Abstract
Suitable animal models are needed to study monkeypox virus (MPXV) as human monkeypox clinically resembles smallpox and MPXV is a zoonotic and potential bioterroristic agent. We have demonstrated that a species of African dormice, Graphiurus kelleni, is susceptible to a lethal infection of MPXV and that MPXV replicated in multiple organs of this species. Following intranasal administration, MPXV replicated locally in the nasal mucosa causing necrosis and hemorrhage with subsequent systemic spread to lymph nodes, spleen, liver, and other tissues where it caused severe necrosis and/or hemorrhage leading to death. The dormouse model was validated for testing prophylactic (Dryvax vaccine) and therapeutic (cidofovir) test articles against intranasal challenges with MPXV.
Keywords: Monkeypox virus, MPXV-ZAI-79, Graphiurus kelleni, Dormouse, Dryvax, Cidofovir, Smallpox, Variola
Introduction
Suitable animal models are needed to study MPXV since human monkeypox clinically resembles smallpox and MPXV is a zoonotic and potential bioterroristic agent (Parker et al., 2007). The black-tailed prairie dog (p.d), Cynomys ludovicianus, and the thirteen-lined ground squirrel (g.s.), Spermophilus tridecemlineatus, have been evaluated and investigated for their suitability as animal models for MPXV research (Guarner et al., 2004, Langohr et al., 2004, Sbrana et al., 2007, Tesh et al., 2004, Xiao et al., 2005). Husbandry issues associated with these species make them challenging to propagate and maintain in a research vivarium. Female p.d. produce only one litter each year (Hoogland, 2001) while g.s. hibernate for part of the year and produce only one litter each year (Vaughan et al., 2006). Consequently, these species are obtained from their natural habitat where their health status is undefined. Another potential experimental host for MPXV is an African dormouse, Graphiurus kelleni, which is a mouse-sized rodent found in areas of Africa where human monkeypox has occurred. Graphiurus spp. were in the shipment of African rodents that introduced MPXV into the U.S. in 2003. The Centers for Disease Control and Prevention (CDC) determined that Graphiurus spp. and other rodents from this shipment tested positive for MPXV (CDC, 2003). Fortunately, this species has many characteristics similar to laboratory mice which enables their propagation and maintenance in a research setting; in a vivarium, they were determined to be non-seasonally polyestrous, litter bearing, and lacking a need for hibernation. Here we show that G. kelleni (dormice/dormouse) is highly susceptible to lethal infections with MPXV-ZAI-79 and can be used to evaluate prophylactics and therapeutics against MPXV.
Results
Morbidity and mortality of dormice following intranasal infection with MPXV-ZAI-79
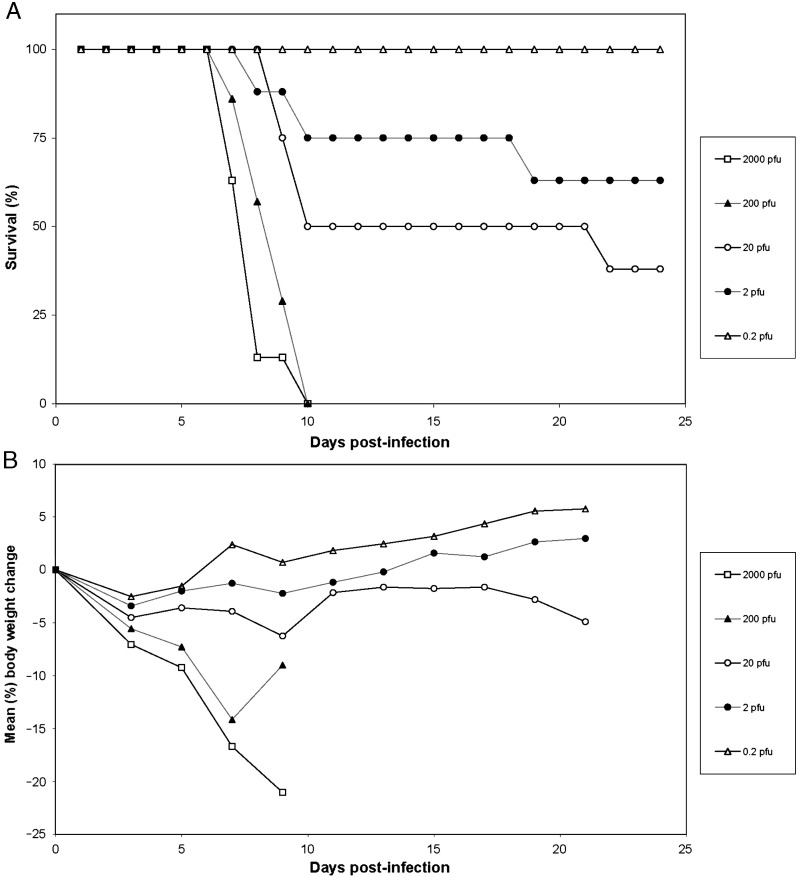
Initially, dormice were infected with MPXV-ZAI-79 (1.4 × 104 PFU/dormouse) via a footpad injection route; mortality was 92% (12 of 13 dormice) which occurred during days 7–10 p.i. Considering that smallpox and monkeypox infections are typically initiated by the deposition of large respiratory droplets containing small quantities of virions on the respiratory epithelium, likely in the upper respiratory tract (Fenner et al., 1988, Parker et al., 2007), we infected dormice (n = 55) with a small (10 μl) volume of MPXV-ZAI-79 virus suspension at various doses by the intranasal (i.n.) route. The dormice were highly susceptible to morbidity and mortality over a range of virus doses (Figs. 1A,B). Virus doses of 2000 and 200 plaque forming units (PFU)/dormouse were lethal for 100% of dormice with a mean time to death of 7.9 ± 1 and 8.7 ± 1 days and highest mean body weight change of − 21% and − 14%, respectively. Infection with 20 PFU/dormouse was 63% lethal (mean time to death: 12.0 ± 5 days) with the highest mean body weight change of − 6%. Infection with 2 PFU/dormouse was 38% lethal (mean time to death: 12.3 ± 5 days) with the highest mean body weight change of + 3%. No mortality was observed at 0.2 PFU/dormouse and the highest mean body weight change was + 6%. Morbidity of dormice that died due to a lethal MPXV-ZAI-79 infection was decreased activity, hunched posture, unkempt hair coat, dehydration, and conjunctivitis. There were no significant differences for day of death attributed to gender (female: 9.64; male: 9.58; p = 0.973). Mortality was 100% for both genders at a virus dose of 2000 and 200 PFU/dormouse; mortality was 50% and 25% for females and 75% and 50% for males at the virus doses of 20 and 2 PFU/dormouse, respectively. The calculated LD50 (Reed and Muench, 1938) of MPXV-ZAI-79 in dormice following i.n. infection was 12 PFU.
Fig. 1.
Mortality and mean body weight of dormice following i.n. infection with increasing doses of MPXV-ZAI-79 (n = 55; female = 28, male = 27). (A) Mortality; (B) mean body weight post-inoculation of survivors.
For comparative purposes, dormice were infected via the intranasal route with MPXV-COP-58, a MPXV strain originating from West Africa and isolated from imported cynomolgus macaques in Copenhagen, Denmark in 1958 (Von Magnus et al., 1959). This strain is less virulent to humans and primates than MPXV-ZAI-79 (Chen et al., 2005). Dormice infected with various doses of MPXV-COP-58 had similar days of death and mortality rates as dormice infected with similar doses of MPXV-ZAI-79.
Kinetics of virus spread and progression of disease in dormice following a lethal intranasal MPXV-ZAI-79 infection
Dormice were infected with 2 × 104 PFU of MPXV-ZAI-79 by the i.n. route. At designated times following infection, dormice were sacrificed and nasal lavages, blood and selected tissues were examined for virus titers and/or histopathology (Fig. 2 ). MPXV-ZAI-79 was first detected in nasal lavages on day 2 p.i. in 6 of 8 dormice (75%). Detectable levels of MPXV-ZAI-79 were noted in the spleen starting day 3 p.i. for 2 of the 8 dormice (25%) sacrificed and in the liver, lung, and blood starting day 4 p.i. for 13, 13, and 25% of the 8 dormice sacrificed, respectively; all positive tissues on day 4 were from different dormice. The highest level of MPXV-ZAI-79 was found in liver tissue on day 8 p.i., with 6 of the 6 dormice demonstrating detectable levels (100%). Day 8 was the last day when surviving dormice were available for evaluation. The temporal similarity of virus detection in spleen, liver, and lung suggest that the lung was infected through lymphatic and/or hematogenous spread of the virus and not through aspiration from the upper respiratory tract.
Fig. 2.
Spread MPXV-ZAI-79 in dormice following an i.n. infection (n = 59; female = 30, male = 29).
After days 3 and 4 p.i., all dormice clinically demonstrated dehydration and conjunctivitis. At necropsy, upper gastrointestinal hemorrhage (Fig. 3 ), hepatomegaly, and lymphadenopathy were observed. Histopathologically, infected dormice sacrificed late in infection (starting days 4–5 p.i.) exhibited rhinitis (Figs. 4B–D), lymphoid necrosis in the submandibular lymph nodes (draining site of infection; Figs. 4F–H), spleen (Figs. 4J–L) and thymus. There was also hepatocellular necrosis in the liver (Figs. 4N–P). Hemorrhage was observed in the lungs, stomach, small intestine and elsewhere while areas of necrosis and myeloid hyperplasia were demonstrated in the bone marrow. In dormice found dead (pooled from many experiments), there was often hemorrhage from the nasal cavity as well as hemorrhage in the gall bladder (Fig. 5A) and brain (Fig. 5B) in addition to the lesions described above. In this and other studies, syncytial cell formation was present in nasal mucosal epithelial cells (Fig. 5C), and intracytoplasmic Guarnieri or basophilic (B)-type viral inclusions were noted in the liver (Fig. 5D), spleen, and nasal mucosa.
Fig. 3.
Left: stomach from an uninfected dormouse; right: stomach from a dormouse that received a lethal dose of MPXV-ZAI-79. Petechial and ecchymotic hemorrhages are evident from the serosal surface. The upper gastrointestinal tract (stomach and small intestine) were filled with blood and devoid of ingesta.
Fig. 4.
Histopathologic changes in selected tissues of dormice following a lethal i.n. infection with MPXV-ZAI-79; same study as in Fig. 2. (A–D) Nasal mucosa, bar = 20 μm. Submucosal inflammation, mucosal necrosis (arrow) and erosion/ulceration were observed with increasing severity over time; (E–H) submandibular lymph node, bar = 100 μm. Necrosis (arrow) increased in severity and distribution over time; (I–L) Spleen, bar = 20 μm. Increased death of lymphocytes were followed by the development of patchy areas of necrosis (arrow) over time; (M–P) Liver, bar = 100 μm. Large necrotic foci (arrows) of hepatic parenchyma were observed at 8 days p.i., which corresponded to maximal viral load in this tissue.
Fig. 5.
(A) Gall bladder, hemorrhage in submucosa, arrow, bar = 100 μm; (B) Brain, hemorrhage, arrow, bar = 100 μm. A and B are from dormice that were found dead on days 8 and 9, respectively, following a lethal infection with MPXV-ZAI-79. (C) Nasal mucosa, syncytia, arrow; numerous necrotic cells with B-type intracytoplasmic viral inclusions, arrowhead, bar = 10 μm; (D) Liver, B-type intracytoplasmic viral inclusions, arrows; hepatic necrosis is demonstrated in the top half of the photo, bar = 10 μm. C and D are from dormice that were sacrificed on days 8 and 6 after receiving a lethal MPXV-ZAI-79 i.n. inoculate, respectively.
In summary, histopathological changes found in dormice (pooled from many experiments) which died due to a lethal MPXV-ZAI-79 infection are listed in Table 1 where necrosis and/or hemorrhage affecting many organs were common features.
Table 1.
Histopathological findings of select tissue from dormicea infected with a lethal dose of MPXV-ZAI-79
| Tissue | Morphological diagnosis | Distribution | Frequency |
|---|---|---|---|
| Nasal mucosa | Necrosis | Diffuse | 100% (16/16) |
| Hemorrhage | Multifocal | ||
| Liver | Necrosis | Mutifocal to coalescing | 100% (16/16) |
| Hemorrhage | Diffuse | ||
| Vacuolar degeneration | Diffuse | ||
| Spleen | Necrosis | Diffuse | 100% (16/16) |
| Hemorrhage | Diffuse | ||
| Lung | Hemorrhage | Multifocal | 100% (16/16) |
| Stomach | Hemorrhage | Multifocal | 100% (16/16) |
| Lymph node (submandibular) | Necrosis | Diffuse | 100% (14/14) |
| Hemorrhage | Multifocal | 71% (10/14) | |
| Adrenal gland | Hemorrhage | Multifocal to coalescing | 100% (16/16) |
| Necrosis | Diffuse | 56% (9/16) | |
| Pancreas | Hemorrhage | Multifocal | 94% (15/16) |
| Thymus | Necrosis | Multifocal to coalescing | 93% (14/15) |
| Bone marrow | Necrosis | Multifocal | 91% (10/11) |
| Heart | Hemorrhage | Multifocal | 87% (13/15) |
| Small intestine | Hemorrhage | Multifocal | 75% (12/16) |
| Gall bladder | Hemorrhage | Diffuse | 75% (9/12) |
| Kidney | Hemorrhage | Multifocal | 63% (10/16) |
| Brain | Hemorrhage | Multifocal | 56% (9/16) |
All dormice (n = 16) were found dead; mean time to death was 9.3 ± 1.5 days.
Use of dormice for evaluating prophylactics and therapeutics against MPXV-ZAI-79
To determine if the dormouse was suitable for efficacy testing of prophylactics and therapeutics, we examined the ability of the Dryvax (smallpox) vaccine and a marketed antiviral, cidofovir, to protect against a lethal i.n. challenge with MPXV-ZAI-79 (Table 2 ). Dormice immunized 4 weeks previously with Dryvax vaccine were solidly protected from mortality when challenged with 2 × 104 PFU of MPXV-ZAI-79, whereas dormice in the non-vaccinated control group experienced uniform mortality. Dormice treated with a single dose of cidofovir 4 h following challenge with MPXV-ZAI-79 (pooled results from 3 independent experiments: infecting doses of 75, 4 × 103, and 5 × 103 PFU) were significantly protected from mortality, whereas dormice in the vehicle treated control group experienced uniform mortality.
Table 2.
| Treatment | Mortality | p value |
|---|---|---|
| Dryvaca | 0/6 (0%) | < 0.0001 |
| Cidofovirb | 7/36 (19%) | < 0.0001 |
| Vehicle | 41/41 (100%) | N/A |
Dormice immunized 4 weeks previously with Dryvax vaccine were solidly protected from mortality when challenged with 2 × 104 PFU of MPXV-ZAI-79 by the i.n. route.
Pooled results from 3 independent experiments using an infectious dose of 75, 4 × 103, or 5 × 103 PFU of MPXV-ZAI-79. Dormice were treated with a single dose of cidofovir 4 h following i.n. challenge with MPXV-ZAI-79. Cidofovir was administered as described in the experimental protocol.
Discussion
Many of the histopathological features of MPXV infected dormice are similar to those observed in smallpox infected humans (Bras, 1952, Councilman et al., 1904, Lillie, 1930). Unfortunately, reports concerning histopathology of humans that died due to a MPXV infection do not exist. Martin (2002) reviewed accessible cases of smallpox pathology and review articles from English language journals written during the last 200 years. He classified smallpox into 3 subtypes: variola vera, modified variola, and hemorrhagic/toxic variola. Variola vera comprised > 90% of cases with a case fatality rate of 30%. Modified variola represented a variable number of cases with a case fatality rate of 3%; this subtype occurred in previously vaccinated humans. Hemorrhagic/toxic variola comprised < 10% of cases with a case fatality rate of nearly 100% and was characterized by lesions with bleeding diathesis and disseminated intravascular coagulation. The MPXV-dormouse model described in this paper most resembles the hemorrhagic/toxic smallpox subtype and provides a more severe disease course than other rodent models of human MPXV disease. The mechanism for severe hemorrhage in dormice infected with a lethal dose of MPXV-ZAI-79 was not determined. Extensive liver and bone marrow necrosis may have resulted in reduced coagulation ability due to the loss of clotting factors and platelets. Additionally, infection and damage of endothelium in affected tissues may have contributed to the multiorgan hemorrhage.
The prairie dog and ground squirrel have been examined as potential rodent models of human MPXV disease. The prairie dog has been experimentally infected with approximately 1 × 105 to 2 × 105 PFU of MPXV via intraperitoneal (i.p.) and i.n. routes (Xiao et al., 2005). The MPXV strain utilized (MPX-2003) was isolated from a skin lesion of a human infected with MPXV during the 2003 outbreak in the United States. Neither route of infection with MPX-2003 produced histopathologic abnormalities in the heart, pancreas, kidneys, adrenal glands, or gastrointestinal tract. The i.p. infection route was uniformly lethal and produced necrosis of the spleen, liver, and abdominal adipose tissue in 3 of 3 prairie dogs. The i.n. infection route did not produce necrosis in the spleen or liver in 5 of 5 prairie dogs, but it did produce necrosis of the thymus and mediastinal lymph nodes in some prairie dogs while necrosis, edema, and hemorrhage were observed in the lungs of 3 of 5 prairie dogs. Death occurred in 3 of 5 prairie dogs infected via the i.n. route; the 2 surviving prairie dogs did not have significant histopathological lesions when their tissues were examined following euthanasia on day 25 p.i.
The ground squirrel was also experimentally infected with approximately 1 × 105 to 2 × 105 PFU of MPX-2003 via i.p. and i.n. routes (Tesh et al., 2004). Both routes were uniformly lethal for the 10 ground squirrels tested. Regardless of the route of infection, all ground squirrels exhibited necrosis of the liver and spleen. Additionally, some i.n. infected ground squirrels demonstrated consolidation and interstitial inflammation of pulmonary tissue and necrosis of peribronchial lymphoid tissue. Necrosis in other lymph nodes was also noted. Another study (Sbrana et al., 2007) utilizing ground squirrels compared the pathology following the subcutaneous injection with 100 PFU of either MPX Z79 (same strain as MPXV-ZAI-79) or MPX US03 (same strain as MPX-2003). Both strains were 100% lethal at the same virus dose for the 40 ground squirrels tested, but the MPXV-ZAI-79 was considered more virulent. Additionally, frequent nosebleeds/bleeding diathesis were observed in ground squirrels infected with MPX Z79 but infrequently observed in ground squirrels infected with MPX US03. However, Tesh et al. (2004) did not report nosebleeds/bleeding diathesis in ground squirrels infected with a higher dose of the MPX-2003 strain administered via i.p or i.n. routes.
Other rodents evaluated for their susceptibility to MPXV were the cotton rat and the multimammate mouse/natal mastomys (Wilson and Reeder, 1993, Wilson and Reeder, 2005). Marennikova et al. (1998) reported the cotton rat (Sigmodon hispidus) as very susceptible to MPXV; MPXV strain and dose was not reported. The cotton rat was described as having acute generalized illness with difficultly breathing, coughing, rhinitis, conjunctivitis, and progressive emaciation following the i.n. administration of MPXV; this route of administration caused 50% mortality. Moreover, a more severe disease course and 100% mortality was noted with this rodent following an intravenous inoculation of MPXV.
Takashi Kitamura (personal comm.) described to the World Health Organization in 1978 the necropsy findings of MPXV (reported as strain MPZ; 1 × 107 PFU) infected multimammate mice (Mastomys natalensis) as hemorrhagic pleuritis or peritonitis and inflammatory hyperemia in multiple organs following the i.p. inoculation of MPXV.
A non-human primate model for human MPXV and possibly smallpox diseases is the cynomolgus monkey (Macaca fascicularis; Zaucha et al., 2001). Histopathology of this species following lethal doses of aerosolized MPXV-ZAI-79 (inhaled doses of 1 × 104 to 1.41 × 105 PFU) demonstrated necrotizing lesions at all affected sites which included: lungs, lymph nodes, thymus, spleen, skin, oral mucosa, gastrointestinal tract, and reproductive systems. Hepatic involvement was uncommon. The monkeys died naturally or were killed 9 to 17 days p.i.; cases of natural death were attributed to fibrinonecrotic bronchopneumonia.
When compared to other rodent models which are presently being explored for MPXV research, dormice have many traits similar to those of laboratory mice. This similarity enables the successful propagation and maintenance of dormice within a research vivarium and allows for an accessible supply of dormice with a defined health status. Dormice can easily utilize customary rodent caging and accessories. In our studies, cidofovir significantly protected against a lethal MPXV-ZAI-79 infection and the Dryvax vaccine when administered 28 days prior to a lethal MPXV-ZAI-79 infection was solidly protective. These findings support the applicability of the dormouse in pathogenesis, vaccine, and therapeutic studies of MPXV.
Materials and methods
Animals
The FDA-Center for Veterinary Medicine granted permission for the procurement of dormice from Ohio and Illinois animal vendors (see Acknowledgements) and the use of this species in MPXV research. The Institutional Animal Care and Use Committee at the Saint Louis School of Medicine approved all experimental protocols. All experimental animal procedures were completed in an ABSL-3 facility where dormice were housed in large ventilated microisolator cages (M.I.C.E. system cages, Animal Care Systems, Centennial, Colorado). A standard rodent diet (Teklad Global 18% Protein Rodent Diet) and water were provided ad libitum. A nesting area (Techniplast Mouse House) and corn cob bedding were provided in each cage where no more than 4 dormice were housed. All dormice were acclimatized to their respective ABSL-3 cages 7 days prior to infection. Dormice utilized in these studies were generally 2–3 months of age.
Taxonomy of the dormouse was completed via morphometric analysis of skulls by Dr. Mary Ellen Holden-Musser. This dormouse species was identified as G. kelleni which is native to Africa; its common name is Kellen's African Dormouse (Wilson, 2005).
Cells and virus
The strain of MPXV used in the majority of studies was MPXV-ZAI-79 which was isolated from a fatal human case in Zaire during 1979 (Democratic Republic of the Congo; Zaucha et al., 2001). In one study, MPXV-COP-58, a West African strain of MPXV isolated from cynomolgus macaques in 1958 was utilized (Von Magnus et al., 1959). The virus strains were propagated on BSC-1 cell monolayers which were grown in Eagle's minimum essential medium (EMEM; Bio-Whittaker, Walkersville, MD) as previously described (Chen et al., 1992).
Antiviral compounds and vaccine
Cidofovir was provided as a gift from Mitch Hitchcock of Gilead Sciences Inc., (lot: A201A1, 75 mg/ml). Dryvax vaccine was provided by the CDC.
Pathology examination
After external and internal gross examination of each dormouse carcass, selected tissues were immersion-fixed in 10% neutral-buffered formalin and then routinely processed to generate paraffin sections which were stained with hematoxylin-and-eosin. Tissues selected for microscopic examination: brain, submandibular lymph node, salivary glands, heart, thymus, lung, kidney, adrenal gland, liver, gall bladder, spleen, bone marrow, stomach, small intestine, colon, and pancreas. Sections of nasal tissue were also routinely processed and stained after fixation and decalcification.
Virus assay
Lung, liver, and spleen were ground with tissue homogenizers which contained a measured amount of phosphate buffered saline (PBS; without Ca+ 2 and Mg+ 2) with 1% fetal calf serum. Nasal lavages and blood were serially diluted in the same state as they were collected. Virus infectivity of all tissue, nasal lavages, and blood was measured by plating serial 10-fold dilutions on monolayers of BSC-1 cells, which were overlayed with carboxylmethyl cellulose as previously described (Chen et al., 1992).
Experimental infection
For all studies involving the i.n. administration of MPXV, dormice were anesthetized with intraperitoneal injections of a ketamine (9 mg/ml) and xylazine (1 mg/ml) mixture at 0.1 ml/10 g of body weight. Once anesthetized, each dormouse was securely positioned in a dorsal recumbent position on a 60 degree incline and one or both nostrils were inoculated with PBS w/o Ca+ 2 and Mg+ 2 alone or containing the specified MPXV strain via a micropipette. The dormice were maintained in this position for 5 min and then returned to their cages for recovery. Dormice were weighed every-other-day and observed daily for morbidity and mortality over the duration of each study. For the study examining virus spread and disease course, 104 dormice were infected with a lethal dose of MPXV-ZAI-79 (2 × 104 PFU). Groups of 6–8 infected dormice were humanely euthanized every 24 h with carbon dioxide; this was continued through day 8 p.i., a point at which there were no remaining dormice that survived infection. Spleen, liver, blood, lung, and nasal lavages were collected for virus infectivity and tissues from 2 of these dormice (both genders) at each time point were collected for histopathology. Each nasal lavage utilized 0.5 ml of PBS containing 1% fetal calf serum. In order to prevent blood contamination of nasal lavage fluid, the lavage was administered retrograde with a syringe and 18 gauge needle via the nasopharyngeal meatus.
During one study, dormice (n = 13) were infected with 1.4 × 104 PFU of MPXV-ZAI-79 via a left footpad injection. These dormice were made temporarily unconscious due to a very brief exposure to a CO2O2 gas mixture immediately before the footpad injection.
Efficacy testing of Dryvax and cidofovir
Dormice in the study testing the efficacy of Dryvax were initially vaccinated with Dryvax (10 μl of a 1:10 dilution containing approximately 1 × 105 PFU) or the vaccine diluent (10 μl) via an injection into the left hind limb footpad. Four weeks later, each dormouse was challenged i.n. with a lethal dose of MPXV-ZAI-79. All dormice were observed daily for morbidity and mortality for the following 21 days.
Dormice in studies involving cidofovir were initially infected i.n. with a lethal dose of MPXV-ZAI-79 and 4 h later they were injected i.p. with 2 mg (0.1 ml of 20 mg/ml solution; 100 mg/kg) of cidofovir or 0.1 ml of sterile saline alone. All dormice were observed daily for morbidity and mortality for the following 21 days.
Statistical methods
Statistical significance of difference was determined by the t test. p < 0.05 was considered significant.
Acknowledgments
We are grateful for Gail Dick of Montpelier, Ohio and Roger Prewett of Hopedale, Illinois for their assistance. We are also grateful for Dr. Mary Ellen Holden-Musser of Charleston, South Carolina for her expertise in the taxonomic identification of the African dormice species maintained in the breeding colony at Saint Louis University. We would like to thank Jill Schriewer, Erin Touchette, Jennifer Stabenow, and the Insectarium at the Saint Louis Zoo for their technical assistance. This work was supported by grant R21-AI061512-01 from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), grant U54-AI057160 from the NAID, the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE), and grant T32-RR007002 from the National Center for Research Resources (NCRR) of the NIH.
References
- Bras G. The morbid anatomy of smallpox. Doc. Med. Geogr. Trop. 1952;4:303–351. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Update: multistate outbreak of monkeypox — Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin. MMWR. 2003;52:616–618. [PubMed] [Google Scholar]
- Chen W., Drillien R., Spehner D., Buller R.M. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992;187:433–442. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., Schriewer J., Buck C., Wang C., Lefkowitz E.J., Esposito J.J., Harms T., Damon I.K., Roper R.L., Upton C., Buller R.M. Virulence differences between monkeypox virus isolates from West Africa and the Congo Basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Councilman W., Magrath G., Brinkerhoff W. The pathological anatomy and histology of variola. J. Med. Res. 1904;11:12–134. [PMC free article] [PubMed] [Google Scholar]
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. World Health Organization; Geneva: 1988. Smallpox and its Eradication. [Google Scholar]
- Guarner J., Johnson B.J., Paddock C.D., Shieh W., Goldsmith C.S., Reynolds M.G., Damon I.K., Regnery R.L., Zaki S.R., and the Veterinary Virus Working Group Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 2004;10:426–431. doi: 10.3201/eid1003.030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland J. Black-tailed, Gunnison's, and Utah Prairie Dogs reproduce slowly. J. Mammal. 2001;82:917–927. [Google Scholar]
- Langohr I., Stevenson G., Thacker H., Regnery R. Extensive lesions of monkeypox in a prairie dog (Cynomys sp.) Vet. Pathol. 2004;41:702–707. doi: 10.1354/vp.41-6-702. [DOI] [PubMed] [Google Scholar]
- Lillie R.D. Smallpox and vaccinia: the pathologic histology. Arch. Pathol. 1930;10:241–291. [Google Scholar]
- Marennikova S.S., Shelukhina E.M., Shenkman L.S. Twelfth International Poxvirus Symposium. St. Thomas; U.S. Virgin Islands: 1998. Cotton rats (Sigmodon hispidus) as an experimental model of monkeypox infection. [Google Scholar]
- Martin D.B. The cause of death in smallpox: an examination of the pathology record. Mil. Med. 2002;167:546–551. [PubMed] [Google Scholar]
- Parker S., Nuara A., Buller R.M., Schultz D.A. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H.A. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Sbrana E., Xiao S., Newman P.C., Tesh R.B. Comparative pathology of North American and Central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med. Hyg. 2007;76:155–164. [PubMed] [Google Scholar]
- Tesh R.B., Watts D.M., Sbrana E., Siirin M., Popov V.L., Xiao S. Experimental infection of ground squirrels (Spermophilus tridecemlineatus) with monkeypox virus. Emerg. Infect. Dis. 2004;10:1563–1567. doi: 10.3201/eid1009.040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D.K., Gruber A.R., Michalski M.L., Seidling J., Schlink S. Capture, care, and captive breeding of 13-lined ground squirrels, Spermophilus tridecemlineatus. Lab. Anim. 2006;35:33–40. doi: 10.1038/laban0406-33. [DOI] [PubMed] [Google Scholar]
- Von Magnus P., Anderson E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta. Path. Microbiol. Scand. 1959;46:156–176. [Google Scholar]
- Wilson D.E., Reeder D.M., editors. Mammal Species of the World: A Taxonomic and Geographic Reference. Smithsonian Institution Press; Washington: 1993. [Google Scholar]
- Wilson D.E., Reeder D.M., editors. Mammal Species of the World: A Taxonomic and Geographic Reference. The Johns Hopkins University Press; Baltimore: 2005. [Google Scholar]
- Xiao S., Sbrana E., Watts D.M., Siirin M., Travassos da Rosa A., Tesh R.B. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 2005;11:539–545. doi: 10.3201/eid1104.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]