Abstract
Naked mole-rats (Heterocephalus glaber) are eusocial rodents that live in large subterranean colonies including a single breeding female and 1-3 breeding males; all other members of the colony, known as subordinates, are reproductively suppressed. We recently found that naked mole-rats lack many of the sex differences in the brain and spinal cord commonly found in other rodents. Instead, neural morphology is influenced by breeding status, such that breeders, regardless of sex, have more neurons than subordinates in the ventromedial nucleus of the hypothalamus (VMH), and larger overall volumes of the bed nucleus of the stria terminalis (BST), paraventricular nucleus (PVN) and medial amygdala (MeA). To begin to understand how breeding status influences brain morphology, we examined the distribution of androgen receptor (AR) immunoreactivity in gonadally intact breeders and subordinates of both sexes. All animals had AR+ nuclei in many of the same regions positive for AR in other mammals, including the VMH, BST, PVN, MeA, and the ventral portion of the premammillary nucleus (PMv). We also observed diffuse labeling throughout the pre-optic area demonstrating that distribution of the AR protein in presumptive reproductive brain nuclei is well-conserved, even in a species that exhibits remarkably little sexual dimorphism. In contrast to other rodents, however, naked mole-rats lacked AR+ nuclei in the suprachiasmatic nucleus and hippocampus. Males had more AR+ nuclei in the MeA, VMH, and PMv than did females. Surprisingly, breeders had significantly fewer AR+ nuclei than subordinates in all brain regions examined (VMH, BST, PVN, MeA, and PMv). Thus, social status is strongly correlated with AR immunoreactivity in this eusocial species.
Keywords: androgen receptor, bed nucleus of the stria terminalis, medial amygdala, naked mole-rat, paraventricular nucleus, plasticity, premammillary nucleus, sex difference, social status, testosterone, ventromedial nucleus of the hypothalamus
Introduction
Naked mole-rats (Heterocephalus glaber) are uniquely social mammals that exhibit the closest mammalian equivalent to eusociality. These small rodents are native to Africa and live in subterranean colonies typically comprised of 60-80 individuals, including a single breeding female (the queen) and 1-3 breeding males (Jarvis, 1981). All other members of the colony are reproductively suppressed, possibly via behavioral intimidation from the queen (Faulkes and Abbott, 1993; Smith et al., 1997), and are socially subordinate to the breeders. Subordinates exhibit no sexual behaviors, but contribute to overall maintenance and survival of the colony, including caring for pups (Brett, 1991; Jarvis, 1981; Lacey and Sherman, 1991; Lacey et al., 1991).
Naked mole-rats are remarkably sexually monomorphic. Among subordinates, there are no sex differences in overall body size, ano-genital distance, or the expression of a large variety of behaviors (Jarvis, 1991; Lacey and Sherman, 1991; Lacey et al., 1991; Pepper et al., 1991; Peroulakis et al., 2002). In addition, we have recently demonstrated that naked mole-rats lack many of the sexual dimorphisms in the brain and spinal cord that are seen in other mammals (Holmes et al., 2007; Peroulakis et al., 2002; Rosen et al., 2007; Seney et al., 2006). We hypothesize that the reduction in sex differences in naked mole-rats may be related to their unique reproductive strategy. In nature, it is estimated that fewer than 5% of all naked mole-rats ever achieve reproductive status (Jarvis et al., 1994).
Social status is not pre-determined in this species, however. Adult subordinates can become breeders given the appropriate social conditions, i.e., if a breeding member of the colony dies, or if the subordinate is removed from the colony and housed with an opposite sex mate (Brett, 1991; Faulkes and Abbott, 1991; Faulkes et al., 1990; Margulis et al., 1995). This suggests the potential for extensive physiological, behavioral, and neural plasticity in adulthood. Indeed, when animals were randomly assigned to remain subordinates within their colony, or to become new breeders, we previously found several changes in nervous system morphology associated with social and reproductive status (Holmes et al., 2007; Seney et al., 2006). Gonadally intact breeders, regardless of sex, had more neurons in the ventromedial nucleus of the hypothalamus (VMH), larger overall volumes of the bed nucleus of the stria terminalis (BST), paraventricular nucleus (PVN) and medial amygdala (MeA), and more motoneurons in Onuf’s nucleus than subordinates. Taken together, these data suggest that social status may be more important than sex in determining differentiation and plasticity in the nervous system of naked mole-rats.
Reproductive status is associated with increases in androgens in adult naked mole-rats of both sexes (Clarke and Faulkes, 1997, 1998; Faulkes et al., 1991). Androgens act via androgen receptors (AR), which are ligand-dependent transcription factors belonging to the superfamily of intracellular nuclear receptors (Prins, 2000). In other species, the AR is expressed in many of the brain regions involved in the control of sex-typical reproductive behaviors (e.g., Choate and Resko, 1992; Commins and Yahr, 1985; Kashon et al., 1996; Lu et al., 1998; Simerly et al., 1990; Wood and Newman, 1995). In addition, sex differences in the brain are organized and/or activated in adulthood by circulating testosterone acting via AR, or after conversion to an estrogen and binding to an estrogen receptor (ER), and both AR and ER are expressed in neural regions that are sexually dimorphic (e.g., Commins and Yahr, 1985; Simerly et al., 1990; Wood and Newman, 1995). No study has previously examined expression of gonadal steroid receptors in either the developing or adult naked mole-rat brain, however.
To begin to understand how social status may influence brain morphology, we investigated AR protein expression in gonadally intact breeding and subordinate naked mole-rats. Based on work in other species (e.g., Burmeister et al., 2007), we predicted that AR expression might be increased in the socially dominant breeders. The study of AR in the naked mole-rat brain also allowed us to explore putative hormone action in the brain of a species with an alternative reproductive strategy and relative absence of sex differences.
Materials and Methods
Animals, Housing and Tissue Collection
Animal history and housing conditions have previously been described (Holmes et al., 2007; Rosen et al., 2007; Seney et al., 2006). Briefly, age-matched animals were randomly assigned to remain subordinate or become breeders. To generate breeders, subordinate naked mole-rats were removed from their colonies and housed with an opposite sex individual from a different colony. All breeders had produced at least one litter. Brains from 5 breeding females, 5 breeding males, 4 subordinate females, and 4 subordinate males were collected; all animals were gonadally intact. Brains were removed and immersion fixed in 5% acrolein for 4 hours, then transferred to 30% sucrose in 0.1M phosphate buffer (PB) for cryoprotection. Frozen, 30 μm coronal sections were collected and stored at -20°C until use. All procedures adhered to NIH guidelines and were approved by the University of Connecticut Animal Care and Use Committee.
AR immunohistochemistry
A one in four series from each animal was processed for AR immunohistochemistry. Briefly, sections were rinsed 3×5 min with 0.05M tris buffered saline (TBS), and incubated in 1% sodium borohydride in 0.1M PB for 30 min. Following a 30 min incubation in blocking serum [0.3% Triton X-100 with 4% normal goat serum (NGS) and 1% H2O2 in TBS], tissue was transferred to the primary antibody solution (blocking serum plus 2 μl/ml of the polyclonal AR antiserum, PG21, a generous gift from Dr. Gail Prins) for 40 hours at 4°C. A 90 min secondary antibody incubation [1:400 goat anti-rabbit (Jackson ImmunoResearch, West Grove, PA) in TBS with 4% NGS] was followed by a 90 min ABC wash as per manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Visualization was achieved using a diaminobenzidine (DAB) reaction for 15 min (1 ml of 1.25% DAB, 10 μl of 30% H2O2, and 120 μl of 8% NiCl in 49 ml TBS). Three 5 min TBS washes were performed between all steps and after the DAB reaction. Tissue was then mounted onto gel coated slides, counter stained with methyl green and coverslipped with Permount (Fisher Scientific).
To confirm specificity of the PG21 antibody in naked mole-rats, several controls were performed. First, preadsorption of PG21 with AR21 peptide (aa 1-21 of the AR; generous gift from Dr. Gail Prins), which was used to generate the PG21 antiserum, completely abolished staining. Second, preadsorption of PG21 with AR462 (aa 462-478 of the AR; generous gift from Dr. Gail Prins), an unrelated peptide, failed to have any effect on staining. Third, omission of the PG21 antibody from the primary antibody solution (no primary control) resulted in no staining. Finally, as a positive control and to facilitate cross-species comparisons, brain sections from an adult male C57Bl/6 mouse were processed alongside the naked mole-rat sections.
AR Distribution and Quantitative Analyses
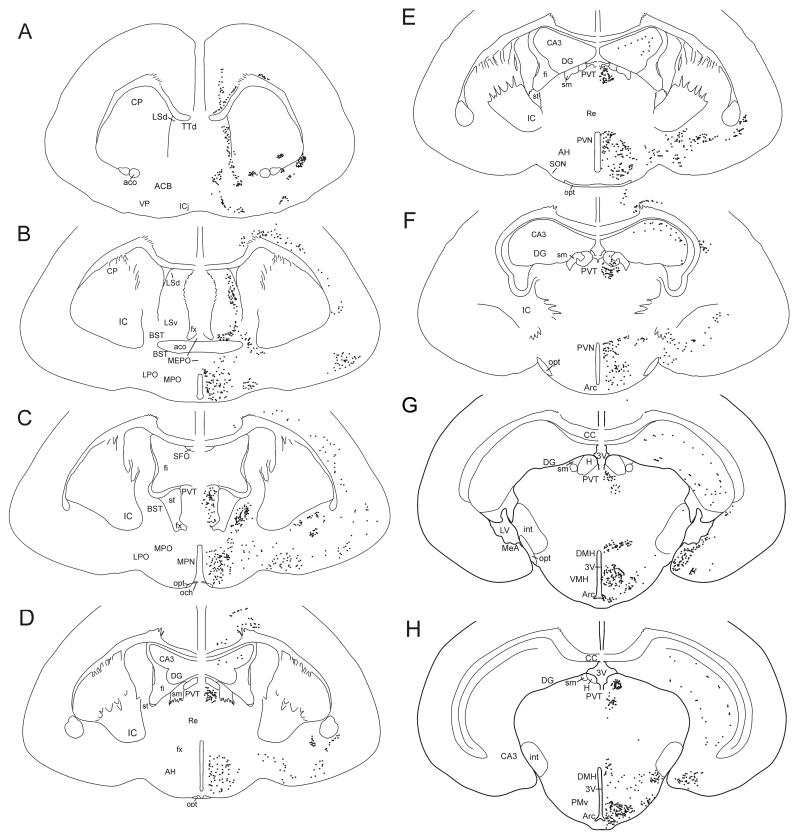
To evaluate the general distribution of AR protein, AR immunoreactivity throughout the forebrain was plotted onto composite camera lucida drawings of the naked mole-rat brain (Figure 1). Tissue caudal to the arcuate nucleus was not available for analyses.
Figure 1.
Camera lucida drawings of frontal sections through the forebrain of the naked mole-rat showing the distribution of AR+ cells (black dots). (A) through (H) represent sequentially more caudal sections. Neuronal structures are indicated on the left, and the location of AR+ cells on the right. The number of dots represents relative density of immunoreactivity. 3V, third ventricle; Aco, anterior commissure; ACB, nucleus accumbens; AH, anterior hypothalamus; Arc, arcuate; BST, bed nucleus of the stria terminalis; CA3, field CA3 Ammon’s horn; CC, corpus callosum; CP, caudate putamen; DG, dentate gyrus; DMH, dorsomedial nucleus of the hypothalamus; fi, septal fimbria; fx, fornix; H, habenula; IC, inferior colliculus; ICj, islands of Calleja; int, internal capsule; LPO, lateral preoptic area; LSd, dorsal lateral septum; LSv, ventral lateral septum; LV, lateral ventricle; MeA, medial nucleus of the amygdala; MEPO, median preoptic nucleus; MPN, medial preoptic nucleus; MPO, medial preoptic area; och, optic chiasm; opt, optic tract; PMv, ventral portion of the premammillary nucleus; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; TTd, tenia tecta, dorsal part; Re, nucleus reuniens; SFO, subfornical organ; sm, stria medullaris; SON, supraoptic nucleus; st, stria terminalis; VMH, ventromedial hypothalamus; VP, ventral pallidum.
Stereological analyses of the percentage of cells positive for AR immunoreactivity in the BST, PVN, VMH, MeA, and PMv were performed using StereoInvestigator software (MicroBrightField, Williston, VT). Cells were initially classified as either darkly labeled, lightly labeled, or unlabeled based on simple visual inspection. Because the pattern of results was the same whether only darkly labeled or all labeled cells were considered, we have combined dark plus light cell counts in the analyses presented here.
Briefly, outlines of each region were traced in each section, and unbiased estimates of the number of labeled (AR+) and unlabeled (AR-) cells were obtained using the optical disector method. Counting frames were either 16 × 16 μm2 or 20 × 20 μm2, depending on the size of the region; frame size was held constant across animals. Similarly, sampling grid size varied from 50 × 50 μm2 to 60 × 60 μm2 depending on brain region, and was held constant for all animals. Cell counts in the BST and PVN were performed bilaterally, whereas counts of the VMH, MeA, and PMv were unilateral (side randomly chosen). For the VMH, the dorsomedial (dm), central (c), and ventrolateral (vl) subregions were analyzed separately.
To account for possible group differences in total cell number in some brain regions (see Holmes et al., 2007), the percentage of AR+ cells was calculated for each region by dividing the number of AR+ cells by the total number of cells (AR+ and AR-cell counts combined). Percentages of AR+ cells were analyzed using two-way ANOVAs with sex and status as independent variables. To ensure that percentage scores were normally distributed, ANOVAs were repeated following an arcsine transformation (Fergusen and Takane, 1989). Transformation did not alter the pattern of significance for any brain region and these F and p values are reported below. Tearing or other tissue artifacts caused some animals to be removed from analyses for specific brain regions. Number of animals per analysis is indicated in the Figures.
Results
General distribution of AR
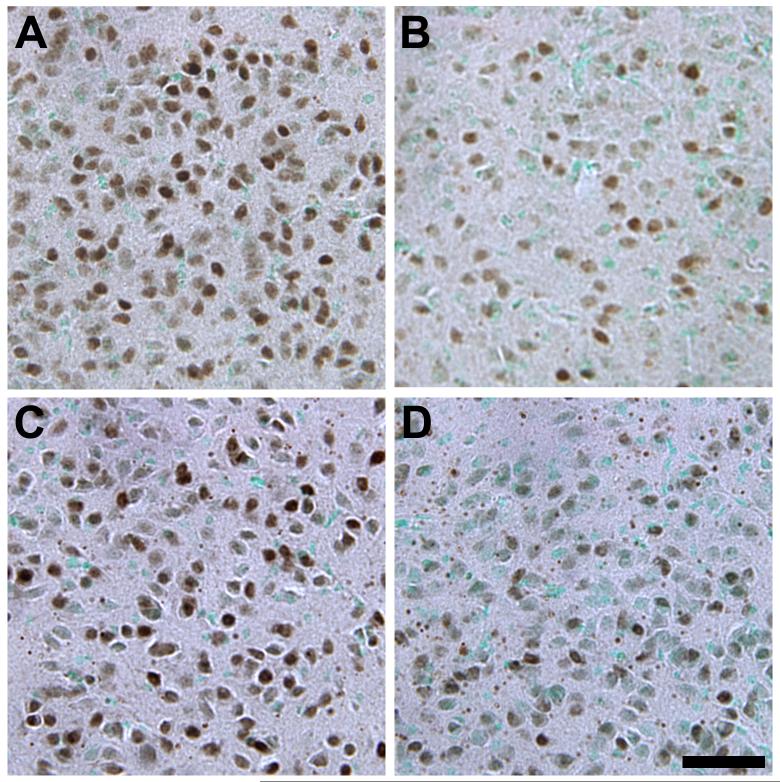
The overall distribution of the AR protein in naked mole-rats was similar in many ways to that reported for other mammals (Figure 1). AR immunoreactivity was predominantly nuclear and in cells with a neuronal morphology (Figure 2). AR nuclear staining was present in the BST, PVN, VMH, MeA, and PMv of all animals; quantification of AR in these regions is presented below. In addition, AR immunoreactivity was also present in cell nuclei in the islands of Calleja, lateral septum, ventral pallidum, medial forebrain bundle, diagonal band of Broca, preoptic area, basolateral amygdala, paraventricular thalamic anterior nucleus, anterior cortical amygdaloid nucleus, and the arcuate nucleus (Figure 1). Sparse labeling in the isocortex and CA2 and CA3 of the hippocampus appeared to be specific to pyramidal neurons. Interestingly, in contrast to sections from the mouse brain processed at the same time, and to previous published studies of AR distribution in other mammals (e.g., Commins and Yahr, 1985; Iqbal et al., 1995; Karatsoreos et al., 2007; Kashon et al., 1996; Simerly et al., 1990; Xiao and Jordan, 2002), naked mole-rats had remarkably little AR immunoreactivity in the suprachiasmatic nucleus (SCN) or CA1 of the hippocampus (Figure 3).
Figure 2.
Photomicrographs of AR immunoreactivity in the VMHdm of a subordinate female (A), breeding female (B), subordinate male (C), and breeding male (D). Sections were counterstained with methyl green. A higher percentage of cells was AR+ in subordinates than in breeders and in males than females. Scale bar = 50μm.
Figure 3.
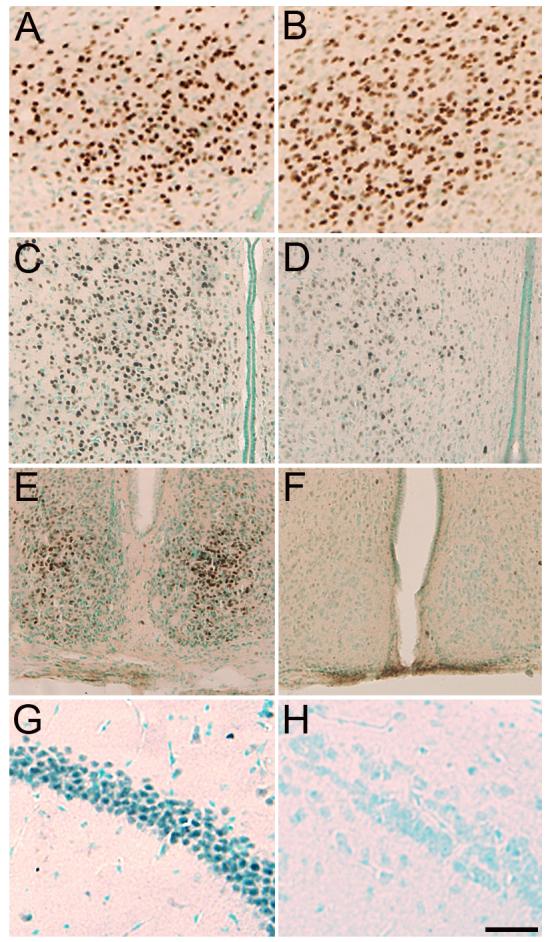
Photomicrographs of AR immunoreactivity in the PMv (A, B), preoptic area (C, D), SCN (E, F), and CA1 of the hippocampus (G, H) of a gonadally intact male mouse (A, C, E, G) and subordinate male naked mole-rat (B, D, F, H). A similar density of AR+ cells is seen in the PMv of naked mole-rats and mice. In contrast to the mouse, AR+ cells were more diffuse in the preoptic area and were markedly reduced in the SCN and CA1 of naked mole-rats. Scale bar = 75μm for A and B, 100μm for C-F, and 50μm for G and H.
Effects of social status and sex on AR immunoreactivity
Subordinates, regardless of sex, had a greater percentage of AR+ cells than breeders. This was true in all regions in which AR was quantified: the BST (F1,14 = 12.29; p = 0.004), PVN (F1,12 = 6.24; p = 0.028), MeA (F1,13 = 7.65; p = 0.016) and PMv (F1,10 = 26.51; p < 0.001), as well as all subregions of the VMH (dm: F1,12 = 26.50; p < 0.001; c: F1,10 = 12.23, p = 0.006; vl: F1,10 = 17.66; p = 0.002; Figures 4 and 5).
Figure 4.
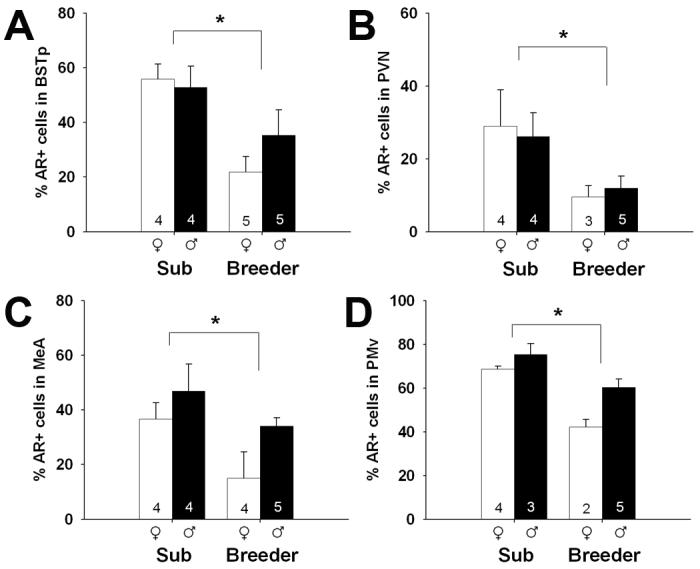
Mean (+/- SEM) percentage of AR+ cells in the BST (A), PVN (B), MeA (C), and PMv (D) of subordinate (Sub) and breeding naked mole-rats. Number of animals per group is noted at the base of each bar. White bars represent females and black bars represent males. Asterisks indicate significant main effects of social status: p = 0.004 (A), p = 0.028 (B), p = 0.016 (C), and p = 0.001 (D). A main effect of sex (favoring males) was also detected in the MeA and PMv. No status by sex interactions were detected on any measure.
Figure 5.
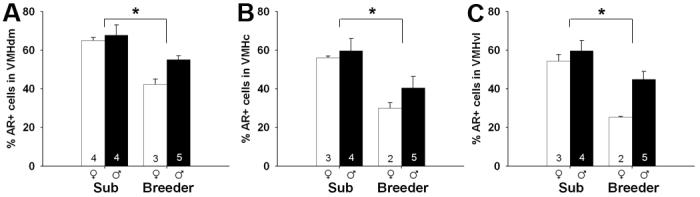
Mean (+/- SEM) percentage of AR+ cells in dorsomedial (A), central (B), and ventrolateral (C) subregions of the VMH. Number of animals per group is noted at the base of each bar. White bars represent females and black bars represent males. Asterisks indicate significant main effects of social status: p = 0.001 (A), p = 0.006 (B), and p = 0.002 (C). A main effect of sex (favoring males) was detected in the VMHdm and VMHvl. No status by sex interactions were detected.
In addition, a sex difference in AR immunoreactivity was found in several regions. Males had a higher percentage of AR+ cells than did females in the MeA (F1,13 = 5.48; p = 0.036), PMv (F1,10 = 9.48; p = 0.012), VMHdm (F1,12 = 4.96, p = 0.046) and VMHvl (F1,10 = 5.68; p = 0.038). We found no effect of sex in the BST, PVN, or the VMHc (all ps > 0.28). Effects of sex, when present, appeared slightly larger in breeders, than in subordinates (Figures 4 and 5), however, there were no significant sex-by-status interactions in any brain region (all ps > 0.24).
Non-nuclear staining
In addition to the well-studied distribution in neuronal nuclei, AR have in recent years been reported in glial cells and in extranuclear locations including the dendrites, axons and terminals of neurons (DonCarlos et al., 2003, 2006; Lorenz et al., 2005; Milner et al., 2007; Tabori et al., 2005). Although not the focus of this study, we did notice punctate staining in the brain regions examined here that also contained nuclear AR. Because this staining was eliminated by preabsorption of the primary antibody with the immunizing peptide, it may be specific. Stereological counts, performed as above, suggest that these puncta were more numerous in the BST of breeders (F1,14 = 4.98; p = 0.043). Breeders also had marginally more puncta in the VMHdm (F1,12 = 4.37, p = 0.058) (see Figure 2). There were no significant differences in the other brain regions examined, and no effect of sex on the number of immunoreactive puncta in any region (data not shown).
Discussion
The present data demonstrate that both social status and sex influence AR expression in the naked mole-rat brain. Of particular interest is that they appear to do so independently. Contrary to our expectation, we found that subordinate mole-rats, regardless of sex, had more AR+ nuclei than breeders in all brain regions examined. Because animals were randomly assigned to become a breeder or remain subordinate, this indicates that social status influences AR expression in the naked mole-rat brain. In addition, males, regardless of status, had more AR+ nuclei than females in the MeA, PMv and two subregions of the VMH (dm and vl), and there were no interactions between social status and sex in any brain region. While increased AR immunoreactivity in males relative to females is a feature common to several mammalian species, the sex differences in AR immunoreactivity seen here are the first sex differences documented in the nervous system of naked mole-rats. We also found evidence for non-nuclear AR immunoreactivity in the naked mole-rat brain. Small, AR+ puncta were variable in number, but found in most brain regions that also contained nuclear AR. Based on size and distribution, these puncta may be glial cells or nerve terminals, both of which have been shown to contain ER or AR in other systems (e.g., Blaustein, 1992; Garcia-Ovejero et al., 2002; Wagner et al., 1998). In the absence of electron microscopy, however, we can only speculate as to what structures these puncta are associated with, and therefore restrict the remainder of our discussion to the more traditional, nuclear AR.
The distribution of AR protein and mRNA is well conserved across mammalian species (Sar et al., 1990; Simerly et al., 1990). As a rule, AR is found throughout the hypothalamus and preoptic area as well as in telencephalic structures that project to these regions. Indeed, AR is found in the same structures investigated here (BST, PVN, VMH, MeA, and PMv) in diverse mammalian species including (but not limited to) rats, mice, hamsters, ferrets, opossums, sheep, and humans (e.g., Clancy et al., 1994; Fernández-Guasti et al., 2000; Herbison et al., 1996; Iqbal et al., 1995; Kashon et al., 1996; Lu et al., 1998; Simerly et al., 1990; Wood and Newman, 1995). The BST, PVN, MeA, VMH, and PMv comprise key nodes of an interconnected neural circuit that regulates neuroendocrine function and couples the expression of reproductive behaviors with appropriate environmental stimuli. Specifically, in other rodent species, the MeA receives sensory input from the accessory olfactory bulb and sends projections to the BST (De Vries and Simerly, 2002; Simerly, 2002). The BST in turn sends both direct and indirect projections to hypothalamic nuclei, including the PVN, VMH, and medial preoptic area (De Vries and Simerly, 2002; Simerly, 2002). The increased AR expression in males as compared to females seen here in the MeA, PMv, and subregions of the VMH, is consistent with a potential role for this circuit in the control of male sex behavior in naked mole-rats. In this sense, the present data demonstrate a conservation of AR distribution, and putative function, even in a species with a highly unusual social and reproductive hierarchy and that exhibits remarkably little sexual dimorphism.
It is interesting to speculate on the significance of the relative absence of AR in the SCN and hippocampus of naked mole-rats. The SCN regulates a wide variety of circadian rhythms in all mammals and birds studied to date (Moore, 1997) and AR in the SCN is associated with sex differences in rhymicity (Iwahana et al., 2007). Naked mole-rats fail to exhibit circadian rhythms of sleep when studied within their colony (Davis-Walton and Sherman, 1994), although a small subset of animals exhibited robust circadian rhythms of locomotor activity in a colony setting (Riccio and Goldman, 2000). These few individuals exhibited a body morph characteristic of ‘dispersers’ (subordinate individuals that are thought to leave their colonies in the field with the potential to become founders of new colonies; O’Riain et al., 1996). Thus, naked mole-rats have retained the capacity for circadian rhythmicity, but rhythms might be expressed only under particular and rare circumstances in this species. Similarly, androgen action in the hippocampus likely contributes to sex differences in hippocampal morphology and associated behaviors (Edinger and Frye, 2007; Jones and Watson, 2005). We do not yet know if there are sex differences in naked mole-rat spatial ability or hippocampal morphology although the absence of sex differences in other brain regions (Holmes et al., 2007) and behaviors (Lacey and Sherman, 1991; Lacey et al., 1991), combined with reduced AR expression in the hippocampal formation reported here, suggests such differences may be absent or reduced compared to other species.
We have previously reported that social status influences the morphology of several reproductive brain regions in naked mole-rats (Holmes et al., 2007). The present data extend our observations to changes in gene and/or protein expression, as have been reported in status-changing fish (Au et al., 2006; Burmeister et al., 2005, 2007). Based on available data, it is not possible to determine whether the relationship between AR and morphology is causal, or simply correlative. For example, it is possible that the shift in social status from subordinate to breeder causes a change in AR expression, which in turn causes the effects on brain morphology seen previously. Alternatively, some as yet unidentified signal(s) associated with the change in status may independently alter both AR and morphology in the naked mole-rat brain. Our method of generating breeders is to pair subordinates of opposite sex following removal from their natal colonies. The time course of neural changes that occur as these animals become breeders is not known, nor is it known what aspect(s) of the new social setting trigger those changes. Breeders experience sexual behavior and birth of a litter. However, it is possible that achievement of full breeding status is not a prerequisite to the neural modifications that we observe in established breeding pairs, and that the mere isolation of a subordinate animal from the colony environment (i.e., removal from the presence of active breeders and/or from the presence of other subordinate kin) is a sufficient condition. It will be of interest in future studies to pinpoint the relevant stimuli leading to the observed neural changes.
Animals in this study were left gonadally intact in order to examine AR expression under the conditions of hormone variation that normally exist within a colony and are associated with differences in brain morphology between subordinates and breeders (Holmes et al., 2007). It is therefore possible that the sex and/or breeding status differences in AR are due to group differences in circulating steroids. While the ability of some antibodies to recognize steroid receptors is influenced by ligand binding (e.g., Meredith et al., 1994), this does not appear to be the case for the PG21 antibody (Lu et al., 1998). Nonetheless, the presence of ligand could indirectly affect counts of AR+ nuclei by changing sub-cellular localization of the receptor. Testosterone rapidly (within 30 minutes) increases nuclear AR immunoreactivity in sexually dimorphic spinal motoneurons, presumably by causing translocation of the receptor from the cytoplasm (Freeman et al., 1995). Because breeding naked mole-rats have higher T levels than do subordinates (Clarke and Faulkes, 1997, 1998; Faulkes et al., 1991), however, changes in sub-cellular location of AR are unlikely to account for our observation that nuclear AR is reduced in breeders.
Circulating hormones might also directly affect the expression of AR. The relationship between androgens and AR expression is complex and differs depending on brain region and species. Nonetheless, testosterone restores AR immunoreactivity following castration in reproductive brain regions in rats (Sar et al., 1990), ferrets (Kashon et al., 1996), guinea pigs (Choate and Resko, 1996) and Siberian hamsters (Bittman et al., 2003). Plasma levels of androgens have not been reported for naked mole-rats, but males have higher urinary testosterone than females (Clarke and Faulkes, 1997, 1998; Faulkes et al., 1991). The greater percentage of AR+ cells in several brain regions of males, as seen here, suggests a positive relationship between urinary androgens and AR. However, the pattern of results we observed defy such a simple hormonal explanation because our data also suggest an inverse relationship with testosterone and AR when considering social status: subordinates had more AR than breeders in all brain regions examined, yet subordinates of both sexes have lower testosterone than their breeding counterparts (Clarke and Faulkes, 1997, 1998; Faulkes et al., 1991). This is in contrast to what is seen in fish, where increased androgen levels in dominant males correlate with increased expression of AR mRNA compared to subordinates (Burmeister et al., 2007). Similarly, estrogens upregulate AR in the adult rat brain (Handa et al., 1996; Lynch and Story, 2000) but breeding females have lower AR expression relative to subordinates, yet presumably have higher estrogen levels.
Evaluating AR expression in naked mole-rats in which testosterone levels are experimentally manipulated could address whether the group differences in AR+ cell number seen here are influenced by circulating gonadal steroids, but several observations suggest that the effects of status on the brain and behavior of naked mole-rats may be independent of the gonads. Gonadectomy does not abolish expression of breeder-specific nuzzling behaviors (Goldman et al., 2006), or eliminate certain status-dependent differences in neural morphology (Holmes, Goldman, and Forger, unpublished). Experience-dependent modulation of AR, which may be independent of gonadal steroids, has been reported. For example, AR in several brain regions is modulated by early social stress in guinea pigs (Kaiser et al., 2003a,b) or by sexual activity in rats (Fernandez-Gausti et al., 2003; Portillo et al., 2006).
The present data are in accord with previous observations that social status influences the nervous system of naked mole-rats (Holmes et al., 2007; Rosen et al., 2007; Seney et al., 2006). The most consistent finding was a reduced percentage of AR+ nuclei in breeders. The next challenge is identifying the physiological mechanisms via which status alters brain structure and gene expression in this eusocial mammal. It is also of interest to speculate on the functional significance of the observed changes. A decrease in the percentage of AR+ nuclei suggests a dampened responsiveness to T in breeders. Given the well-established relationship between T and aggression (Wingfield, 2005), it is possible that AR is decreased in breeding naked mole-rats to facilitate life in a highly social and cooperative society, in which many animals live in close quarters with remarkably few agonistic encounters (Clarke and Faulkes, 1997).
Acknowledgements
This work was funded by NSF grants 0344312 and 0642050 (NGF and BDG), and a CIHR postdoctoral fellowship (MMH). Dr. Gail Prins and Lynn Birch generously provided the PG21 antibody and the AR21 and AR462 peptides. Sharry Goldman is thanked for outstanding animal husbandry and care, Greta Rosen for assistance with tissue collection, and Lynn Bengston and Jill McCutcheon for assistance with the brain maps. Geert de Vries and Marianne Seney provided helpful discussion and advice.
References
- Au TM, Greenwood AK, Fernald RD. Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behav. Brain Res. 2006;170:342–346. doi: 10.1016/j.bbr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Ehrlich DA, Ogdahl JL, Jetton AE. Photoperiod and testosterone regulate androgen receptor immunostaining in the Siberian hamster brain. Biol. Reprod. 2003;69:876–84. doi: 10.1095/biolreprod.102.010900. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinol. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Brett RA. The population structure of naked mole-rat colonies. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; New Jersey: 1991. pp. 97–136. [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Horm. Behav. 2007;51:164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate JVA, Resko JA. Effects of androgen on brain and pituitary androgen receptors and LH secretion of male guinea pigs. J. Steroid Biochem. Mol. Biol. 1996;59:315–322. doi: 10.1016/s0960-0760(96)00122-7. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Whitman C, Michael RP, Albers HE. Distribution of androgen receptor-like immunoreactivity in the brains of intact and castrated male hamsters. Brain Res. Bull. 1994;33:325–332. doi: 10.1016/0361-9230(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Clarke FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc. Biol. Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke FM, Faulkes CG. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc. Biol. Sci. 1998;265:1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J. Comp. Neurol. 1985;231:473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Connolly PB, Resko JA. Progestins affect reproductive behavior and androgen receptor dynamics in male guinea pig brain. Brain Res. 1989;503:312–316. doi: 10.1016/0006-8993(89)91681-8. [DOI] [PubMed] [Google Scholar]
- Connolly PB, Handa RJ, Resko JA. Progesterone modulation of androgen receptors in the brain and pituitary of male guinea pigs. Endocrinol. 1988;122:2547–2553. doi: 10.1210/endo-122-6-2547. [DOI] [PubMed] [Google Scholar]
- Davis-Walton J, Sherman PW. Sleep arrhythmia in the eusocial naked mole-rat. Naturwissenschaften. 1994;81:272–275. doi: 10.1007/BF01131581. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Moss RL, Rubin RT, editors. Hormones, Brain, and Behavior. IV. Academic Press; San Diego: 2002. pp. 137–191. [Google Scholar]
- DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura L-M, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinol. 2003;144:3632–3638. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer G, Garcia-Segura L-M. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neurosci. 2006;138:801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiol. Learn. Mem. 2007;87:201–208. doi: 10.1016/j.nlm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH. Social control of reproduction in breeding and non-breeding male naked mole-rats (Heterocephalus glaber) J. Reprod. Fertil. 1991;93:427–35. doi: 10.1530/jrf.0.0930427. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH. Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats (Heterocephalus glaber) J. Reprod. Fertil. 1993;99:225–230. doi: 10.1530/jrf.0.0990225. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J. Reprod. Fertil. 1990;88:559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, Jarvis JU. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J. Reprod. Fertil. 1991;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- Ferguson GA, Takane Y. Statistical Analysis in Psychology and Education. 6th Ed. McGraw-Hill, Inc; USA: 1989. pp. 264–267. [Google Scholar]
- Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J. Comp. Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Swaab D, Rodríguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendo. 2003;28:501–512. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Freeman LM, Padgett BA, Prins GS, Breedlove SM. Distribution of androgen receptor immunoreactivity in the spinal cord of wild-type, androgen-insensitive and gonadectomized male rats. J. Neurobiol. 1995;27:51–59. doi: 10.1002/neu.480270106. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Veiga S, Garcia-Segura L-M, DonCarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- Goldman SL, Forger NG, Goldman BD. Influence of gonadal sex hormones on behavioral components of the reproductive hierarchy in naked mole-rats. Horm. Behav. 2006;50:77–84. doi: 10.1016/j.yhbeh.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Res. Mol. Brain Res. 1996;39:57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinol. 1996;63:120–131. doi: 10.1159/000126948. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Rosen GJ, Jordan CL, De Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc. Natl. Acad. Sci. 2007;104:10548–10552. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Moore TO, Ferris CF, Mougey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Horm. Behav. 1991;25:206–216. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Swanson JJ, Prins GS, Jacobson CD. Androgen receptor-like immunoreactivity in the Brazilian opossum brain and pituitary: distribution and effects of castration and testosterone replacement in the adult male. Brain Res. 1995;703:1–18. doi: 10.1016/0006-8993(95)00983-3. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm. Behav. 2007 doi: 10.1016/j.yhbeh.2007.11.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JUM. Eusociality in a mammal: Cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- Jarvis JUM. Reproduction in naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; New Jersey: 1991. pp. 384–425. [Google Scholar]
- Jarvis JUM, O’Riain MJ, Bennett NC, Sherman PW. Mammalian eusociality: A family affair. Trends Ecol. Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol. Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Kruijver FP, Straub RH, Sachser N, Swaab DF. Early social stress in male Guinea-pigs changes social behaviour, and autonomic and neuroendocrine functions. J. Neuroendocrinol. 2003a;15:761–769. doi: 10.1046/j.1365-2826.2003.01055.x. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Kruijver FP, Swaab DF, Sachser N. Early social stress in female guinea pigs induces a masculinization of adult behavior and corresponding changes in brain and neuroendocrine function. Behav. Brain Res. 2003b;144:199–210. doi: 10.1016/s0166-4328(03)00077-9. [DOI] [PubMed] [Google Scholar]
- Kashon ML, Arbogast JA, Sisk CL. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J. Comp. Neurol. 1996;376:567–586. doi: 10.1002/(SICI)1096-9861(19961223)376:4<567::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lacey EA, Sherman PW. Social organization of naked mole-rat colonies: Evidence for divisions of labor. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; New Jersey: 1991. pp. 275–336. [Google Scholar]
- Lacey EA, Alexander RD, Braude SH, Sherman PW, Jarvis JUM. An ethogram for the naked mole-rat: nonvocal behaviors. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; New Jersey: 1991. pp. 209–242. [Google Scholar]
- Lorenz B, Garcia-Segura L-M, DonCarlos LL. Cellular phenotype of androgen receptor-immunoreactive nuclei in the developing and adult rat brain. J. Comp. Neurol. 2005;492:456–468. doi: 10.1002/cne.20763. [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinol. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Lynch CS, Story AJ. Dihydrotestosterone and estrogen regulation of rat brain androgen-receptor immunoreactivity. Physiol. Behav. 2000;69:445–453. doi: 10.1016/s0031-9384(99)00257-7. [DOI] [PubMed] [Google Scholar]
- Margulis SW, Saltzman W, Abbott DH. Behavioral and hormonal changes in female naked mole-rats (Heterocephalus glaber) following removal of the breeding female from a colony. Horm. Behav. 1995;29:227–247. doi: 10.1006/hbeh.1995.1017. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behav. Brain Sci. 1998;21:353–363. [PubMed] [Google Scholar]
- Meredith JM, Auger CJ, Blaustein JD. Down-regulation of estrogen receptor immunoreactivity by 17 beta-estradiol in the guinea pig forebrain. J. Neuroendocrinol. 1994;6:639–648. doi: 10.1111/j.1365-2826.1994.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Milner TA, Hernandez FJ, Herrick SP, Pierce JP, Iadecola C, Drake CT. Cellular and subcellular localization of androgen receptor immunoreactivity relative to C1 adrenergic neurons in the rostral ventrolateral medulla of male and female rats. Synapse. 2007;61:268–278. doi: 10.1002/syn.20370. [DOI] [PubMed] [Google Scholar]
- Moore RY. Circadian rhythms: Basic neurobiology and clinical applications. Ann. Rev. Med. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- O’Riain MJ, Jarvis JUM, Faulkes CG. A dispersive morph in the naked mole-rat. Nature. 1996;380:619–621. doi: 10.1038/380619a0. [DOI] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav. Brain Res. 2006;1662:291–295. doi: 10.1016/j.bbr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Pepper JW, Braude SH, Lacey EA, Sherman PW. Vocalizations of the naked mole-rat. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; New Jersey: 1991. pp. 243–274. [Google Scholar]
- Peroulakis ME, Goldman B, Forger NG. Perineal muscles and motoneurons are sexually monomorphic in the naked mole-rat (Heterocephalus glaber) J. Neurobiol. 2002;51:33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- Portillo W, Díaz NF, Cabrera EA, Fernández-Guasti A, Paredes RG. Comparative analysis of immunoreactive cells for androgen receptors and oestrogen receptor alpha in copulating and non-copulating male rats. J. Neuroendocrinol. 2006;18:168–176. doi: 10.1111/j.1365-2826.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- Prins GS. Molecular biology of the androgen receptor. Mayo Clin. Proc. 2000;75(Suppl):S32–35. [PubMed] [Google Scholar]
- Reeve HK, Westneat DF, Noon WA, Sherman PW, Aquadro CF. DNA “fingerprinting” reveals high levels of inbreeding in colonies of the eusocial naked mole-rat. Proc. Natl. Acad .Sci. 1990;87:2496–2500. doi: 10.1073/pnas.87.7.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio AP, Goldman BD. Circadian rhythms of locomotor activity in naked mole-rats (Heterocephalus glaber) Physiol. Behav. 2000;71:1–13. doi: 10.1016/s0031-9384(00)00281-x. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, DeVries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J. Comp. Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinol. 1990;127:3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O’Malley B, Levine JE. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinol. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- Seney ML, Goldman BD, Forger NG. Breeding status affects motoneuron number and muscle size in naked mole-rats: Recruitment of perineal motoneurons? J. Neurobiol. 2006;66:1354–1364. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smith TE, Faulkes CG, Abbott DH. Combined olfactory contact with the parent colony and direct contact with nonbreeding animals does not maintain suppression of ovulation in female naked mole-rats (Heterocephalus glaber) Horm. Behav. 1997;31:277–288. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J. Comp. Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neurosci. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and - adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Wagner C, Silverman A, Morrell J. Evidence for estrogen receptor in cell nuclei and axon terminals within the lateral habenula of the rat: regulation during pregnancy. J. Comp. Neurol. 1998;392:330–342. [PubMed] [Google Scholar]
- Wingfield JC. A continuing saga: the role of testosterone in aggression. Horm. Behav. 2005;48:253–255. doi: 10.1016/j.yhbeh.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinol. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Horm. Behav. 2002;42:327–336. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]