Abstract
Zinc sensitive fluorescent probes have become increasingly important in the investigation of the cellular roles of zinc. There is however little information on how the other transition metals in cells may influence zinc measurements. We have characterized in-vitro the interaction of the nominal zinc indicators, FluoZin-3 and Newport Green with all the cationic transition metals found within cells, Cr, Mn, Fe, Co and Cu, as well as Ni and Cd, by measuring their dissociation constants. In addition, we have shown how FluoZin-3 can be used to quantify the concentration of copper in a cell-free assay and report that the fluorescence of NPG is boosted by both Cu(I) and Fe(II). Furthermore, we have introduced diagnostics for detecting the interference of metals other than zinc with its measurement within cells.
Introduction
Metal ions are cofactors in many biological processes, acting as catalytic centers in some proteins and as structural factors in others [1; 2]. In order for a metal ion to be incorporated into a protein, the ion has to be transported from the cytoplasm to a site within the protein. In the case of copper, the ion is ferried by specific copper chaperones to the appropriate protein and the levels of the free hydrated-metal are kept vanishingly low [3]. In other cases (e.g. zinc [4] and iron [5; 6]) the metal may be drawn from a pool of chelatable (or ‘free’ metal). To estimate the size of the chelatable pool, fluorimetric probes are typically used that change their fluorescence quantum yield upon metal binding. A large number of fluorescent zinc indicators have been synthesized [7; 8], which are best described as nominal zinc-sensors since they invariably bind other transition metals.
Fluorescent probes provide the only known means for measuring both the temporal and spatial dynamics of free ion concentrations within live cells [9]. Most other analytical techniques are only capable of assessing the total metal concentration, oftentimes only in fixed tissue. While electrochemical methods (volttammetry or ion-sensitive electrodes) can sample free ion levels, they can only do so at a single point and are difficult to deploy intracellularly.
In general the order of prevalence of transition metals in mammalian cells is Fe≈Zn>Cu>Mn>Co≈Cr, with ~3000 times less Co than zinc [10; 11]. In this manuscript we have determined the likely interference of these biological transition metals with two widely used zinc probes, FluoZin-3 [12] and Newport Green (NPG) [13]. Our approach was to characterize the affinity of these probes for biological transition metals and then to use chelators to determine which metals might interferwith the detection of zinc.
No synthetic chelators are known that exclusively bind a single transition metal [14]. In chelators with open architectures similar to EDTA the strength of the ligand-metal interaction typically follows the Irving-Williams (IW) series: Mn(II)<Fe(II)<Co(II)<Ni(II)<Cu(II) >Zn(II) [1]. Note that the series is written in the order of elements in the periodic table and Cu(II) has the highest affinity. Since fluorimetric zinc probes are based on such chelators (BAPTA [15] in the case of FluoZin-3 and di-(2-picolyl)amine (DPA) for NPG) this restricts their specificity. To anticipate how biological metals might perturb measurements of zinc in cellular systems we have characterized the broader metal responsiveness of FluoZin-3 and NPG.
Most fluorimetric metal indicators signal metal chelation by the bound metal suppressing an electron transfer in the excited state which in the metals absence quenches the fluorescence of the fluorophore [16]. The activation of the probe is essentially the inhibition of an intrinsic quenching mechanism. In some cases the binding of a metal with unpaired electrons further quenches the intrinsic fluorescence of the probe. However, with the exception of bathocuproine disulphonate (BCS) and Cu(II)-NPG, in all the cases discussed below, all of the metals tested reduce the fluorescence by displacing zinc from the indicator, but do not quench the intrinsic fluorescence. We shall refer to this as ‘squelching’ and reserve ‘quenching’ for cases where the intrinsic fluorescence is diminished. If the level of fluorescence in the presence of a strong metal chelator is equal to that in the presence of a metal that decreases the fluorescence, we can be confident that the metal is instead squelching the fluorescence.
Materials and methods
Fluorimetry
Excitation-emission spectra and fluorescent time-courses were determined on a Hitachi F-4500 spectrofluorimeter using a stirred methacrylate cuvette (Fisher Scientific) whose temperatures was controlled by a circulating water bath at 26 °C. The monochromator slit widths were set at 5 nm and data was sampled at 1 Hz. All experiments were performed in a Hepes buffered saline containing (in mM): 140 NaCl, 2.5 KCl and 10 Hepes (pH 7.4) with FluoZin-3 at a concentration of 500 nM unless otherwise specified. Deionized water was prepared by a Barnstead Nanopure system and the precautions detailed in [17] were observed to avoid metal contamination.
Cu(I) was added in the form of [Cu(I)(CH3CN)4]PF6 in acetonitrile [18], purging oxygen from the solution by the addition of 1.5 % (w/v) Na2SO3.
At a concentration of 10 μM ascorbate had no effect on the fluorescence of NPG or FluoZin-3 in the presence or absence of zinc. The reducing agent disulfite (2.5%) diminished the fluorescence of both NPG and FluoZin-3 by ~40% in the presence of saturating zinc levels.
Bathocuproine disulfonate (BCS) has an excitation peak at 290 nm and two emission peaks at 780 and 402 nm. It has previously been reported that BCS has an excitation peak at 580nm [19], however we found that this apparent peak originates from light at ~ 290nm that escapes from the monochromator by second-order diffraction. To demonstrate this we used a long-pass filter with a cutoff at 380nm (Hoya) in the excitation pathway.
The excitation and emission wavelengths used were as follows: BCS (ex 289nm, em 403nm), FluoZin-3 (ex 494 nm, em 518 nm) and Newport Green (ex 506nm, em 535 nm).
To estimate the approximate dissociation constants between a metal (X) and a fluorimetric probe (F) we considered the following two equilibria:
| (1) |
| (2) |
Two methods were used:
-
For a metal that increases the fluorescence of the probe by more than ~10% the following equation, which is an exact analytical relationship derived from the mass-action equation for the formation of a 1:1 complex between probe and metal, was used to estimate the dissociation constant, Kd.X [20]:
(3) Where f is the measured fluorescence intensity in the presence of metal, fmax the fluorescence in the presence of saturating metal and fmin the fluorescence in the absence of metal. In all cases a low concentration (500 nM) of probe was used and we assumed that the free metal concentration was equal to the added metal concentration.
- For cases where the metal, X, squelches the fluorescence of the probe, a competitive titration method was used. Excess zinc (2 μM) was added to 500nM of the probe and the metal, X was titrated on. The following relationship was used to estimate the dissociation constant with the assumption that Kd.Zn = 15 nM [12]:
(4)
Reagents
CdCl2, CoCl2, CrNO3, CuSO4, Cu(I)(CH3CN)4]PF6, FeNO3, FeSO4, MnCl2, and NiCl2, (Aldrich). All metals used had contaminating metals < 5mg/Kg.
BCS, EDTA and N,N,N’,N’-Tetrakis-(2-pyridylmethyl)-Ethylenediamine (TPEN) (Fluka). Hepes (Calbiochem), FluoZin-3 and Newport Green (Molecular Probes).
The following prepared solutions were used: 1 M CaCl2 (Fluka, 2115), 1 M MgCl2 (Sigma, M-1028) and 51 mM ZnSO4 (Aldrich, 31,962-7). All other reagents were from Sigma.
RESULTS
We first provide a detailed characterization of the interactions of copper in both its biologically relevant oxidation states with the free-acid forms of FluoZin-3 and NPG since according to the IW series it is the ion that is most likely to have the strongest interactions with the indicators.
Cu(II)
FluoZin-3 has a single excitation peak at 494 nm and an emission peak at 518 nm, neither of which shift on the addition zinc or any other biological transition metals. Addition of Cu(II) leads to a diminution of the fluorescence, that results from the displacement of trace amounts of zinc from the indicator. This can be seen in Fig. 1 a where the addition of copper (500 nM) diminishes the fluorescence of FluoZin-3 and then the addition of a higher concentration of zinc (50 μM) displaces copper restoring the fluorescence. The initial fluorescence of the probe originates largely from contaminating metal and to lesser degree from the intrinsic fluorescence of the probe. The fluorescence of a given concentration of FluoZin-3 without exogenous zinc has the same fluorescence when saturated with Cu(II) or Cu(I) and in the presence of EDTA. Hence copper squelches the fluorescence of FluoZin-3 (see above).
Fig. 1.
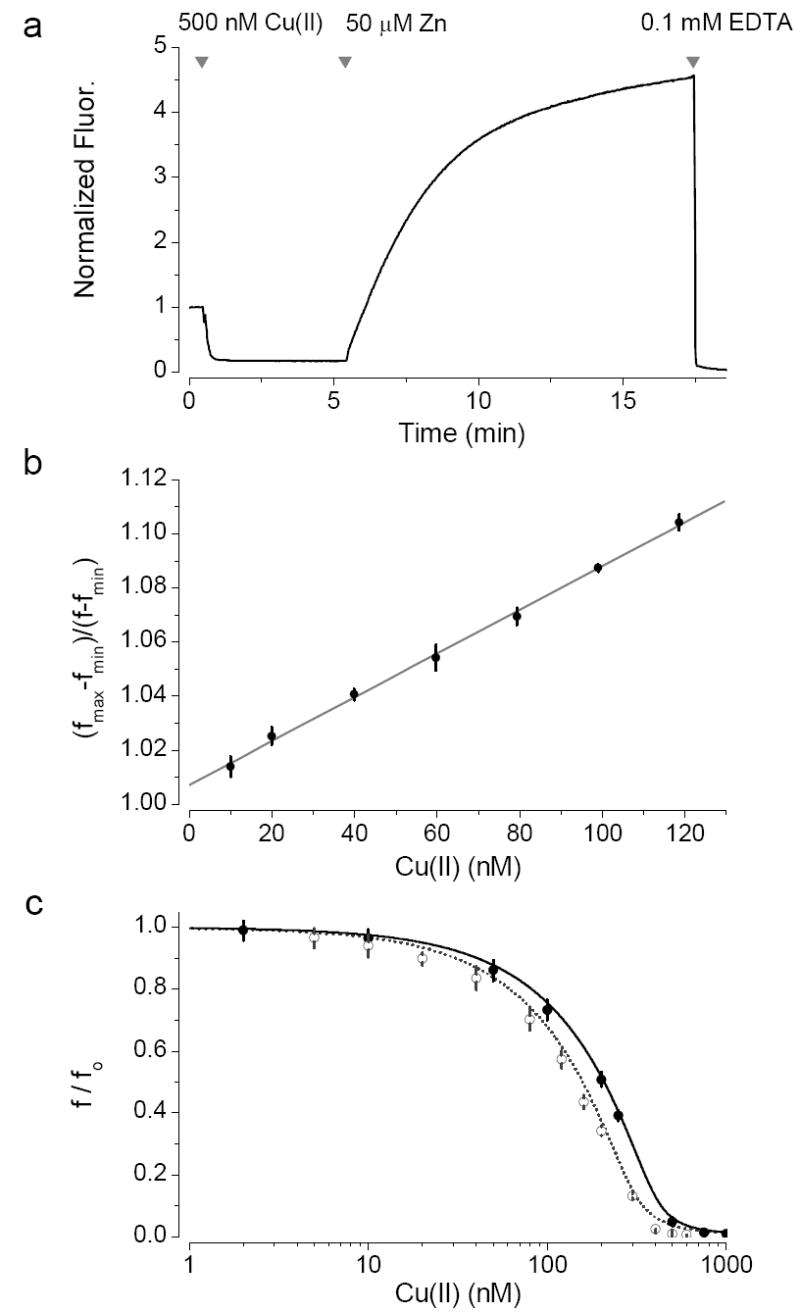
Response of FluoZin-3 to Cu(II). (a) Cu(II) squelches FluoZin-3. Addition of higher concentration of zinc then displaces Cu(II) increasing the fluorescence. EDTA then chelates all the metal. (b) vs Cu(II) with 1μM FluoZin-3 and 40 μM ZnSO4. (n=5) (c) f/ fo vs Cu(II) at different concentrations of FluoZin-3. ● 1.6 μM Zn, 0.3 μM FluoZin-3 ; ○ 1.6 μM Zn, 0.4 μM FluoZin-3 An equation (B13 in the appendix) was derived from equilibria 1 and 2 for f/ fo as a function of the added copper, f0 is the measured fluorescence intensity in the absence of copper.
To measure the dissociation constant (Kd) for the interaction of copper and FluoZin-3, zinc was added to saturate the probe and then low concentrations of Cu(II) were added to displace the zinc and squelch the fluorescence. This approach has the effect of increasing the dynamic range of the sensor, making the mathematics more tractable and minimizing the influence of contaminating zinc and other transition metals. Moreover, the addition of low concentrations of copper perturbs neither the free FluoZin-3 nor zinc concentrations.
The fluorescence intensity is proportional to [F.Zn] (where F=FluoZin-3). If fmax is the measured fluorescence intensity in the presence of saturating zinc without copper, fmin the fluorescence intensity in the presence of saturating copper; f is the measured fluorescence intensity with different concentration of copper ion, Cut the total copper concentration, Kd.Zn the dissociation constant for zinc and FluoZin-3 and Kd.Cu the dissociation constant for copper and FluoZin-3 then (see appendix A for the derivation):
| (5) |
Under our experimental conditions, on the addition of low concentrations of copper, we can assume that the [Zn] and [F.Zn] are not perturbed. Under these conditions a plot of as a function of the Cut should be linear with slope, S:
| (6) |
The dissociation constant for FluoZin-3 and Cu(II) was estimated to be 91±5 pM(n=8) from plots of f0/f as a function of the Cut (see Fig. 1 b).
Using our estimates of the equilibrium constants we were able to successfully fit the data derived for copper concentrations in the range 1 – 1000 nM and two different probe concentrations (Fig. 1c), to an analytically derived equation based on equilibria 1 and 2 (Appendix B). The completeness of the fit also suggests that little if any collisional quenching occurs.
In contrast to FluoZin-3 the fluorescence of NPG was quenched by Cu(II) rather than squelched.
Response of NPG to Cu(I)
We found that the fluorescence of NPG was boosted by Cu(I) and this proved useful in the characterization of the interaction of these ions with FluoZin-3. Application of Cu(I) in the form of [Cu(CNCH3)]PF6 led to a rapid and sustained increase in fluorescence of NPG, when the oxidation state was preserved in the cuprous state by the presence of sulfite (Fig. 2c). The fluorescence was rapidly diminished by the application of 20 μM TPEN but 5 μM EDTA did not quench the fluorescence, consistent with the low affinity of the chelator for the cuprous ion (data not shown).
Fig. 2.
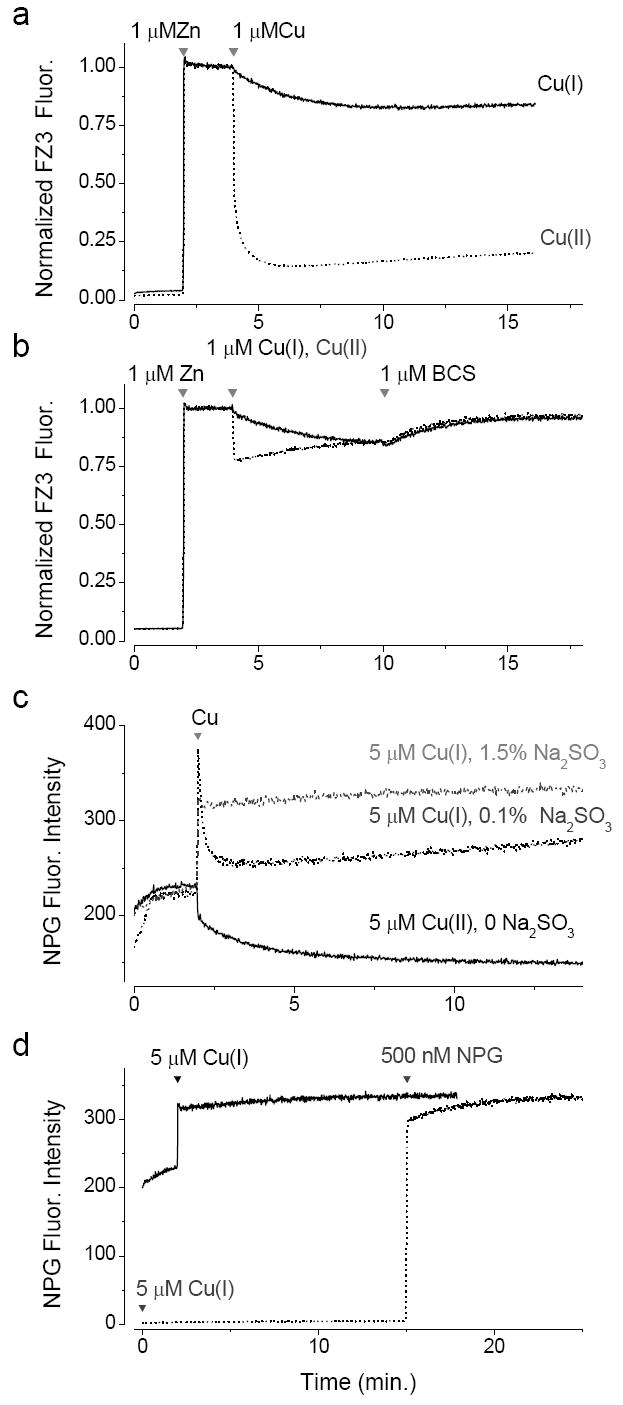
Responsiveness of fluorescent indicators to Cu(I) (a) Cu(II) associates more rapidly and more tightly with FluoZin-3 than Cu(I). Cu(I) experiment in the presence of 1.5% Na2SO3 and Cu(II) in its absence. (b) Addition of Cu(I) or Cu(II) to FluoZin-3 under reducing conditions (1.5 %Na2SO3). (c) Response of NPG (500nM) to Cu(I) under different redox conditions. (d) Cu(I) is stabilized by the presence of Na2SO3. Black line, NPG is added prior to Cu(I) addition at 1 min. Dotted line, Cu(I) is added at time zero and the NPG is added at 15 min.
Although NPG has been in use for over ten years its pH dependence has not been characterized. Protonation of the carboxyl group of the fluorophore with a pKa of ~4.5 quenches the NPG fluorescence (see Supplementary data. While protonation of the aliphatic nitrogen (pKa ~ 6.9) competes with zinc binding.
Cu(I)
Cu(I) was added in the form of [Cu(I)(CH3CN)4]PF6 in acetonitrile [18], purging oxygen from the solution by the addition of 1.5% Na2SO3. When Cu(II) was added to a solution of FluoZin-3 and zinc the fluorescence was rapidly squelched (τ = 0.22 min) whereas the addition of Cu(I) squelched the fluorescence far more slowly (τ=1.28min) (Fig. 2b). The difference in kinetics suggests that the copper is indeed in the monovalent oxidation state. Increasing the sulfite concentration from 0.5 to 2% did not change the rate of equilibration, suggesting that sulfite effectively removes oxygen from the solution. It is worth noting that the fluorescence intensity of FluoZin-3 in the absence of zinc, saturated with Cu(II) or Cu(I) was indistinguishable from that of the metal-free form of the indicator.
Addition of Cu(II) to FluoZin-3 in the presence of 1.5% sulfite led to a rapid but partial squelching of the fluorescence as it is abbreviated by the rapid conversion of Cu(II) to Cu(I) under reducing conditions. The level of squelching under these conditions was indistinguishable for Cu(I) and Cu(II), suggesting that the amount of Cu(I) at equilibrium after the addition of either form of copper was the same (Fig. 2b). Addition of the chelator bathocuproine disulfonate (BCS), which has a higher affinity for Cu(I) than Cu(II), leads to the restoration of the fluorescence with a slow time course, limited by the dissociation rate of the FluoZin-3.Cu(I) complex.
We have interpreted the Cu(I)-induced slow decline of FluoZin-3 fluorescence as resulting from the cuprous ion binding to the FluoZin-3 directly. However, the slow conversion to the cupric state could also account for our results. Cu(II) could form either from trace amounts of oxygen or via the disproportionation reaction (i.e. 2Cu(I) <-> Cu + Cu(II)) which can occur under anaerobic conditions. How can this possibility be excluded? To address this question we used the sensitivity of NPG to Cu(I). Addition of Cu(I) to NPG in the presence of 1.5% sulfite leads to the persistent elevation of the fluorescence by ~ 42%, while the addition of Cu(II) squelches it by ~34% (Fig. 2c). This suggests that addition of Cu(I) in the form of [Cu(I)(CH3CN)4]PF6 in the presence of sulfite can sustain stable levels of the cuprous ion in solution. If the sulfite levels are low conversion from the cuprous to cupric ions does occur and can be monitored with NPG (Fig. 2c). These experiments confirm that Cu(I) levels can be sustained in the presence of a high concentration of sulfite. Moreover the rapidity of the response of NPG to Cu(I) corroborates our hypothesis that in Fig. 2a FluoZin-3 responds directly to Cu(I) rather than to its conversion to Cu(II).
It could be argued that Cu(I) is stabilized by NPG or FluoZin-3. In order to test the stability of Cu(I) in 1.5% sulfite, we assayed its persistence in the presence and absence of NPG. In the former, 5μM Cu(I) was added to a solution containing 500 nM NPG (Fig. 2d, black line) while in the latter 5μM Cu(I) was added to the buffer solution and 500 nM NPG added 15 minutes later (dotted line). The rapid increase of the latter suggests that over a period of 15 min the cuprous species endures. The slight suppression of the initial response to NPG suggests that a small degree of disproportionation may occur.
In the absence of a glove box, sodium sulfite (1.5%) proved to be more effective in purging the solution of oxygen than superfusion of the solution with argon gas. Even if the solution was evacuated three times, ultrasonicated between evacuations and then bubbled with Ar gas for 30 min, there was enough trace oxygen to oxidize the copper as monitored by NPG, within a minute after addition (data not shown).
Our experiment thus far suggests the following: both species of copper squelch the fluorescence of FluoZin-3, but Cu(II) has a higher affinity and it binds more rapidly than Cu(I). To confirm our hypotheses on the interaction of zinc and copper with FluoZin-3 we used BCS as a fluorescent sensor for copper. The fluorescence of BCS is quenched by both states of copper but has a higher affinity for Cu(I) than Cu(II) and little affinity for zinc. In a solution containing 500 nM FluoZin-3 and 1 μM BCS we monitored the fluorescence of both fluorophores. Addition of 1 μM ZnSO4 increased the FluoZin-3 signal but did not change that of BCS (Fig. 3). On addition of 0.5 μM Cu(II) the FluoZin-3 fluorescence was rapidly squelched, consistent with the high affinity of the probe for Cu(II), whereas the quenching of BCS was incomplete. Addition of 10 μM ascorbic acid rapidly quenched the BCS fluorescence, because the ascorbate reduces Cu(II) to Cu(I) which has a higher affinity for BCS. This was then followed by a slow linear increase in the FluoZin-3 signal, that recovered after the free copper concentration declines and the Cu(II) bound to FluoZin-3 is displaced by the free zinc. The slow decline in BCS fluorescence after the addition of ascorbate also mirrors the release of Cu(II) from FluoZin-3 and its conversion to Cu(I). Finally, the application of 40 μM TPEN reduced the FluoZin-3 fluorescence and restored that of BCS. The almost complete recovery of the fluorescence of BCS shows that it is not degraded during the course of the experiment.
Fig. 3.
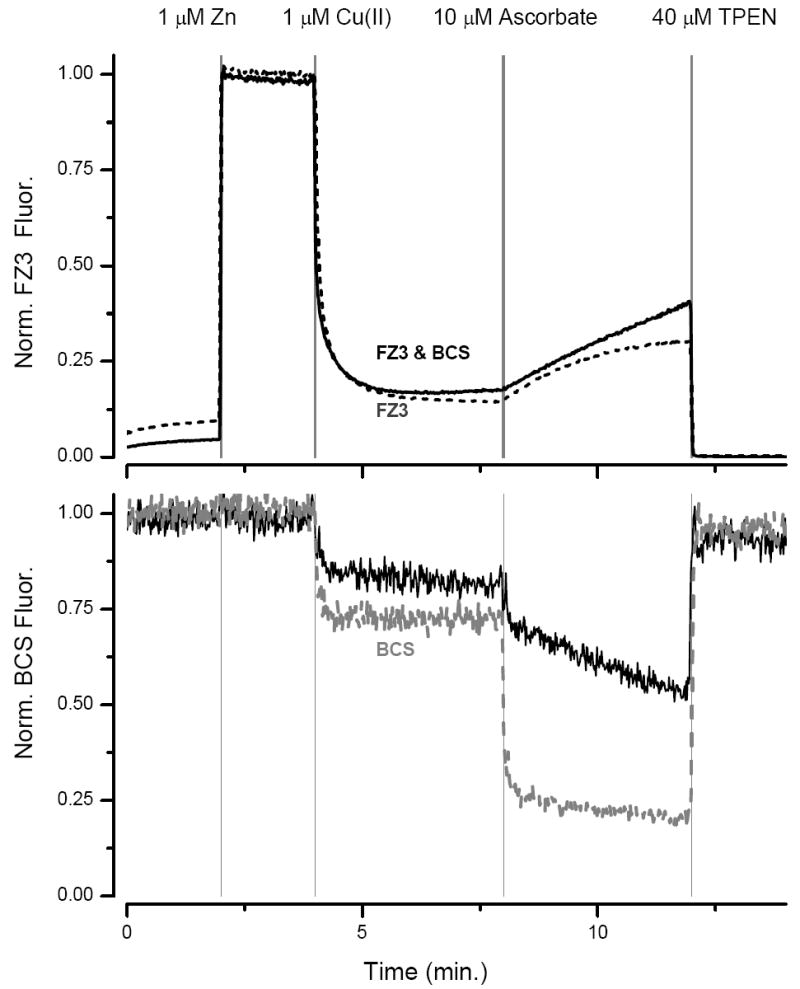
Detecting Cu(I) and Cu(II) with FluoZin-3 and BCS. Top panel FluoZin-3 channel, black line, in presence of both FluoZin-3 and BCS (1μM), dashed line: only FluoZin-3. Bottom panel BCS channel (ex 289nm, em 403nm), black line in presence of FluoZin-3 and BCS, gray line, only BCS.
Using the same strategy as described above for Cu(II) we have estimated the Kd of FluoZin-3 for Cu(I) as 180±27 nM (n=3), which is about one thousand-fold higher than that of Cu(II). The dissociation constants of Cu(I) and Cu(II) for NPG are 17±1 μM and 0.79±0.04 nM respectively.
The interaction of Cr, Mn, Fe, Co, Ni & Cd with FluoZin-3 and NPG
Titrations of FluoZin-3 and NPG with Cr, Mn, Fe(II), Fe(III), Co, Ni and Cd are shown in Fig. 4 a&b, respectively. In the absence of exogenous zinc, none of the metals exhibited any quenching of the fluorophore, all boosted the fluorescence, except Fe(III) that was without effect. In the case of FluoZin-3 the maximum fluorescence was elicited by Cd but was only 30% of that evoked by zinc. For NPG the maximal fluorescence evoked by saturating concentrations of Co, Ni and Fe(II) exceeded that of zinc. Unlike FluoZin-3, Mn had no effect on the fluorescence of NPG within the concentration range 1 μM to 300 μM. The measured dissociation constants for FluoZin-3 and NPG are listed in table 1 and both probes adhered to the IW series.
Fig. 4.
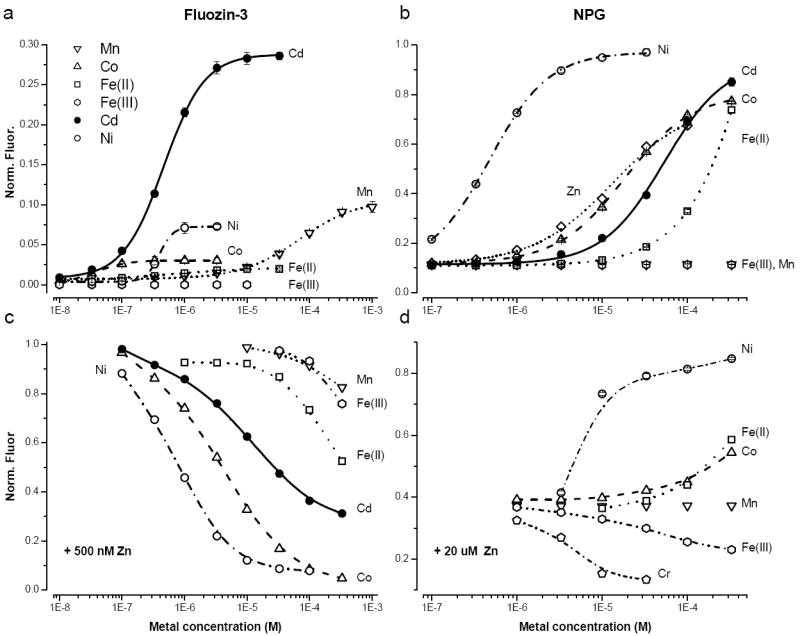
Response of FluoZin-3(a) and NPG (b) to different transition metals. The fluorescence was normalized by the maximum fluorescence, which was for zinc in the case of FluoZin-3 and nickel in the case of NPG. All points are the average of 5 experiments; mean ± SD. Squelching of the FluoZin-3 (c) and NPG (d) by different metals. The probe concentration was 500 nM in all cases.
Table 1.
Metal-indicator dissociation constants. Mean ± SD (n=5).
| FluoZin-3 (M) | NPG (M) | |
|---|---|---|
| Mn | 4.12±0.0.05×10-6 b | - |
| Fe(II) | 1.41±0.09×10-6 b | 2.97±0.07×10-4 a |
| Co | 1.54±0.03×10 -8 b | 1.58±0.01×10-5 a |
| Ni | 2.85±0.03×10 -9 b | 3.21±0.07×10-7 a |
| Cu(I) | 1.8±0.2×10-7 b | 1.7±0.1×10-5 a |
| Cu(II) | 9.1±0.5×10-11 b | 7.9±0.4×10-10 b |
| Zn | 1.5×10-8 * | 1.24±0.03×10-5 a |
| Cd | 1.88±0.01 ×10-7 a | 3.91±0.07×10-5 a |
a or b indicates the method used to calculate the dissociation constant (see ‘Materials and Methods’),
from [12]
Fe(III) tends to form precipitate at pH 7.4 making it rather difficult to assess its affinity, fortunately most intracellular free iron is in the ferrous state.
The squelching of the fluorescence of FluoZin-3 and NPG Cr, Mn, Fe(II), Fe(III), Co, Ni and Cd are shown in Fig. 4 c&d, where the metal was titrated on at a fixed zinc concentration (500 nM for FluoZin-3 and 20 μM for NPG).
Of the metals tested with NPG, nickel forms the brightest complexes, however, the binding of the metal is very slow (τ ~ 6.5 min, see Supplementary Data). The slowness is a function of the DPA, as ZnAF-2 [21], which also has this moiety, binds nickel slowly, while the binding to the BAPTA based FluoZin-3 is rapid (<1s).
Discussion
Fluorimetric probes provide the only means available for probing the spatiotemporal dynamics of weakly chelated metals within biological tissue. To guard against artifacts associated with other biological metals it is important carry out detailed characterizations of metal-probe interactions in cell-free assays, which allow precise and quantitative determinations of probe affinity while eliminating potential confounding factors inherent in live-cell experiments.
In this communication we have delved into the interactions of FluoZin-3 and NPG with all the cationic transition metals that are likely to be encountered in the intracellular milieu as well as some noxious metals. In a previous publication we showed that the predominant divalent ions, calcium and magnesium do not interfere with measurements by FluoZin-3 [22] and this is also true for NPG. Our results provide a firmer framework for interpreting the signals of these probes in living cells, where multiple metals may influence probe signal simultaneously. Also we show that FluoZin-3 has potential as a copper sensor, and NPG as a nickel sensor.
Along with zinc, iron and copper are the most prevalent intracellular transition metals and most likely sources of interference with zinc measurements. Cells do maintain what has been termed a labile iron (LI) pool, that can quench probes like calcein. Estimates of the LI are in the range of ~ 10 μM [6]. With regard to FluoZin-3 the changes in fluorescence induced by iron are very small and are unlikely to be registered. In the case of NPG only very high concentrations of Fe(II) can induce a substantial increase in fluorescence.
Copper has a higher affinity for both NPG and FluoZin-3 than iron, however, its intracellular concentration is likely to be considerably lower than iron, indeed in unicellular organisms there are indications that its concentration is vanishingly low [3]. The labile copper in the cell is likely to be in the cuprous form [23; 24] for which FluoZin-3 has a lower affinity. The probe is thus unlikely to perturb the intracellular copper equilibrium. However, if the probe partitions into intracellular stores, where copper might be predominantly in the cupric state things might be very different.
How can one determine if copper is having any effect on the fluorescence FluoZin-3 or NPG? Unfortunately, there are no shifts in the peaks of the excitation or emission curves associated with different metals. However, the use of chelators can provide a distinction. For example, the membrane permeant chelator bathocuproine (BC) has a very high affinity for copper particularly in its cuprous form but a much lower affinity for iron and zinc. What might one expect on the application of BC to cells? If an intracellular compartment has a high concentration of Cu(II), the application of BC to cells loaded with NPG and FluoZin-3 would give rise to a desquelching of the fluorescence. If on the contrary the compartment contained Cu(I), in the case of FluoZin-3, BC would increase fluorescence, whereas in NPG loaded cells, it would induce a small decrease in fluorescence.
FluoZin-3 can be used with the equations described above to quantify copper, in the simplified setting of a cuvette. However, in cells there are a variety of natural chelators and variations in redox state, as result the interpretation of changes in the fluorescence of FluoZin-3 becomes rather difficult [25].
Regardless of these caveats, our results add important confirmations and extensions of previous studies. Experiments using fluorescent indicators on living cells showed that oxidative stress mobilizes metals from intracellular stores, and that transition metal chelation, usually with the membrane-permeant TPEN, reverses the dye signal and protects against oxidant toxicity [26; 27; 28]. Because dyes sensitive to, but not necessarily selective for zinc respond consistently in such paradigms, it has been largely accepted that zinc is the major transition metal mobilized. However, questions regarding probe selectivity and the identity of metal species have lingered. For instance, it might be argued that iron or copper is similarly mobilized during oxidative stress. But as the data in this report show, iron is incapable of eliciting a strong response from FluoZin-3, while Cu(II), which predominates over Cu(I) in an oxidizing environment, strongly squelches a FluoZin-3 response to zinc. Although iron may indeed be labile, it cannot account for the dye response, while intracellular free copper must remain very low, if it rises at all.
The sensitivity of NPG to nickel is rather unique and has proved useful in detecting the release of nickel from stainless steel electrodes [17] and nickel accumulation within cells [29] and on cells [30]. In principle, the kinetics of the response of NPG to nickel could be used to distinguish it from the other transition metals. The tardiness of nickel binding to DPA, which has been described elsewhere [31], could arise from a slow rearrangement of DPA to satisfy energetic constraints, coupled with the very slow water exchange of the primary hydration shell of nickel [32]. However, with NPG, and fluorescent sensors in general, it is prudent to be alert to fluorescence changes unrelated to alterations in metal concentration. For example peroxynitrite can react with NPG, in increasing its fluorescence [33]. Such non-metal induced changes would of course be impervious to metal chelation. Similarly, changes in the environment, for example changes in the hydrophobicity, could alter the quantum yield and hence the fluorescence intensity and would also be unchanged by a metal chelator [34].
Both FluoZin-3 and NPG are available in membrane permeant forms that can be used to load the cytoplasm. As has been shown for other probes, this AM loading procedure does not exclusively target the cytoplasm, inevitably dye will load into vesicular compartment within the cell [35]. We have evidence that FluoZin-3 and NPG load such compartments (Rumschik and Kay, unpublished observations). In interpreting the results of fluorimetric experiments it is thus important to establish the location of the probe with for example cellular compartment markers [36].
A further complication in live-cell measurements results from over- accumulation of the probe in the cytoplasm, which is often a consequence of passive diffusion loading protocols for cell-permeant forms (e.g. acetoxymethyl ester). When the probe concentration is high enough to deplete the free metal pool, the equation used to estimate the free metal concentration will systematically underestimate it [37; 38]. Such problems underscore the value of carefully determining in vitro probe properties, while at the same time illustrating the kinds of errors that can arise when that data is uncritically extrapolated to in vivo situations.
It is worth pointing out that if exogenous metal is applied to cells it could give rise to an increase in intracellular concentration of a different metal. For example, if copper is applied it may rapidly bind to MT and displace zinc (Zhao and Kay, unpublished observations).
Fluorimetric metal sensors must operate in the complex environment within cells. To aid the interpretation of the changes that occur within cells loaded with FluoZin-3 or NPG we have provided a detailed characterization of their responses to the full range of cationic transition metals found within cells. With the judicious use of chelators it is possible to detect the interference of Cu or Fe on zinc measurements. This would be made simpler if specific probes could be developed for Fe, Cu, Mn and Co. Progress has been made on Cu and Fe [8], and it does not seem inconceivable that it could be extended to Mn and Co, particularly by mimicking biological metal binding peptides. Reaching these goals is sure to stretch the ingenuity of chemists for a while yet.
Supplementary Material
pH dependence of the fluorescence of NPG. The titration was performed in Hepes buffered saline with 40 μM zinc (●) or no zinc (○), both with 40 μM NPG.
Nickel binds slowly to NPG. Arrows indicate additions of nickel to solution with 500 nM NPG.
Acknowledgments
We thank Irma Nydegger for helpful comments on an earlier version of this manuscript and Dr. Christoph Fahrni for helpful discussions. This work was supported in part by a grant from NINDS (NS47508 to ARK) and NIEHS through the University of Iowa Environmental Health Sciences Research Center (NIEHS/NIH P30 ES0560). MJD and KED acknowledge support from and discussions with Dr. Ian J. Reynolds (AG20899).
Abbreviations used
- BCS
bathocuproine disulfonate
- DPA
Di-(2-picolyl)amine
- TPEN
N,N,N’,N’-Tetrakis-(2-pyridylmethyl)-Ethylenediamine
A. Estimation of dissociation constants
In order to estimate Kd.X in a competitive titration between X and Zn we will consider two situations. Firstly when Kd.X >Kd.Zn and secondly with Kd.X < Kd.Zn.
Kd.X >Kd.Zn
Considering equilibria 1 and 2.
| (A1) |
| (A2) |
Where a and bX are proportionality constants relating the species concentration to their fluorescent intensities and Ft is the total concentration of the fluorimetric probe. fmax is the fluorescent intensity in the presence of saturating zinc and fmin is the fluorescent intensity in the presence of saturating X.
Since there is a saturating metal concentration
| (A3) |
| (A4) |
| (A5) |
| (A6) |
| (A7) |
this equation is only applicable when Kd.X >Kd.Zn and when [X] > Ft.
Kd.X < Kd.Zn
Estimating the affinity of copper for FluoZin-3
When the total zinc concentration is greater than FluoZin-3 most of the fluorophore will be in association with the metal. For example with 40 μM zinc and 1 μM FluoZin-3 the free FluoZin-3 concentration will be ~ 3.9 10-10 M.
Considering equilibria 1 and 2.
| (A8) |
| (A9) |
Substituting A9 into A8 and rearranging:
| (A10) |
| (A9) |
Under our experimental condition for low concentrations of copper, a plot of against Xt, will be linear with slope (S) from which Kd.X can be estimated.
| (A10) |
B. Derivation of the relationship between f/fo and total metal concentration
Considering equilibria 1 and 2.
| (B1) |
| (B2) |
| (B3) |
| (B4) |
| (B5) |
Znt is the total zinc concentration; Xt is total concentration of X added; Ft is the initial concentration of the fluorescent probe.
The fluorescence intensity is proportional to the concentration of the F.Zn complex, so:
| (B6) |
Where, f is the measured fluorescence intensity with different concentrations of X ion; f0 is the measured fluorescence intensity in the absence of X; [Zn.F]0 is concentration of the complex without X ions.
To simplify the equation, set [Zn.F]0 = C0, so:
| (B7) |
| (B8) |
| (B9) |
| (B10) |
| (B11) |
Substitute B11, B7 and B9 to B3, then
| (B12) |
Solving equation B12 for Xt :
| (B13) |
and C0 can be calculated from B1 and B4 when Xt=0:
| (B14) |
From Equation B13 one can then calculate f/fo as a function of Xt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frausto da Silva JJR, Williams RJP. The Biological Chemistry of the Elements: The inorganic chemistry of life. Oxford University Press; Oxford: 2001. [Google Scholar]
- 2.Thiele DJ, Gitlin JD. Assembling the pieces. Nat Chem Biol. 2008;4:145–7. doi: 10.1038/nchembio0308-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finney LA, O’Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–6. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 4.Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem. 2006;11:1049–62. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 5.Petrat F, de Groot H, Sustmann R, Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol Chem. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- 6.Breuer W, Shvartsman M, Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–4. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Jiang P, Guo Z. Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord Chem Rev. 2004;248:205–229. [Google Scholar]
- 8.Que EL, Domaille DW, Chang CJ. Metals in Neurobiology: Probing Their Chemistry and Biology with Molecular Imaging. Chemical Reviews. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 9.Tsien RY. Imagining imaging’s future. Nat Rev Mol Cell Biol Suppl. 2003:SS16–21. [PubMed] [Google Scholar]
- 10.Bowen HJM. Environmental chemistry of the elements. Academic Press; 1979. [Google Scholar]
- 11.Tarohda T, Yamamoto M, Amamo R. Regional distribution of manganese, iron, copper, and zinc in the rat brain during development. Anal Bioanal Chem. 2004;380:240–6. doi: 10.1007/s00216-004-2697-8. [DOI] [PubMed] [Google Scholar]
- 12.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–8. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 13.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–9. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martell AE, Hancock RD. Metal Complexes in Aqueous Solution. Plenum Press; New York, NY: 1996. [Google Scholar]
- 15.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 16.Valeur B, Leray I. Design principles of fluorescent molecular sensors for cation recognition. Coordination Chemistry Reviews. 2000;205:3–40. [Google Scholar]
- 17.Kay AR. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiol. 2004;4:4. doi: 10.1186/1472-6793-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron x-ray fluorescence microscopy. Proc Natl Acad Sci U S A. 2005;102:11179–84. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapisarda VA, Volentini SI, Farias RN, Massa EM. Quenching of bathocuproine disulfonate fluorescence by Cu(I) as a basis for copper quantification. Anal Biochem. 2002;307:105–9. doi: 10.1016/s0003-2697(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 21.Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. Highly Zinc-selective fluorescent sensor molecules suitable for biological applications. J Am Chem Soc. 2000;122:12399–12400. [Google Scholar]
- 22.Zhao J, Bertoglio BA, Gee KR, Kay AR. The zinc indicator FluoZin-3 is not perturbed significantly by physiological levels of calcium or magnesium. Cell Calcium. 2008 doi: 10.1016/j.ceca.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 24.Davis AV, O’Halloran TV. A place for thioether chemistry in cellular copper ion recognition and trafficking. Nat Chem Biol. 2008;4:148–51. doi: 10.1038/nchembio0308-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys. 2007 doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of Neuronal Apoptosis by Thiol Oxidation: Putative Role of Intracellular Zinc Release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 27.St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol Lung Cell Mol Physiol. 2002;282:L185–92. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- 28.Tatsumi T, Fliss H. Hypochlorous acid and chloramines increase endothelial permeability: possible involvement of cellular zinc. Am J Physiol. 1994;267:H1597–607. doi: 10.1152/ajpheart.1994.267.4.H1597. [DOI] [PubMed] [Google Scholar]
- 29.Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T. Nickel carcinogenesis: epigenetics and hypoxia signaling. Mutat Res. 2005;592:79–88. doi: 10.1016/j.mrfmmm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Thierse HJ, Helm S, Pink M, Weltzien HU. Novel fluorescence assay for tracking molecular and cellular allergen-protein interactions. J Immunol Methods. 2007;328:14–20. doi: 10.1016/j.jim.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard CD. The kinetics of interaction of Nickel (II) and Cobalt(II) with bis(2-pyridylmethyl)amine. Inorganic Chemistry. 1971;10:2340–2343. [Google Scholar]
- 32.Diebler HM, Eigen M, Ilgenfritz G, Mass G, Winkler R. Kinetics and mechanism of reactions of main group metal ions with biological carriers. Pure Appl Chem. 1969;20 [Google Scholar]
- 33.Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, Rosenberg PA. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–27. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snitsarev V, Budde T, Stricker TP, Cox JM, Krupa DJ, Geng L, Kay AR. Fluorescent detection of Zn(2+)-rich vesicles with Zinquin: mechanism of action in lipid environments. Biophys J. 2001;80:1538–46. doi: 10.1016/S0006-3495(01)76126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–23. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- 36.Muylle FA, Adriaensen D, De Coen W, Timmermans JP, Blust R. Tracing of labile zinc in live fish hepatocytes using FluoZin-3. Biometals. 2006;19:437–50. doi: 10.1007/s10534-005-4576-y. [DOI] [PubMed] [Google Scholar]
- 37.Dineley KE, Malaiyandi LM, Reynolds IJ. A reevaluation of neuronal zinc measurements: artifacts associated with high intracellular dye concentration. Mol Pharmacol. 2002;62:618–27. doi: 10.1124/mol.62.3.618. [DOI] [PubMed] [Google Scholar]
- 38.Kay AR. Imaging synaptic zinc: promises and perils. Trends in Neuroscience. 2006;29:200–206. doi: 10.1016/j.tins.2006.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
pH dependence of the fluorescence of NPG. The titration was performed in Hepes buffered saline with 40 μM zinc (●) or no zinc (○), both with 40 μM NPG.
Nickel binds slowly to NPG. Arrows indicate additions of nickel to solution with 500 nM NPG.


