Abstract
Exposure to ozone induces airway hyperresponsiveness (AHR) mediated partly by SP released from nerve terminals of intrinsic airway neurons. Our recent studies showed that IL-1, an important multifunctional proinflammatory cytokine, increases synthesis and release of SP from intrinsic airway neurons. The purpose of this study is to investigate the possible involvement of endogenous IL-1 in modulating neural responses associated with ozone-enhanced airway responsiveness. Ferrets were exposed to 2 ppm ozone or filtered air for 3 hrs. IL-1 in the bronchoalveolar lavage (BAL) fluid was significantly increased in ozone-exposed animals and responses of tracheal smooth muscle to methacholine (MCh) and electrical field stimulation (EFS) were elevated significantly. Both the SP nerve fiber density in tracheal smooth muscle and the number of SP-containing neurons in airway ganglia were significantly increased following ozone exposure. Pretreatment with IL-1 receptor antagonist (IL-1 Ra) significantly diminished ozone-enhanced airway responses to EFS as well as ozone-increased SP in the airway. To selectively investigate intrinsic airway neurons, segments of ferret trachea were maintained in culture conditions for 24 hrs to eliminate extrinsic contributions from sensory nerves. The segments were then exposed to 2 ppm ozone in vitro for 3 hrs. The changes of ozone-induced airway responses to MCh and EFS, and the SP levels in airway neurons paralleled those observed with in vivo ozone exposure. The ozone-enhanced airway responses and neuronal SP levels were inhibited by pretreatment with IL-1 Ra. These findings show that IL-1 is released during ozone exposure enhances airway responsiveness by modulating SP expression in airway neurons.
Keywords: airway smooth muscle contraction, muscarinic agonists, neurokinin receptor, airway innervation
1. INTRODUCTION
Exposure to ozone produces epithelial injury and inflammation in the airways, accompanied by airway hyperresponsiveness (AHR) to a variety of bronchoactive substances in several species including rat, guinea pig and ferrets as well as in human subjects (Hazbun, et al., 1993;Lee, et al., 1979;Joad, et al., 1996;Koto, et al., 1995;Wu, et al., 2001;Wu, et al., 1997;Wu, et al., 2003;Dye, et al., 1999). SP, a member of the tachykinin family, has potent effects on airway smooth muscle tone, vascular permeability to protein and mucus secretion (Barnes, et al., 1991;Lundberg, et al., 1983;Lundberg, et al., 1984). Recent studies have shown that SP is synthesized and released from airway nerves and plays an important role in ozone-induced AHR (Lee, et al., 1979;Joad, et al., 1996;Koto, et al., 1995;Wu, et al., 2001;Wu, et al., 1997;Wu, et al., 2003). Most of these studies focus on SP released by sensory neurons located in the nodose and jugular ganglion following ozone exposure (Joad, et al., 1996;Koto, et al., 1995;Lee, et al., 1979). A few studies, including our own, have found that SP levels in nerve cell bodies located in intrinsic airway ganglia as well as nerve fibers originating from neurons of airway ganglia are increased after ozone exposure (Wu, et al., 2001;Wu, et al., 2003).
SP is localized in the peripheral endings of nerves innervating the airways and originates in nerve cell bodies located both in sensory neurons and in neurons of airway ganglia (Lundberg, et al., 1984;Dey, 1995;Dey, et al., 1993;Dey, et al., 1999). The current study focuses on neurons in airway ganglia. In some species, airway ganglia are organized to form two layers. In the ferret trachea (Baker, et al., 1986), one layer of ganglia is associated with the a nerve plexus termed the longitudinal trunk (LT), a pair of nerve trunks that extends nearly the entire length of the trachea. A second group of ganglia is associated with a plexus closely associated with the dorsal surface of the trachealis muscle arranged as a diffuse network named the superficial muscular plexus (SMP). Almost all neurons in the LT ganglia contain acetylcholine (Dey, et al., 1996) although some express SP (Dey, et al., 1996;Wu, et al., 2002). Cell bodies in the SMP contain predominantly vasoactive intestinal peptide (VIP) and nitric oxide (NO), with a small population containing SP (Dey, et al., 1993;Dey, et al., 1996). Ozone exposure increases SP levels in nerve cell bodies of both LT and SMP ganglia as well as SP innervation of tracheal smooth muscle (Wu, et al., 2001;Wu, et al., 2003). Interstingly, communications between ganglia have been demonstrated (Zhu and Dey, 2001) suggesting reciprocal synaptic regulation. The neuroanatomy and structure of the ferret trachea is similar to human and should be considered an acceptable model of human airway responses (Fisher, 1964;Vinegar, et al., 1985).
Irritant- or antigen-induced release of inflammatory mediators, such as arachidonic acid metabolites and cytokines, including IL-1, are known to enhance the sensitivity of sensory nerve endings in the lung (Ho, et al., 2000;Yu, et al., 2007). However, the intrinsic mechanisms involved in transduction of ozone exposure to enhance SP production remains to be established, but the cytokine IL-1 has been implicated as a potential signaling molecule affecting SP synthesis in sympathetic neurons (Shadiack, et al., 1994). Our recent study showed that IL-1 treatment increases the number of SP-positive neurons of airway ganglia with a concomitant increase in innervation of airway smooth muscle (Wu, et al., 2002), suggesting that IL-1 plays a role in the regulation of SP synthesis in intrinsic airway neurons. Levels of IL-1 in the lung are elevated after inhalation of irritants (Becker, et al., 1999;Arsalane, et al., 1995;Pendino, et al., 1994), and ozone exposure increases IL-1 release from alveolar macrophages (Arsalane, et al., 1995;Pendino, et al., 1994). Although IL-1β has been implicated in the development of AHR in animal models and isolated human bronchi (Tsukagoshi, et al., 1994;Hakonarson, et al., 1999;Barchasz, et al., 1999), the exact pathway through which IL-1β modifies smooth muscle reactivity has not been fully elucidated. Given the effects of IL-1β on SP production in neurons, it is possible that IL-1β regulates SP production in airway neurons as well. In fact, our previous studies demonstrated that exogenous IL-1 increases airway hyperresponsiveness by enhancing SP expression in airway ganglia (Wu, et al., 2002), raising the possibility that endogenous release of IL-1 may be involved in upregulation of SP in neurons of airway ganglia. The present experiments use a recently developed IL-1 receptor antagonist to address the hypothesis that enhanced airway responsiveness during ozone exposure results from increased SP in airway neurons mediated by the action of endogenously released IL-1.
2. METHODS
Female ferrets (Marshall Farms, North Rose, New York) from 250 to 500 g were housed two to four per cage with access to food and water ad libitum in an AAALAC accredited facility. Female ferrets are used because they are easily handled and can be housed together in groups. Ferrets were anesthetized with ketamine (25 mg/kg) and xylazine (2 mg/kg) in a single intraperitoneal injection and sacrified by CO2 inhalation. All procedures were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, and were approved by the WVU Animal Care and Use Committee #03-1105.
2.1 IL-1Ra Intratracheal Instillation (i.t.)
Ferrets were anesthetized with ketamine (25 mg/kg) and xylazine (2 mg/kg) in a single intraperitoneal injection. An 18-gauge steel tube 15 cm in length was marked to indicate when the tip reached the carina and connected to a 1 ml tuberculin syringe filled with IL-1Ra (3 μg/0.3ml) or saline. The tube was inserted through the oral cavity and pharynx into the trachea and IL-1Ra or saline was instilled into the trachea and deposited at four equal intervals along the trachea from immediately superior to the carina to immediately inferior to the larynx. 30 min after the last IL-1 Ra or saline treatment, ferrets were exposed to in vivo ozone for 3 hrs.
2.2 In vivo Ozone Exposure
All in vivo ozone exposures were done at 2 ppm in a 12×12 inch stainless steel and glass chamber for 3 hrs. Ozone was produced by passing hospital-grade air through a drying and high-efficiency particle (HEPA) filter and then through an ultraviolet light source. The ozone concentration in the chamber was measured by chemiluminescence with a calibrated ozone analyzer (OA 350-2R model; Forney Corporation; Carrollton, TX) and adjusted every 10 sec by an on-line computer. A separate group of animals was exposed to filtered air using procedures identical to those described above, except that ozone was not delivered to the mixing chamber. To determine the possible involvement of endogenously released IL-1, some ferrets were given one intraperitoneal injection (i.p) of IL-1 Ra (10 mg/kg) 30 min prior to ozone or air exposure. The IL -1Ra concentration was based on suggestion of Amgen Inc.
2.3 Organotypic Cultures of Ferret Trachea
In order to examine the effects of ozone on nerves originating from the neurons in airway ganglia, tracheal segments were placed in organotypic cultures for 24 hrs. We have characterized the validity of using tracheal explants in previous studies (Dey, et al., 1991). We characterized a timeline of nerve fiber survival for up to 7 days (Dey, et al., 1999) in culture and showed that that nerve fibers originating from neurons outside the lung (sympathetic, sensory, preganglionic parasympathetic) degenerated in the culture and that cholinergic contractions (Wu, et al., 2002) and iNANC relaxations (Canning, et al., 1996) are well maintained for 1 to 3 days in culture. The methods for organotypic cultures of tracheas from normal ferrets were modified from our previously described technique (Dey, et al., 1999;Wu, et al., 2002;Wu, et al., 2001;Wu, et al., 2006). Under sterile conditions, tracheas were removed and washed with cold culture medium (described below). The trachea was then placed in a petri dish with culture medium and cut into 100 mm long segments for in vitro ozone exposure or 30 mm long for IL-1 treatment. After a second wash, the segments were placed directly on the bottom of petri dishes containing fresh culture medium. The culture media consisted of CMRL 1066 containing 0.1 μg/ml hydrocortisone hemisuccinate, 1 μg/ml recrystalized bovine insulin, 60 μg/ml penicillin G (100 units/ml), 10 μg/ml amphotericin B, 100 μg/ml streptomycin, and 5% heat-inactivated fetal calf serum. The petri dishes were then placed in a controlled atmosphere culture chamber and gassed with 95% O2 and 5% CO2. The chamber was placed on a rocker and incubated at 37°C for 24 hrs. After culture, the tracheal segments were exposed to 2.0 ppm ozone using the in vitro ozone exposure system described below.
2.4 Verification of IL-1 Ra Concentration in Culture
Our previous studies have shown that 10 ng/ml IL-1 enhanced airway smooth muscle responses to EFS in cultured trachea (Wu, et al., 2002). In order to verify IL-1 Ra concentration in culture, tracheal segments were maintained in organotypic culture with IL-1β or saline for 24 h and the different IL-1Ra concentrations were added to the culture media prior to adding IL-1 in five separate experimental groups: 1) segments cultured with 10 ng/ml IL-1and 0 ng/ml IL-1 Ra; 2) segments cultured with10 ng/ml IL-1and 10 ng/ml IL-1 Ra; 3) segments cultured with10 ng/ml IL-1and 50 ng/ml IL-1 Ra; 4) segments cultured with10 ng/ml IL-1and 200 ng/ml IL-1 Ra; 5) segments cultured with saline (control) and no IL-1Ra. After culture for 24 hrs, tracheal smooth muscle reactivity was evaluated by measuring contractile responses to methacholine (MCh) and electical field stimulation (EFS).
2.5 In vitro Ozone Exposure
After culture for 24 hrs, the tracheal segments were exposed to ozone by a modification of our previously described technique (Wu, et al., 2003). Briefly, the segments were mounted vertically in Krebs solution, securely tied to upper and lower hose connectors and stretched to normal resting tension in a glass exposure vessel. The upper hose connector was connected to the mixing chamber that is a plexi-glass cylinder used to mix the incoming humidified air and ozone streams prior to being administered to the tracheal lumen. Ozone was produced by an ozone generator (ENMET Corp, Blairsville, PA) that utilizes a low-pressure mercury vapor lamp with high output ultra-violet radiation of 254 nm wavelength producing up to 150 ppm of ozone at 1 liter per minute (0.294 mg / liter) with ambient air as the input gas. To avoid decomposition of ozone, all the tubes exposed to ozone were made of glass or Teflon. Humidified air was generated and regulated by a humidifier (Electro-tech systems Inc.; Glenside, PA), a relative humidity controller (Electro-tech systems Inc. Glenside, PA), and peristaltic pump (Barnant Corp, Barrington, IL), which work in conjunction to maintain a set range of percent relative humidity as well as a constant downstream pressure to prevent back-flow of ozone into the humidifier assembly. The relative humidity controller was set at 79%. The peristaltic pump speed was set to allow mixing of humidified air into the mixing chamber resulting in an ozone concentration of 2.00 ppm. The lower hose connector was connected to an ozone analyzer (OA 350-2R model; Forney Corporation; Carrollton, TX) that continuously measured the ozone concentration in the tracheal lumen.
All in vitro ozone exposures were done at 2 ppm for 3 hrs. A separate group of tracheas was subjected to air exposure in which procedures identical to those described above were followed, except that humidified air without ozone was not delivered to the mixing chamber. In some experiments, IL-1 Ra (final concentration 200 ng/ml) or the vehicle was added to the Krebs solution 30 minutes prior to ozone or air exposure and maintained throughout the experiment in order to determine the role of IL-1 in airway neurons.
2.6 Inflammatory Cell Analysis in Bronchoalveolar Lavage (BAL) Fluid
BAL fluid was obtained 3 hrs after in vivo ozone exposure by injecting 20 ml of sterile saline (10ml, twice) via the tracheal cannula. The collected BAL fluid (approximately 15 ml) was centrifuged at 1,500 rpm for 10 min. The supernatant was aliquoted and frozen at −80°C for subsequent assays, and the pelleted cells were treated with TRIS-buffered ammonium chloride solution (pH 7.2) to lyse red blood cells. The remaining cells were washed once with phosphate-buffered saline supplemented with 1% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin (GIBCO, Grand Island, NY). Total cell counts were determined by using a hemocytometer. Differential leukocyte counts were then performed on cytospin slides. At least 200 leukocytes were counted using standard morphologic criteria to identify each cell type.
2.7 IL-1 Enzyme-Linked Immunosorbent Assay
The BAL fluid supernatant samples (initial 3 ml) were frozen at −80°C. The concentration of IL-1 (15.6–1000 pg/ml) in each sample was assayed using the IL-1 Emax ImmunoAssay System according to manufacturer’s instructions (R/D System). IL-1 was detected using an antibody sandwich format in 96-well plates. Each well was initially coated with 50 μl of anti-IL-1 Ab (Assay Diluent RD1-21), then was added either 50 μl of lavage supernatant or IL-1 standard (15.6–1000 pg/ml). The plate was incubated for two hours at room temperature. Each well was aspirated and washed for four times, followed by two hours incubation with rat-IL-1 conjugate (100 μl/well) at room temperature. Then, each well was washed and incubated with 100 μl substrate solution for 30 minutes, followed by stop solution. The absorbance of each well was measured at 540 nm on a Spectra Max 340pc plate reader (Molecular Devices, Sunnyvale, CA). The concentration of IL-1 in each lavage sample was extracted from an IL-1 standard curve. All samples were run in duplicate or triplicate, and as a negative control, a PBS sample was run with each assay.
2.8 Measurement of Tracheal Smooth Muscle Contraction
Tracheal smooth muscle reactivity was evaluated by measuring contractile responses to methacholine (MCh) or electrical field stimulation (EFS). MCh responses measure smooth muscle responses to the applied agonist, whereas EFS evaluates cholinergic responses resulting from the release of acetylcholine (ACh) from airway nerves. Tracheal segments were cut into 3 mm wide strips at 3 hrs after in vivo or in vitro ozone exposure with IL-1 Ra or saline, mounted in holders and maintained in gassed (95%O2-5%CO2) modified Krebs-Henseleit (MKH) solution at 37°C with a composition of NaCl 113 mM, KCl 4.8 mM, CaCl 2.5 mM, MgSO4 1.2 mM, NaHCO3 24 mM, KH2PO4 1.2 mM, and glucose 5.7 mM, pH 7.4. The detail procedures are described in previous studies (Wu, et al., 2003;Wu, et al., 2002). Briefly, the strips were tied at each end with 4-O silk and positioned between the rings of platinum electrodes attached to tissue holders and equilibrated for 60 min at a resting tension of 1.0 g. After equilibration, cumulative concentration-response curves for MCh were constructed for separate strips by adding a series of concentrations of MCh to the bath in half log increment concentrations ranging from 10−9 to 10−3 M. The next concentration was not added until the previous response reached a plateau. After the MCh solution was completely washed out and smooth muscle tension returned to baseline, EFS experiments were carried out. Frequency response curves were constructed by increasing the frequency from 1 to 30 Hz, using a submaximum voltage of 120 V, 0.2-ms pulse duration, and 10-s train duration. Between each stimulation period, 10 min was allowed for the previous response to return to baseline. EFS-induced contractions were normalized as a percentage of the response to 10−3 M MCh (% MCh response). In previous studies (Wu, et al., 2002), we have shown that all EFS-induced responses are cholinergic in nature (blocked by atropine), but that blocking NK1 receptors inhibited the ozone-enhanced increase in smooth muscle contractility, demonstrating the modulatory action of SP. Further, we have shown that capsaicin treatment of the cultured airway segments did not produce contractions in controls or alter contractions after IL-1 treatment (Wu, et al., 2002), suggesting that sensory nerves were not involved in the EFS-response and were functionally eliminated from the explants during the 24 hrs culture period, probably due to degeneration.
2.9 Morphometric Analysis
The goal of the morphometric analysis was to evaluate SP levels in nerve cell bodies of neurons in airway ganglia and to measure changes in nerve fiber density (NFD) in tracheal smooth muscle. This approach involves immunocytochemical processing of tracheal tissues, followed by separate morphometric approaches to measure 1) the number of SP positive nerve cell bodies in airway ganglia and 2) NFD in tracheal smooth muscle.
Immunocytochemistry
The procedures for immunocytochemical demonstration of SP-like immunoreactivity were described previously (Dey, et al., 1999;Wu, et al., 2003;Wu, et al., 2002). Briefly, tracheal segments at 3 hrs after in vivo or in vitro ozone exposure were fixed in picric acid-formaldehyde (PAF) fixative for 3 h and rinsed 3 times with a 0.1 M phosphate-buffered saline containing 0.3% Triton-X-100 (PBS-Tx), frozen in isopentane, cooled with liquid nitrogen, and stored at −80°C. Cryostat sections (12 μm thickness) were collected on gelatin-coated cover slips and dried briefly at room temperature. Then, cryostat sections were covered with SP antibody diluted 1:200, incubated in a humid chamber at 37°C for 30 min, and rinsed with a 1% bovine serum albumin-PBS-TX solution three times. The sections were then covered with fluorescein isothiocyanate-labeled goat anti-rabbit antibody diluted 1:100, incubated at 37°C for 30 min, and rinsed. After all immunocytochemical procedures were conducted, the coverslips were mounted with fluoromount and observed with a fluorescence microscope.
Number of SP-containing nerve cell bodies
Fluorescence images of SP-like immunofluorescence were recorded for LT and SMP ganglion cell bodies. Images were recorded with the Olympus AX 70 microscope (Olympus Corporation, Melville, NY) with SPOT 2 (Diagnostic Instruments, Sterling Heights, MI), and fluorescence intensity was measured with Optimas 6.5 software. The intensity recordings were calibrated with the InSpeck Green (505/515) microscope image intensity calibration kit (Molecular Probes, Eugene, OR). The cell bodies were identified by drawing the perimeter of the cell, and the fluorescence intensity was reported as gray level on a scale of 256 for each neuron. Neurons with a gray level < 50 were considered negative because they were at or below the general background. Fluorescence intensities of ≥50 were counted as labeled neurons. Labeled neurons were clearly identifiable due to the positive immunocytochemical labeling. Unlabled neurons are also visible due to variable levels of backscattered fluorescent lighting creating contrast between different tissues in the section. Thus, all identifiable neurons in longitudinal trunk (LT) and superficial muscular plexus (SMP) ganglia were evaluated in non-adjacent sections, usually amounting to a total of 10–15 sections analyzed per ferret. The data are expressed separately for each ganglion as the percentage of SP-positive neurons compared with total number of neurons observed.
NFD in tracheal smooth muscle
For measurement of NFD in tracheal smooth muscle, images of SP-containing nerve fibers were collected in series with the Zeiss LSM 510 confocal microscope. A series of images representing all of the tracheal smooth muscle in a section were collected in digital files, saved to an internal database, and measured with Optimas software (Optimas 6.5, Media Cybernetics Inc, Bethesda, MD). The smooth muscle regions were outlined to measure total cross-sectional area of smooth muscle. SP-positive nerve fibers were identified by segmentation using threshold gray levels with the Optimas software. NFD was then calculated as percentage of SP-immunoreactive nerve fiber area based on the total cross-sectional area of smooth muscle. At least 10 measurements were made for each section, and 15 sections were measured in each animal.
2.10 Data Analysis
Unless otherwise stated, results are expressed as mean ± SE. Contractions elicited by EFS were expressed as a percentage of the maximal contraction elicited by MCh. Contractions to MCh were normalized as a percentage of the respective maximal responses for each agonist. The half–maximal effective concentration (EC50) represents half concentration and refers to the concentration of a drug which induces a response halfway between the baseline and maximum. EC50 for MCh were calculated using a four parameter logistic curve fit (Sigmoidal, SigmaPlot 2000) and are presented with 95% confidence interval in parentheses. Force development was expressed by normalizing force (g) divided by the wet weight of the tissue. SMP and LT neurons were expressed as % SP-positive cell bodies. Nerve fiber density was expressed as % area of SP-immunoreactive nerve fibers in the total area of the smooth muscle. Statistical analyses of inflammatory cell and IL-1 release in BAL fluid were performed using student t-test. Statistical analyses of immunocytochemisty and EC50 were performed with two ways ANOVA. Statistical analysis of EFS was performed with two-way repeated-measures ANOVA. One factor was ozone exposure, and another factor was IL-1 Ra treatment. When the main effect was considered significant at P ≤ 0.05, pairwise comparisons were made with a post hoc analysis (Fisher’s least significant difference). A P value ≤0.05 was considered significant, and n represents the number of animals studied.
2.11 Materials
MCh chloride, hydrocortisone hemisuccinate, amphotericin B, and recrystalized bovine insulin were obtained from Sigma (St. Louis, MO). Penicillin G, streptomycin, fetal calf serum, and CMRL 1066 were obtained from GIBCO (Grand Island, NY). IL-1 Ra was obtained from Amgen Inc (Thousand Oaks, CA). SP antibody was obtained from Peninsula Inc (Belmont, CA). Fluorescein isothiocyanate-labeled goat anti-rabbit antibody was obtained from ICN Immunobiologicals (Costa Mesa, CA).
3. RESULTS
3.1 Effect of ozone on airway inflammation and endogenous IL- release
The total number of leukocytes in the BAL fluid collected from ozone-exposed animals exceeded that of air-exposed animals by 56%, indicating that airway inflammation was induced by the ozone exposure. In particular, neutrophils in the BAL fluid of ozone animals were markedly increased (Table 1).
Table 1.
Effect of in vivo ozone on leukocyte counts in BAL fluid
| Cell counts, ×105 |
|||||
|---|---|---|---|---|---|
| Group | Total cell | Neutrophils | Eosinophils | Lymphocytes | Macrophages |
| Air | 20.68 ±1.77 | 0.62±0.16 (3.0%) | 0.38±0.13 (1.8%) | 0.49±0.27 (2.4%) | 19.19±3.28 (92.7%) |
| Ozone | 32.21 ±2.39* | 3.68 ±0.32* (11.4%) | 0.49±0.21 (1.5%) | 0.72±0.26 (2.2%) | 27.32±5.12 (84.9%) |
N = 5.
Significant difference between air and ozone exposure; p≤ 0.05.
The level of IL-1 in the BAL fluid obtained from ozone-exposed animals was 90.45 ± 11.43 pg/ml, which was significantly higher than that obtained from air-exposed animals (43.68 ± 10.80 pg/ml, Figure 1).
Figure 1.
Effect of ozone exposure on IL-1 release in bronchoalveolar lavage fluid obtained from air and ozone exposed animals. Values are means ± SE; n = 5 in each group. IL-1 was measured by ELISA. * Significant difference between in vivo air and ozone exposed animals, P ≤0.05.
3.2 Effect of IL-1 Ra on in vivo ozone-enhanced airway smooth muscle responses
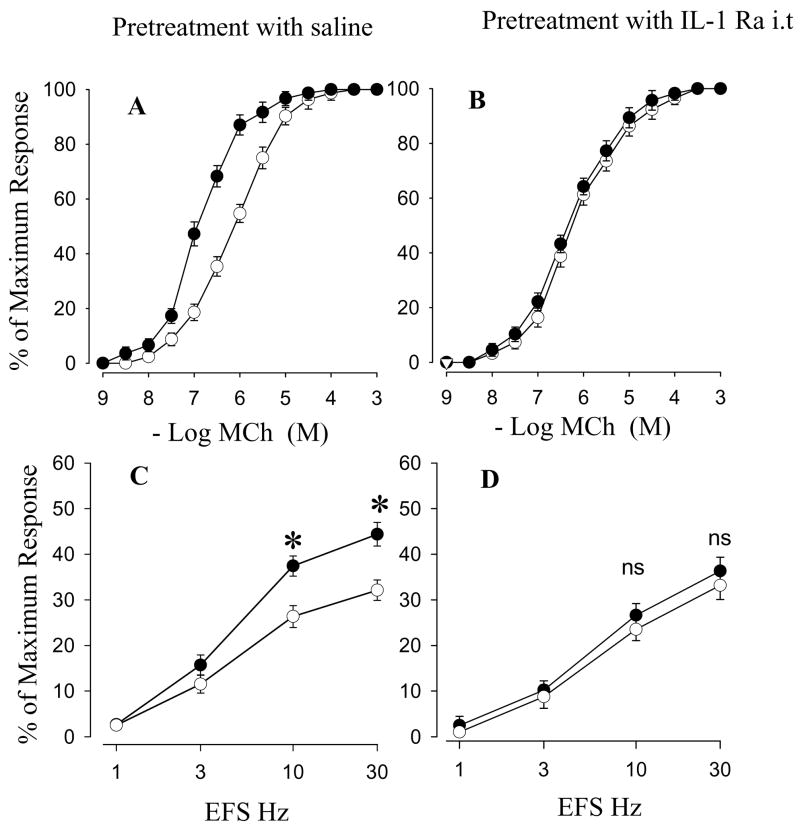
The initial experiments were intended to test the effect of IL-1 Ra i.v on airway smooth muscle responses in in vivo ozone exposure. MCh dose-response curves were markedly shifted to the left and the EC50 value was significantly decreased in animals pretreated with saline after exposure to ozone (Figure 2A, Table 2). Significant increases in EFS-stimulated contractions at 10 Hz and 30 Hz were also observed after ozone exposure (Figure 2C). There were no effects of IL-1 Ra on MCh dose-response curves and EFS-induced contractions in air-exposed animals (Table 2). However, pretreatment with IL-1 Ra markedly attenuated the increases in reactivity to MCh (Figure 2B, Table 2) and EFS at 10 Hz and 30 Hz (Figure 2D). There is no significant difference in EC50 and EFS values between the ozone exposure group with IL-1 Ra pretreatment and the air exposure group.
Figure 2.
Effects of saline (A and C) or IL-1 Ra i.v (B and D) on cumulative concentration-response curves for MCh (A and B) and frequency-response curves for EFS (C and D) in tracheal smooth muscle after in vivo exposure to air (○) or ozone (●). Values are means ± SE; n = 5. The difference in MCh cumulative concentration-response curves between air and ozone exposure are presented in Table 2. *Significant difference in EFS between air and ozone exposure, P ≤ 0.05.
Table 2.
Effect of IL-1 Ra i.v on cumulative concentration-response curves for MCh in tracheal smooth muscle after in vivo ozone exposure.
| Air
|
Ozone
|
|||
|---|---|---|---|---|
| EC50 (M) | Tmax | EC50 (M) | Tmax | |
| MCh | ||||
| Saline (95% CI) | 6.22 ×10−7 (4.12 ~ 8.3) | 122.1±16.4 | 1.24×10−7* (0.15 ~ 2.33) | 124.2±11.1 |
| IL-1 Ra (95% CI) | 5.69 ×10−7 (3.68~7.70) | 118.5±17.1 | 4.62×10−7 (3.14 ~ 6.10) | 111.8±13.8 |
EC50 values are given in molar concentration with 95% confidence interval (95% CI) in parentheses. Maximum tension (Tmax) values are given as g of tension per g of tissue.
N=5.
Significant difference between air and ozone exposure; p≤ 0.05.
The next experiments examined the effect of intratracheal (i.t) pretreatment with IL-1 Ra on tracheal smooth muscle responses in in vivo ozone exposure. Cumulative concentration-response curve for MCh and the EFS-stimulated contractions in i.t IL-1Ra or saline pretreated groups after ozone exposure (Figure 3A, Table 3) demonstrated similar changes as those described above i.p pretreated groups. IL-1 Ra did not affect MCh dose-response curves and EFS-induced contraction in air-exposed animals. But the IL-1 Ra significantly inhibited the ozone-enhanced smooth muscle responses to MCh and EFS at 10 and 30 Hz (Figure 3B, 3D and Table 3). Theses results indicated that pretreatment with IL-1 Ra attenuates in vivo ozone-enhanced responses to MCh and EFS.
Figure 3.
Effects of saline (A and C) or IL-1 Ra i.t (B and D) on cumulative concentration-response curves for MCh (A and B) and frequency-response curves for EFS (C and D) in tracheal smooth muscle after in vivo exposure to air (○) or ozone (●). Values are means ± SE; n = 5. The difference in MCh cumulative concentration-response curves between air and ozone exposure are presented in Table 2. *Significant difference in EFS between air and ozone exposure, P ≤0.05.
Table 3.
Effect of IL-1 Ra i.t on cumulative concentration-response curves for MCh in tracheal smooth muscle after in vivo ozone exposure.
| Air
|
Ozone
|
|||
|---|---|---|---|---|
| EC50 (M) | Tmax | EC50 (M) | Tmax | |
| MCh | ||||
| Saline (95% CI) | 7.45 ×10−7 (4.95 ~ 9.95) | 116.4±12.5 | 1.26×10−7* (0.10 ~ 2.32) | 112.2±13.7 |
| IL-1 Ra (95% CI) | 6.23 ×10−7 (3.51~8.95) | 114.5±11.2 | 4.87×10−7 (3.16 ~ 6.58) | 116.2±12.4 |
EC50 values are given in molar concentration with 95% confidence interval (95% CI) in parentheses.
N=5.
Significant difference between air and ozone exposure; p≤ 0.05.
3.3 Effect of IL-1 Ra on in vitro IL-1- and ozone-enhanced airway smooth muscle responses
The initial experiments tested effects of the different IL-1 Ra concentrations on IL-1-enhanced airway contraction to EFS in cultured trachea. Our previous studies have shown that 10 ng/ml IL-1 enhances airway smooth muscle responses to EFS in cultured trachea (Wu, et al., 2002). Tracheal segments were maintained in organotypic culture with IL-1β or saline for 24 h and the different IL-1Ra concentrations were added to the culture media prior adding IL-1. Contractions produced by EFS at 10 Hz and 30 Hz were significantly increased in tracheal strips cultured with IL-1β without pretreatment with IL-1 Ra (concentration: 0 ng/ml) compared with control group (no IL-1β and no IL-1 Ra treatment) (Figure 4A). Addition of low concentration (10 ng/ml) and medium concentration (50 ng/ml) did not significantly inhibit IL-1-enhanced airway smooth muscle responses to EFS at 10 Hz and 30 Hz (Figure 4B and C). However, addition of high concentrations (200 ng/ml) significantly inhibited the IL-1-enhanced smooth muscle responses to EFS at 10 and 30 Hz (Figure 4A, B and C).
Figure 4.
Effects of the different concentrations of IL-1 Ra on frequency-response curves for EFS in organotypic cultured tracheal smooth muscle prior to IL-1 treatment. Figure 4A shows that 10 ng/ml IL-1 enhanced airway smooth muscle responses to EFS and 200 ng/ml IL-1 Ra totally inhibited IL-1-enhanced airway smooth muscle responses to EFS. Figure 4B shows that effects of the different concentrations of IL-1 Ra on IL-1-enhanced airway smooth muscle responses to EFS at 10 Hz. Figure 4C shows the effects of the different concentrations of IL-1 Ra on IL-1-enhanced airway smooth muscle responses to EFS at 30 Hz. Values are means ± SE; * Significant difference in EFS between IL-1 with IL-1 Ra 0 ng/ml treatment and control (no IL-1 and no IL-1 Ra treatment) or IL-1 with IL-1 Ra 200 ng/ml treatment, P ≤0.05.
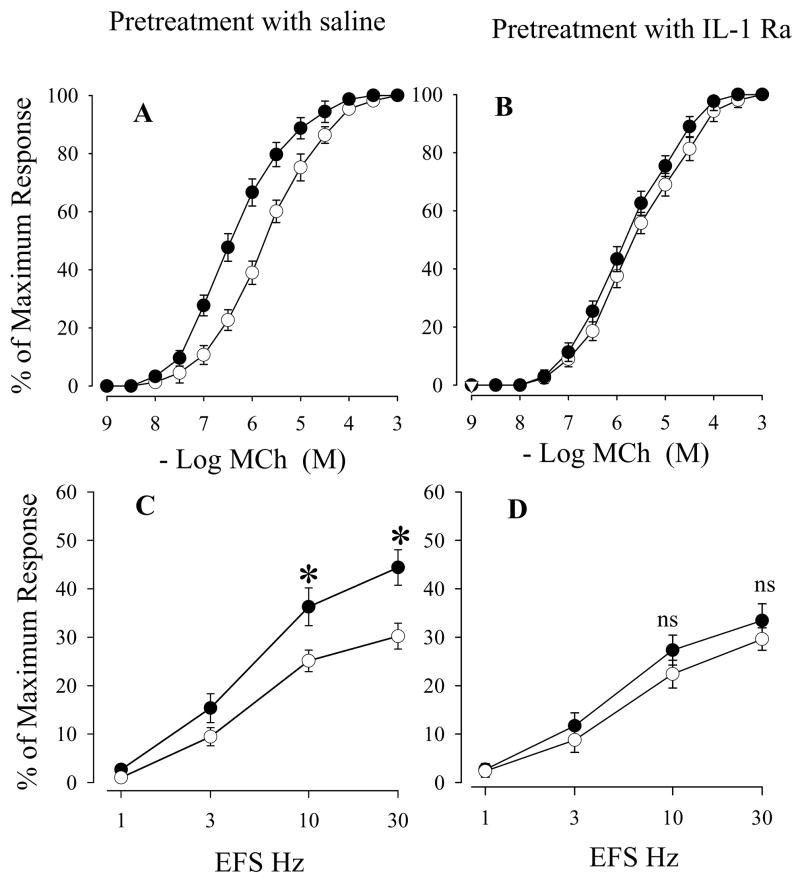
The next experiments examined the effect of IL-1 Ra on tracheal smooth muscle responses in in vitro ozone exposure. Cumulative concentration-response curves for MCh and the EFS- induced contractions in cultured tracheal segments after in vitro ozone exposure in the group pretreated with saline demonstrated similar changes as in vivo ozone exposure; i.e., MCh dose-response curves were markedly shifted to the left (Figure 5A, Table 4) and smooth muscle contractions to EFS 10 Hz and 30 Hz were significantly increased after exposure to in vitro ozone (Figure 5C). IL-1 Ra did not affect MCh dose-response curves and EFS-induced contraction in air-exposed animals. But the IL-1 Ra significantly inhibited the ozone-enhanced smooth muscle responses to MCh and EFS at 10 and 30 Hz (Figure 5B, 5D and Table 4). These results indicated that IL-1 Ra blocked in vitro ozone exposure-enhanced airway smooth muscle contractions to MCh and EFS.
Figure 5.
Effects of saline (A and C) or IL-1 receptor antagonist (B and D) on cumulative concentration-response curves for MCh (A and B) and frequency-response curves for EFS (C and D) in organotypic cultured tracheal smooth muscle after in vitro exposure to air (○) or ozone (●). Values are means ± SE; n = 5. The difference in MCh cumulative concentration-response curves between air and ozone in vitro exposure are presented in Table 3 *Significant difference in EFS between in vitro air and ozone exposure, P ≤0.05.
Table 4.
Effect of IL-1 Ra on cumulative concentration-response curves for MCh in cultured tracheal smooth muscle after in vitro ozone exposure.
| Control
|
Ozone
|
|||
|---|---|---|---|---|
| EC50 (M) | Tmax | EC50 (M) | Tmax | |
| MCh | ||||
| Saline (95% CI) | 1.91 ×10−6 (1.24 ~ 2.58) | 105.5±11.2 | 3.81×10−7* (1.97 ~5.65) | 113.4±10.9 |
| IL-1 Ra (95% CI) | 2.63 ×10−6 (1.86~3.40) | 109.2±10.3 | 1.65×10−6 (1.14~2.167) | 119.2±12.4 |
EC50 values are given in molar concentration with 95% confidence interval (95% CI) in parentheses.
N=5.
Significant difference between air and ozone exposure; p≤ 0.05.
3.4 Effect of IL-1 Ra on ozone-induced changes of immunoreactive SP-containing neurons in airway ganglia
First, the effect of in vitro IL-1 Ra on SP nerves in smooth muscle and neurons of intrinsic airway in in vitro ozone exposure were examined. SP nerve fibers and SP-containing cell bodies were present within the neural plexuses of trachea in ozone exposed tracheal segments pretreated with saline (Figure 6). In ferrets pretreated with saline, the percentage of SP-containing cell bodies in SMP and LT were significantly increased from 28% and 35% to 53% and 64%, respectively, by in vitro ozone exposure (Figure 6 and 7). These findings indicate that SP positive neurons in airways exposed to by ozone were significantly increased compared to tracheal segments exposed to air. However, pretreatment with the IL-1Ra attenuated the ozone-enhanced SP positive neurons in airway; only about 33% of the cell bodies in the SMP (Figure 7A) and about 41% of the cell bodies in the LT neurons contained SP(Figure 7A and 7B) in animals pretreated with IL-1 Ra after ozone exposure. There was no significant difference in SP positive neurons between air exposure animals treated with saline and ozone exposure animals pretreated with IL-1 Ra. Also, SP NFD was significantly increased from 0.14% in air exposure animals treated with saline compared to 0.29% in ozone exposure animals treated with saline. Pretreatment with the IL-1Ra attenuated the ozone-enhanced SP NFD (Figure 7C).
Figure 6.
Fluorescence photomicrographs of substance P (SP)-immunoreactive nerve cell bodies within superficial muscular plexus and SP-immunoreactive nerve fiber density within tracheal smooth muscle in IL-1 Ra or saline-treated tracheal segments after in vitro exposure to air or ozone. A (air exposure with IL-1 Ra pretreatment): few SP-immunoreactive nerve fibers are present in tracheal smooth muscle (NFD of this micrograph is 0.20). B (ozone exposure with saline pretreatment): increased SP-immunoreactive nerve fibers in tracheal smooth muscle (NFD of this micrograph is 0.41). C (ozone exposure with IL-1 Ra pretreatment) decreased SP-immunoreactive nerve fibers in tracheal smooth muscle (NFD of this micrograph is 0.21). D (air exposure with IL-1 Ra pretreatment): negative SP-immunoreactive neurons are present in the SMP. E (ozone exposure with saline pretreatment): SP-immunoreactive cell bodies are increased in the SMP. F (ozone exposure with IL-1 Ra pretreatment): negative SP-immunoreactive neurons are seen. The images of the SP-containing nerve cell bodies in SMP and SP NFD in air exposure group with saline pretreatment (not shown) are the same as air exposure with IL-1 pretreatment. Magnification: x285.
Figure 7.
Effects of IL-1 receptor antagonist treatment on SP-containing nerve cell bodies in SMP and LT and SP nerve fiber density in tracheal smooth muscle after in vitro exposure to air or ozone. Values are means ± SE; n = 5. *Significant difference between saline/ozone and other groups, P ≤0.05.
The next studies were done to examine the effect of IL-1 Ra on SP in in vivo ozone exposure. The changes in ozone induced-SP nerve fibers and SP-containing cell bodies after in vivo IL-1 Ra treatment were similar to the changes that occurred with in vitro IL-1 Ra treatment. In ferrets pretreated with saline, the percentages of SP-containing cell bodies in SMP and LT were significantly increased from 42% and 44% to 68% and 73%, respectively, by in vivo ozone exposure (Figure 8 A and B). However, pretreatment with the IL-1Ra attenuated both the ozone-enhanced SP positive neurons in airway ganglia; there was no significant difference in SP positive neurons between air exposed animals treated with saline and in vivo ozone exposed animals pretreated with IL-1 Ra. Also, SP NFD was significantly increased from 0.23% in air exposure animals treated with saline to 0.38% in ozone exposure animals treated with saline. Pretreatment with the IL-1Ra attenuated the ozone-enhanced SP NFD in airway smooth muscle (Figure 8C). By contrast, pretreatment with IL-1 Ra either in vivo or in vitro did not modify SP levels after air exposure (Figure 6, 7 and 8).
Figure 8.
Effects of IL-1 receptor antagonist i.v on SP-containing nerve cell bodies in SMP and LT and SP nerve fiber density in tracheal smooth muscle after in vivo exposure to air or ozone. Values are means ± SE; n = 5. *Significant difference between saline/ozone and other groups, P ≤0.05.
4. DISCUSSION
Results obtained from this study show that ozone exposure induces airway inflammation, as exemplified by the marked increases in total cell number, neutrophil percentage and IL-1 levels in the BAL fluid. Indeed, it is well documented that airway inflammation and mucosal injury occur after irritant inhalation (Hazbun, et al., 1993;Wu, et al., 1999;Becker, et al., 1999). Further, our study shows that the ozone exposure enhances airway smooth muscle reactivity to MCh and EFS increases in the number of SP neurons in airway ganglia, and increases SP nerve fiber density in tracheal smooth muscle. These results were observed after either in vivo or in vitro ozone exposure and are consistent with our previous studies using exogenous IL-1 (Wu, et al., 2003;Wu, et al., 2001). The novel findings in the current study are that the blockade of endogenous IL-1 effects by pretreatment with an IL-1 receptor antagonist significantly diminished the ozone-induced airway responsiveness to nerve stimulation, and mitigated the ozone-induced increase in SP-containing neurons in airway ganglia and SP nerve fiber density in tracheal smooth muscle. These inhibitory effects of the IL1 receptor antagonist on airway innervation were observed after either in vivo or in vitro ozone exposure. The findings support the conclusion that endogenous IL-1 is released after ozone exposure and enhances airway responsiveness through actions that augment SP levels in neurons of airway ganglia.
Ozone exposure induces epithelial cell damage and rapid development of airway inflammation in humans and monkeys (Plopper, et al., 1998;Hatch, et al., 1994). In the present study, the number of total cells and neutrophils in BAL fluid after ozone exposure were markedly increased indicating that ozone exposure induces airway inflammation in ferrets. IL-1 is produced by neutrophils, alveolar macrophages and tracheal epithelial cells, and levels are elevated in BAL fluid during airway injury in animal and human airways (Dye, et al., 1999;McKinney, et al., 1998). Recent studies also showed that ozone exposure caused IL-1 release from alveolar macrophages (Arsalane, et al., 1995;Pendino, et al., 1994). Thus, it is not surprising in the present study that the level of IL-1in BAL fluid significantly increased after in vivo ozone exposure. Our in vitro exposure experiments showed that the inhibition of endogenous IL-1 attenuated the neural and smooth muscle responses. This suggests that the airway epithelium may provide an important source of IL-1 since macrophage and neutrophil influx would not occur extensively in by the in vitro preparation. Therefore, it is possible that epithelial release of IL-1 initiates the early responses subsequent to ozone exposure, promoting neural and smooth muscle responses. As inflammation progresses, neutrophils and macrophages may perpetuate the inflammatory responses in the airway by maintaining enhanced levels of SP synthesis and release associated with the initial ozone exposure (Wu, et al., 2003;Wu, et al., 2001).
Our previous study showed that the application of exogenous IL-1 in the airways enhanced SP expression in intrinsic airway neurons (Wu, et al., 2002). SP has been associated with inflammation and interacts with other cells in the lung (e.g., mast cells, leukocytes, epithelial cells) to trigger the release of inflammatory mediators. We also reported previously that SP production and release is increased in nerve endings originating from neurons of airway ganglia in the lung after ozone exposure (Wu, et al., 2003;Wu, et al., 2001) and that the neurotrophin, nerve growth factor (NGF), was involved in mediating the increase in SP expression in airway ganglia (Wu and Dey, 2006). Induction of SP expression by cytokines in autonomic ganglia has been reported previously. In cultures of sympathetic ganglia, IL-1 has been shown to induce SP expression (Shadiack, et al., 1993). Interestingly, pure cultures of sympathetic neurons do not produce SP in response to IL-1, but sympathetic neuron cultures which include non-neuronal cells produced SP after IL-1 treatment (Freidin, et al., 1991). These findings suggest that IL-1-enhanced SP expression may involve additional intermediate signaling molecules that are produced by non-neuronal cells. A recent study has found that leukemia inhibitory factor (LIF) is a signaling molecule regulating IL-1-enhance SP expression in sympathetic ganglia (Shadiack, et al., 1993). In the lung, IL-1 increases NGF release from several cell types, including bronchial epithelium, airway smooth muscle, and inflammatory cells (Frossard, et al., 2005). Furthermore, IL-1 increases SP-induced airway smooth muscle contraction, a response that is blocked by NGF antibody (Frossard, et al., 2005). Therefore, it seems likely that the ozone-induced increases in neuronal SP levels and smooth muscle reactivity to nerve stimulation may result from an initial release of IL-1, possibly from the airway epithelium which stimulates the release of NGF, which upgregulates SP expression in airway neurons
Although the duration for the ozone exposure is three hours, the experiments are conducted an additional at 3 hrs hours after the end of the ozone exposure. This 6 hour time frame is sufficient to allow for the inflammatory process to peak in the airway (Joad, et al., 1993) for intranuronal transcription of SP to increase (Lai, et al., 2003). The rapid increase in SP level may be facilitated in neurons of airway ganglia because the length of these intramural neurons is short, the longest being several millimeters (Mitchell, et al., 1992), compared to sensory, postganglionic sympathetic or pregangllionic parasympathetic neurons, Therefore, transport distances for peptides are relative short and would be easily accomplished in a short timeframe. An additional consideration is that since the airway neurons are bathed directly in the interstitial fluid, cytokines released into the airway mucosa or submucosa after ozone exposure could directly affect the cell body without requiring transport along an axon.
Our recent studies found that ozone-enhanced airway responses to EFS are attenuated by treatment of ferrets with the neurokinin-1 (NK1) receptor antagonist CP99994 (Wu, et al., 2003;Wu, et al., 2001), suggesting that ozone-induced airway hyperresponsiveness involves enhanced SP release from airway neurons. In the present study, the contractile responses to EFS at 10 and 30 Hz were 32% and 40%, respectively, in ozone exposure animals; and contractions to EFS decreased to 25% and 31%, respectively, after treatment of IL-1Ra. The magnitude of these changes are almost identical to the changes observed with ozone exposure animals treated by NK1 antagonist CP99994 (Wu, et al., 2003;Wu, et al., 2001). Thus, one likely mechanism by which IL-1 Ra attenuates ozone-enhanced tracheal smooth muscle responsiveness to EFS is through the downstream inhibition of ozone-increased SP levels in airway neurons. SP enhances cholinergic responsiveness either through a direct effect on sensitivity of airway smooth muscle (Tanaka, et al., 1990) or by enhancing acetylcholine (ACh) release from parasympathetic nerve terminals (Larsen, et al., 2004;Watson, et al., 1993). Our present findings show that IL-1 Ra abolished ozone-enhanced tracheal smooth muscle responsiveness to EFS and to exogenous cholinergic agonists, suggesting that both sensitivity of airway smooth muscle and ACh release from parasympathetic nerve terminals were involved in ozone-induced hyperresponsiveness. For the neuronal effects, SP may act as a neuromodulator increasing ACh release from cholinergic nerve terminals. Since NGF is known to promote smooth muscle hyperresponsiveness (De Vries, et al., 1999) and influx of inflammatory cells (Path, et al., 2002), IL-1 may exert additional inflammatory effects through NGF as well.
In conclusion, the current results clearly show that ozone exposure increases airway inflammation and IL-1 release. At the same time, SP levels in intrinsic airway neurons and sensitivity of airway smooth muscle to EFS are increased. Administration of IL-1 receptor antagonist attenuates the ozone-induced airway responses to EFS and ozone-enhanced SP level in airway. The findings indicate that IL-1 released during ozone exposures enhances airway responsiveness by modulating SP expression in airway neurons.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-35812. The authors are grateful to Dr. G. Hobbs in the Department of Statistics, West Virginia University, for statistical analysis. The authors also thank Amgen Inc (Thousand Oaks, CA) for the supply of IL-1 Ra.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arsalane K, Gosset P, Vanhee D, Voisin C, Hamid Q, Tonnel AB, Wallaert B. Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am J Respir Cell Mol Biol. 1995;13:60–68. doi: 10.1165/ajrcmb.13.1.7598938. [DOI] [PubMed] [Google Scholar]
- Baker DG, McDonald DM, Basbaum CB, Mitchell RA. The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol. 1986;246:513–526. doi: 10.1002/cne.902460408. [DOI] [PubMed] [Google Scholar]
- Barchasz E, Naline E, Molimard M, Moreau J, Georges O, Emonds-Alt X, Advenier C. Interleukin-1beta-induced hyperresponsiveness to [Sar9,Met(O2)11]substance P in isolated human bronchi. Eur J Pharmacol. 1999;379:87–95. doi: 10.1016/s0014-2999(99)00484-7. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Baraniuk JN, Belvisi MG. Neuropeptides in the respiratory tract. Am Rev Respir Dis. 1991;144:1391–1399. doi: 10.1164/ajrccm/144.6.1391. [DOI] [PubMed] [Google Scholar]
- Becker S, Clapp WA, Quay J, Frees KL, Koren HS, Schwartz DA. Compartmentalization of the inflammatory response to inhaled grain dust. Am J Respir Crit Care Med. 1999;160:1309–1318. doi: 10.1164/ajrccm.160.4.9901062. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Undem BJ, Karakousis PC, Dey RD. Effects of organotypic culture on parasympathetic innervation of guinea pig trachealis. Am J Physiol Lung Cell Mol Physiol. 1996;271:L698–L706. doi: 10.1152/ajplung.1996.271.5.L698. [DOI] [PubMed] [Google Scholar]
- De Vries A, Dessing MC, Engels F, Henricks PAJ, Nijkamp FP. Nerve growth factor induces a neurokinin-1 receptor-mediated airway hyperresponsiveness in guinea pigs. Am J Respir Crit Care Med. 1999;159:1541–1544. doi: 10.1164/ajrccm.159.5.9808058. [DOI] [PubMed] [Google Scholar]
- Dey RD. Airways Ganglia. In: Raeburn D, Giembycz MA, editors. Airways Smooth Muscle: Structure, Innervation and Neurotransmission. Birkhauser Verlag; Basel: 1995. pp. 79–101. [Google Scholar]
- Dey RD, Altemus JB, Michalkiewicz M. Distribution of vasoactive intestinal peptide- and substance P-containing nerves originating from neurons of airway ganglia in cat bronchi. J Comp Neurol. 1991;304:330–340. doi: 10.1002/cne.903040213. [DOI] [PubMed] [Google Scholar]
- Dey RD, Altemus JB, Rodd AB, Mayer B, Said SI, Coburn RF. Neurochemical characterization of intrinsic neurons in ferret tracheal plexus. Am J Respir Cell Mol Biol. 1996;14:207–216. doi: 10.1165/ajrcmb.14.3.8845170. [DOI] [PubMed] [Google Scholar]
- Dey RD, Mayer B, Said SI. Colocalization of vasoactive intestinal peptide and nitric oxide synthase in neurons of the ferret trachea. Neuroscience. 1993;54:839–843. doi: 10.1016/0306-4522(93)90578-4. [DOI] [PubMed] [Google Scholar]
- Dey RD, Satterfield B, Altemus JB. Innervation of tracheal epithelium and smooth muscle by neurons in airway ganglia. Anat Rec. 1999;254:166–172. doi: 10.1002/(SICI)1097-0185(19990201)254:2<166::AID-AR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Dye JA, Madden MC, Richards JH, Lehmann JR, Devlin RB, Costa DL. Ozone Effects on Airway Responsiveness, Lung Injury, and Inflammation. Comparative Rat Strain and In Vivo/In Vitro Investigations. Inhal Toxicol. 1999;11:1015–1040. doi: 10.1080/089583799196664. [DOI] [PubMed] [Google Scholar]
- Fisher AWF. The intrinsic innervation of the trachea. J Anat. 1964;98:117–124. [PMC free article] [PubMed] [Google Scholar]
- Freidin M, Kessler JA. Cytokine regulation of substance P expression in sympathetic neurons. Proc Natl Acad Sci USA. 1991;88:3200–3203. doi: 10.1073/pnas.88.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard N, Naline E, Olgart HC, Georges O, Advenier C. Nerve growth factor is released by IL-1beta and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J. 2005;26:15–20. doi: 10.1183/09031936.05.00047804. [DOI] [PubMed] [Google Scholar]
- Hakonarson H, Maskeri N, Carter C, Chuang S, Grunstein MM. Autocrine interaction between IL-5 and IL-1beta mediates altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1999;104:657–667. doi: 10.1172/JCI7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- Hazbun ME, Hamilton R, Holian A, Eschenbacher WL. Ozone-induced increased in substance P and 8-epi-prostaglandin F2a in the airways of human subjects. Am J Respir Cell Mol Biol. 1993;9:568–572. doi: 10.1165/ajrcmb/9.5.568. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E(2) enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med. 2000;162:528–533. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- Joad JP, Bric JM, Pino MV, Hyde DM, McDonald RJ. Effects of ozone and neutrophils on function and morphology of the isolated rat lung. Am Rev Respir Dis. 1993;147:1578–1584. doi: 10.1164/ajrccm/147.6_Pt_1.1578. [DOI] [PubMed] [Google Scholar]
- Joad JP, Kott KS, Bric JM. The local C-fiber contribution to ozone-induced effects on the isolated guinea pig lung. Toxicol Appl Pharmacol. 1996;141:561–567. doi: 10.1006/taap.1996.0323. [DOI] [PubMed] [Google Scholar]
- Koto H, Aizawa H, Takata S, Inoue H, Hara N. An important role of tachykinins in ozone-induced airway hyperresponsiveness. Am J Respir Crit Care Med. 1995;151:1763–1769. doi: 10.1164/ajrccm.151.6.7767518. [DOI] [PubMed] [Google Scholar]
- Lai YL, Yu SC, Chen MJ. RNA interference prevents lipopolysaccharide-induced preprotachykinin gene expression. Toxicol Appl Pharmacol. 2003;193:47–54. doi: 10.1016/s0041-008x(03)00295-3. [DOI] [PubMed] [Google Scholar]
- Larsen GL, Loader J, Nguyen DD, Fratelli C, Dakhama A, Colasurdo GN. Mechanisms determining cholinergic neural responses in airways of young and mature rabbits. Pediatr Pulmonol. 2004;38:97–106. doi: 10.1002/ppul.20060. [DOI] [PubMed] [Google Scholar]
- Lee L-Y, Dumont C, Djokic TD, Menzel TE, Nadel JA. Mechanism of rapid, shallow breathing after ozone exposure in the conscious dogs. J Appl Physiol. 1979;46:1108–1109. doi: 10.1152/jappl.1979.46.6.1108. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T, Martling C-R, Saria A, Cuello C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tiss Res. 1984;235:251–261. doi: 10.1007/BF00217848. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Saria A, Brodin E, Rosell S, Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci USA. 1983;80:1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WJ, Jaskot RH, Richards JH, Costa DL, Dreher KL. Cytokine mediation of ozone-induced pulmonary adaptation. Am J Respir Cell Mol Biol. 1998;18:696–705. doi: 10.1165/ajrcmb.18.5.2928. [DOI] [PubMed] [Google Scholar]
- Mitchell HW, Coburn RF. Multiple motor pathways to single smooth muscle cells in the ferret trachea. J Physiol (Lond) 1992;456:557–574. doi: 10.1113/jphysiol.1992.sp019353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Path G, Braun A, Meents N, Kerzel S, Quarcoo D, Raap U, Hoyle GW, Nockher WA, Renz H. Augmentation of allergic early-phase reaction by nerve growth factor. Am J Respir Crit Care Med. 2002;166:818–826. doi: 10.1164/rccm.200202-134OC. [DOI] [PubMed] [Google Scholar]
- Pendino KJ, Shuler RL, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-alpha, and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11:279–286. doi: 10.1165/ajrcmb.11.3.8086166. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Hatch GE, Wong V, Duan XC, Weir AJ, Tarkington BK, Devlin RB, Becker S, Buckpitt AR. Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose, and glutathione depletion in rhesus monkeys. Am J Respir Cell Mol Biol. 1998;19:387–399. doi: 10.1165/ajrcmb.19.3.3183. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Carlson CD, Ding M, Hart RP, Jonakait GM. Lipopolysaccharide induces substance P in sympathetic ganglia via ganglionic interleukin-1 production. J Neuroimmunol. 1994;49:51–58. doi: 10.1016/0165-5728(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Hart RP, Carlson CD, Jonakait GM. Interleukin-1 induces substance P in sympathetic ganglia through the induction of leukemia inhibitory factor (LIF) J Neurosci. 1993;13:2601–2609. doi: 10.1523/JNEUROSCI.13-06-02601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka DT, Grunstein MM. Maturation of neuromodulatory effect of substance P in rabbit airways. J Clin Invest. 1990;85:345–350. doi: 10.1172/JCI114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H, Sakamoto T, Xu W, Barnes PJ, Chung KF. Effect of interleukin-1β on airway hyperresponsiveness and inflammation in sensitized and nonsensitized Brown-Norway rats. J Allergy Clin Immunol. 1994;93:464–469. doi: 10.1016/0091-6749(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Vinegar A, Sinnett EE, Kosch PC, Miller ML. Pulmonary physiology of the ferret and its potential as a model for inhalation toxicology. Lab Anim Sci. 1985;35:246–250. [PubMed] [Google Scholar]
- Watson N, Maclagan J, Barnes PJ. Endogenous tachykinins facilitate transmission through parasympathetic ganglia in guinea-pig trachea. Br J Pharmacol. 1993;109:751–759. doi: 10.1111/j.1476-5381.1993.tb13638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZX, Dey RD. Nerve growth factor-enhanced airway responsiveness involves substance P in ferret intrinsic airway neurons. Am J Physiol Lung Cell Mol Physiol. 2006;291:L111–L118. doi: 10.1152/ajplung.00377.2005. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Lee LY. Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea pigs: role of tachykinins. J Appl Physiol. 1999;87:1621–1628. doi: 10.1152/jappl.1999.87.5.1621. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Maize DF, Jr, Satterfield BE, Frazer DG, Fedan JS, Dey RD. Role of intrinsic airway neurons in ozone-induced airway hyperresponsiveness in ferret trachea. J Appl Physiol. 2001;91:371–378. doi: 10.1152/jappl.2001.91.1.371. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Morton RF, Lee LY. Role of tachykinins in ozone-induced airway hyperresponsiveness to cigarette smoke is guinea pigs. J Appl Physiol. 1997;83:958–965. doi: 10.1152/jappl.1997.83.3.958. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1beta-induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol. 2002;283:L909–L917. doi: 10.1152/ajplung.00363.2001. [DOI] [PubMed] [Google Scholar]
- Wu Z-X, Satterfield BE, Dey RD. Substance P release from intrinsic airway neurons contributes to ozone-enhanced airway hyperresonsiveness in ferret trachea. J Appl Physiol. 2003;95:742–750. doi: 10.1152/japplphysiol.00109.2003. [DOI] [PubMed] [Google Scholar]
- Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol. 2007;156:116–119. doi: 10.1016/j.resp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Zhu W, Dey RD. Projections and pathways of VIP- and nNOS-containing airway neurons in ferret trachea. Am J Respir Cell Mol Biol. 2001;24:38–43. doi: 10.1165/ajrcmb.24.1.4255. [DOI] [PubMed] [Google Scholar]