: 10.1176/ajp.154.9.1195. [DOI] [PubMed] [Google Scholar]
- 238.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 239.O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15(4):355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- 240.O'Dell LE, Manzardo AM, Polis I, Stouffer DG, Parsons LH. Biphasic alterations in serotonin-1B (5-HT1B) receptor function during abstinence from extended cocaine self-administration. J Neurochem. 2006;99(5):1363–1376. doi: 10.1111/j.1471-4159.2006.04163.x. [DOI] [PubMed] [Google Scholar]
- 241.Otano A, Garcia-Osta A, Ballaz S, Frechilla D, Del RJ. Facilitation by 8-OH-DPAT of passive avoidance performance in rats after inactivation of 5-HT(1A) receptors. Br J Pharmacol. 1999;128(8):1691–1698. doi: 10.1038/sj.bjp.0702974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102(12):1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Parsons LH, Weiss F, Koob GF. Serotonin1b receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacology (Berl) 1996;128(2):150–160. doi: 10.1007/s002130050120. [DOI] [PubMed] [Google Scholar]
- 244.Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18(23):10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Patkar AA, Berrettini WH, Mannelli P, Gopalakrishnan R, Hoehe MR, Bilal L, Weinstein S, Vergare MJ. Relationship between serotonin transporter gene polymorphisms and platelet serotonin transporter sites among African-American cocaine-dependent individuals and healthy volunteers. Psychiatr Genet. 2004;14(1):25–32. doi: 10.1097/00041444-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 246.Pavlov IP. An Investigation of the Physiological Activity of the Cerebral Cortex. Oxford University Press; 1927. Conditioned Reflexes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38(6):863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 248.Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology (Berl) 1993;110(4):390–394. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- 249.Perez-Cuesta LM, Hepp Y, Pedreira ME, Maldonado H. Memory is not extinguished along with CS presentation but within a few seconds after CS-offset. Learn Mem. 2007;14(1):101–108. doi: 10.1101/lm.413507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Petrakis IL, Trevisan L, Boutros NN, Limoncelli D, Cooney NL, Krystal JH. Effect of tryptophan depletion on alcohol cue-induced craving in abstinent alcoholic patients. Alcohol Clin Exp Res. 2001;25(8):1151–1155. [PubMed] [Google Scholar]
- 251.Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, Molnar E. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology. 2001;41(6):700–713. doi: 10.1016/s0028-3908(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 252.Pitsikas N, Rigamonti AE, Cella SG, Muller EE. The 5-HT 1A receptor antagonist WAY 100635 improves rats performance in different models of amnesia evaluated by the object recognition task. Brain Res. 2003;983(1–2):215–222. doi: 10.1016/s0006-8993(03)03091-9. [DOI] [PubMed] [Google Scholar]
- 253.Pitsikas N, Tsitsirigou S, Zisopoulou S, Sakellaridis N. The 5-HT1A receptor and recognition memory. Possible modulation of its behavioral effects by the nitrergic system. Behav Brain Res. 2005;159(2):287–293. doi: 10.1016/j.bbr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 254.Pittenger C, Kandel ER. A genetic switch for long-term memory. Comptes Rendus de l'Academie des Sciences - Series III - Sciences de la Vie. 1998;321(2–3):91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- 255.Pollack MH, Rosenbaum JF. Fluoxetine treatment of cocaine abuse in heroin addicts. J Clin Psychiatry. 1991;52(1):31–33. [PubMed] [Google Scholar]
- 256.Prado-Alcala RA, Solana-Figueroa R, Galindo LE, Medina AC, Quirarte GL. Blockade of striatal 5-HT2 receptors produces retrograde amnesia in rats. Life Sci. 2003;74(4):481–488. doi: 10.1016/j.lfs.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 257.Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12(2):133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 258.Przegalinski E, Filip M. Stimulation of serotonin (5-HT)1A receptors attenuates the locomotor, but not the discriminative, effects of amphetamine and cocaine in rats. Behav Pharmacol. 1997;8(8):699–706. doi: 10.1097/00008877-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 259.Przegalinski E, Golda A, Frankowska M, Zaniewska M, Filip M. Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur J Pharmacol. 2007;559(2–3):165–172. doi: 10.1016/j.ejphar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 260.Przegalinski E, Papla I, Siwanowicz J, Filip M. Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on the locomotor and sensitizating effects of cocaine in rats. Eur Neuropsychopharmacol. 2004;14(3):217–225. doi: 10.1016/S0924-977X(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 261.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92(2–3):179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 263.Reid MS, Palamar J, Raghavan S, Flammino F. Effects of topiramate on cue-induced cigarette craving and the response to a smoked cigarette in briefly abstinent smokers. Psychopharmacology (Berl) 2007;192(1):147–158. doi: 10.1007/s00213-007-0755-6. [DOI] [PubMed] [Google Scholar]
- 264.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 265.Robbe D, Alonso G, Manzoni OJ. Exogenous and endogenous cannabinoids control synaptic transmission in mice nucleus accumbens. Ann N Y Acad Sci. 2003;1003:212–225. doi: 10.1196/annals.1300.013. [DOI] [PubMed] [Google Scholar]
- 266.Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 268.Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393(6681):175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 269.Rohsenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68(5):641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- 270.Rothman RB, Blough BE, Baumann MH. Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci. 2006;27(12):612–618. doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 271.Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28(1):12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 272.Roy A, Roy M, Smelson DA. Risperidone, ERG and cocaine craving. Am J Addict. 1998;7(1):90. doi: 10.1111/j.1521-0391.1998.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 273.Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learn Mem. 2006;13(1):1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- 274.Ruotsalainen S, Sirvio J, Jakala P, Puumala T, MacDonald E, Riekkinen P., Sr Differential effects of three 5-HT receptor antagonists on the performance of rats in attentional and working memory tasks. Eur Neuropsychopharmacol. 1997;7(2):99–108. doi: 10.1016/s0924-977x(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 275.Sakurai Y, Wenk GL. The interaction of acetylcholinergic and serotonergic neural systems on performance in a continuous non-matching to sample task. Brain Res. 1990;519(1–2):118–121. doi: 10.1016/0006-8993(90)90068-m. [DOI] [PubMed] [Google Scholar]
- 276.Salamone JD. Will the last person who uses the term 'reward' please turn out the lights? Comments on processes related to reinforcement, learning, motivation and effort. Addict Biol. 2006;11(1):43–44. doi: 10.1111/j.1369-1600.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- 277.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 278.Sanchis-Segura C, Cline B, Jurd R, Rudolph U, Spanagel R. Etomidate and propofol-hyposensitive GABAA receptor beta3(N265M) mice show little changes in acute alcohol sensitivity but enhanced tolerance and withdrawal. Neurosci Lett. 2007;416(3):275–278. doi: 10.1016/j.neulet.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 279.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 280.Sattar SP, Bhatia SC. Olanzapine for cocaine cravings and relapse prevention. J Clin Psychiatry. 2003;64(8):969. doi: 10.4088/jcp.v64n0819a. [DOI] [PubMed] [Google Scholar]
- 281.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20(18):RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Schenk S. Effects of the serotonin 5-HT(2) antagonist, ritanserin, and the serotonin 5-HT(1A) antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacol Biochem Behav. 2000;67(2):363–369. doi: 10.1016/s0091-3057(00)00377-4. [DOI] [PubMed] [Google Scholar]
- 283.Schiapparelli L, Del RJ, Frechilla D. Serotonin 5-HT receptor blockade enhances Ca(2+)/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation. J Neurochem. 2005;94(4):884–895. doi: 10.1111/j.1471-4159.2005.03193.x. [DOI] [PubMed] [Google Scholar]
- 284.Schiapparelli L, Simon AM, Del RJ, Frechilla D. Opposing effects of AMPA and 5-HT1A receptor blockade on passive avoidance and object recognition performance: correlation with AMPA receptor subunit expression in rat hippocampus. Neuropharmacology. 2006;50(7):897–907. doi: 10.1016/j.neuropharm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 285.Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21(7):RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526(1–3):65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 287.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71(3):517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 288.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526(1–3):140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 289.Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63(1):90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- 290.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11(5):648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 292.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54(1):1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 293.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8(4):209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- 294.Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29(10):1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- 295.Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105(7):825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 296.Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, Roll J, Shapiro B, Rotheram-Fuller E, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(1):12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 297.Shutoh F, Hamada S, Shibata M, Narita M, Shiga T, Azmitia EC, Okado N. Long term depletion of serotonin leads to selective changes in glutamate receptor subunits. Neurosci Res. 2000;38(4):365–371. doi: 10.1016/s0168-0102(00)00184-x. [DOI] [PubMed] [Google Scholar]
- 298.Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiol Learn Mem. 2006;86(3):305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 299.Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 2007;190(4):569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- 300.Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am. 2004;27(4):627–648. doi: 10.1016/j.psc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 301.Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin Emerg Drugs. 2006;11(1):91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- 302.Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994;643(1–2):10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 303.Substance Abuse and Mental Health Services Administration. 2006 National Survey on Drug Use & Health: National Results. SAMHSA. 2006
- 304.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421(6918):70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 305.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24(20):4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Suzuki WA, Amaral DG. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 2004;40(1):220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- 307.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 308.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76(1):1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 309.Tambour S, Quertemont E. Preclinical and clinical pharmacology of alcohol dependence. Fundam Clin Pharmacol. 2007;21(1):9–28. doi: 10.1111/j.1472-8206.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 310.Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nat Neurosci. 2001;4(8):813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- 311.Torres C, Morales A, Candido A, Maldonado A. Differential effect of buspirone and diazepam on negative contrast in one-way avoidance learning. Eur J Pharmacol. 1995;280(3):277–284. doi: 10.1016/0014-2999(95)00210-c. [DOI] [PubMed] [Google Scholar]
- 312.Tran-Nguyen LT, Baker DA, Grote KA, Solano J, Neisewander JL. Serotonin depletion attenuates cocaine-seeking behavior in rats. Psychopharmacology (Berl) 1999;146(1):60–66. doi: 10.1007/s002130051088. [DOI] [PubMed] [Google Scholar]
- 313.Tran-Nguyen LT, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;157(4):340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- 314.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8(4):262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 315.Turner JH, Garnovskaya MN, Coaxum SD, Vlasova TM, Yakutovich M, Lefler DM, Raymond JR. Ca2+-calmodulin and janus kinase 2 are required for activation of sodium-proton exchange by the Gi-coupled 5-hydroxytryptamine 1a receptor. J Pharmacol Exp Ther. 2007;320(1):314–322. doi: 10.1124/jpet.106.112581. [DOI] [PubMed] [Google Scholar]
- 316.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 317.Udo H, Jin I, Kim JH, Li HL, Youn T, Hawkins RD, Kandel ER, Bailey CH. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron. 2005;45(6):887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 318.Ueda S, Sakakibara S, Yoshimoto K. Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning. Neuroscience. 2005;135(2):395–402. doi: 10.1016/j.neuroscience.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 319.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411(6837):583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 320.United Nations Office on Drugs and Crime. 2007 World Drug Report,Volume 1 : Analysis. 2007 http://www.unodc.org/unodc/en/data-and-analysis/WDR-2007.html.
- 321.Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Herve D. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26(18):4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Van den Oever MC, Spijker S, Li KW, Jimenez CR, Koya E, Van der Schors RC, Gouwenberg Y, Binnekade R, de Vries TJ, Schoffelmeer AN, Smit AB. A proteomics approach to identify long-term molecular changes in rat medial prefrontal cortex resulting from sucrose self-administration. J Proteome Res. 2006;5(1):147–154. doi: 10.1021/pr050303y. [DOI] [PubMed] [Google Scholar]
- 323.Vickers SP, Dourish CT. Serotonin receptor ligands and the treatment of obesity. Curr Opin Investig Drugs. 2004;5(4):377–388. [PubMed] [Google Scholar]
- 324.Villareal G, Li Q, Cai D, Glanzman DL. The role of rapid, local, postsynaptic protein synthesis in learning-related synaptic facilitation in aplysia. Curr Biol. 2007;17(23):2073–2080. doi: 10.1016/j.cub.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102 Suppl 1:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 326.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 Suppl 1:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 327.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 329.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292(5519):1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 330.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 331.Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997;130(1):41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 332.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25(3):635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 333.Williams JM, Guevremont D, Kennard JT, Mason-Parker SE, Tate WP, Abraham WC. Long-term regulation of N-methyl-D-aspartate receptor subunits and associated synaptic proteins following hippocampal synaptic plasticity. Neuroscience. 2003;118(4):1003–1013. doi: 10.1016/s0306-4522(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 334.Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47 Suppl 1:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 335.Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80(2–3):65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- 336.Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28(2):157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]