Abstract
Voltage gated proton channels and NADPH oxidase function cooperatively in phagocytes during the respiratory burst, when reactive oxygen species are produced to kill microbial invaders. Although these molecules are distinct entities, with no proven physical interaction, their presence and activity in many cells appears to be coordinated. We describe these interactions and discuss several types of mechanisms that might explain them.
Keywords: phagocyte, respiratory burst, pH, ion channels, gating, leukocytes
Voltage gated proton channels
Voltage gated proton channels are proton selective ion channels with several unusual properties [1]. They are perfectly selective for protons and have single-channel currents in the low femtoampere range [2], which is ∼103 smaller than most ion channels. Although they open preferentially with membrane depolarization, their voltage dependence is not absolute, but instead is strongly modulated by pHo and pHi. Increasing the pH gradient, ΔpH (pHo – pHi) by one unit shifts the gH-V relationship by 40 mV to more negative voltages [3]. This elaborate pH sensitive gating mechanism results in the channel opening when there is an outward electrochemical driving force for protons, so that opening proton channels results in acid extrusion from cells. The most potent inhibitor of proton channels is Zn2+ [4], although other polyvalent cations are also effective [5]. Zinc slows channel opening and shifts the gH-V relationship positively, by binding competitively with protons at two His residues that are accessible to the external solution [6], recently identified as His140 and His193 in the human channel [7].
In 2006, proton channel genes were identified in human [7], mouse, and Ciona intestinalis [8]. Exogenously expressed murine and human proton channels closely resemble native proton channels, but activate at ∼30 mV more negative voltages, for reasons that remain unknown [9]. The proton channel molecule exhibits surprising similarity to the first four membrane-spanning regions, S1-S4, of other voltage-gated ion channels [7,8]. In other channels, S1-S4 comprise the putative voltage sensor, whereas the S5-S6 domains from each of the four monomers form a single central pore where ion conduction occurs. The proton channel lacks S5-S6 and hence has no obvious pore. Recent evidence indicates that the proton channel exists as a dimer, not a tetramer like many other channels, and that each monomer has a separate conduction pathway [10-12].
Proton channel knockout mice have been generated, but a full phenotypic description has not yet been published. Impaired reactive oxygen species (ROS) production by B lymphocytes from knockout mice has been observed [13], which supports the proposed role for proton channels during NADPH oxidase activity (described next).
NADPH oxidase
NADPH oxidase is a multi-component enzyme complex that has been studied most extensively in phagocytes (neutrophils, eosinophils, and macrophages), but which is present in a variety of isoforms in many other cells. NADPH oxidase activity is much greater in phagocytes, where it is thought to facilitate the killing of microbial invaders [14-17]. In numerous other cells, ROS produced by various NADPH oxidase isoforms act as signaling molecules, and consequently do not need to be produced at such high levels [18-20]. The components of NADPH oxidase (in this review, we discuss only the phagocyte isoform, Nox2) are physically separated in resting cells, and assemble upon stimulation by a variety of agonists [21,22]. Because the phorbol ester PMA (phorbol myristate acetate) appears to activate NADPH oxidase maximally and in nearly every cell, this artificial agonist is used widely; the PMA response is a standard by which other (and more physiological) agonists can be evaluated [23].
The consequences of genetic elimination of NADPH oxidase activity have been explored in humans afflicted with the rare chronic granulomatous disease (CGD) [24,25]. CGD results from any of several hundred known mutations of the genes that encode essential components of the NADPH oxidase complex [26,27]. Humans with CGD are susceptible to recurrent infections, especially with Staphylococcus aureus, Aspergillus sp., Klebsiella sp., and Escherichia coli [25,26]. Without medical treatment, CGD patients often die in early childhood of chronic, recurrent infections [26], hence the name “fatal granulomatous disease of childhood” [25]. Models for CGD include knockout cell lines [28] and knockout mice [29,30].
What is the basis of the symbiotic relationship between NADPH oxidase and voltage gated proton channels in phagocytes?
The existence of proton channels in human neutrophils was first proposed by Henderson, Chappell, and Jones [31] on the basis of pH and membrane potential changes observed during the PMA response. This seminal study (a) identified the electrogenic nature of NADPH oxidase activity, (b) proposed that the observed electrogenic proton efflux compensated this charge movement, and (c) speculated that the pathway might resemble the proton channels described earlier in snail neurons by Thomas & Meech [32]. All three conclusions have proven to be correct! The electrogenic nature of NADPH oxidase activity is directly observable as electron current [33,34]. Demonstration that Cd2+ or Zn2+ inhibited both H+ efflux and NADPH oxidase activity [35] established the necessity for proton current to compensate charge. Proton currents were subsequently identified by voltage-clamp in human neutrophils [36] and other leukocytes [37-42], and in each cell type are present at levels that could easily compensate for the charge translocation by NADPH oxidase (Fig. 11 in Ref. 43; Fig. 26 in Ref. 1).
The hypothesis that proton channels compensate charge for NADPH oxidase requires not only kinetic competence, but also that both proton channels and NADPH oxidase become activated together in a coordinated manner. Under normal physiological conditions, the threshold voltage for opening proton channels is somewhat positive to 0 mV, whereas neutrophils (as well as most other cells) appear to have a rather more negative resting membrane potential, with a dozen estimates ranging from -100 mV to -27 mV (p. 541, Ref. 1). That NADPH oxidase activity itself causes profound depolarization ameliorates this dilemma considerably. Nevertheless, demonstration that depolarization reaches +50 or +60 mV in activated human neutrophils [44-46], clearly sufficient to turn on the proton conductance, gH, provided welcome support for the hypothesis. The main mechanisms that result in coordinated activity of proton channels and NADPH oxidase are described in the next section.
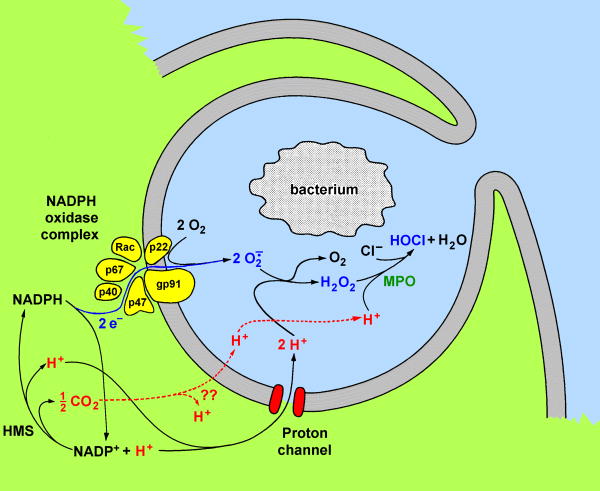
Beyond charge compensation, the intracellular proton concentration, pHi, provides another functional link between proton channels and NADPH oxidase. Oxidation of NADPH releases one proton into the cytoplasm (Fig. 1). Resynthesis of NADPH from the product NADP also generates one intracellular proton. NADPH synthesis must occur continuously in phagocytes; otherwise the entire cytoplasmic NADPH content in eosinophils would be consumed in ∼0.1-0.2 s [47]. Thus, each of the two electrons that leave the cell per NADPH molecule consumed effectively leaves one proton behind in the cytoplasm. These protons must be extruded not only for charge compensation, but also to prevent a drop in pHi. NADPH oxidase activity in phagocytes generates large quantities of cytoplasmic protons [48]. In an eosinophil during the respiratory burst, assuming a buffering capacity of 50 mM, pHi would decrease by ∼0.3 units/minute if there were no proton extrusion (p. 542, Ref. 1). NADPH oxidase activity is so great in phagocytosing neutrophils that no more than ∼5% could be compensated by ions other than H+ without leading to implausible pH changes [49]. Correspondingly, estimated K+ flux [50] is roughly of the magnitude predicted by this analysis.
Figure 1.
Involvement of proton channels in the phagocyte respiratory burst. Phosphorylation enhances the opening of proton channels and phosphorylation of cytosolic subunits results in assembly of the NADPH oxidase complex. Each NADPH molecule donates two electrons, which cross the membrane through a redox pathway contained within gp91phox and reduce two O2 molecules to superoxide anion, O2·-. Two protons are effectively left behind. Electron flux depolarizes the membrane, opening proton channels, which extrude the bulk of the protons. (From Ref. 49).
How is the activation of proton channels and NADPH oxidase coordinated?
At least two obvious mechanisms help to coordinate the activity of proton channels and NADPH oxidase. First, to some extent, the fact that the same agonists appear to activate both NADPH oxidase and proton channels inevitably means that their activity will appear to be coordinated. Second, in intact phagocytes (as opposed to voltage-clamped cells): rapid and profound depolarization occurs almost immediately upon activation of even a small fraction of the available NADPH oxidase complexes [46,49] and depolarization directly opens proton channels in phagocytes.
The term “activation” applied to proton channels has two distinct meanings. Since Hodgkin and Huxley [51], the traditional term for the opening of any ion channel has been “activation;” channel closing is called “deactivation,” and closing into a refractory state is called “inactivation.” This terminology seems reasonable, because the main point of an ion channel is to open and conduct ions across the membrane; voltage-gated channels are opened or activated by voltage. The conversion of any molecule into a state in which it performs its duties is called activation. However, the voltage gated proton channel has the unusual property of existing in two profoundly different operational states, or “gating modes.” In resting cells, proton channels have predictable behavior, but in “activated” phagocytes (meaning cells with NADPH oxidase turned on), proton channels exhibit very different gating kinetics. Despite the strong temptation to call proton channels in this gating mode “activated,” we will try to avoid confusion by using different terminology, and will refer to these proton channels as exhibiting “enhanced gating mode” behavior. The enhanced gating mode encompasses a constellation of four characteristic changes, each of which increases the probability of a proton channel being open at any given voltage. Compared with resting mode, the maximum conductance increases (larger gH,max), channel opening during depolarizing pulses becomes faster (smaller τact), deactivation or channel closing upon repolarization becomes slower (larger τtail), and the entire conductance-voltage relationship (gH-V) shifts negatively. The main biological effect of enhanced gating during the respiratory burst is that less depolarization is necessary to elicit sufficient proton current to compensate for the electron flux through NADPH oxidase. Because depolarization impairs oxidase function [52], the smaller depolarization required when proton channel gating is enhanced improves the efficiency of NADPH oxidase by 18% [49].
Enhanced gating of proton channels appears to result from phosphorylation of the channel by PKC [53-55]. PKC also activates NADPH oxidase; phosphorylation of NADPH oxidase components, especially p47phox, triggers assembly of the complex [21,22,56]. After both molecules are activated, their activity can be reversed by PKC inhibition [54,57]. This observation suggests that ongoing phosphorylation is necessary for sustained activity of either molecule.
Paradoxical interactions
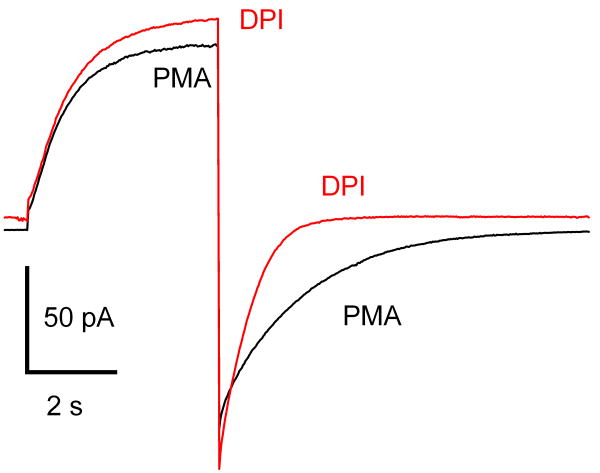
Some of the interactions between NADPH oxidase and proton channels seem to defy explanation, such as the phenomenon in Fig. 2. As mentioned above, tail current decay is profoundly slowed (larger τtail) in the enhanced gating mode. Inhibition of PKC by GFX or staurosporine partially reverses this slowing, along with the other enhanced gating properties [54], presumably by preventing phosphorylation of the proton channel. An astonishing result, however, is that inhibition of NADPH oxidase and its direct manifestation, electron current, by DPI (diphenylene iodonium), largely reversed the slowing of τtail [34]. DPI does not influence any of the other enhanced gating features. DPI has no effect on proton currents in unstimulated cells [34]; hence, its effects on τtail must be a consequence of the loss of NADPH oxidase activity.
Figure 2.
Inhibiting NADPH oxidase reverses the slowing of proton channel tail current decay that occurs in the enhanced gating mode. This human eosinophil was voltage-clamped in perforated-patch configuration and stimulated with PMA. Illustrated is the current during a pulse from -60 mV to +40 mV in the presence of PMA, and then after addition of DPI. DPI eliminated ∼6 pA of inward current at -60 mV, which indicates this was electron current generated by NADPH oxidase activity. The proton current activation kinetics and amplitude were not affected; the outward current was shifted upwards by the loss of electron current, which is inward up to +200 mV [52]. However, the tail current decay was greatly accelerated.
Certain observations can be interpreted to indicate that NADPH oxidase assembly may somehow facilitate the activation (or conversion to enhanced gating mode) of proton channels. Nanda et al. [58] found that the Zn2+ sensitive H+ efflux that occurs during the respiratory burst (i.e., NADPH oxidase activity) failed to occur in CGD patients lacking either gp91phox or p47phox and concluded that the activation of proton efflux could occur only in the presence of a functional oxidase complex. DPI, which inhibits NADPH oxidase activity, but does not impair assembly of the complex, did not prevent activation of proton efflux by PMA, thus oxidase activity is not required, consistent with an earlier study [59]. These measurements were made in the presence of high [K+]o with valinomycin to depolarize the cells to ∼0 mV and 3 μM DPI. However, 3 μM DPI might not have fully inhibited NADPH oxidase, and the activity of even a small fraction of NADPH oxidase is sufficient to produce strong depolarization [46,49]. Rada et al [46] showed that PMA still depolarized the membrane potential beyond 0 mV in the presence of 5 μM DPI that reduced O2·- production by >95%. Thus, the greater H+ efflux of normal cells may simply reflect residual NADPH oxidase activity in the presence of DPI. A further study revealed that CGD patients with a mutation resulting in normal expression levels of gp91phox (in its heterodimeric form of cytochrome b), but lacking enzymatic function, exhibited normal activation of H+ efflux [60]. Partial activation of H+ efflux was observed in patients whose mutation resulted in reduced assembly (low cytochrome b levels) or reduced stability of the complex (absence of p67phox). The authors proposed that proper assembly, but not function, of NADPH oxidase was required to activate proton efflux.
Another peculiar phenomenon that is evident in Table 1 is the apparent correlation between expression of NADPH oxidase in various cells and the ability of proton channels in these cells to respond to stimulation by PMA or other agonists. Proton channels in cells with a high level of NADPH oxidase activity exhibit profound enhancement of gating upon stimulation by PMA. At the other extreme, there is no convincing proton channel response to PMA in some cells with little or no detectable NADPH oxidase activity, such as HEK-293 cells transfected with Hv1 [9] or rat alveolar epithelial cells [34]. In human alveolar epithelial cells measurable H2O2 is produced by DUOX1, a relative of the phagocyte NADPH oxidase [61]; however, the production rate is 2-3 orders of magnitude lower than that for neutrophils. Intriguingly, cells that lack NADPH oxidase activity, but are related to phagocytes, including human basophils and CGD neutrophils with genetically dysfunctional NADPH oxidase, exhibit a clear PMA response, but one that is distinctly weaker than that of normal phagocytes. This intermediate response includes increased gH,max, faster activation, and a moderate negative shift of the gH-V relationship, but there is negligible slowing of τtail. So, without exception, a slowing of τtail is a universal feature of the proton channel response in cells with functional NADPH oxidase.
Table 1. NADPH Oxidase Activity and Proton Channel Gating Enhancement by PMA.
| Cell type | Ie (pA) | gH,max | ΔgH-V | τact | τtail | Reference |
|---|---|---|---|---|---|---|
| Human eosinophil | -6.0 | - | -42.6 | 4.2 | 5.4 | 68 |
| Human eosinophil | -5.9 | 3.9 | -36.6 | 3.4 | 3.1 | 53 |
| Human neutrophil | -2.3 | 1.9 | -38.8 | 3.7 | 5.5 | 34 |
| Mouse osteoclast | -8.4 | 2.7 | -35 | 2.4 | 1.9 | 69§ |
| Mouse granulocyte | -2.6 | 3.8 | -20 | 1.9 | 3.3 | 53 |
| Human monocyte | -1.4 | 3.6 | -28.3 | 4.4 | 4.0 | 75 |
| Same, with glucose | -4.1 | 2.7 | -32.5 | 2.3 | 3.5 | 75 |
| PLB-985 | -2.4 | 2.43 | -32 | 3.7 | 2.56 | 63 |
| PLBKO (no gp91phox) | 0 | 2.06 | -7.9 | 2.1 | 1.09 | 63 |
| CGD neutrophil | 0 | - | -13.3 | 1.9 | 1.15 | 63 |
| Human basophil | 0 | 2.86 | -19 | 5.04 | 1.32 | 56 |
| HEK-293 + HVCN1* | 0 | - | -5.5 | 1.38 | 1.21 | 9 |
| Rat alveolar epithelial | 0 | 1 | 0 | 1 | 1 | 34 |
Activity of NADPH oxidase is given as its equivalent electron current (Ie) in cells stimulated by PMA. In cells with measureable NADPH oxidase activity (Ie), PMA profoundly enhances proton channel opening. In leukocytes that lack Ie, there is a clear PMA response, but of distinctly smaller amplitude. In non-leukocytes that lack Ie, there is no little or no PMA response. The proton current parameters are expressed as the ratio of the maximal effect after PMA to the value before stimulation, except for τact which is the inverse of this ratio and ΔgH-V which is the shift of the gH-V relationship after PMA.
Values for Hv1-transfected HEK-293 cells include only cells that appeared to respond to PMA; a majority of cells did not respond; hence these values overstate any genuine effect.
In this study, pHi was not controlled and decreased during the PMA (>1 μM) response. Thus some of the effects reflect the lower pHi.
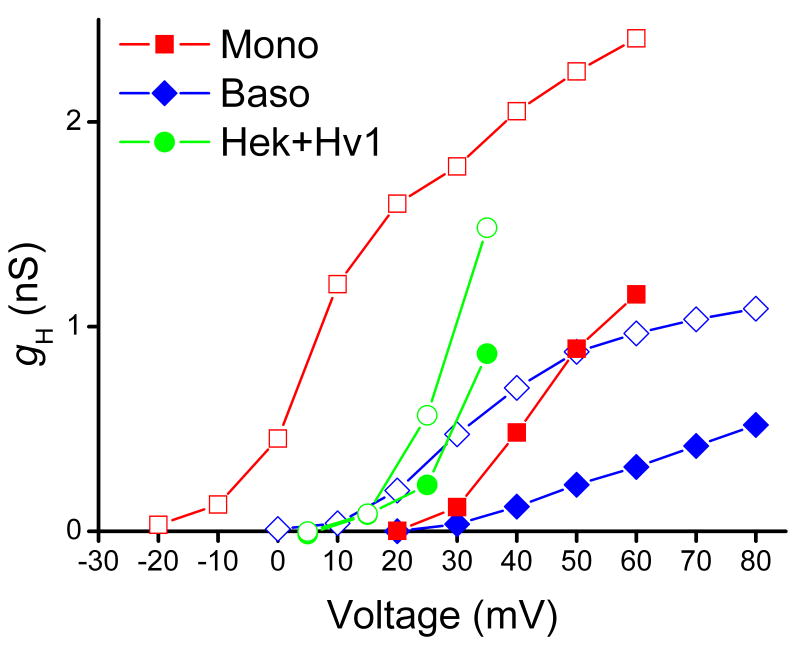
In Fig. 3, gH-V relationships are shown in three types of cells before and after stimulation with PMA. In the monocyte, representing professional phagocytes, the gH-V relationship is shifted by -40 mV. The basophil exhibits a distinct shift that is only half as large, -20 mV. In HEK-293 cells transfected with Hv1, there is at best a small response and in many cells there was no response [9].
Figure 3.
The effect of PMA on the gH-V relationship in a human monocyte (■, □), a human basophil (◆, ◇), and a HEK-293 cells transfected with the human proton channel gene, Hv1 (●, ○). In each case, the open symbol is after PMA. The gH values were calculated from the current at the end of 8-s pulses using the measured reversal potential.
Speculative explanations for the paradoxical interactions between proton channels and NADPH oxidase
The following is an attempt to list the types of interactions that might conceivably explain the phenomena described here.
Identity. If proton channels were part of the active NADPH oxidase complex, many interactions could be imagined. However, the idea that the gp91phox component of NADPH oxidase functions as a voltage gated proton channel [62,63] has been largely abandoned [1,64-67].
Direct physical interaction. Perhaps proton channels co-assemble with NADPH oxidase. Because there appear to be 1-2 orders of magnitude more active NADPH oxidase complexes in stimulated human neutrophils or eosinophils than proton channels (pp. 543-544, 549, Ref. 1), stoichiometric interaction is improbable. However, every proton channel could still conceivably be associated with an NADPH oxidase complex. Such co-assembly might occur only in the resting or the activated states of either or both molecules. If, as suggested by Koch et al [10], enhanced gating of proton channels results from interconversion between monomeric and dimeric assemblies of proton channels, one of these forms might preferentially bind to a component of NADPH oxidase.
Parallel regulation. A remarkable number of NADPH oxidase activators also enhance proton channel gating: PMA, AA (arachidonic acid), oleic acid, LTB4 (leukotriene B4), IL-5 (interleukin-5), fMLF (formyl-methionyl-leucyl-phenylalanine, a chemotactic peptide), and spontaneous activation presumably due to adherence [34,53,54,64,68-70]. One might conclude on teleological grounds that cells that need a high level of charge compensation would want to maximize proton channel gating enhancement; which has been estimated to improve the efficiency of NADPH oxidase by 15-20% in neutrophils and eosinophils [49]. Such cells might have evolved signaling pathways that coordinate the activity of both molecules. Although parallel signaling pathways may explain much, it is difficult to conceive how inhibiting electron current by DPI could be communicated to proton channels by this means.
-
A consequence of the activity of one molecule affects the other. In an attempt to explain the slower tail current decay when NADPH oxidase is active, we proposed that the intracellular generation of protons by NADPH oxidase activity lowered the local pH near proton channels [34]. According to a mechanism proposed for pH regulation of gating, lowering pHi stabilizes the open conformation of the proton channel [3], a logical manifestation of which is a slower channel closing (larger τtail). Calculations assuming that both molecules are randomly distributed in the plasma membrane predict an average distance too great for the proton channel to sense substantial local accumulation of protons (p. 553-554, Ref. 1). The proton accumulation hypothesis thus seems to require a special mechanism (e.g., nonrandom distribution in the membrane, rapid surface conduction of protons, etc.). Another complication is that if τtail is sensitive to local pH, why do the other enhanced parameters not change when τtail responds to changes in Ie, as in Fig. 2?
To generalize from this example, any other consequence of NADPH oxidase activity might modulate proton channel properties. NADPH oxidase tends to lower pHi, increase pHo (or phagosomal pH), depolarize the membrane, and produce enormous quantities of reactive oxygen species. In addition, intermediaries, such as Na+/H+ antiport activity associated with NADPH oxidase activation, might conceivably influence proton channels. With the exception of τtail, enhanced proton channel gating [70] or H+ efflux [58,59] still occurs in the presence of DPI.
The counterpart to this class of effect would be feedback of the effects of proton channel activity onto NADPH oxidase function. Intuitively, it seems that NADPH oxidase drives the respiratory burst and that proton channels respond to the resulting depolarization and pH changes, but it is clear that effects of proton channel activity will directly affect oxidase activity. One study even suggested that proton efflux might activate NADPH oxidase in renal medullary cells [71]. Proton channel activity certainly changes the pH on both sides of the membrane as well as the membrane potential. NADPH oxidase activity is sensitive to pHi, being optimal at pHi 7.5 and decreasing with changes in either direction [Ref. 47 and references therein]. Interestingly, pHi mainly regulates the number of active NADPH oxidase complexes, and only weakly influences the activity of assembled complexes [47,72]. The oxidase is also influenced by membrane potential, with profound inhibition by membrane depolarization [52]. The effect of membrane potential seems to be directly on the activity (rather than the assembly) of the NADPH oxidase complex [52].
Regulation of proton channels by NADPH oxidase assembly. Nanda et al [73] suggested that the oxidase could affect proton channel behavior by electrostatic interactions, provided that the two molecules were close together. “Multiple phosphorylation of p47phox and assembly of the oxidase in the vicinity of the channel may modify the inner surface potential, shifting the current-voltage relationship to more negative potentials.” In retrospect, by this mechanism, NADPH oxidase assembly might convert H+ channels into their enhanced gating mode. However, an analogous electrostatic mechanism could explain how phosphorylation of the proton channel itself could produce the characteristic negative shift of the gH-V relationship, without invoking proximity to NADPH oxidase. Petheő et al [74] concluded that assembly of NADPH oxidase was a prerequisite for enhanced gating of proton channels, and proposed that association of p47phox with membrane lipids sustains proton channel activity.
Some combination of the above. Most likely, several of the listed mechanisms contribute, which may explain the difficulty in understanding the precise nature of the relationship.
Conclusion
NADPH oxidase and voltage gated proton channels often coexist in the same cells, both are activated by phosphorylation, both produce rapid and profound effects on membrane potential and pH, and both are extremely sensitive to pH and membrane potential. Whether these common properties explain all of their interactions is not clear. However, in phagocytes at least, their destinies are inextricably linked.
Acknowledgments
This work was supported by the Schmidtmann Foundation (B.M.), by the Heart, Lung and Blood Institutes of Health (research grant HL61437 to T.D.) and by Philip Morris USA Inc. and Philip Morris International (T.D.).
Abbreviations
- CGD
chronic granulomatous disease
- DPI
diphenylene iodonium
- GFX
GF109203X
- gH
proton conductance
- gH,max
maximum gH
- gH-V
proton conductance-voltage relationship
- Ie
electron current
- O2·-
superoxide anion
- pHo
external pH
- pHi
internal pH
- ΔpH
pH gradient (pHo – pHi)
- PMA
Phorbol myristate acetate
- ROS
reactive oxygen species
- τact
activation time constant
- τtail
deactivation (tail current) time constant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Boris Musset, Email: Boris_Musset@rsh.net.
Vladimir V. Cherny, Email: vcherny@rush.edu.
Deri Morgan, Email: Deri_Morgan@rush.edu.
Thomas E. DeCoursey, Email: tdecours@rush.edu.
References
- 1.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 2.Cherny VV, Murphy R, Sokolov V, Levis RA, DeCoursey TE. Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and direct measurement. J Gen Physiol. 2003;121:615–28. doi: 10.1085/jgp.200308813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–96. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahaut-Smith M. The effect of zinc on calcium and hydrogen ion currents in intact snail neurones. J Exp Biol. 1989;145:455–64. doi: 10.1242/jeb.145.1.455. [DOI] [PubMed] [Google Scholar]
- 5.DeCoursey TE, Cherny VV. Pharmacology of voltage-gated proton channels. Curr Pharm Des. 2007;13:2406–20. doi: 10.2174/138161207781368675. [DOI] [PubMed] [Google Scholar]
- 6.Cherny VV, DeCoursey TE. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J Gen Physiol. 1999;114:819–38. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–6. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–92. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 9.Musset B, Cherny VV, Morgan D, Okamura Y, Ramsey IS, Clapham DE, DeCoursey TE. Detailed comparison of expressed and native voltage-gated proton channel currents. J Physiol. 2008;586:2477–86. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–6. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SY, Letts JA, MacKinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–56. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capasso M, Bhamrah M, Boyd RS, Cain K, Pulford K, Musset B, Cherny VV, Morgan D, DeCoursey TE, Gascoyne RD, Dyer MJS. The voltage-gated proton channel HVCN1 co-localizes with B cell receptor and is involved in class switch recombination. Blood. 2008 abstract, in press. [Google Scholar]
- 14.Babior BM. Oxygen-dependent microbial killing by phagocytes. New Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 15.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 16.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 17.Rada B, Hably C, Meczner A, Timár C, Lakatos G, Enyedi P, Ligeti E. Role of Nox2 in elimination of microorganisms. Semin Immunopathol. 2008;30:237–53. doi: 10.1007/s00281-008-0126-3. [DOI] [PubMed] [Google Scholar]
- 18.Buetler TM, Krauskopf A, Ruegg UT. Role of superoxide as a signaling molecule. News Physiol Sci. 2004;19:120–3. doi: 10.1152/nips.01514.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–47. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 21.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase components. J Leukocyte Biol. 1996;60:677–91. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 22.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeCoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–93. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berendes H, Bridges RA, Good RA. A fatal granulomatosus of childhood: the clinical study of a new syndrome. Minn Med. 1957;40:309–12. [PubMed] [Google Scholar]
- 25.Holmes B, Quie PG, Windhorst DB, Good RA. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966;1(7449):1225–8. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- 26.Dinauer MC, Newburger PE, Nauseef WM. Inherited Disorders of Phagocyte Killing. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th. Vol. 3. McGraw-Hill Inc.; New York: 2001. pp. 4857–4887. Chap. 189. [Google Scholar]
- 27.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–84. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhen L, King AA, Xiao Y, Chanock SJ, Orkin SH, Dinauer MC. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc Natl Acad Sci USA. 1993;90:9832–6. doi: 10.1073/pnas.90.21.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollock JD, Williams DA, Gifford MAC, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–9. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 30.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–8. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson LM, Chappell JB, Jones OTG. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987;246:325–9. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–8. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- 33.Schrenzel J, Serrander L, Bánfi B, Nüsse O, Fouyouzi R, Lew DP, Demaurex N, Krause KH. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–7. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 34.DeCoursey TE, Cherny VV, Zhou W, Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci USA. 2000;97:6885–9. doi: 10.1073/pnas.100047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson LM, Chappell JB, Jones OTG. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988;255:285–90. [PMC free article] [PubMed] [Google Scholar]
- 36.DeCoursey TE, Cherny VV. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys J. 1993;65:1590–8. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demaurex N, Grinstein S, Jaconi M, Schlegel W, Lew DP, Krause KH. Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J Physiol. 1993;466:329–44. [PMC free article] [PubMed] [Google Scholar]
- 38.Kapus A, Romanek R, Qu AY, Rotstein OD, Grinstein S. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J Gen Physiol. 1993;102:729–60. doi: 10.1085/jgp.102.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eder C, Fischer HG, Hadding U, Heinemann U. Properties of voltage-gated currents of microglia developed using macrophage colony-stimulating factor. Pflügers Arch. 1995;430:526–33. doi: 10.1007/BF00373889. [DOI] [PubMed] [Google Scholar]
- 40.Gordienko DV, Tare M, Parveen S, Fenech CJ, Robinson C, Bolton TB. Voltage-activated proton current in eosinophils from human blood. J Physiol. 1996;496:299–316. doi: 10.1113/jphysiol.1996.sp021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrenzel J, Lew DP, Krause KH. Proton currents in human eosinophils. Am J Physiol. 1996;271:C1861–71. doi: 10.1152/ajpcell.1996.271.6.C1861. [DOI] [PubMed] [Google Scholar]
- 42.Schilling T, Gratopp A, DeCoursey TE, Eder C. Voltage-activated proton currents in human lymphocytes. J Physiol. 2002;545:93–105. doi: 10.1113/jphysiol.2002.028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eder C, DeCoursey TE. Voltage-gated proton channels in microglia. Prog Neurobiol. 2001;64:277–305. doi: 10.1016/s0301-0082(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 44.Geiszt M, Kapus A, Nemet K, Farkas L, Ligeti E. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–8. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- 45.Jankowski A, Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. J Biol Chem. 1999;274:26098–104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 46.Rada BK, Geiszt M, Káldi K, Tímár C, Ligeti E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood. 2004;104:2947–53. doi: 10.1182/blood-2004-03-1005. [DOI] [PubMed] [Google Scholar]
- 47.Morgan D, Cherny VV, Murphy R, Katz BZ, DeCoursey TE. The pH dependence of NADPH oxidase in human eosinophils. J Physiol. 2005;569:419–31. doi: 10.1113/jphysiol.2005.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Zwieten R, Wever R, Hamers MN, Weening RS, Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981;68:310–3. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy R, DeCoursey TE. Charge compensation in phagocytes. Biochim Biophys Acta: Bioenergetics. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–7. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 51.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–44. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–4. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 53.Bankers-Fulbright JL, Kita H, Gleich GJ, O'Grady SM. Regulation of human eosinophil NADPH oxidase activity: a central role for PKCδ. J Cell Physiol. 2001;189:306–15. doi: 10.1002/jcp.10022. [DOI] [PubMed] [Google Scholar]
- 54.Morgan D, Cherny VV, Finnegan A, Bollinger J, Gelb MH, DeCoursey TE. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J Physiol. 2007;579:327–44. doi: 10.1113/jphysiol.2006.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musset B, Capasso M, Cherny VV, Morgan D, Dyer M, DeCoursey TE. Identification of phosphorylation sites that activate voltage gated proton channels in leukocytes. Biophys J. 2009 abstract, in press. [Google Scholar]
- 56.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–59. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musset B, Morgan D, Cherny VV, MacGlashan DW, Jr, Thomas LL, Ríos E, DeCoursey TE. A pH-stabilizing role of voltage gated proton channels in IgE-mediated activation of human basophils. Proc Natl Acad Sci USA. 2008;105:11020–5. doi: 10.1073/pnas.0800886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nanda A, Grinstein S, Curnutte JT. Abnormal activation of H+ conductance in NADPH oxidase-defective neutrophils. Proc Natl Acad Sci USA. 1993;908:760–4. doi: 10.1073/pnas.90.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapus A, Szászi K, Ligeti E. Phorbol 12-myristate 13-acetate activates an electrogenic H+-conducting pathway in the membrane of neutrophils. Biochem J. 1992;281:697–701. doi: 10.1042/bj2810697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nanda A, Curnutte JT, Grinstein S. Activation of H+ conductance in neutrophils requires assembly of components of the respiratory burst oxidase but not its redox function. J Clin Invest. 1994;93:1770–5. doi: 10.1172/JCI117162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1506–14. doi: 10.1152/ajplung.00029.2007. [DOI] [PubMed] [Google Scholar]
- 62.Henderson LM, Banting G, Chappell JB. The arachidonate-activable [sic], NADPH oxidase-associated H+ channel. Evidence that gp91-phox functions as an essential part of the channel. J Biol Chem. 1995;270:5909–16. [PubMed] [Google Scholar]
- 63.Bánfi B, Schrenzel J, Nüsse O, Lew DP, Ligeti E, Krause KH, Demaurex N. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med. 1999;190:183–94. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeCoursey TE, Cherny VV, Morgan D, Katz BZ, Dinauer MC. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J Biol Chem. 2001;276:36063–6. doi: 10.1074/jbc.C100352200. [DOI] [PubMed] [Google Scholar]
- 65.Morgan D, Cherny VV, Price MO, Dinauer MC, DeCoursey TE. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J Gen Physiol. 2002;119:571–580. doi: 10.1085/jgp.20018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Touret N, Grinstein S. Voltage-gated proton “channels”: a spectator's viewpoint. J Gen Physiol. 2002;120:767–71. doi: 10.1085/jgp.20028706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–61. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 68.DeCoursey TE, Cherny VV, DeCoursey AG, Xu W, Thomas LL. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J Physiol. 2001;535:767–81. doi: 10.1111/j.1469-7793.2001.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cherny VV, Henderson LM, Xu W, Thomas LL, DeCoursey TE. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J Physiol. 2001;535:783–94. doi: 10.1111/j.1469-7793.2001.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M. Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Miner Res. 2003;18:2069–76. doi: 10.1359/jbmr.2003.18.11.2069. [DOI] [PubMed] [Google Scholar]
- 71.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol. 2005;289:F1048–56. doi: 10.1152/ajprenal.00416.2004. [DOI] [PubMed] [Google Scholar]
- 72.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979;53:1133–9. [PubMed] [Google Scholar]
- 73.Nanda A, Romanek R, Curnutte JT, Grinstein S. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J Biol Chem. 1994;269:27280–5. [PubMed] [Google Scholar]
- 74.Petheő GL, Girardin NC, Goossens N, Molnár GZ, Demaurex N. Role of nucleotides and phosphoinositides in the stability of electron and proton currents associated with the phagocytic NADPH oxidase. Biochem J. 2006;400:431–8. doi: 10.1042/BJ20060578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Musset B, Cherny VV, DeCoursey TE. Electron current and proton current in human monocytes - glucose dependence of electron current. Biophys J. 2009 doi: 10.1152/ajpcell.00335.2011. abstract, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]