Summary
The hypothalamic orexin neuropeptide acutely promotes appetite, yet orexin deficiency in humans and mice is associated with obesity. Prolonged effects of increased orexin signaling upon energy homeostasis have not been fully characterized. Here we examine the metabolic effects of orexin gain-of-function utilizing genetic and pharmacologic techniques in mice. Transgenic orexin overexpression confers resistance to high fat diet-induced obesity and insulin insensitivity by promoting energy expenditure and reducing consumption. Genetic studies indicate that orexin receptor-2 (OX2R) rather than OX1R signaling predominantly mediates this phenotype. Likewise, prolonged central administration of an OX2R-selective peptide agonist inhibits diet-induced obesity. While orexin overexpression enhances the anorectic-catabolic effects of central leptin administration, obese leptin-deficient mice are completely resistant to the metabolic effects of orexin overexpression or OX2R agonist infusion. We conclude that enhanced orexin-OX2R signaling confers resistance to diet-induced features of the metabolic syndrome through negative energy homeostasis and improved leptin sensitivity.
Introduction
Animals employ genetically and physiologically determined homeostatic mechanisms to prevent excess weight gain; however, this regulation may be circumvented by environmental and hedonic factors. The ready availability of palatable calorically dense food and the reduced need for physical activity in modern life have resulted in a pandemic of obesity. Rodent models have elucidated the mechanisms and modulators of body weight homeostasis such as the fat tissue-derived satiety hormone leptin (Enriori et al., 2007; Myers et al., 2008). Leptin centrally regulates body weight by suppressing food intake and permitting energy expenditure. While absence of leptin in rodents causes hyperphagia, obesity, and diabetes, most human obesity is associated with hyperleptinemic leptin resistance.
Orexins (also known as hypocretins) are lateral hypothalamic neuropeptides that are upregulated with fasting and can acutely promote appetite when administered into the central nervous system (Sakurai et al., 1998). The two receptors for orexin, type 1 (OX1R) and type 2 (OX2R) show differential affinity for the products of the prepro-orexin gene, orexin-A and orexin-B (Sakurai et al., 1998). OX1R and OX2R exhibit distinct expression patterns indicating distinct roles in behavior and metabolism. The arcuate nucleus of hypothalamus (ARH) is a point of convergence for both orexin and leptin signaling which modulate the activities of neuropeptide regulators of food intake and metabolism such as neuropeptide Y (NPY), agouti-related peptide (AGRP), and proopiomelanocortin (POMC). Pathologic leptin resistance may be mediated by changes in second messengers including the long form of leptin receptor (LEPR), downstream signal transducer and activator of transcription-3 (STAT3), or the feedback suppressor of cytokine signal-3 (SOCS3) (Horvath 2005; Myers et al., 2008).
Central administration of orexin neuropeptides to rodents acutely promotes appetite, and prepo-orexin deficiency or post-gestational ablation of orexin neurons in mice cause modest reductions in food intake. However, orexin-deficient mice also exhibit narcolepsy, inactivity, and obesity, indicating that orexin may exert an overall catabolic influence upon energy balance (Hara et al., 2001, 2005; Willie et al., 2001). Narcoleptic human individuals (the majority of which is orexin-deficient) have also been reported to have greater body mass index and higher incidence of metabolic syndrome (Nishino, 2007). This effect of orexin upon energy balance may be primary since orexin-deficient narcoleptic patients showed higher body mass index than otherwise clinically indistinguishable narcoleptics with normal orexin levels (Nishino et al., 2001).
As the conclusion that orexin promotes negative energy balance derives indirectly from loss-of-function studies, we utilized genetic and pharmacological methods to directly examine whether increased orexin signaling promotes negative energy balance. First, using CAG/orexin transgenic mice (Mieda et al., 2004) that overproduce orexin neuropeptides from an ectopically expressed transgene, we examined the effects of constitutively increased orexin signaling upon diet-induced obesity by measuring adiposity, locomotor activity, and other metabolic parameters. To differentiate the role of each receptor pathway, we also examined the effects of the CAG/orexin transgene upon OX1R knockout mice (OX1R−/−, Kisanuki et al., 2000) and OX2R knockout mice (OX2R−/−, Willie et al., 2003). Results of genetic studies were then verified and extended pharmacologically using a selective agonist for OX2R. Finally, we sought to determine the effect of the CAG/orexin transgene upon leptin deficient ob/ob mice, we examined the sensitivity of CAG/orexin mice to central leptin administration, and we investigated the effects of selective OX2R agonism upon ob/ob mice.
Results
Expression of orexin peptide in CAG/orexin mouse
Previous results with CAG/orexin transgenic mice revealed multifold increases in both orexin-A and orexin-B peptides in whole brain extracts (Mieda et al., 2004). Immunohistochemical localization of orexin-A in the brain of CAG/orexin mice demonstrates ectopic peptide production in medial, basal, lateral, and suprachiasmatic hypothalamic nuclei, nucleus accumbens, globus pallidus, hippocampal formation, ventral tegmental area, and locus coerulus (Figures S1, 2, Table S1). All of these locations have previously been implicated as participants in networks controlling various homeostatic, circadian, learned, and/or hedonistic aspects of food intake, taste preference, or energy homeostasis (Saper et al., 2002; Willie and Woolsey, 2008). Previous results demonstrated that CAG/orexin transgene insertion was sufficient to rescue the narcolepsy/cataplexy phenotype of mice lacking endogenous orexinergic neurons (Mieda et al., 2004). Thus, the CAG/orexin transgene produces functional peptides that can activate orexin receptors.
The physiological relevance of peripheral actions of orexins, if any, remains controversial (Heinonen et al., 2008). In spite of the use of a general promoter for orexin overexpression, we found that CAG/orexin mice exhibited ectopic orexin-A immunoreactivity in a limited set of peripheral tissues including thyroid gland, adrenal cortex, and some pancreatic islets. No evidence of ectopic expression was encountered in other metabolic tissues such as brown and white adipose, liver, or skeletal muscle (Figure S3, Table S2, 3).
CAG/orexin Mice are Resistant to Diet-Induced Obesity
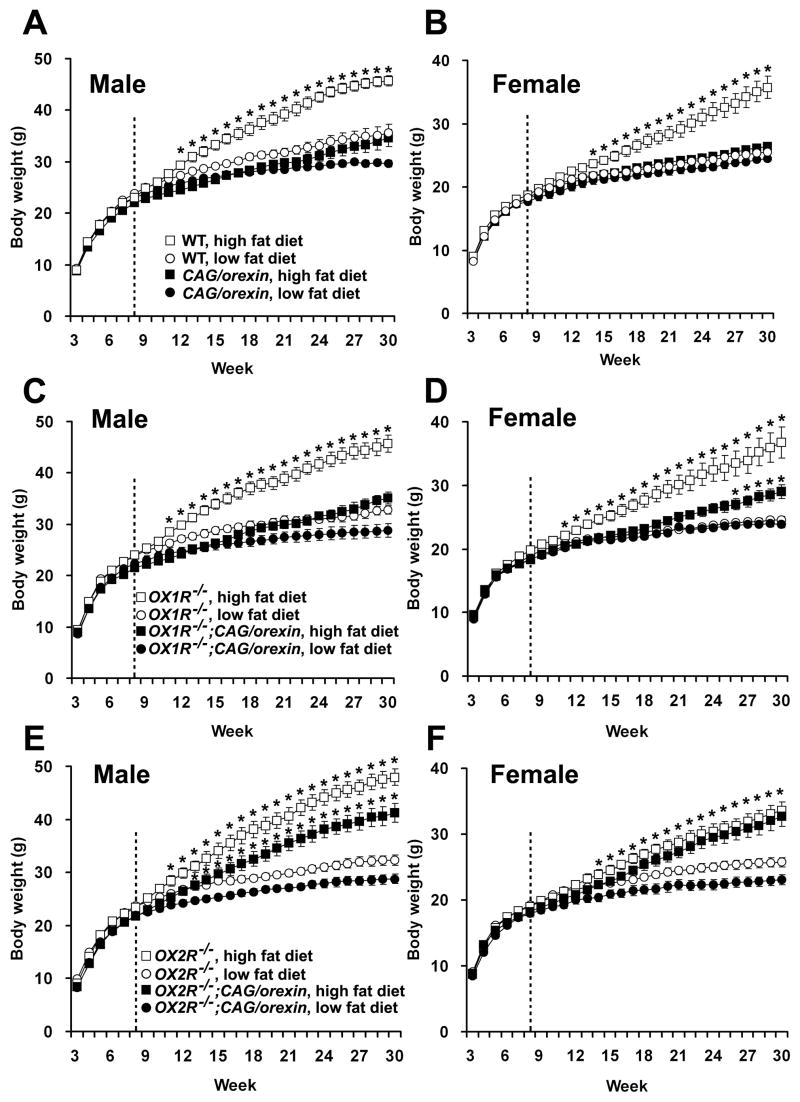
To examine the effect of increased orexin on body weight, CAG/orexin transgenic mice and wild-type littermate mice were fed either a low or a high fat diet. In both male and female mice, the body weights of wild-type mice were significantly higher when fed a high fat diet compared to a low fat diet. However, mice overexpressing orexin did not show a significant difference in body weight growth between a low fat diet and a high fat diet (Figure 1A, B). Thus, wild-type mice are susceptible to diet-induced obesity, whereas CAG/orexin mice are quite resistant.
Figure 1. Growth curves of genetically modified mice fed low or high fat diet.
(A, B) Body weight curves of wild-type male (A) and female (B) mice on a high fat diet were significantly higher than those of wild-type mice on a low fat diet (p<0.0005, both sexes). CAG/orexin mice did not differ significantly under low fat and high fat dietary conditions (p= 0.51 male; p=0.13 female).
(C, D) Body weight curves of OX1R−/− male (C) and female (D) mice on a high fat diet were significantly higher than those of OX1R−/− mice on a low fat diet (p<0.0001 in male; p<0.01 in female). OX1R−/−; CAG/orexin male mice did not differ significantly between low fat and high fat dietary conditions (p=0.21). The body weights of OX1R−/−; CAG/orexin female mice on a high fat diet were significantly less than those of OX1R−/− mice on a high fat diet (p<0.01), in spite of no body weight difference between OX1R−/−; CAG/orexin female mice and OX1R−/− female mice on a low fat diet (p=0.50).
(E,F) The body weight growths of OX2R−/− male (E) and female (F) mice on a high fat diet were significantly higher than those of OX2R−/− mice on a low fat diet (p<0.0001 in male; p<0.005 in female). Likewise, OX2R−/−; CAG/orexin male (E) and female (F) mice showed significant weight gain on a high fat diet compared to those on a low fat diet (p<0.0005 in male; p<0.001).
Body weights of mice measured weekly from the age of 3 weeks to 30 weeks. A high fat diet started at the age of 8 weeks (dotted line). The numbers of mice are 10–14 mice per group. * indicates significant difference between different diet condition for each genotypic group according to post-hoc analysis at each time point. Significant difference in (F) for OX2R−/− mice and OX2R−/−; CAG/orexin are indicated by one asterisk. Data are expressed as means ± SEM.
To determine which receptor pathway mediates the anti-obesity effect of orexin overexpression, we crossed CAG/orexin transgenic mice to OX1R−/− and OX2R−/− lines. We compared the effects of isolated orexin-OX2R signaling (in OX1R−/− mice and OX1R−/−; CAG/orexin mice) versus isolated orexin-OX1R signaling (in OX2R−/− mice and OX2R−/−; CAG/orexin mice) upon growth curves. Figure 1C and 1D show that increased OX2R activation is sufficient to mediate the preponderance of resistance to diet-induced obesity. On the other hand, in both sexes, increased OX1R activation alone does not significantly protect from development of obesity (Figure 1E, F). Unlike differences in body weight, there were no significant differences in linear growth among the various genotypic groups (data not shown). Thus, OX2R signaling selectively mediates the anti-obesity effect of orexin overexpression in mice challenged with a high fat diet.
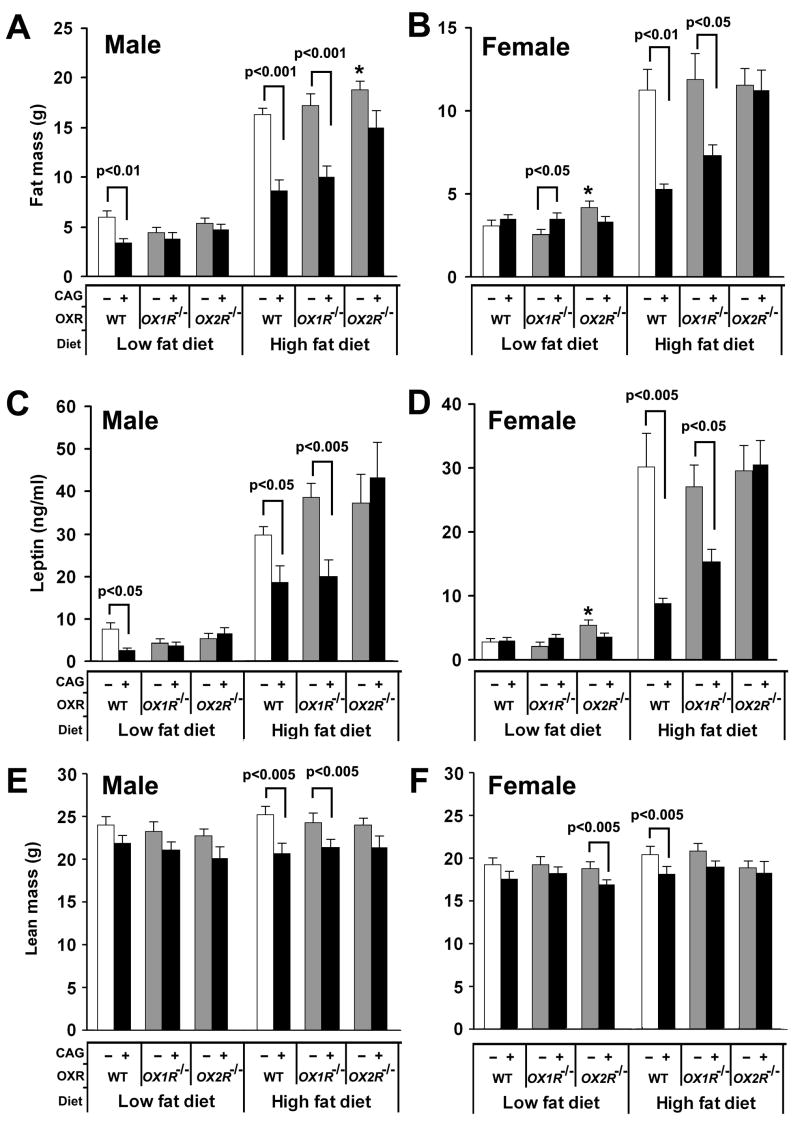
CAG/orexin transgenic male mice were also resistant to ageing-related adiposity while wild-type male mice fed a low fat diet showed continuous weight gain during ageing (Figure 1A). In spite of similar growth curves before 18 weeks of age, the growth curve between 19 weeks and 30 weeks of age of wild-type mice fed a low fat diet was significantly larger than that of CAG/orexin transgenic mice (p=0.0016). Likewise, OX1R−/− male mice fed a low fat diet showed larger body weight growth between 17 weeks and 30 weeks of age than OX1R−/−; CAG/orexin mice despite no significant difference in the growth curves before 16 weeks of age (p=0.036). OX2R−/− male mice fed a low fat diet showed larger body weight than OX2R−/−; CAG/orexin mice through the whole observation period (p=0.005), however, the fat mass and serum leptin of OX2R−/−; CAG/orexin male mice were similar to those of OX2R−/− male mice (Figure 2).
Figure 2. Fat mass, serum leptin levels, and lean mass of orexin signaling-modified mice.
(A and B) The fat masses of male (A) and female (B) mice at 28 weeks of age on different diets.
(C and D) The serum leptin of male (C) and female (D) mice at 30 weeks of age on different diets.
(E and F) The lean masses of male (A) and female (B) mice at 28 weeks of age on different diets. * indicates significant (p<0.05) increase compared to wild-type mice under the same food condition. The numbers of mice are 8–14 mice per group. Data are expressed as means ± SEM.
CAG/orexin Transgene Reduces Fat Mass and Leptin
Consistent with body weight data, at 28 weeks of age, CAG/orexin male mice showed a significant reduction of fat mass on a low fat diet as compared with wild-type male mice (Figure 2A). The fat mass of CAG/orexin male mice and OX1R−/−; CAG/orexin mice fed a high fat diet was significantly less than those of wild-type mice and OX1R−/− mice, respectively, for both sexes (Figure 2A, B). There was no significant difference in fat mass between OX2R−/− mice and OX2R−/−; CAG/orexin mice on both a low fat and a high fat diet for either sex. OX2R−/−male mice exhibited a significant tendency toward increased fat mass under high fat, and OX2R−/− female mice exhibited a mild but significant tendency toward increased fat mass under even low fat conditions compared to wild-type mice, which is consistent with previously described adiposity of narcoleptic mice (Hara et al., 2001) and a physiological role of OX2R signaling in suppressing adiposity.
Next, we measured serum leptin which is typically correlates with fat mass. Concordance between fat mass and leptin levels was confirmed in each genotype. Specifically, the leptin levels of CAG/orexin mice and of OX1R−/−; CAG/orexin mice were significantly lower than those of wild-type mice and of OX1R−/− mice fed a high fat diet, respectively, whereas there was no significant difference in serum leptin levels between OX2R−/−; CAG/orexin mice and OX2R−/−mice on both low fat and high fat diets for both sexes (Figure 2C, D). Compared to differences observed in fat mass, the CAG/orexin transgene was associated with small but significant reductions in lean mass of male mice having functional orexin receptors and those deficient in OX1R under high fat conditions (Figure 2E). The CAG/orexin transgene was similarly associated with a significant mild reduction in lean mass of female mice having functional receptors under high fat conditions, but a significant mild reduction of lean mass by the transgene under OX2R deficient low fat conditions was also observed (Figure 2F).
Increased Energy Expenditure of CAG/orexin mice
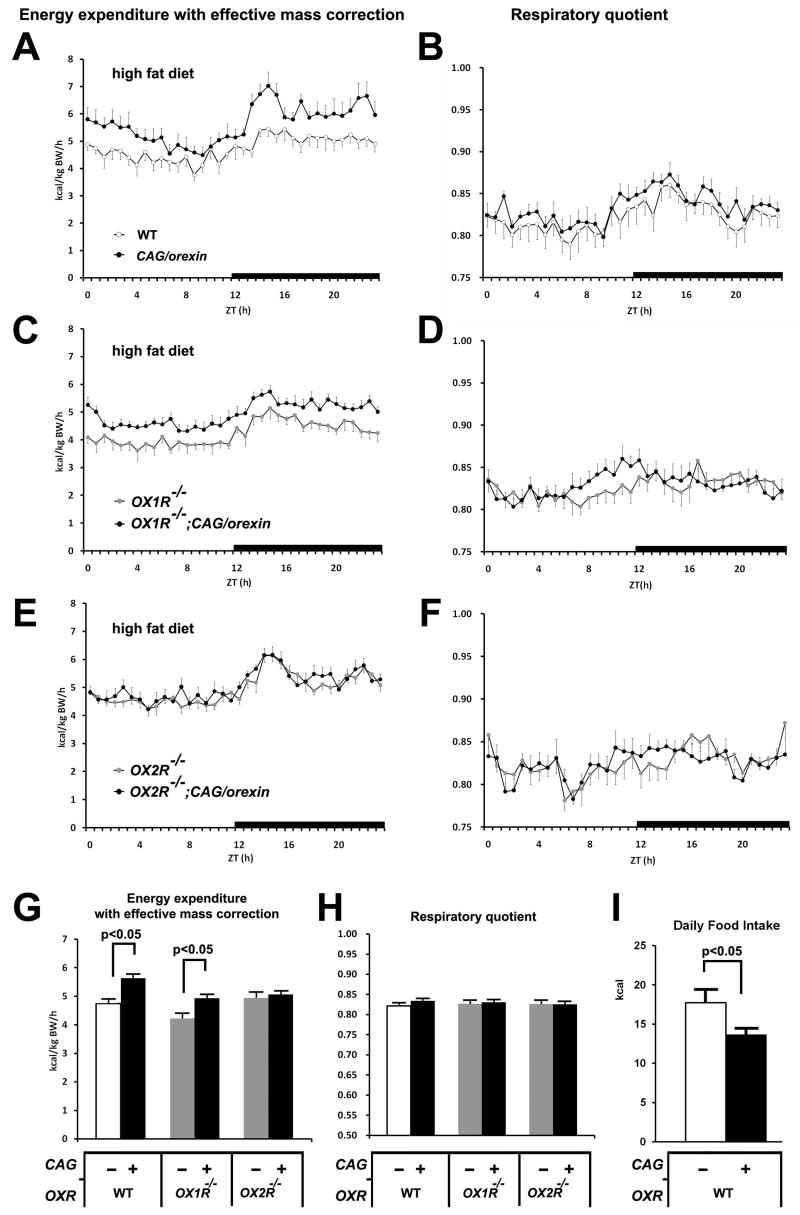
To explore the underlying cause of differential resistance to diet-induced obesity in mice overexpressing orexin, we housed mice from each genotypic group in metabolic cages in order to measure oxygen consumption, carbon dioxide production, and locomotor activity. The effective mass-corrected energy expenditures of CAG/orexin male mice and OX1R−/−; CAG/orexin mice on a high fat diet were consistently elevated over those of wild-type mice and OX1R−/− mice, respectively (Figure 3A, C, G), while the energy expenditures of OX2R−/−; CAG/orexin mice resembled those of OX2R−/− mice (Figure 3E, G). In contrast, we observed no consistent differences in respiratory quotient (an indirect indicator of lipid versus carbohydrate utilization) among different genotypic groups on a high fat diet (Figure 3B, D, F, H). The CAG/orexin transgene induced no differences in energy expenditure or respiratory quotient among any genotypic groups on a low fat diet, regardless of the presence or absence of orexin receptors (Figure S4). Low fat-fed OX1R−/− mice showed reduced energy expenditure compared to wild-type controls (Figure S4G). Importantly, CAG/orexin transgenic mice did not exhibit hyperactivity, regardless of diet or receptor status (Figure S5), although OX2R−/− mice fed a low fat diet showed some reduced locomotion compared to wild-type mice (Figure S5G), which is consistent with previous data from narcoleptic mice (Hara et al., 2001, 2005). Basal core body temperature in CAG/orexin mice on a high fat diet tended to be higher than in wild-type controls, but this difference did not reach significance (wild-type low fat diet: 36.6±0.1°C; CAG/orexin low fat diet: 36.7±0.1°C; wild-type high fat diet: 36.8±0.1°C; CAG/orexin high fat diet: 37.0±0.1°C; n = 5–6).
Figure 3. The metabolic parameters of orexin signaling-modified mice on a high fat diet.
(A, B) The energy expenditure with effective mass correction (A) and respiratory quotient (B) sampled every 40 min over 24 hr of CAG/orexin mice and wild-type mice at 16–20 weeks of age.
(C, D) The energy expenditure with effective mass correction (A) and respiratory quotient (B) over 24 hr of OX1R−/−; CAG/orexin mice and OX1R−/− mice.
(E, F) The energy expenditure with effective mass correction (A) and respiratory quotient (B) over 24 hr of OX2R−/−; CAG/orexin mice and OX2R−/− mice.
(G, H) The averaged energy expenditure with effective mass correction (G) and respiratory quotient (H).
(I) Averaged daily high fat diet intake of CAG/orexin mice and wild-type mice for 14 days.
The numbers of mice are 6–9 mice per group. Data are expressed as means ± SEM.
Both total high fat diet intake for 14 days (Figure 3I) and body weight-adjusted daily food intake (data not shown) were significantly reduced in CAG/orexin mice compared to wild-type controls. Critically, this did not result from abnormal taste preferences: compared to wild-type mice, CAG/orexin and wild-type mice similarly exhibited greater preferences for high fat over low fat chow and for 10% sucrose over 1% sucrose solutions (Figure S6).
Glucose Metabolism of CAG/orexin Mice
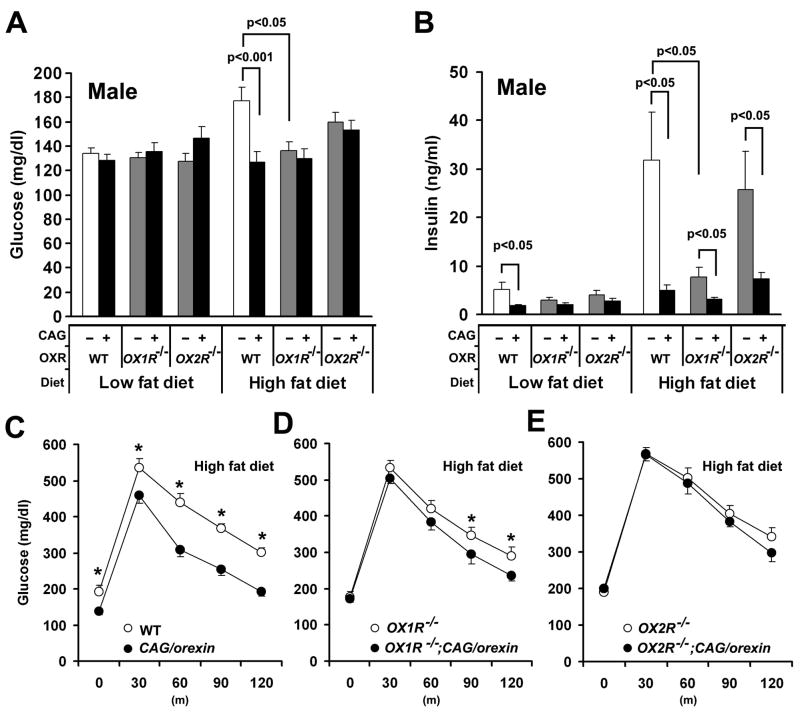
To examine the effect of orexin overexpression on glucose metabolism, we first measured blood glucose and serum insulin of fed mice at 30 weeks of age. When maintained on a low fat diet, we observed no significant difference in fed glucose level among genotypes (Figure 4A). On a high fat diet, however, wild-type mice exhibit hyperglycemia that is attenuated in CAG/orexin, OX1R−/−, and OX1R−/−; CAG/orexin mice, but not OX2R−/− or OX2R−/−; CAG/orexin mice. Thus, the protective effect depends upon functional OX2R, but can be mediated by endogenous orexin levels even without orexin overexpression. Notably, these data also show that OX1R deficiency alone can prevent high fat diet-induced hyperglycemia (see below).
Figure 4. Glucose metabolism of orexin signaling-modified mice on different fat diets.
(A) Blood glucose levels of orexin-related gene mutant mice on different fat diets.
(B) Serum insulin levels of orexin-related gene mutant mice on different fat diets.
(C) Glucose tolerance test showed that the blood glucose levels of CAG/orexin mice were significantly lower than those of wild-type littermate mice on a high fat diet after the administration of glucose.
(D) Glucose tolerance test showed that the blood glucose levels of OX1R−/−; CAG/orexin mice were significantly lower than those of OX1R−/− mice on a high fat diet after the administration of glucose. Data are expressed as means ± SEM.
(E) Glucose tolerance test showed that there is no significant difference in the blood glucose levels between OX2R−/−; CAG/orexin mice and OX2R−/− mice on a high fat diet after the administration of glucose (p=0.33).
The numbers of mice are 8–14 mice and 6–10 mice per group for blood glucose and insulin measurement, and glucose tolerance test, respectively. Data are expressed as means ± SEM. *p<0.05
Increased serum insulin levels with obesity or ageing indicate mounting insulin resistance and sensitively predict deteriorating glucose control in human metabolic syndrome. When compared to wild-type mice, the CAG/orexin transgene reduced serum insulin levels on low fat diet and conferred protection from hyperinsulinemia on high fat diet (Figure 4B). Notably, a similar protective effect occurred in OX1R−/− mice (Figure 4B), despite relative obesity under these conditions (Figure 1C,D; 2C,D), suggesting that endogenous orexin-OX1R signaling can play a specific permissive role in development of hyperinsulinemia. However, the CAG/orexin transgene conferred protection from hyperinsulinemia upon all three genetic backgrounds on a high fat diet, suggesting that both OX1R and OX2R mediate protective effects of orexin overexpression on insulin sensitivity.
We next examined the effects of orexin overexpression upon fasting glucose and glucose tolerance after glucose administration in mice. On a low fat diet, orexin overexpression did not significantly affect glucose homeostasis (p=0.47, Figure S7). On a high fat diet, however, CAG/orexin mice exhibited significantly reduced basal fasting glucose levels as well as improved glucose tolerance at all time points tested relative to wild-type controls (Figure 4C). Despite absence of basal differences in fasting serum glucose between OX1R−/−and OX1R−/−; CAG/orexin mice, the CAG/orexin transgene conferred mild but significant improvements in glucose tolerance onto the OX1R−/− background at later time points (Figure 4D). Improved glucose tolerance in the setting of reduced insulin levels indicates that the transgene confers improved insulin sensitivity. By contrast, we observed no significant differences in fasting glucose or glucose tolerance between OX2R−/− mice and OX2R−/−; CAG/orexin mice (Figure 4E). Thus, while OX1R may also influence circulating insulin levels, orexin overexpression improves insulin sensitivity by a predominantly OX2R-dependent mechanism.
Effects of CAG/orexin Transgene on Peripheral Tissues
Ectopic orexin production in thyroid tissue raises the possibility that abnormal activity of the thyroid axis contributes to leanness in CAG/orexin mice. We measured serum thyroid stimulating hormone (TSH), triiodothyronine (T3), and thyroxine (T4) on low and high fat diets. High fat diet increased serum T3 and T4 levels of CAG/orexin mice to a similar extent as wild-type mice despite significant differences in adiposity between the groups (Figure S8). Serum TSH levels of CAG/orexin mice on high fat diet were significantly elevated over those on a low fat diet, while high fat diet did not significantly affect serum TSH levels of wild-type mice. Importantly, the levels of serum TSH, T3, and T4 of CAG/orexin mice were similar to those of wild-type mice when maintained on a low fat diet.
To determine whether increased energy expenditure of CAG/orexin mice was associated with increased mitochondrial uncoupling proteins, we examined mRNA levels of major uncoupling proteins in brown fat and skeletal muscle (Figure S9). High fat diet resulted in comparable increases in UCP1 mRNA in brown fat but not skeletal muscle in both genotypes. In contrast, UCP2 and UCP3 mRNA levels did not differ significantly by genotype or dietary condition, consistent with previous report (Surwit et al., 1998).
Despite detection of ectopic orexin peptide in adrenal gland, CAG/orexin mice and wild-type mice had similar total daily urinary levels of epinephrine and norepinephrine, and similar serum corticosterone levels (Figure S10). In addition, CAG/orexin transgene did not affect systolic blood pressure either in low fat or high fat diet (Figure S10).
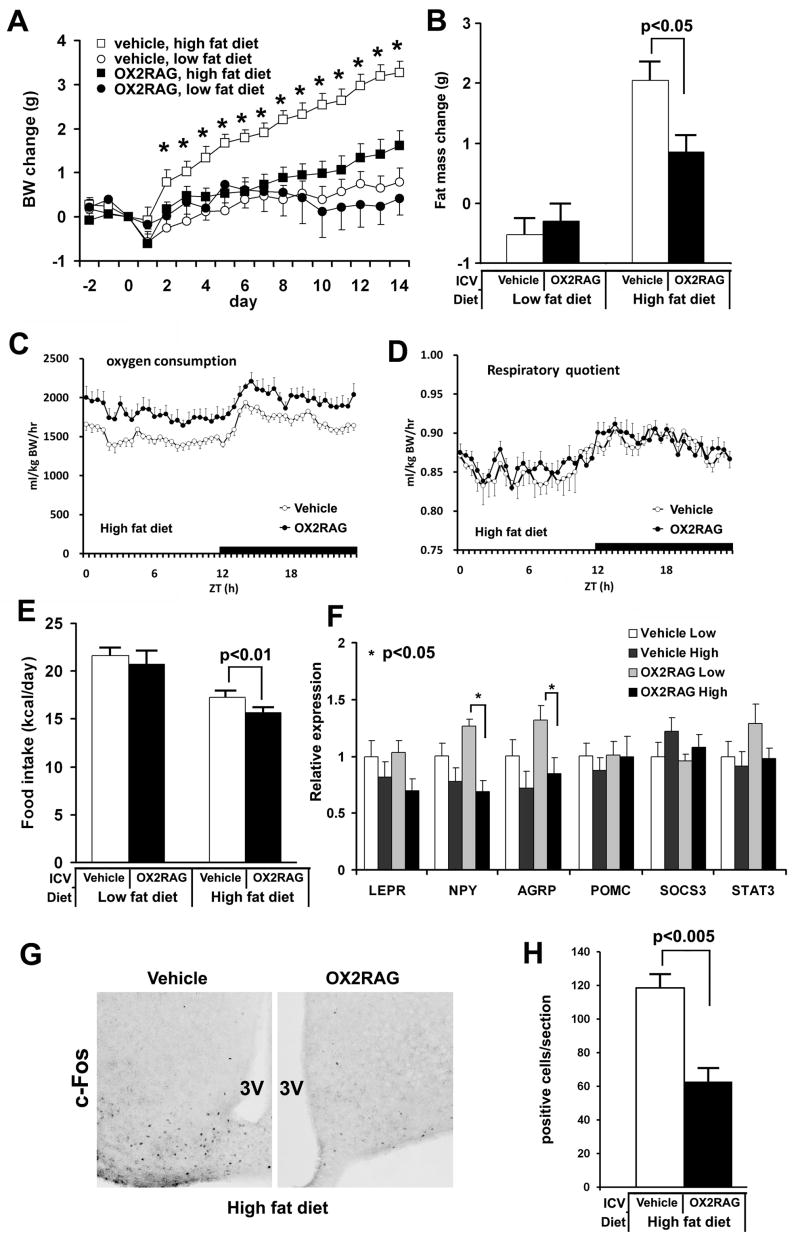
OX2R Agonist Prevents Diet-Induced Obesity
Our genetic studies implicate the OX2R pathway as mediator of the effects of orexin overexpression upon energy homeostasis. To further test the hypothesis that central enhancement of orexin-OX2R signaling confers resistance to diet-induced obesity, an OX2R selective agonist [Ala11, D-Leu15] Orexin-B (Asahi et al., 2003) was continuously infused in the lateral ventricles of wild-type mice for 14 days. The administration of the OX2R selective agonist suppressed weight gain on a high fat diet without altering weight homeostasis on a low fat diet (Figure 5A). Importantly, the OX2R selective agonist had no obvious effect upon OX2R-deficient mice on a high fat diet (n=4, weight gain 3.33±0.61 g, P=0.67), verifying the specificity of the agonist in vivo. Following 14 days, the agonist-infused wild-type mice gained significantly less fat mass than did the vehicle-injected mice on a high fat diet, and no effect was observed on a low fat diet (Figure 5B). When centrally-infused mice fed high fat diet were monitored in metabolic chambers, OX2R agonist infusions resulted in consistently greater energy expenditures (Figure 5C) but not respiratory quotients (Figure 5D) or locomotor activity (data not shown), over vehicle-infused controls.
Figure 5. Effect of OX2R selective agonist on diet-induced obesity.
(A) The daily body weight changes of chronically ICV injected mice. ICV and high fat diet begin at day 0. The body weight growths of the OX2R selective agonist-injected mice (0.5 nmol/d) are significantly lower than those of vehicle-injected mice on a high fat diet (p<0.0005), whereas there is no significant difference in the body weight growth between them on a low fat diet (p=0.45).
(B) The fat masses change of mice administered with the OX2R-selective agonist or vehicle on different fat diets during 14 days.
(C) The oxygen consumption with effective mass correction of OX2R agonist-infused mice on a high fat diet were higher than vehicle-infused mice (p<0.0005, repeated ANOVA). Data were sampled every 30 min.
(D) The respiratory quotient of OX2R agonist-infused mice on a high fat diet was similar to that of vehicle-infused mice.
(E) The averaged daily food intake of mice injected with the OX2R selective agonist or vehicle on different fat diets during 14 days.
(F) Hypothalamic gene expressions at the end of OX2R selective agonist administration are determined using q-PCR. Gene expressions are normalized by GAPDH.
(G) Immunostaining for c-Fos in ARH region of mouse on a high fat diet during central administration of OX2R agonist or vehicle; 3V, third ventricle.
(H) The number of c-Fos positive cells in ARH region.
The numbers of mice per group are 7–14 mice, 6–7 mice, and 5–6 mice for (A, B, C), (D, G, H), and (E,F), respectively. Data are expressed as means ± SEM.
As sleep/wake disturbances could affect food intake and energy expenditure, we recorded EEG/EMG signals during central OX2R agonist or vehicle infusions. Mice receiving OX2R agonist exhibited total wake or sleep times during both light and dark phases that closely resembled vehicle controls, irrespective of dietary condition (Figure S11). As predicted from previous studies (Willie et al., 2003), OX2R agonism continued to promote consolidation of behavioral states as demonstrated by increased wake and NREM episode durations in mice maintained on low fat diet. As this consolidation was not evident under high fat fed conditions, sleep/wake change cannot be the primary cause in metabolic effects of enhanced orexin signaling observed predominantly under high fat conditions.
We observed an expected homeostatic reduction of food intake in mice maintained on high fat diet compared to low fat diet (West et al., 1992), and administration of the agonist significantly enhanced this effect by further suppressing food intake selectively in mice fed high fat diet (Figure 5E). After 14 days of OX2R agonist administration, we observed reduced hypothalamic mRNA expression of orexigenic factors, NPY and AGRP on a high fat diet, compared to those on a low fat diet (Figure 5F). Indeed, the number of c-Fos-positive cells in ARH region was significantly reduced in OX2R agonist-administered mice on a high fat diet (Figure 5G, H). The reduction of c-Fos-positive cell number was particularly notable in the ventromedial aspect of ARH (Figure 5G) in which orexigenic NPY/AGRP neurons are located (Horvath, 2005), consistent with the observed reduction in food intake and in NPY/AGRP mRNAs we observed under this condition.
Leptin Mediates Anti-Obesity Effects of Orexin
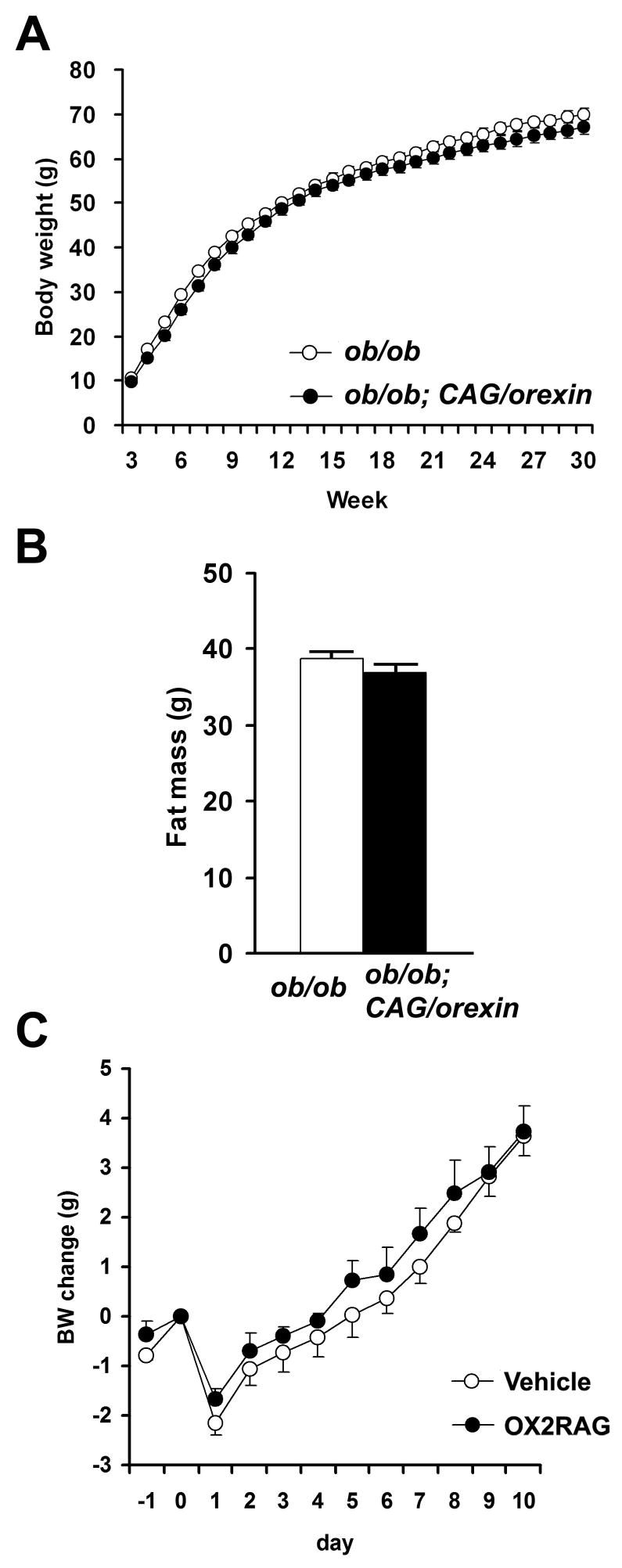
Leptin negatively regulates body weight, suppresses food intake, and increases energy expenditure by inhibiting NPY/AGRP neurons and activating POMC neurons of ARH. Diet-induced obesity is associated with leptin resistance resulting from signal transduction abnormalities in ARH (Myers et al., 2008). OX2R is highly expressed in ARH, and the effects of circulating leptin upon ARH resemble some effects of increased orexin-OX2R signaling that we observed. We hypothesized, therefore, that leptin signaling mediates some of the metabolic effects of orexin. To examine the consequences of orexin signaling enhancement on mice in the absence of leptin activity, we crossed CAG/orexin transgenic and leptin-deficient ob/ob lines. Remarkably, the CAG/orexin transgene had no impact upon weight gain or fat mass of leptin-deficient ob/ob mice (Figures 6A, B), suggesting that indeed the anti-obesity effect of CAG/orexin depends upon leptin activity. We then centrally administered OX2R agonist to ob/ob mice and similarly found no significant effect upon weight gain under low or high fat dietary conditions (Figures 6C). We also observed no effect of OX2R agonist compared to vehicle administration upon core body temperature of ob/ob mice (data not shown).
Figure 6. No effect of orexin overexpression on the weight gain of ob/ob mouse.
(A) The body weight growth of ob/ob; CAG/orexin male mice was similar to that of ob/ob male mice (p=0.76).
(B) Fat mass of 28 week-old ob/ob male mice with or without CAG/orexin transgene. There is no significant difference in fat mass for both male and female. 13–20 mice per group.
(C) The body weight growth of OX2R agonist-infused ob/ob mice was similar to that of vehicle-injected ob/ob mice maintained on a low fat (C) and a high fat diet. Data are expressed as means ± SEM.
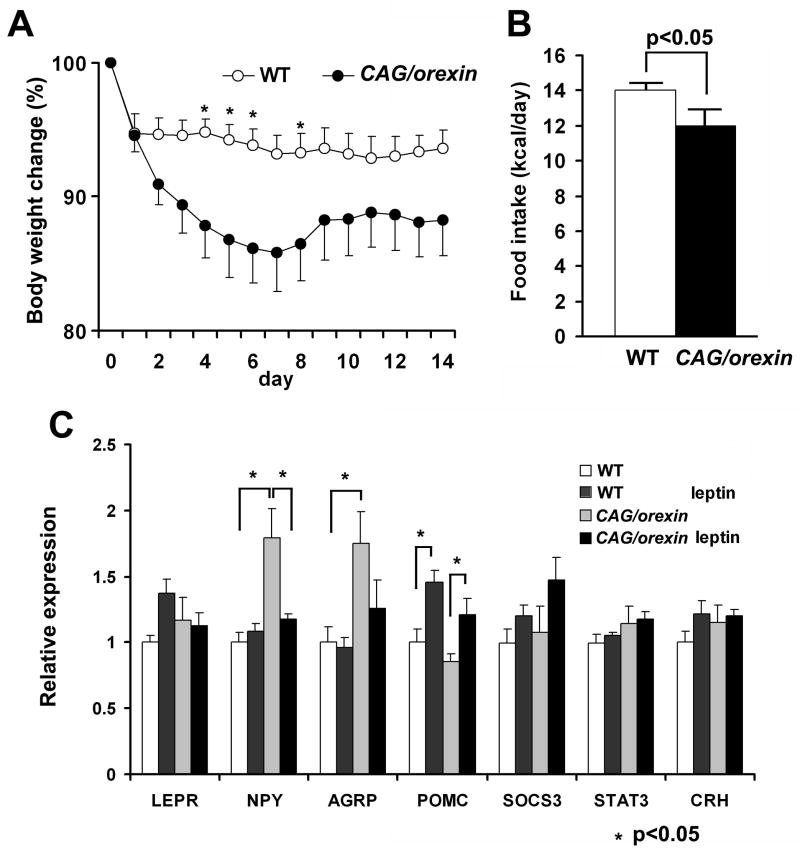
We directly examined whether orexin overexpression alters sensitivity to leptin. Leptin was continuously administered in the lateral ventricles of CAG/orexin and wild-type littermate pairs. Mice (3–4 month-old) were maintained on a low fat diet in order to initially match as to body weight (WT 29.7±3.6ng/ul and CAG/orexin 27.0±3.1ng/ul). Both wild-type mice and CAG/orexin mice lost weight during the administration of leptin, but CAG/orexin mice showed significantly enhanced weight loss and anorexia compared to wild-type mice on a low fat diet (Figure 7A, B), indicating that increased orexin signaling is associated with a more leptin-sensitive state.
Figure 7. Increased sensitivity of orexin overexpression mouse to leptin.
(A) The body weight changes during chronic ICV injection of leptin (2ug/day) for 14 days, CAG/orexin mice on a low fat diet show larger weight loss compared with wild-type mice (p<0.05).
(B) Daily food intake of CAG/orexin mice during chronic injection of leptin is smaller than that of wild-type mice.
(C) Hypothalamic gene expressions at the end of leptin administration. 6–8 mice per group. Data are expressed as means ± SEM. *p<0.05
Compared to control wild-type mice, 14 days of central leptin administration resulted in basal hypothalamic expression levels of NPY and AGRP and an expected induction of POMC mRNA (Figure 7C). In contrast, under basal conditions, CAG/orexin transgenic mice showed increased expression of NPY and AGRP, but not POMC mRNA. While we detected no significant changes in the expression of LEPR, SOCS3, or STAT3 gene products, the overall profile of altered hypothalamic gene expression we detected are consistent with the physiological state of anorexia and weight loss observed in mice undergoing leptin administration.
Discussion
When challenged with high fat diet, CAG/orexin mice maintain elevated energy expenditure, decreased food intake, and resistance to diet-induced obesity, hyperleptinemia, and hyperinsulinemia, although these mice show normal adiposity and energy homeostasis under low fat diet. Molecular genetic dissection of the metabolic phenotype utilizing CAG/orexin; OX1R−/− and CAG/orexin; OX2R−/− mice indicated that OX2R predominantly mediates these anti-adipogenic effects and improved insulin sensitivity. Central infusion of an OX2R agonist confirms the role of central orexin-OX2R signaling in protection from high fat diet-induced obesity. Furthermore, the anti-adipogenic effects of genetic or pharmacologic enhancement of orexin signaling require leptin, and CAG/orexin mice exhibit increased sensitivity to exogenous leptin infusion.
Technical Considerations Regarding CAG/orexin Transgene
The CAG promoter is a universal, constitutively active promoter, yet ectopic orexin production was restricted to a limited number of tissues, likely due to the necessity of lineage-specific enzymatic machinery required for neuropeptide production. Likewise, immunohistochemical localization of orexin-A peptide does not demonstrate homogenous presence of the antigen throughout brain parenchyma, but restricted presence at specific brain regions. In CAG/orexin mice, the appearance and number of strongly orexin-A-positive neurons in LHA (endogenous orexin cells) and the density of orexin-A-positive fibers in brain regions to which endogenous orexin neurons normally project resemble wild-type mice (Figure S2). Additionally, we observe a diffuse background ectopic orexin-A immunoreactivity especially in the medial basal hypothalamus including the ARH (Figures S1, S2). Ectopically expressed orexin confers physiologic signaling, which is demonstrated by our previous result that the CAG/orexin transgene rescues the narcolepsy-cataplexy phenotype (Mieda et al., 2004). Moreover, dependency of the CAG/orexin phenotype upon the intact OX2R gene demonstrates that the metabolic phenotype of CAG/orexin mice is not an artifact, but the physiological effect of increased orexin-OX2R signaling.
Although we cannot rule out a contribution of ectopic orexin production in the thyroid, adrenal medulla, and pancreatic islets to the phenotype of CAG/orexin mice, we found no indication of primary peripheral endocrine disturbance in CAG/orexin mice. CAG/orexin mice have normal levels of serum TSH, T3, T4, corticosteroid, and urinary catecholamines, and we observed improved glucose metabolism consistent with increased insulin sensitivity and reduced leptin levels that correspond in the expected manner to reduced adiposity. Furthermore, replication of anti-adipogenic effects with central OX2R agonist administration suggests that the metabolic effects of enhanced orexin signaling originate centrally.
Orexin Signaling Promotes Negative Energy Balance
Energy balance is a function of caloric intake and energy expenditure, and our data indicate that both orexin overexpression and OX2R agonist infusion increase energy expenditure and further suppress consumption of a high fat diet, providing the mechanistic rationale for observed resistance to adiposity. While this result seems at odds with the well-documented acute pharmacologic orexigenic activity of orexin (Sakurai et al., 1998; Willie 2001), chronic central administration of orexin-A does not support increased food consumption or anabolism in rats (Yamanaka et al., 1999). This suggests that the acute appetite-promoting effects of orexin peptides may be temporary, or progressively overwhelmed by counter-regulatory mechanisms that oppose weight gain.
Low fat-fed mice carrying the CAG/orexin transgene or treated with OX2R agonist for two weeks demonstrated elevated expression of the orexigenic genes NPY and AGRP. These changes could represent a direct effect as orexin acutely stimulates neurons of the ARH when microinjected (van den Top et al., 2004; Yamanaka et al., 2000), and orexin-stimulated food consumption depends pharmacologically upon NPY signaling (Yamanaka et al., 2000). An alternative mechanism for up-regulated NPY and AGRP transcription could be a compensatory response to relative negative energy balance since we noted significant differences from controls only when mice were maintained on calorie-poor rather than calorie-dense chow.
Despite differences in orexigenic effects across different experimental paradigms, consistent and unifying results from pharmacologic and genetic studies indicate that orexin gain-of-function promotes energy expenditure while loss-of-function promotes energy conservation. Just as the orexin system is believed to orchestrate disparate circuits of the ascending arousal system to maintain a consolidated state of arousal, it may also normally serve to consolidate the activity in parallel reward and metabolic networks that control behavioral and homeostatic responses to support energy expenditure. The exact peripheral (downstream) mechanisms for the orexin-mediated increases of energy consumption remain unclear. Although we did not detect significant increase in urinary catecholamines or basal blood pressure, the data do not exclude the possibility of a subtly increased sympathetic tone in certain peripheral tissues. Indeed, we speculate that the sympathetic pathways are one of likely downstream mechanisms for the increased metabolic rate under enhanced orexin signaling.
Interactions of Orexin and Leptin Signals
Anti-adipogenic effects of orexin-OX2R signaling require the presence of leptin, and orexin overexpressing mice showed increased sensitivity to catabolic-anorectic effects of exogenous leptin. These findings suggest that leptin mediates the suppressive effect of enhanced OX2R signaling on diet-induced obesity. Leptin-responsive neurons are found in ARH, VMH, DMH, LHA, and tuberomamillary nucleus of the hypothalamus (Elmquist, 2000). These nuclei receive orexin innervations, express high levels of OX2R, and exhibit ectopic orexin immunostaining in CAG/orexin transgenic mice. Among these, ARH is a particularly critical nexus for body weight regulation that monitors peripheral energy storages and enteral feeding status through integration of circulating leptin and insulin, metabolites, and vagal relays. Through outputs to other hypothalamic and brainstem sites, ARH modulates the thresholds triggering drives to eat and expend energy, and it influences insulin secretion and sensitivity (Horvath 2005; Coppari et al 2005; Meyer et al., 2008; Willie and Woolsey, 2008). Moreover, ARH harbors cellular abnormalities underlying acquired leptin resistance (Kievit et al., 2006), and reduced leptin sensitivity in ARH has been causally linked with diet-induced obesity (Enriori et al., 2007). While ARH neurons project to orexin neurons of the LHA, ARH receives dense reciprocal orexin fiber innervation and expresses mainly OX2R receptor (Cluderay et al., 2002; Peyron et al., 1998; Marcus et al., 2001). Acute microinjections of orexin-A into ARH increase oxygen consumption and body temperature under anesthesia (Wang et al., 2003).
The mechanism by which orexin and leptin signals interact remains unclear. Neurons expressing both LEPR and OX2R may have convergent intracellular second messenger signaling including extracellular factor-regulated kinase (ERK) and the Janus kinase JAK2/STAT3 pathways (Myers et al., 2008; Zhu et al., 2003). In ARH, leptin-responsive neurons such as those expressing NPY/AGRP are directly excited by orexin while POMC neurons are directly inhibited by orexin (Muroya et al., 2004), but inhibitory GABAergic interneurons in ARH may also be activated via postsynaptic OX2R (Burdakov et al., 2003), predicting complexity in up- or down-regulation of these circuits. Fos immunostaining reveals reduced neuronal activity in a population of ARH neurons following two weeks of OX2R agonist administration on high fat diet, but the true molecular identity of these cells and the direct versus indirect nature of this effect requires investigation.
Orexin neurons could directly sense lipids through kinetics of fatty acid metabolites to alter feeding behavior and energy homeostasis. However, we observed CAG/orexin transgenic mice to be also resistant to aging-associated adiposity, even when maintained on a low fat diet, and we detected no significant differences in circulating cholesterol or fatty acids among genotypes or dietary conditions (data not shown). Therefore, abnormal kinetics of hypothalamic fatty acid metabolism alone is unlikely to explain the obesity resistant CAG/orexin phenotype. Furthermore, endogenous orexin neurons are themselves unlikely to play a crucial lipid-sensing role in energy homeostasis as CAG/orexin mice in which endogenous orexin neurons have been selectively eliminated remain lean (JTW and TS, unpublished observations).
Potential Role of OX1R on Glucose Metabolism
The unifying scheme in the present study is that OX2R, but not OX1R, is the primary receptor that mediates the beneficial effects of orexin gain-of-function under high fat diet. The observed improvements in glucose metabolism and insulin sensitivity could largely be explained by the OX2R-mediated reduction of body adiposity. However, there is a notable exception: we observed that OX1R deficiency alone, without orexin overexpression, can improve glycemia and insulin sensitivity on high fat diet (Figures 4A, 4B), despite the fact that OX1R−/−and wildtype mice are similarly obese under high fat diet (Figures 1C, 2C). This suggests that endogenous levels of orexin acting on OX1R may in part mediate the deleterious effects of high fat diet on glucose metabolism. Indeed, OX1R is expressed in the solitary tract nucleus and dorsal motor nucleus of the vagus (Marcus et al., 2001), which participates in the regulation of hepatic glucose production (Pocai, et al., 2005). Although OX1R is also detected in beta cells of pancreatic islets, role for orexin, if any, in the pancreatic islet is controversial (Heinonen et al., 2008). At any rate, under orexin overexpression, the OX2R-mediated effects prevail and the presence or absence of OX1R does not affect (the improvement of) glycemia or insulinemia. The specific role of OX1R on glucose regulation merits further investigation.
Therapeutic Implications
The robust innervation by the orexin system of the whole brain and the multiple phenotypic aspects of orexin-deficient animals such as cataplexy, attenuated morphine dependence, and diminished stress response has led to conceptualization of the orexin system as a hypothalamic output pathway controlling arousal, motivational behavior, and autonomic responses (Chemelli et al., 1999; Sakurai, 2007). Our results demonstrate that orexin signaling also has the capacity to primarily promote energy expenditure via leptin sensitization. Augmentation of OX2R signaling or its downstream targets beneficially alters hypothalamic setpoints controlling metabolic rate, food intake, and leptin and insulin sensitivity. Similar interventions in humans might prevent or reverse the effects of consumption of calorie-dense food that promote or maintain pathological adiposity and metabolic syndrome. From a therapeutic standpoint, it is important to note that orexin gain-of-function did not overtly alter the basal blood pressure, or the thyroid, glucocorticoid, and catecholiamine status in our models.
While continuous orexin gain-of-function did not induce locomotor hyperactivity or perturb overall amounts of sleep and wakefulness in CAG/orexin mice or OX2R agonist-infused mice, further sleep/wake characterization of these models is warranted. The metabolic syndrome is a disorder not only of obesity and insulin insensitivity, but possibly also inactivity, sleep/wake disturbances, and co-morbid depression (Fabricatore and Wadden, 2007). Daytime administration of an OX2R agonist to such individuals could have multiple beneficial effects by maintaining elevated metabolic rate while also promoting daytime wakefulness and consolidating sleep/wake states. The orexin system has emerged as a key target for therapeutic intervention in disorders associated with hypothalamic dysfunction, including not only narcolepsy and hypersomnia, but now also the metabolic syndrome.
Experimental Procedures
Animals
All mice were backcrossed more than 10 generations to the C57BL/6J strain. In CAG/orexin transgenic mice, the expression of preproorexin is controlled by the chimeric CAG promoter constructed from the chicken beta actin promoter and the cytomegalovirus immediate early gene enhancer (Mieda et al., 2004). Each genotypic group was compared by pairing of littermates as follows: wild-type with CAG/orexin, OX1R−/− (Kisanuki et al., 2000) with OX1R−/−; CAG/orexin, OX2R−/−(Willie et al., 2003) with OX2R−/−; CAG/orexin, ob/ob with ob/ob; CAG/orexin. Ob/ob (Zhang et al., 1994) mice were obtained from The Jackson Laboratory. Mice were provided food and water ad lib, maintained on a 12 hr light dark cycle at all times, and were housed at 2 or 3 mice per cage under controlled temperature and humidity unless otherwise specified. All procedures were approved by the appropriate institutional animal care and use committees and were carried out in strict accordance with NIH guidelines.
Body weight study
For diet-induced obesity, all mice were fed a low fat diet (standard chow 8664 F6 Rodent Diet; Harlan Teklad) until 8 weeks of age. At 8 weeks of age, mice were assigned randomly to feed either a low fat or a high fat diet (D12451 Research Diet). A low fat diet provided 4.1 kcal/g of energy (67% carbohydrate, 20% protein, and 13% fat). A high fat diet provided 4.7 kcal/g of energy (35% carbohydrate, 20% protein, and 45% fat). Body weight was measured weekly until 30 weeks of age. At 28 weeks of age, mice were subjected to NMR (Minispec NMR Analyzer, Bruker) to measure fat and lean mass per the manufacturer’s instructions. At 30 weeks of age, mice were euthanized to collect blood and measure blood glucose. Serum was collected from centrifuged blood and stored at -80C until use. Ob/ob and ob/ob; CAG/orexin mice were fed a low fat diet until 30 weeks of age.
Blood Analysis
For analysis of serum, we used Mouse Leptin and Ultra Sensitive Rat Insulin ELISA kits with Mouse Insulin Standard (Crystal Chem). Whole blood glucose levels were measured using a standard clinical glucometer (Elite, Bayer). For glucose tolerance tests, 21 to 25 week-old mice were fasted for 12 hours from ZT16 and then injected glucose (1.5 g/kg of body weight, i.p.) at ZT4. Tail blood was collected at 0, 15, 30, 60, and 90 min after injection.
Metabolic Cage Study
Indirect calorimetry and locomotor data were simultaneously measured using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments). For genetic studies, 16–20 week-old animals were individually housed in calorimeter chambers and 3 days of data collection followed a 4-day acclimatization period. For pharmacologic studies, ICV cannulation surgery was performed at 10–12 weeks of age and high fat feeding initiated in an acclimatization chamber. After 4 days of acclimatization, each animal was housed in a metabolic chamber for 4.5 days. We calculated metabolic parameters based on the following equations:
Chronic ICV Injection
Three to four month-old male C57B/6J mice were single-housed one week before surgery and fed a low fat diet. Mice were anesthetized with ketamine and xylazine (100 mg/Kg and 10 mg/Kg, respectively, i.p.). A cannula (Brain infusion kit III, Alzet) was implanted into the right lateral ventricle (0.3 mm posterior from the bregma, 0.9 mm lateral from the midline, and 2.4 mm from the surface of skull) using standard sterile stereotactic techniques. An osmotic minipump (model 2001, Alzet) was attached to the cannula and implanted in the subcutaneous space during the same surgical session. The OX2R selective agonist ([Ala11, D-Leu15] Orexin-B, Asahi et al 2003; American Peptide) or vehicle was continuously injected in the lateral ventricle for 14 days (0.5 nmol/day). The agonist was diluted with vehicle (Dulbecco’s PBS, Sigma) immediately before use. At the day of surgery, the implanted mice were randomly assigned to a low fat diet or a high fat diet. Body weight and food intake were monitored daily for 14 days, and fat mass was detected by NMR immediately after surgery and again at day 14. 12 week-old ob/ob male mice were used for chronic ICV infusion of OX2R agonist for 10 days as described above. For leptin administration experiments, weight-matched 3–4 month-old CAG/orexin and wild-type littermates were continuously injected with leptin (2ug/day, PreproTech) as described above while maintained on a low fat diet. Body weight and food intake were monitored daily for 14 days.
Quantitative PCR
Hypothalamus was dissected microscopically and included tissue caudal from of the optic chiasm, rostral from the mammillary bodies, 1 mm bilateral from the midline and 1.5 mm dorsal from the ventral surface. This dissected tissue includes ARH, VMH, DMH, PVN, anterior hypothalamic area, and a part of LHA. Total RNA was isolated using RNeasy Mini kit and used for cDNA synthesis by random hexamer and Omniscript reverse transcriptase (Qiagen). Real-time quantitative PCR reactions were performed on cDNA with ABI Prism 7000 Sequence Detection System using SYBR GREEN PCR Master Mix (Applied Biosystems) according to the manufacturer’s manual. GAPDH mRNA level was used for normalization.
Immunohistochemistry
After 2 weeks of continuous administration of OX2R agonist or PBS, mice on a high fat diet were harvested during early dark phase under a red light. The immunohistochemistry for c-Fos was performed using free-floating method as described previously (Chemelli et al., 1999) utilizing anti-c-Fos polyclonal antisera (Ab-5; Oncogene). Fos-positive cells in ARH region of two sections per animal were counted by an observed blinded to treatment group.
Data Analysis
Body weight growths and glucose tolerance tests were examined using repeated measure analysis of variance (ANOVA) followed by Tukey’s post-hoc test except where otherwise specified.
Supplementary Material
Acknowledgments
We thank J Horton, N Anderson, D Smith, L Brule, A Skach, C Lee, HC Lee, S Dixon, R Floyd, M Thornton and the O’Brien Kidney Research Core Center for technical support; T Motoike, C Sinton, Y Ikeda, H Kumagai, S Baldock, I Chang, A Chang, A Shaito, S Ogawa, J Long, and other lab members for critical discussions and manuscript review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asahi S, Egashira S, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, Ihara M, Morishima H. Development of an orexin-2 receptor selective agonist [Ala(11), D-Leu(15)]orexin-B. Bioorg Med Chem Lett. 2003;13:111–113. doi: 10.1016/s0960-894x(02)00851-x. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Liss B, Ashcroft FM. Orexin Excites GABAergic Neurons of the Arcuate Nucleus by Activating the Sodium–Calcium Exchanger. J Neurosci. 2003;23:4951–4957. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC., Jr The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Elmquist JK. Anatomic basis of leptin action in the hypothalamus. Front Horm Res. 2000;26:21–41. doi: 10.1159/000061020. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol. 2006;2:357–377. doi: 10.1146/annurev.clinpsy.2.022305.095249. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–242. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Heinonen MV, Purhonen AK, Mäkelä KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiol. 2008;192:471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Kisanuki Y, Chemelli RM, Tokita S, Willie JT, Sinton CM, Yanagisawa M. Behavioral and polysomnographic characterization of orexin-1 receptor and orexin-2 receptor double knockout mice. Sleep. 2001;24:A22. [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. ProcNatlAcadSciUSA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Münzberg H. Mechanisms of Leptin Action and Leptin Resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, Okun M, Rogers W, Brooks S, Mignot E. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–388. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8:373–399. doi: 10.1016/j.sleep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. ProcNatlAcadSciUSA. 1998;95:4061–4065. doi: 10.1073/pnas.95.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AGRP pacemaker neurons in the hypothalamic arcuate nucleus. NatNeurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Wang J, Osaka T, Inoue S. Orexin-A-sensitive site for energy expenditure localized in the arcuate nucleus of the hypothalamus. Brain Res. 2003;971:128–34. doi: 10.1016/s0006-8993(03)02437-5. [DOI] [PubMed] [Google Scholar]
- West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Willie JT, Woolsey TA. Central Pathways: Targets for Treating Obesity and Metabolic Syndrome. AANS Neurosurgeon. 2008;17:17. [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai A, Matsuzaki I, Miwa Y, Goto K, Sakurai T. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000;859:404–409. doi: 10.1016/s0006-8993(00)02043-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, Sakurai T, Goto K. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–266. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.