Abstract
The NOD-like receptor (NLR) family of proteins is involved in the regulation of innate immune responses and cell death pathways. Recent findings show that the NLR family member NLRC4 (also known as IPAF) has important roles in innate immune responses to Gram-negative bacteria. Macrophages infected with Legionella pneumophila, Salmonella typhimurium, Shigella flexneri, or Pseudomonas aeruginosa activate caspase-1 in an NLRC4-dependent manner leading to macrophage cell death and the release of proinflammatory cytokines. This review will discuss these findings as well as the role of bacterial type III and type IV secretion systems and flagellin in NLRC4-mediated caspase-1 activation.
Keywords: Innate Immunity, NOD-like receptors, NLRC4, Caspase-1, Inflammasome, Macrophage, Flagellin, Type 3 Secretion System, type 4 Secretion System
Introduction
The innate immune system possesses numerous germline-encoded pattern recognition receptors that are capable of recognizing highly conserved pathogen-associated molecular patterns (PAMPs). These receptors include Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like RNA helicases (RLHs) and C-type lectin receptors (CLRs). In addition to PAMPs, NLRs can sense endogenous danger signals (danger-associated molecular patterns; DAMPs) released by cellular damage or stress in response to either invading pathogens or sterile inflammatory responses.
The importance of the NLRs in innate immunity can be demonstrated by the finding that mutations within a number of the NLR genes have been associated with autoimmune and autoinflammatory disorders in humans (Table 1). Mutations in NOD2 have been associated with Crohn’s disease and Blau syndrome [14,15]. Mutations in NLRP3 (also known as NALP3, CIAS1 and cryopyrin) are responsible for the autoinflammatory syndromes, Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and neonatal-onset multisystem inflammatory disease [14,15]. Most recently sequence variants in the NLRP1 (also known as NALP1 and DEFCAP) gene have been linked to autoimmune and autoinflammatory diseases associated with vitiligo [7].
Table 1.
Overview of human NLR associated diseases
| Synonyms | Associated Disease | Reference | |
|---|---|---|---|
| NOD2 | NLRC2; CARD15; CLR16.3 | Crohn’s disease; Blau Syndrome; Early-onset sarcoidosis | [1–6] |
| NLRP1 | NALP1; DEFCAP; NAC; CARD7; CLR17.1 | Vitiligo-related autoimmune disorders | [7] |
| NLRP3 | NALP3; CIAS1; PYPAF1; Cryopyrin; CLR1.1 | Muckle Wells Syndrome; Familial Cold Autoinflammatory Syndrome; CINCA | [8–9] |
| NLRP7 | NALP7; PYPAF3; NOD12; PAN7; CLR19.4 | Hydatiform mole | [10] |
| NLRP12 | NALP12; PYPAF7; Monarch1; PAN6; CLR19.3 | Hereditary periodic fevers | [11] |
| NAIP | Birc1; CLR5.1; NLRB1 | Spinal muscular atrophy | [12] |
| CIITA | MHCIITA; NLRA | Bare lymphocyte syndrome | [13] |
The NLR family
The human NLR family of intracellular proteins contains 22 members including 14 NLRP members, 5 members of the NLRC subfamily, NAIP, NLRX, and CIITA [16]. Currently, a number of different names have been assigned to the members of the NLR family. Recently a standardized nomenclature has been proposed which we will use throughout this review [17]. NLRs have a unique nucleotide-binding domain called NACHT, which is located at the center of the molecule between an N-terminal protein-binding domain (CARD (caspase-recruitment domain), PYD (pyrin domain) or BIR (Baculovirus IAP repeat)) and a C-terminal LRR (Leucine-rich repeat) domain.
A number of the NLR molecules have been shown to interact with and modulate the function of the cysteine protease caspase-1. Based on elegant biochemical analysis of three NLR proteins, NLRP1, NLRP2 and NLRP3, a model was proposed in which caspase-1 was activated within a multiprotein complex termed the inflammasome [18]. The structure and components of these inflammasomes have recently been reviewed [16]. In addition to NLRP1, NLRP2, and NLRP3, a number of other NLR molecules including, NLRC4, NLRP6, NLRP7, NLRP10 and NLRP12 have also been reported to modulate caspase-1 activity [16]. However, so far only NLRP1, NLRP3 and NLRC4 have been shown to have clear physiologic roles.
The NLRC4 inflammasome
NLRC4 (also known as IPAF, Card12 and CLAN) is expressed in myeloid cells where it has been shown to regulate caspase-1 activation and IL-1β processing. NLRC4 contains an N-terminal CARD, a central NACHT domain and C-terminal LRRs. Deletion of the LRR domain of NLRC4 results in a constitutively active form of the molecule suggesting that NLRC4 activation occurs in a similar manner to other NLRs that also gain activity upon deletion of their LRR domain [19]. NLRC4, similar to other NLRs such as NLRP3, NOD1 and NOD2, also interacts with the chaperone protein heat-shock protein 90 (HSP90) and the ubiquitin ligase-associated protein SGT1 [20,21]. HSP90 and SGT1 have been shown to be required for NLRP3, NOD1 and NOD2 activation and are predicted to also be required for NLRC4 [20,21].
Despite the many similarities between NLRC4 and other NLR proteins there are also striking differences. Activation of caspase-1 through an NLRC4 dependent pathway is closely associated with the subsequent death of the cell. However, stimuli that activate caspase-1 through NLRP3 predominantly result in the processing and secretion of IL-1β and IL-18 without necessarily resulting in cell death. Hence, NLRP3 and NLRC4 may play an important role in directing the final fate of caspase-1 activity either towards processing of proinflammatory cytokines or initiation of cell death pathways and may therefore add a layer of specificity to the innate immune response against specific subgroups of pathogens.
Many studies have demonstrated the importance of a potassium efflux in the activation of the NLRP3 inflammasome [22–24]. Activation of caspase-1 through NLRC4 however does not require potassium, further demonstrating the differences between these two pathways [25].
Bacterial activation of the NLRC4 inflammasome
NLRC4-mediated activation of caspase-1 has been shown to be important in host defense for a number of pathogens. Infection of macrophages with Salmonella, Shigella, Legionella, and Pseudomonas all lead to caspase-1 activation, release of IL-1β and rapid cell death. The first study to demonstrate NLRC4’s role in host defense examined the pathogen S. typhimurium [26]. NLRC4-deficient macrophages infected with S. typhimurium had a marked defect in their ability to activate caspase-1 and secrete IL-1β and IL-18. In addition, S. typhimurium-induced macrophage death was also retarded in NLRC4-deficient macrophages [26]. However, despite the dramatic in vitro effects of NLRC4-deficiency on S. typhimurium-induced macrophage caspase-1 activation, NLRC4-deficient mice infected orally with S. typhimurium did not display enhanced susceptibility to infection [27]. Caspase-1-deficient mice infected with S. typhimurium were however more susceptible to infection with S. typhimurium [27]. The in vivo difference seen between caspase-1-deficient mice and NLRC4-deficient mice may be due to additional undefined pathways that lead to Salmonella-induced caspase-1 activation that are not mediated through NLRC4.
Legionella pneumophila is also capable of mediating macrophage cell death through a caspase-1-dependent manner. The activation of caspase-1 by L. pneumophila has been demonstrated to be dependent on NLRC4 [28]. L. pneumophila is unique in that another NLR member, Naip5 (Birc1e), is also involved in susceptibility to infection with L. pneumophila. Naip5 and NLRC4 have been shown to physically interact linking Naip5 to the caspase-1 pathway [28], however the role of Naip5 in caspase-1 activation remains unclear as discussed below. NLRC4 has also been shown to play an important role in L. pneumophila mediated phagosome maturation. L. pneumophila-containing phagosomses avoid fusion with the lysosome in NLRC4- and caspase-1-deficient macrophages hence allowing bacterial replication [29]. These findings demonstrate a novel function for the NLRC4 inflammasome in response to intracellular bacteria.
S. flexneri has also been shown to activate caspase-1 in an NLRC4 dependent manner [30]. This intriguing study also shows that shigella-induced autophagy was increased in the absence of NLRC4 or caspase-1 and that autophagy may protect macrophages from caspase-1-mediated pyroptosis [30]. Finally, three recent studies have demonstrated a clear role for NLRC4 in host defense against P. aeruginosa [31–33]. NLRC4-deficient macrophages were markedly diminished in their ability to activate caspase-1 and secrete IL-1β in response to P. aeruginosa. Additionally, in both a pulmonary and a peritoneal in vivo model of P. aeruginosa infection NLRC4-deficient mice were more susceptible to infection [31,32]. Interestingly, a subset of P. aeruginosa strains that express the effector molecule ExoU were able to inhibit caspase-1 activation through ExoU phospholipase activity [32]. The P. aeruginosa effector molecule ExoS has also been shown to inhibit IL-1β maturation in a manner dependent on its ADP ribosyltransferase activity [34]. The fact that P. aeruginosa appears to have evolved mechanisms to inhibit NLRC4-mediated caspase-1 activation further suggests that this pathway is important for host defense against Gram-negative bacterial infections.
ASC and Naip5 in NLRC4 mediated caspase-1 activation
As NLRC4 can interact directly with pro-caspase-1 through a CARD-CARD interaction, the role of the adaptor molecule ASC in NLRC4-mediated caspase-1 activation is unclear. In a number of infectious models including Salmonella, Shigella and Pseudomonas, ASC-deficient macrophages demonstrated defective caspase-1 activation and IL-1β secretion; however, these infected ASC-deficient macrophages still underwent cell death in response to infection with similar kinetics compared to WT macrophages [26,30,32]. These observations suggest that ASC is in fact crucial for NLRC4 mediated caspase-1 activation and IL-1β secretion but does not play a role in NLRC4-mediated cell death. As there is no detectable caspase-1 activation in ASC-deficient macrophages infected with Salmonella, Shigella or Pseudomonas, it remains to be determined the mechanism by which ASC-deficient macrophages are undergoing cell death.
Macrophages from A/J mice have a mutant Naip5 allele and are permissive for the intracellular growth of L. pneumophila. Given that caspase-1- and IPAF-deficient macrophages were also permissive for replication of L. pneumophila and that IPAF and Naip5 could physically interact, it was postulated that Naip5 played a role in the IPAF inflammasome pathway [28,35]. A number of studies have found that the restriction of Legionella growth is far more complex and requires IPAF-dependent activation of caspase-1 as well as Naip5 signaling [36,37]. Miao and colleagues also examined the role of Naip5 in caspase-1 activation and found that A/J (Naip5-deficient) macrophages were able to secrete IL-1β in response to infection with S. typhimurium, P. aeruginosa, and Listeria monocytogenes suggesting that Naip5 is dispensable for caspase-1 activation [33].
Does cytosolic flagellin trigger NLRC4-mediated caspase-1 activation?
Recent studies have begun to elucidate how the NLRC4-inflammasome is activated. One component that is critical for pathogens, such as Salmonella, Legionella, Shigella and Pseudomonas, to activate capase-1 is a functional bacterial type III (T3SS) or type IV (T4SS) secretion system [28,32,38,39]. The T3SS is a complex macromolecular structure that spans both bacterial membranes and includes a long needle-like structure through which effector molecules pass. Access of the effector molecules into the cytoplasm of the host cell requires disruption of the plasma membrane of the host cell by a proteinaceous pore called the translocon. L. pneumophila possesses a Dot-Icm T4SS that is structurally unrelated but functionally similar to the T3SS. Bacteria defective in T3SS or T4SS activity were also found to be unable to activate macrophage caspase-1. S. typhimurium and L. pneumophila strains deficient in flagellin are also defective in their ability to activate caspase-1 following macrophage infection [28,35,40–42]. In two independent studies, by the groups of Aderem and Núñez, delivery of purified flagellin into the macrophage cytosol by transfection was capable of activating caspase-1 in an NLRC4-dependent manner [41,42].
These findings led to the hypothesis that flagellin monomers gain entry into the cytosol of infected cells through T3SS or T4SS and lead to the activation of caspase-1 [41,42]. Recently Sun et al. demonstrated that the T3SS can in fact serve as a conduit for flagellin to pass into the cytosol of the infected cell (Figure 1) [43]. However, the activation of NLRC4 by cytosolic flagellin is not the complete story. The non-flagellated bacterium S. flexneri is capable of activating capase-1 in an NLRC4-dependent manner [18]. We have also demonstrated that the P. aeruginosa mutant PAKΔfliC, which is deficient in flagellin, is still capable of activating caspase-1 also in an NLRC4-dependent manner [32]. The groups of Aderem and Núñez did not observe caspase-1 activation using flagellin-deficient P. aeruginosa strains [31,33], but this may reflect differences in multiplicities of infection and infection times between these studies. Miao et al. showed that at a high multiplicity of infection flagellin-deficient S. typhimurium strains were still capable of inducing macrophage secretion of IL-1β [41]. These data suggest that the NLRC4 inflammasome can also be activated independently of flagellin (Figure 1). Although flagellin may augment NLRC4-mediated caspase-1 activation, the direct ligand for NLRC4, as for most of the NLR family members, remains to be determined. Identification of the ligand for NLRC4 will help resolve many of the above questions and will enhance our understanding of the specificity built into the innate immune system.
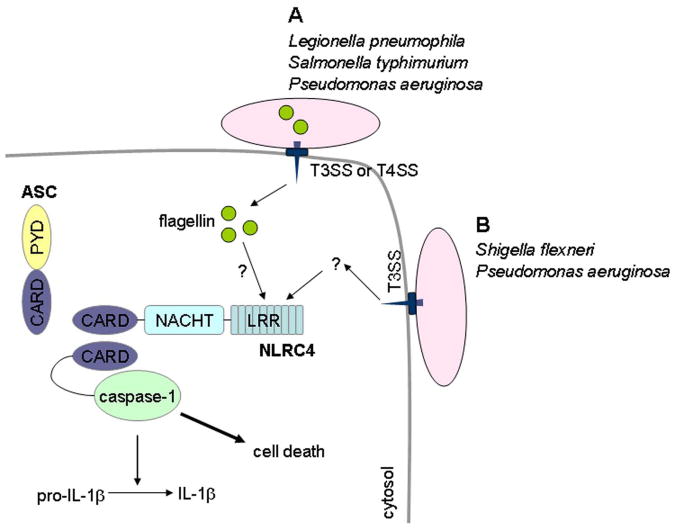
Figure 1.
Activation of the NLRC4 inflammasome by Gram negative bacteria. Activation of caspase-1 following infection of macrophages with S. typhimurium, P. aeruginosa, S. flexneri or L. pneumophila requires a functional type III or type IV secretion system. Infection causes NLRC4 to undergo a conformational change by an unknown mechanism, which allows NLRC4 to oligomerize. Following oligomerization, NLRC4 recruits pro-caspase-1 via homophilic CARD-CARD interactions, which leads to activation of caspase-1. ASC is required for NLRC4-mediated caspase-1 activation although its exact role remains unclear. (A) Bacterial-derived cytosolic flagellin augments caspase-1 activation following infection with L. pneumophila, S. typhimurium, and P. aeruginosa. (B) Activation of caspaspe-1 following infection of macrophages with S. flexneri and P. aeruginosa can occur independently of flagellin.
Acknowledgments
We thank Stephanie Eisenbarth for critical review of the manuscript. This work was supported by an Ellison Foundation grant to R.A.F. F.S.S. was supported by NIH grant K08 AI065517.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 2.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 3.Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ, Bridger S, van Deventer S, Forbes A, Nikolaus S, Lennard-Jones JE, Foelsch UR, Krawczak M, Lewis C, Schreiber S, Mathew CG. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 4.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Häfner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 5.Rosé CD, Doyle TM, McIlvain-Simpson G, Coffman JE, Rosenbaum JT, Davey MP, Martin TM. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol. 2005;32:373–375. [PubMed] [Google Scholar]
- 6.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, Nakajima M, Kobayashi H, Fujiwara I, Tsutsumi H, Utani A, Nishigori C, Heike T, Nakahata T, Miyachi Y. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-κB activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murdoch S, Djuric U, Mazhar B, Seoud M, Khan R, Kuick R, Bagga R, Kircheisen R, Ao A, Ratti B, Hanash S, Rouleau GA, Slim R. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet. 2006;38:300–302. doi: 10.1038/ng1740. [DOI] [PubMed] [Google Scholar]
- 11.Jéru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105:1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy N, Mahadevan MS, McLean M, Shutter G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, Salih M, Aubry H, Tamai K, Guan X, Ioannou P, Crawford TO, de Jong PJ, Surh L, Ikeda J-E, Korneluk RG, MacKenzie A. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 13.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 14.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 15.Rosenstiel P, Till A, Schreiber S. NOD-like receptors and human diseases. Microbes Infect. 2007;9:648–657. doi: 10.1016/j.micinf.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 17.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Núñez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR Gene Family: A Standard Nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 19.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–13. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 20.Mayor A, Martinon F, De Smedt T, Pétrilli V, Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Correia J, Miranda Y, Leonard N, Ulevitch R. SGT1 is essential for Nod1 activation. Proc Natl Acad Sci U S A. 2007;104:6764–6769. doi: 10.1073/pnas.0610926104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1β in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perregaux D, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 24.Hogquist KA, Unanue ER, Chaplin DD. Release of IL-1 from mononuclear phagocytes. J Immunol. 1991;147:2181–2186. [PubMed] [Google Scholar]
- 25.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 26.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 27.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galán JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 29.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozören N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Núñez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Núñez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Núñez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 32.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galle M, Schotte P, Haegman M, Wullaert A, Yang HJ, Jin S, Beyaert R. The Pseudomonas aeruginosa Type III secretion system plays a dual role in the regulation of Caspase-1 mediated IL-1β maturation. J Cell Mol Med. 2007 doi: 10.1111/j.1582-4934.2007.00190.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coers J, Vance RE, Fontana MF, Dietrich WF. Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol. 2007;9:2344–2357. doi: 10.1111/j.1462-5822.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 37.Lamkanfi M, Amer A, Kanneganti TD, Muñoz-Planillo R, Chen G, Vandenabeele P, Fortier A, Gros P, Núñez G. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol. 2007;178:8022–8027. doi: 10.4049/jimmunol.178.12.8022. [DOI] [PubMed] [Google Scholar]
- 38.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 40.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 42.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Núñez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 43.Sun YH, Rolán HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]