Abstract
Luminance signals mediated by the magnocellular (MC) pathway play an important role in vernier tasks. MC ganglion cells show a phase advance in their responses to sinusoidal stimuli with increasing contrast due to contrast gain control mechanisms. If the phase information in MC ganglion cell responses were utilized by central mechanisms in vernier tasks, one might expect systematic errors caused by the phase advance. This systematic error may contribute to the contrast paradox phenomenon, where vernier performance deteriorates, rather than improves, when only one of the target pair increases in contrast. Vernier psychometric functions for a pair of gratings of mismatched contrast were measured to seek such misestimation. In associated electrophysiological experiments, MC and parvocellular (PC) ganglion cells’ responses to similar stimuli were measured to provide a physiological reference. The psychophysical experiments show that a high-contrast grating is perceived as phase advanced in the drift direction compared to a low-contrast grating, especially at a high drift rate (8 Hz). The size of the phase advance was comparable to that seen in MC cells under similar stimulus conditions. These results are consistent with the MC pathway supporting vernier performance with achromatic gratings. The shifts in vernier psychometric functions were negligible for pairs of chromatic gratings under the conditions tested here, consistent with the lack of phase advance both in responses of PC ganglion cells and in frequency-doubled chromatic responses of MC ganglion cells.
Keywords: Contrast paradox, Vernier, Contrast gain, Magnocellular, Parvocellular
Introduction
Vernier performance improves as the contrast of a target pair (e.g., a pair of bars or gratings) increases (Bradley & Skottun, 1987; Krauskopf & Farell, 1991; Waugh & Levi, 1993). On the other hand, if the contrast of only one of the target pair increases and that of the other remains unchanged, vernier performance deteriorates rather than improves. This is called the contrast paradox (Stevenson & Cormack, 2000). The contrast paradox also occurs with stereo vision and apparent motion tasks (Halpern & Blake, 1988; Legge & Gu, 1989; Schor & Heckmann, 1989; Cormack et al., 1997). Stevenson and Cormack proposed that the contrast paradox is due to contrast mismatch in a single cortical filter. Here it is shown that for a vernier task with moving targets, a spatial mismatch due to phase shifts arising from magnocellular (MC) cells’ contrast gain control mechanism (Benardete et al., 1992; Yeh et al., 1995) can cause systematic errors in positional alignment, which may also contribute to the contrast paradox.
In primate retina, MC ganglion cells’ response amplitudes increase rapidly with contrast, but then saturates. This is thought to be due to a contrast gain control mechanism, also found in cat ganglion cells (Shapley & Victor, 1979a, 1979b), which causes the response phase of MC cells to advance as grating contrast increases. Parvocellular (PC) cells’ response amplitudes increase more slowly with stimulus contrast but show little saturation at high contrast levels (Benardete et al., 1992; Yeh et al., 1995). The response phase of PC cells remains constant with contrast.
Other studies have shown that signals from the MC pathway play an important role in vernier tasks (Rüttiger et al., 2002; Sun et al., 2003, 2004). If the phase information in MC ganglion cells’ responses is utilized by central mechanisms in such tasks, and if there is no specific cortical mechanism that corrects for such phase shifts, then systematic errors in positional coding due to the MC cell phase advance might be expected. To look for such systematic misestimation, vernier psychometric functions for pairs of drifting gratings were measured with method of constant stimuli. Any shifts of these functions for grating pairs of mismatched contrast might indicate a systematic misestimation due to spatial mismatch caused by phase differences in MC ganglion cells’ responses to the two targets. In associated electrophysiological experiments, MC and PC ganglion cells’ responses were measured with similar stimuli and the amount of phase advance with increasing stimulus contrast was estimated.
There was a clear shift in vernier psychometric curves for sinusoidal and square-wave luminance gratings with mismatched contrasts drifting at 8 Hz and a lesser shift at 2 Hz, both consistent with the high-contrast grating being perceived phase advanced compared to the low-contrast grating. The size of the phase advance was correlated to that seen in MC cells under similar stimulus conditions. The results are consistent with MC cells being responsible for vernier performance with luminance gratings. The shifts in vernier psychometric functions were negligible for pairs of chromatic gratings under the conditions tested, consistent with the lack of phase advance both in responses of PC ganglion cells and in frequency-doubled chromatic responses of MC cells.
Materials and methods
Psychophysics
Stimulus
Visual stimuli were generated via a VSG ViSaGe system (Cambridge Research Systems Ltd., Rochester, UK) and presented on a CRT monitor (LaCie Electron 22, frame rate 100 Hz) 0.878 m from the eye. The luminance and chromaticity of the monitor was calibrated with OptiCal Colorimeter (Cambridge Research Systems Ltd., Rochester, UK) and PR650 Spectra Colorimeter (Photo Research Inc., Chatsworth, MA). The vernier stimulus consisted of a pair of 0.8 cpd horizontal sinusoidal or square-wave gratings (5° × 25°) drifting randomly upward or downward at either 2 or 8 Hz. The grating pair was separated horizontally by a 30-inch gap. The stimulus was presented for a duration of 150 ms, ramped on and off with a 10-ms raised-cosine envelope to reduce any transient artifact. The gratings were either luminance gratings (60% vs. 30%, 30% vs. 15%, 30% vs. 30% Michelson contrast) or equiluminance chromatic gratings (12% vs. 6% M-cone contrast). The luminance and chromatic gratings were obtained by modulating the red and green guns either in-phase or out-of-phase of each other. The equiluminant point between the red and green guns were estimated with the minimum motion technique for each observer (Anstis & Cavanagh, 1983). Mean chromaticity of the stimulus and the background (30° × 25°) was (0.45, 0.47) in CIE x, y coordinates for a CIE standard observer (it varied slightly with the equiluminance setting); mean luminance of the stimulus and background was 31 cd/m2.
Observers
Two observers (HS, RCB) participated in the experiments. Both are authors and experienced psychophysical observers and both have normal color vision as assessed with Rayleigh anomaloscopy, Ishihara pseudoisochromatic plates, and Farnsworth-Munsell 100-Hue Test. Both observers had corrected-to-normal visual acuity. This study followed the tenets of the Declaration of Helsinki, and was approved by the Institutional Review Board of the SUNY State College of Optometry and South-Eastern Norway Regional Committee for Medical Research Ethics.
Procedure
Psychometric functions for vernier performance were measured using the method of constant stimuli. In each trial, a grating pair with a spatial phase shift was presented, and the observer indicated which grating appeared shifted upward. When grating a pair had mismatched contrast, the high contrast one was randomly presented to the left or right of the low contrast one. There were 11 phase shifts varying from −50° to +50° for each psychometric function and 20 trials at each phase shift. Two psychometric functions, one for upward drifting and one for downward drifting gratings, were measured simultaneously in one session. In different experimental sessions, the grating contrast, drift rate and grating type (sinusoidal vs. square-wave, luminance vs. chromatic) were varied. Each psychometric function was measured at least twice. For all experiments, the observer viewed the targets monocularly and foveally with the aid of a fixation point presented at the center of the stimuli.
Physiology
Stimulus
Visual stimuli were generated via a VSG 203 system (Cambridge Research Systems Ltd., Rochester, UK) and presented on a CRT monitor (Sony Trinitron GDM-F500, 150 Hz frame rate) 2.28 m away from the monkey. The stimulus was similar to that in the psychophysical experiments except it was a single horizontal grating drifting upward, and the spatial frequency was 0.2 cpd, to scale the stimulus appropriately for the parafoveal recording location. Other stimulus parameters were as in the psychophysical experiments.
Procedure
All procedures strictly conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the SUNY State College of Optometry Animal Care and Use Committee. The animals (M. fascicularis) were initially sedated with an intramuscular injection of ketamine (10 mg/kg). Anesthesia was induced with sodium thiopental (10 mg/kg), and maintained with inhaled isoflurane (0.2–2%) in a 70:30 N2O-O2 mixture. Local anesthetic was applied to points of surgical intervention. EEG and ECG were monitored continuously to ensure animal health and adequate depth of anesthesia. Muscle relaxation was maintained by an infusion of gallamine triethiodide (5 mg/kg/h i.v.) with accompanying dextrose Ringer solution (5 ml/kg/h). Body temperature was kept close to 37.5°C. End-tidal CO2 was kept close to 4% by adjusting the rate and depth of respiration.
Neuronal activity was recorded directly from retinal ganglion cells by an electrode inserted through a cannula entering the eye behind the limbus. The details of the preparation can be found elsewhere (Lee et al., 1989). The eccentricities of the cells recorded were between 4° and 12°. Cell identification was achieved through standard tests (Lee et al., 1989). PC cells can generally be identified by their tonic responses and spectral opponency, and MC cells by their phasic responses and lack of spectral opponency. For each cell, the locus of the receptive field center was determined and the stimulus was centered on this point. Times of spike occurrence were recorded to an accuracy of 0.1 ms, and averaged histograms of spike trains were simultaneously accumulated with 64 bins per cycle of modulation.
Results
Psychophysics
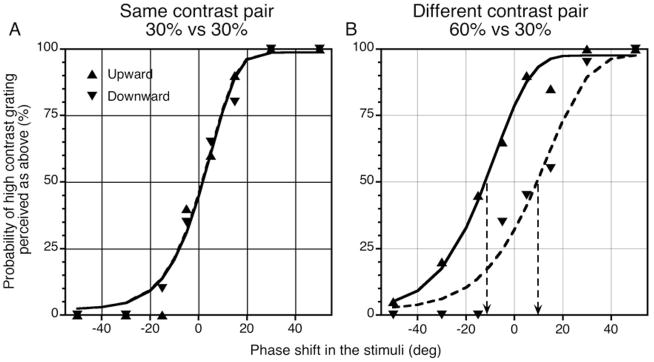
Fig. 1 shows psychometric functions for a pair of luminance sinusoidal gratings drifting at 8 Hz. The x-axis represents the phase shift between the two gratings: +20° phase shift means the high-contrast grating was shifted upward relative to the low-contrast grating (or the right grating was shifted upward relative to the left one when both have the same contrast), while −20° phase shift means the high-contrast grating was shifted downward relative to the low-contrast grating. The y-axis represents the probability of the high-contrast grating being perceived as shifted upward (or the right grating was perceived as shifted upward when both gratings have the same contrast). 100% means the high-contrast grating was always perceived as above the low-contrast grating; 0% means the high-contrast grating was always perceived as below the low-contrast grating; 50% means the two gratings were perceived as aligned.
Fig. 1.
Psychometric functions for a pair of luminance sinusoidal gratings drifting at 8 Hz for observer HS. Erected and downward triangles represent experimental data for upward and downward drifting conditions; solid and dashed curves represent Weibull fits. (A) The grating pair has the same contrast. Psychometric functions for upward and downward drifting overlap and the slopes of the curves are steep. (B) The grating pair has mismatched contrast. Psychometric functions for upward and downward drifting shift along the x-axis in opposite directions, and the slopes of the curves become shallower.
When both gratings had 30% contrast (Fig. 1A), the psychometric functions for upward and downward drifting gratings overlapped, and they intercepted the y-axis at 50% with 0° phase shift in the stimuli, which means the two gratings were perceived as aligned when physically aligned. When the contrast of one grating was doubled to 60% (Fig. 1B), the two psychometric functions were separated along the x-axis. The upward-drift psychometric curve intercepted the y-axis above 50%, which indicates that, when the two gratings were physically aligned, the higher contrast one was actually perceived as shifted upward, along the direction of the motion, relative to the low-contrast grating. The opposite is the case for the downward-drift psychometric curve. In both situations, the shifts in vernier psychometric functions are consistent with the high-contrast grating being perceived phase advanced in the drift direction compared to the low-contrast grating, in this case by ~ 10°, at 0° phase shift in the stimuli.
In addition to systematic phase shifts, the slope of the psychometric functions became shallower for grating pairs of mismatched contrast compared with those of the same contrast (the slope parameters for the Weibull function fits were 3.6 and 4.45 for mismatched and matched contrast conditions, respectively), which implies an increase in vernier threshold. This indicates that, although systematic phase misestimation may contribute to the contrast paradox, additional factors also contribute as suggested by Stevenson and Cormack (2000).
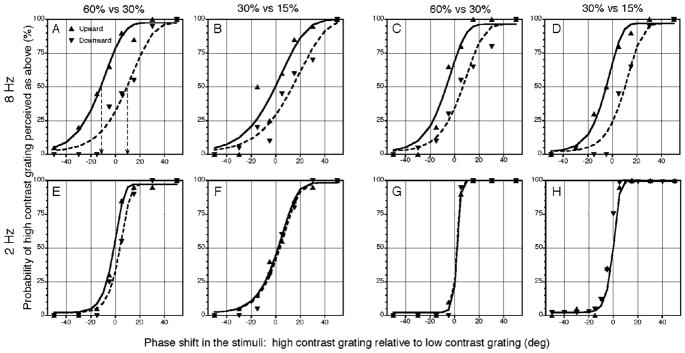
Other factors which may influence the phase shift seen in the psychometric curves, such as, drift rate, contrast, and grating type, were further explored in order to establish a link between the psychophysical shift and physiological data. Fig. 2 shows data for two types of luminance grating pairs with different contrast combinations at two drift rates. The top row represents data for sinusoidal or square-wave gratings drifting at 8 Hz, and the bottom row represents data for 2 Hz. The psychometric phase shift was smaller at 2 Hz and the psychometric curves were steeper, which implies a decrease in vernier threshold (Levi, 1996; Sun et al., 2004).
Fig. 2.
Psychometric functions for luminance grating pairs of different contrast combinations, drift speeds and grating types for observer HS. (A–D) Luminance sinusoidal or square-wave gratings at 8 Hz. There are clear phase shifts between the upward and downward conditions. (E–H)Luminance sinusoidal or square-wave gratings at 2 Hz. There is little or no significant phase shift between the upward and downward conditions. The format is the same as in Fig. 1.
The psychometric results for luminance gratings for two observers are summarized in Table 1, where the phase shifts were averaged for upward and downward drifting directions for each condition. Data from the two observers show similar patterns: clear phase shifts at 8 Hz and little phase shift at 2 Hz. Observer RCB, however, found the task too difficult to perform with 8 Hz sinusoidal gratings when the contrast was low (30% vs. 15%).
Table 1.
The amount of perceived phase shift between gratings of high and low contrasts estimated from the psychometric functions for observers HS and RCB in various stimulus conditions
| 8 Hz
|
2 Hz
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Grating Type | Sine | Square | Sine | Square | ||||
| Contrast combination (%) | 60–30 | 30–15 | 60–30 | 30–15 | 60–30 | 30–15 | 60–30 | 30–15 |
| Estimated Phase shift (deg) | ||||||||
| HS | −10.8 | −8.4 | −6.2 | −8.7 | −1.8 | 1.1 | 0.4 | −0.8 |
| RB | −9.3 | −5.2 | −8.9 | −0.8 | −0.8 | 1.2 | 0.2 | |
Psychometric functions using red-green equiluminant gratings were also measured. Vernier performance deteriorates markedly for red-green equiluminance gratings (Morgan & Aiba, 1985), and the task became even more difficult when the contrasts of the grating pair were mismatched. Neither observer could perform the task at 8 Hz. At 2 Hz, there was little shift between the upward and downward psychometric curves.
Physiology
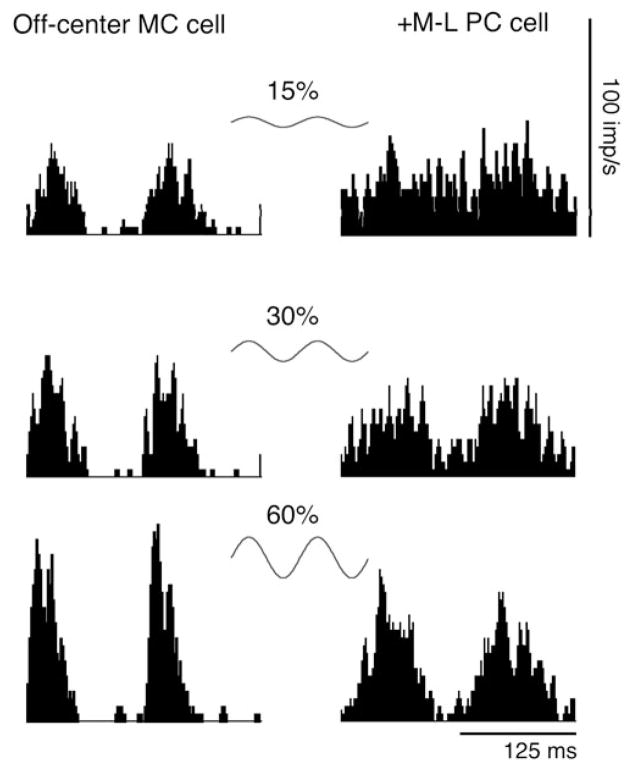
The phase shifts seen in psychometric functions were compared with the phase advance of MC ganglion cells with increasing contrast. MC and PC ganglion cells’ responses were measured with similar grating stimuli as in psychophysics. Fig. 3 shows histograms of a typical MC cell’s response to luminance gratings and a typical PC cell’s response to chromatic gratings. In both cases, the gratings were sinusoidal, drifting at 8 Hz, and the contrast varied from 15% to 60% (or 3% to 12% M-cone contrast for equiluminant gratings). Both the MC and the PC cell’s responses increased with contrast. The MC cell’s responses showed a clear phase advance with contrast with the peak response shifted toward to the left, while the PC cell’s responses showed negligible phase change with contrast.
Fig. 3.
Histograms for a typical MC cell’s responses to luminance gratings and a typical PC cell’s responses to chromatic gratings at three contrast levels (15% to 60% luminance contrast or 3% to 12% M-cone contrast). All gratings are drifted at 8 Hz. MC cell responses show phase advance as contrast increases, while PC cell responses show little phase change as contrast increases.
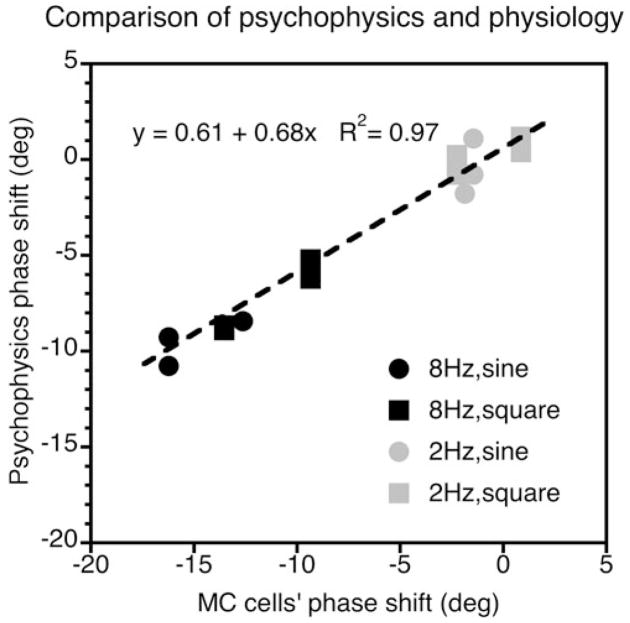
From the phase of the Fourier first harmonic, the amount of phase advance was calculated when grating contrast was doubled from 15% to 30% and from 30% to 60%. The averaged phase shifts for MC cells are plotted against the psychophysical data (of both observers) in Fig. 4. The pattern of MC cells’ phase shift is very similar to the psychophysical phase shift: it is prominent at 8 Hz for both sinusoidal and square-wave gratings and for both contrast conditions tested, and it is much reduced at 2 Hz. The correlation between psychophysical and physiological data is significant (R2 > 0.97, P < 0.001 for combined 8 and 2 Hz data; R2 > 0.90, P < 0.001 for 8 Hz data alone), with the phase differences for MC cells being somewhat larger.
Fig. 4.
Comparison of the MC cells’ phase shift with the psychophysical phase shift. The data can be fitted with a straight line of slope 0.68 indicting that the phase differences for the MC cells are larger than in the psychophysical measurements (some data points are occluded due to clustering).
The phase difference of PC cells’ responses to chromatic gratings of increasing contrast was also estimated. When chromatic contrast was doubled, the phase difference varied from −1.7° to 1.5° for the same stimulus conditions as mentioned above. The lack of phase difference was consistent with the lack of phase shift for chromatic psychometric curves at 2 Hz (not measurable at 8 Hz).
In the physiological experiment, the response phase of the ganglion cells was estimated using the response phases of the first harmonic. This may not be appropriate for square-wave gratings due to the presence of higher harmonic components. The cells’ response phases to square-wave gratings was therefore reanalyzed with a template-matching method (Rüttiger et al., 2002). These two methods gave very similar results.
Discussion
The psychophysical experiments show that high-contrast gratings are estimated as phase advanced in the drift direction compared to low-contrast gratings. The size of the phase advance was comparable to that seen in MC cells under similar stimulus conditions. The shifts in vernier psychometric functions were negligible for pairs of chromatic gratings at conditions tested here, consistent with the lack of phase advance in responses of PC ganglion cells (see below for frequency-doubled chromatic responses of MC ganglion cells).
The contrast paradox was originally reported in stereopsis (Halpern & Blake, 1988; Legge & Gu, 1989; Schor & Heckmann, 1989; Cormack et al., 1997). Stevenson and Cormack (2000) further extended the paradox into vernier and motion performance. They used the method of constant stimuli and measured psychometric functions for vernier, stereopsis and motion. A shallower slope of the psychometric curve indicated an increase in threshold. It is shown here that, with moving targets, there are not only changes in the slopes of the psychometric functions, but also systematic misestimation of grating location. It is uncertain how far this will apply with stationary targets and free viewing condition. No positional misestimation would be expected for briefly presented targets, but the size of phase misestimation with fixational eye movements and longer presentations remains uncertain. Since the direction of eye movements with regard to stationary targets is unknown, any phase shifts would be of unspecified direction and be indistinguishable from a change in psychometric function slope. There is, however, clearly a change in psychometric function slope as well as positional misestimation for moving vernier targets in our data, and the models presented by Stevenson and Cormack (2000) remain applicable.
The positional misestimation with luminance gratings was consistent with an origin in the phase shifts in MC ganglion cells’ responses. It is uncertain why the phase shifts measured psychophysically were smaller than those seen physiologically. The psychophysical measurements were foveal, and the physiological recordings parafoveal, but spatial frequency was scaled for 4° parafoveal presentation (Virsu & Rovamo, 1979) in the physiological experiments to compensate for this.
For chromatic gratings, there was little phase shift of psychometric functions at 2 Hz. Nonetheless the possibility that there might have been an effect at 8 Hz cannot be excluded, but the task was too difficult to be done. In any event, PC ganglion cells from old-world primates show little change in response phase with contrast at both 8 and 2 Hz. This is also the case for PC cells of the lateral geniculate nucleus (Benardete et al., 1992). MC cells gave frequency-double responses to chromatic stimuli which could provide positional information (Sun & Lee, 2004), although response amplitudes were much smaller than their luminance responses. Any phase advances of MC cells frequency-doubled chromatic responses with contrast were small and variable, and not statistically significant. Hence, for chromatic gratings, the lack of phase shift in the psychophysical experiment is consistent with the lack of phase advance both in responses of PC ganglion cells and in frequency-doubled chromatic responses of MC cells.
It is well-known that the perceived speed of a low-contrast grating is slowed compared to a grating of high contrast moving at the same velocity (Thompson, 1982; Stone & Thompson, 1992; Hawken et al., 1994; Smith & Derrington, 1996). It is tempting to postulate that the positional mismatch for gratings of mismatched contrasts is somehow related to the perceived speed change. The slowing of perceived speed, however, is present with successive presentation and is greatest for slowly moving targets. This would not be expected on the basis of the results presented here, and so the two phenomena are probably unrelated.
Acknowledgments
This work was supported by National Eye Institute grants EY 13112 to BBL.
References
- Anstis S, Cavanagh P. A minimum motion technique for judging equiluminance. In: Mollon JD, Sharpe LT, editors. Colour Vision Physiology and Psychophysics. London: Academic Press; 1983. pp. 155–166. [Google Scholar]
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Visual Neuroscience. 1992;8:483–486. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- Bradley A, Skottun BC. Effects of contrast and spatial frequency on vernier acuity. Vision Research. 1987;27:1817–1824. doi: 10.1016/0042-6989(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Cormack LK, Stevenson SB, Landers DD. Interactions of spatial frequency and unequal monocular contrasts in stereopsis. Perception. 1997;26:1121–1136. doi: 10.1068/p261121. [DOI] [PubMed] [Google Scholar]
- Halpern DL, Blake RR. How contrast affects stereoacuity. Perception. 1988;17:483–495. doi: 10.1068/p170483. [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Gegenfurtner KR, Tang C. Contrast dependence of colour and luminance motion mechanisms in human vision. Nature. 1994;367:268–270. doi: 10.1038/367268a0. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Farell B. Vernier acuity: Effects of chromatic content, blur and contrast. Vision Research. 1991;31:735–749. doi: 10.1016/0042-6989(91)90012-t. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. Journal of Physiology. 1989;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Gu YC. Stereopsis and contrast. Vision Research. 1989;29:989–1004. doi: 10.1016/0042-6989(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Levi DM. Pattern perception at high velocities. Current Biology. 1996;6:1020–1024. doi: 10.1016/s0960-9822(02)00647-4. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Aiba TS. Positional acuity with chromatic stimuli. Vision Research. 1985;25:689–695. doi: 10.1016/0042-6989(85)90175-0. [DOI] [PubMed] [Google Scholar]
- Rüttiger L, Lee BB, Sun H. Transient cells can be neurometrically sustained: The positional accuracy of retinal signals to moving targets. Journal of Vision. 2002;2:232–242. doi: 10.1167/2.3.3. [DOI] [PubMed] [Google Scholar]
- Schor C, Heckmann T. Interocular differences in contrast and spatial frequency: Effects on stereopsis and fusion. Vision Research. 1989;29:837–847. doi: 10.1016/0042-6989(89)90095-3. [DOI] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. Nonlinear spatial summation and the contrast gain control of cat retinal ganglion cells. Journal of Physiology. 1979a;290:141–160. doi: 10.1113/jphysiol.1979.sp012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. The contrast gain control of the cat retina. Vision Research. 1979b;19:431–434. doi: 10.1016/0042-6989(79)90109-3. [DOI] [PubMed] [Google Scholar]
- Smith DR, Derrington AM. What is the denominator for contrast normalisation? Vision Research. 1996;36:3759–3766. doi: 10.1016/0042-6989(96)00100-9. [DOI] [PubMed] [Google Scholar]
- Stevenson SB, Cormack LK. A contrast paradox in stereopsis, motion detection, and vernier acuity. Vision Research. 2000;40:2881–2884. doi: 10.1016/s0042-6989(00)00164-4. [DOI] [PubMed] [Google Scholar]
- Stone LS, Thompson P. Human speed perception is contrast dependent. Vision Research. 1992;32:1535–1549. doi: 10.1016/0042-6989(92)90209-2. [DOI] [PubMed] [Google Scholar]
- Sun H, Lee BB. A single mechanism for both luminance and chromatic grating vernier tasks: Evidence from temporal summation. Visual Neuroscience. 2004;21:315–320. doi: 10.1017/s0952523804213232. [DOI] [PubMed] [Google Scholar]
- Sun H, Lee BB, Rüttiger L. Coding of position of achromatic and chromatic edges by retinal ganglion cells. In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and Defective Colour Vision. Oxford: Oxford University Press; 2003. pp. 79–87. [Google Scholar]
- Sun H, Rüttiger L, Lee BB. The spatiotemporal precision of ganglion cell signals: A comparison of physiological and psychophysical performance with moving gratings. Vision Research. 2004;44:19–33. doi: 10.1016/j.visres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Thompson P. Perceived rate of movement depends on contrast. Vision Research. 1982;22:377–380. doi: 10.1016/0042-6989(82)90153-5. [DOI] [PubMed] [Google Scholar]
- Virsu V, Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Experimental Brain Research. 1979;37:475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Waugh SJ, Levi DM. Visibility, luminance and vernier acuity. Vision Research. 1993;33:527–538. doi: 10.1016/0042-6989(93)90256-v. [DOI] [PubMed] [Google Scholar]
- Yeh T, Lee BB, Kremers J. The temporal response of ganglion cells of the macaque retina to cone-specific modulation. Journal of the Optical Society of America A. 1995;12:456–464. doi: 10.1364/josaa.12.000456. [DOI] [PubMed] [Google Scholar]