Abstract
African trypanosomes are unable to synthesize purines de novo and must salvage preformed purine nucleosides and nucleobases from their hosts. The Trypanosoma brucei genome project has identified 12 members of the equilibrative nucleoside transporter family, most of which have been characterized previously as nucleoside and/or nucleobase transporters. Here the eleventh member of this family, TbNT11.1, has been functionally expressed in null mutants of Leishmania that are deficient in purine nucleoside or nucleobase uptake and identified as a high affinity purine nucleobase transporter. Expression of TbNT11.1 in Xenopus oocytes revealed that it is also a transporter for the diamidine drug pentamidine that is the principal drug employed to treat early stage human African trypanosomiasis and may thus contribute to the uptake of this therapeutically important compound. In addition, characterization of the twelfth member of the family, TbNT12.1, reveals that it is an adenine/pentamidine transporter
1. Introduction
The African trypanosome Trypanosoma brucei, like all other known parasitic protozoa, is unable to synthesize purines de novo and thus expresses a plethora of purine transporters capable of salvaging purines from the host [1] as well as various purine salvage enzymes [2] that inter-convert different purines and generate purine nucleotides from nucleobases or nucleosides. Examination of the T. brucei genome [1,3] revealed 12 distinct equilibrative nucleoside transporter (ENT) family members (SLC29 family in the Human Genome Organization Database (http://bioparadigms.org) or 2.A.57 family in the Transporter Classification Database (http://www.tcdb.org)), integral membrane proteins with 11 predicted transmembrane segments that mediate the uptake of nucleosides, nucleobases, or both [4]. It is not clear why this parasite expresses so many ENTs, but ongoing studies are attempting to define the transport properties of all these permeases to illuminate their distinct functions (Table 1). TbAT1, the first ENT to be identified in African trypanosomes [5], is the so-called P2 transporter originally identified in intact bloodstream form parasites [6] that is responsible for uptake of adenosine and adenine. A cluster of genes on chromosome 2 encodes TbNT2-TbNT7, which are P1 type transporters [6] that mediate the uptake of adenosine, guanosine, inosine, and in some cases hypoxanthine [7,8], although TbNT3 and TbNT4 did not exhibit uptake activity for purines when their cRNAs were injected into Xenopus laevis oocytes or when overexpressed in purine nucleobase or nucleoside transport deficient null mutants of Leishmania and thus have unknown substrates. TbNT8.1, TbNT8.2, and TbNT8.3 are encoded by a cluster of 3 very closely related genes; TbNT8.1 is a high affinity nucleobase permease that transports hypoxanthine, adenine, guanine, and xanthine [9], and TbNBT1/TbNT8.2 also transports guanosine and inosine with somewhat lower affinity than the nucleobases [10]. In addition TbNT9 [1,11] and TbNT10 [11,12] are also purine nucleoside transporters with P1 type activity. Thus two more family members, TbNT11 and TbNT12, remain to be characterized. TbNT11 is represented by two closely related isoforms, differing by 4 amino acids, whose genes TbNT11.1 and TbNT11.2 are located on chromosome 9. In this report we have examined the function of TbNT11.1. While TbNT11.2 is likely to function similarly to TbNT11.1, this presumption remains to be tested. We have also examined the function of TbNT12, which is likewise represented by two closely related isoforms TbNT12.1 and TbNT12.2.
Table 1.
ENT family members from T. brucei.
| TbNT Designation | Alternate Designation | GeneDB Systematic Name | Substrates | References |
|---|---|---|---|---|
| TbAT1 | Tb927.5.286b | Ado, Ade, Pentamidine, Melarsoprol, DB75 | [5,14,15] | |
| TbNT2 | Tb927.2.6150 | Ado, Guo, Ino | [7,8] | |
| TbNT3 | Tb927.2.6200 | Unknown | [8] | |
| TbNT4 | Tb927.2.6220 | Unknown | [8] | |
| TbNT5 | Tb927.2.6240 | Ado, Guo, Ino, Hyp | [8] | |
| TbNT6 | Tb927.2.6320 | Ado, Guo, Ino, Hyp | [8] | |
| TbNT7 | Tb927.2.6280 | Ado, Guo, Ino, Hyp | [8] | |
| TbNT8.1 | Tb11.02.1100 | Hyp, Ade, Gua, | [9,10] | |
| TbNT8.2 | TbNBT1 | Tb11.02.1105 | Xan | |
| TbNT8.3 | Tb11.02.1106 | (Guo, Ino) | ||
| TbNT9 | AT-D | Tb927.6.220 | Ado, Guo, Ino | [1,11] |
| TbNT10 | AT-B | Tb09.160.5480 | Ado, Guo, Ino | [11,12] |
| TbNT11.1 | Tb09.244.2020 | Hyp, Ade, Xan, Pentamidine | This report | |
| TbNT11.2 | Tb09.v4.0106 | Not examined | ||
| TbNT12.1 | Tb927.3.590 | Ade, Pentamidine | This report | |
| TbNT12.2 | None | Ade, Pentamidine | This report |
In addition to its role in purine uptake, TbAT1 also mediates the uptake of unrelated anti-trypanosomal drugs such as the diamidine pentamidine and the arsenical melarsoprol [6,13–15]. Pentamidine is still the drug of choice for treatment of early stage African trypanosomiasis [16], and other aromatic diamidines such as DB75 and its orally available prodrug DB289 [17] offer considerable promise for improved treatment of this infection and are currently in phase III trials. Nonetheless, there are at least two other transport activities, designated HAPT1 (high-affinity pentamidine transporter) and LAPT1 (low-affinity pentamidine transporter), that serve as alternative routes for uptake of pentamidine [16,18]. However, the permeases associated with these latter uptake activities have not been identified. Since TbAT1 is a member of the ENT family, it is possible that other ENTs in T. brucei might also mediate uptake of pentamidine and related diamidines.
In the studies reported here, we have expressed TbNT11.1 in recently developed null mutants of the related kinetoplastid parasites Leishmania major or L. donovani that are deficient in uptake of either nucleobases [19] or nucleosides [20]. The results indicate that TbNT11.1 is a second high affinity nucleobase transporter that displays differences in kinetic and pharmacological properties compared to the previously characterized nucleobase transporters of the TbNT8 family. Furthermore functional expression of TbNT11.1 in Xenopus oocytes, a system that provides low background levels of pentamidine uptake, indicates that this permease can also transport this anti-trypanosomal drug. Similar studies with TbNT12.1 and TbNT12.2 revealed that they transport the nucleobase adenine and, similar to TbNT11.1, also mediate the uptake of pentamidine when expressed in Xenopus oocytes. These results suggest that 3 related nucleobase transporters, TbAT1, TbNT11, and TbNT12 are involved in uptake of pentamidine but that the nucleobase transporters of the more divergent TbNT8 family are not pentamidine transporters.
2. Methods
2.1 Chemical and reagents
[2,8-3H]hypoxanthine (36.4 Ci/mmol), [8-3H]xanthine (18 Ci/mmol), [2,8-3H]adenine (50 Ci/mmol), [8-3H]guanine (7 Ci/mmol), [3H]allopurinol (1.2 Ci/mmol) were purchased from Moravek Biochemicals. [14C]pentamidine isethionate (54 μCi/mmol) was a gift from Research Triangle Institute International obtained under support of the Drug Synthesis and Chemistry Branch, National Cancer Institute. All other chemicals and reagents were of the highest commercial quality available.
2.2 Growth and transfection of parasites and isolation of nucleic acids
T. brucei 927 PF and BF parasites were grown as described previously [8]. PF cell line 29-13-6 and BF cell line SM, both expressing the tetracycline repressor and T7 RNA polymerase [21], were grown in medium containing 25 μg/ml hygromycin and 15 μg/ml G418, and 2.5 μg/ml G418 respectively. 5 to 10 μg of linearized DNA was used to transfect mid-log phase PF or BF parasites following the Cross laboratory protocols (http://tryps.rockefeller.edu/crosslab_protocols_index.html). Leishmania major strain Friedlin VI (MHOM/IL/80Friedlin) and Δlmant3 null mutants were cultured as described [19], and Leishmania transfection was performed as described elsewhere [19]. For isolation of genomic DNA and total RNA preparations, DNAzol and TRIzol regents (Invitrogen) were used according to manufacturer’s instructions.
2.3 Cloning and sequence determination of TbNT11 and TbNT12 genes
TbNT11 (Tb09.244.2020) and TbNT12 (Tb927.3.590) open reading frames (ORFs) were PCR amplified from genomic DNA using Pfu turbo (Stratagene) and specific oligonucleotides. Forward oligonucleotides TbNT11F (ccaccatgcttggcttcggttctgtg) or TbNT12F (ccaccatgatgctcgggttcgaatcggtttctgag) representing the first 7 or 10 amino acids of each ORF including a Kozak consensus [22] sequence (underlined) were used as forward primers, and TbNT11R or (ttactgtgactcatttttcgggagagc) TbNT12R (ctattgaggaagtccctccttgacggcaag) encompassing the complement of the last 9 or 10 amino acids of each ORF including the stop codon (in italics) were used as reverse primers. PCR amplification and cloning were carried out as described in [12]. Resulting clones were characterized by DNA restriction analysis and sequencing by the Core Facility of the Department of Molecular Microbiology and Immunology at the Oregon Health & Science University using an ABI model 377 DNA sequencer (Perking-Elmer).
2.4 Deduced amino acid sequence analysis
For DNA sequence analysis of TbNT11.1 and TbNT12.1 and amino acid sequence alignments, MacVector software (Intelligenetics) was used. Transmembrane segments were predicted using the TMHMM Server v. 2.0 (www.cbs.dtu.dk/services/TMHMM).
2.5 Expression of TbNT11.1 and TbNT12.1 in Leishmania null mutants
To generate the episomal expression constructs, the TbNT11.1 or TbNT12.1 ORF was amplified by PCR and subcloned into EcoR I restriction site within pX63NEORI [23]. Undigested plasmids (10 μg) were used for parasite’s transfection as previously described [24].
2.6 TbNT11.1 and TbNT12.1 expression in Xenopus oocytes
The TbNT11.1 and TbNT12.1 ORFs were sub-cloned into EcoR I site of the Xenopus expression vector pL2-5 [25], linearized, and in vitro transcribed using the mMESSAGE mMACHINE T7 Ultra Kit (Ambion) according to the manufacturer’s instructions. Stage V–VI Xenopus laevis oocytes were injected with 23 nL of cRNA (~ 10 ng) or water and then incubated for 3 days at 16°C in ND96 buffer [26] before performing uptake assays.
2.7 Uptake assays
Uptake of [3H]substrates was assayed for Leishmania promastigotes as described [27] and for Xenopus oocytes as described [26]. EC50 and Ki values were estimated by fitting in the data to one site competition equation employing Prism 4.0b GraphPad Software, Inc. [28] employing a Km value of 141 μM for uptake of hypoxanthine and 2.7 μM for uptake of adenine by TbNT11.1. Substrate saturation curves were generated by incubating for 1 min (Leishmania cells) or 1 h (oocytes) with various concentrations of radiolabeled compounds. These data were fitted to the Michaelis-Menten equation by non-linear regression using Prism 4.0b GraphPad Software, Inc.
2.8 Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from 5 × 108 cells using 1 ml of TRIzol (Invitrogen). RNA was treated with TURBO DNase (Ambion) for 30 min at 37°C and purified by RNeasy® Mini Kit (Invitrogen). To make qRT-PCR templates, 1 μg of RNA was in vitro transcribed using the iScript™cDNA Synthesis Kit (BIO-RAD) in a 20 μl reaction. Each experiment also included a reaction without reverse transcriptase as a negative control. For each 25 μl qRT-PCR, 12.5 μl of SYBR Green Master Mix (Applied Biosystems) was combined with 5 μl 1.5 μM forward oligonucleotide, 5 μl reverse oligonucleotide, and 2.5 μl of 10-fold diluted cDNA template per well of a 96-well plate. An Applied Biosystems Prism 7000 Thermocycler was employed using the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Thermal dissociation confirmed PCR generated a single amplicon. Primers for amplification of 18S rRNA were used as internal controls for normalization of RNA from PF and BF trypanosomes. TbNT11.1 and TbNT12.1 primers were designed from the most divergent sequences within the ORF using Applied Biosystems PRIMER EXPRESS 2.0 Software and were: (TbNT11) cttgtcgttcctggagttcttcct (forward) and tccgtctccagcttgatgttctc (reverse), producing a 78 bp amplification product, and (TbNT12) gcggatccctctggctg (forward) and cgacgaaaactgccaaaattg (reverse), producing a 65 bp amplification product, while primers for 18S rRNA were: cggaatggcaccacaagac (forward) and tggtaaagttccccgtgttga (reverse), producing a 64 bp amplification product. Quantitative differences in target amplicons were determined by the Pfaffl method [29].
2.9 Inducible expression of TbNT11.1- 3HA-COOH and NH2-Ty-TbNT12.1 in 29-13-6 and SM cell lines and immunofluorescence microscopy
To generate epitope-tagged transporter proteins, the luciferase ORF from pLew82 [21] was replaced by a triple hemagglutinin epitope (3HA) [30] or a Ty epitope [31] to produce plasmids p82M3HA and p82MTy that allow the inducible expression of tagged proteins driven by the T7 promoter upon addition of doxycyclin. TbNT11.1 and TbNT12.1 ORFs flanked by specific enzyme restriction sites were PCR amplified and subcloned into p82M3HA and p82MTy and sequenced.
29-13-6 or SM parasites were transfected with 5 μg of linear NotI digested constructs as described (http://tryps.rockefeller.edu/crosslab_protocols_index.html), selected on 2.5 μg/ml phleomycin and 2.5 ng/ml doxycyclin, and cloned. To overexpress TbNT11.1-3HA-COOH or NH2-Ty-TbNT12.2, 1 μg/ml doxycyclin was added to each cell line culture and incubated for 48 h. Induced PF cell lines were analyzed by immunofluorescence microscopy using anti-HA monoclonal antibodies (CONVANCE) or anti-Ty monoclonal antibodies [31], as previously described [19]. Briefly, for SM induced parasites, 5 × 106 parasites were centrifuged and washed twice at room temperature with PBS containing 10 mM glucose. The cell pellet was resuspended in 3.7% paraformaldehyde in PBS, pH 7.2 and incubated for 15 min at room temperature, cells were centrifuged and washed once with 10 mM glycine in PBS and once with PBS, resuspended in 100 μl PBS, spotted onto poly-L-lysine coated coverslips, and treated as described for PFs. Fluorescence images were obtained using a wide field deconvolution system (Applied Precision Instruments, Inc.) consisting of an inverted Nikon TE 200 Eclipse microscope, a Kodak CH350 CCD camera, and the Deltavision operating system. Images were acquired using a 60X objective in a 1024×1024 format and deconvolved with 9 iteration using SoftWoRx software. Adobe Photoshop 7 and Adobe Illustrator 10 (Adobe Systems Inc.) were used to create image compositions.
3. Results and Discussion
3.1 Comparison of TbNT11.1 and TbNT12.1 to other ENTs in T. brucei
The 12 ENT family members within the T. brucei genome, designated TbAT1 and TbNT2 – TbNT12 according to their order of discovery, are listed in Table 1 along with their updated Systematic Names (http://www.genedb.org), alternate designations in other publications, and experimentally defined substrate specificities. In this study we have investigated the functions of the two family members, TbNT11 and TbNT12, whose transport activities were unknown previously; the studies performed herein reveal that these novel permeases mediate the uptake of purine nucleobases (see below). A sequence comparison with other TbNT family members (Fig. 1 and Fig. 2A) revealed that TbNT11 and TbNT12 (represented by the alleles TbNT11.1 and TbNT12.1, see below) are most closely related to each other and to the adenosine/adenine transporter TbAT1. Furthermore, among the alignments scored in Fig. 2A, TbAT1, TbNT11.1, and TbNT12.1 are more closely related to each other in a phylogenetic tree than they are to another purine nucleobase transporter TbNT8.1 (Fig. 2B), suggesting the possibility that TbAT1, TbNT11.1, and TbNT12.1 might share functional properties distinct from TbNT8.1.
Fig. 1.
Alignment of the amino acid sequences of TbAT1, TbNT11.1, and TbNT12.1. Identities are indicated by the black background, similarities by gray background, and predicted transmembrane helices by heavy lines above the sequence labeled TM1-TM11. Boxed sequences indicate regions of TbNT11.1 that are unique when compared to all other members of this family in T. brucei (Table 1), and amino acids in the sequence of TbNT12.1 with an asterisk underneath indicate residues unique to this permease. Dashes indicate gaps introduced to maximize alignment. The CLUSTALW program (MacVector, Intelligenetics) was used for alignments. Transmembrane helices were predicted using TMHMM software (http://www.cbs.dtu.dk/services/TMHMM-2.0/).
Fig. 2.
Sequence relationships between purine nucleobase transporters in T. brucei. (A) The percent amino acid identities between TbAT1, TbNT8.1, TbNT11.1, and TbNT12.1 are presented. Pairwise alignment was performed using MacVector software (Intelligenetics) employing an open gap penalty of 10.0, an extend gap penalty of 0.1, and the BLOSUM similarity matrix. (B) Phylogenetic tree for the purine nucleobase transporters from T. brucei. Branch lengths indicate evolutionary distances (values over the branches). As an approximate guide, a value of 0.1 corresponds to a difference of ~10% between sequences. The tree was generated using MacVector software employing the Neighbor Joining Best Tree method.
Of additional interest, a multi-alignment of all 12 family members in T. brucei revealed 3 segments of sequence, of greater than 3 amino acids, that are unique to TbNT11 (Fig. 1): TEEQKF (amino acids 55–60 in the exofacial hydrophilic loop between predicted transmembrane domains TM1 and TM2), KRTEDRVASR (amino acids 189–198 in the exofacial loop between TM5 and TM6 according to the experimentally determined topology for human hENT1 [32]), and the intrafacial COOH-terminal sequence DESQ. These divergent segments could be determinants of distinct functional properties of TbNT11.1 and may be worthy of investigation in future studies. TbNT12.1 exhibited several individual residues that were unique to this permease (asterisks in Fig. 1) but did not possess any unique sequence segments.
3.2 Functional expression in nucleobase or nucleoside transport-deficient null mutants of Leishmania reveals that TbNT11.1 is a purine nucleobase transporter
Recently we have generated a null mutant in the NT3 nucleobase transporter gene of L. major, designated Δlmant3, that is deficient in the uptake of hypoxanthine and guanine and strongly impaired in uptake of adenine and xanthine [19], and Liu et al. have generated a dual null mutant in the NT1 and NT2 nucleoside transporter genes of L. donovani, Δldnt1/Δldnt2, that is deficient in uptake of the adenosine, guanosine, inosine, and xanthosine [20]. These two null mutants provide opportune genetic backgrounds for expression and functional characterization of ENT permeases. Since Leishmania are more closely related to T. brucei than the Xenopus oocytes or mutant yeast strains that have been used to characterize several ENT genes from trypanosomes and thus may provide a preferable heterologous expression system, we have complemented the Δlmant3 and Δldnt1/Δldnt2 mutants with the TbNT11.1 open reading frame (ORF) encompassed within an episomal expression vector.
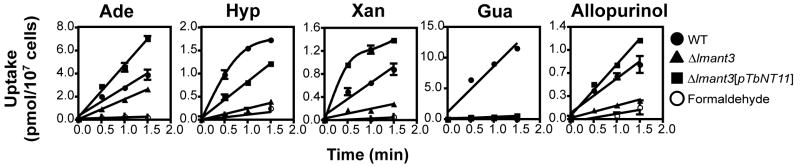
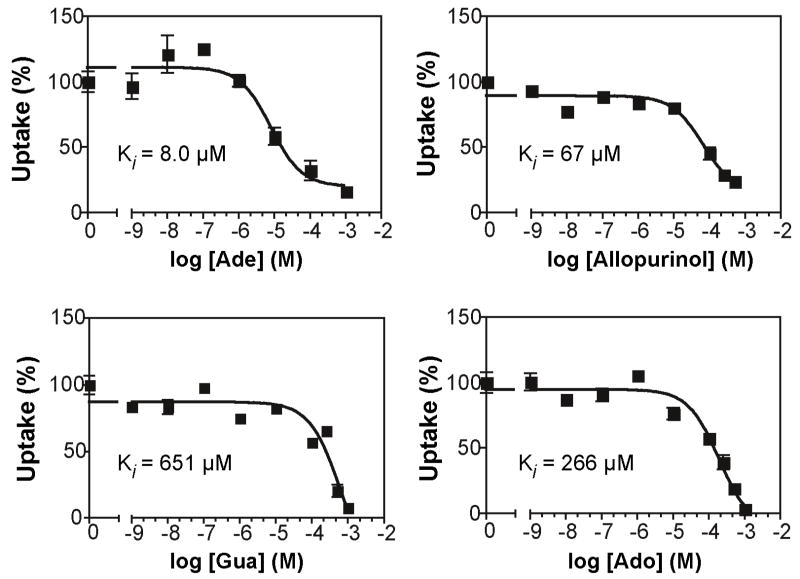
Radiolabel uptake assays using the complemented Δlmant3[pTbNT11] line (Fig. 3) revealed that TbNT11.1 mediated uptake of 1 μM adenine, hypoxanthine, and xanthine but not guanine. Hence, TbNT11.1 is a purine nucleobase transporter but is distinct from TbNT8.1 that mediates uptake of all 4 nucleobases [9] or TbNBT1/TbNT8.2 that mediates the uptake of the 4 nucleobases plus guanosine and inosine [10]. Furthermore TbNT11.1 also promoted uptake of the pyrazolopyrimidine analog of hypoxanthine, 4-hydroxypyrazolo[3,4-d]-pyrimidine-4-one or allopurinol [33], that is employed as an anti-leishmanial drug [34], and it has been suggested that the multiple allopurinol uptake systems in T. brucei [35] present a potential for use of such purine antimetabolites for treatment of African trypanosomiasis. In addition, inhibition of uptake of 1 μM [3H]hypoxanthine by graded concentrations of unlabeled adenine or allopurinol revealed single component inhibition curves (Fig. 4) with estimated Ki values of 8.0 and 67 μM respectively, consistent with permeation of these substrates through TbNT11.1. Although guanine is not a substrate for TbNT11.1 (Fig. 3), it is an inhibitor of this permease (Fig. 4). In contrast, the pyrimidine nucleobases uracil and cytosine did not inhibit uptake of hypoxanthine at concentrations up to 1 mM (data not shown) indicating the transporter’s specificity for purine nucleobases. By comparison, the complemented Δldnt1/Δldnt2[pTbNT11.1] line did not take up 0.5 μM or 100 μM [3H]adenosine (data not shown), although adenosine is an inhibitor of TbNT11.1 (Fig. 4), and failure of 1 mM inosine, guanosine, or xanthosine to inhibit uptake of [3H]hypoxanthine in Δlmant3[pTbNT11.1] parasites (data not shown) confirmed that TbNT11.1 is not a nucleoside transporter.
Fig. 3.
Uptake of [3H]nucleobases by TbNT11.1 expressed from an episomal expression vector in the nucleobase transport deficient null mutant of Leishmania major Δlmant3 [19]. The Δlmant3 cell line (▲) was transfected with the TbNT11.1 ORF cloned into the pX63NEORI vector (Δlmant3[pTbNT11.1]) (■) and both lines were assayed for uptake of 1 μM [3H]adenine (Ade), [3H]hypoxanthine (Hyp), [3H]xanthine (Xan), [3H]guanine (Gua) or [3H]-allopurinol (Allopurinol). Uptake of wild type (WT) L. major promastigotes before (●) and after (○) treatment with 1% formaldehyde was also measured. For each time course, background values of uptake at t=0 were subtracted from all data points. Data points represent the mean and error bars the standard deviation for uptake assays performed in triplicate. Similar results were obtained in 3 independent experiments.
Fig. 4.
Inhibition of transport of [3H]hypoxanthine by TbNT11.1 by graded concentrations of unlabeled adenine (Ade), guanine (Gua), allopurinol (Allopurinol), and adenosine (Ado). Uptake of 1 μM [3H]hypoxanthine by Δlmant3[pTbNT11.1] parasites was measured over the course of 1 min in the presence or absence of inhibitor, and uptake by Δlmant3 parasites was subtracted to obtain TbNT11.1-specific transport. Results are reported as percentage of uptake compared to assays performed in the absence of inhibitor. Each data point represents the mean and standard deviation of triplicate measurements. Similar results were obtained in 2 independent experiments.
Uptake assays performed over a range of substrate concentrations (Fig. 5) revealed Km values of 2.7 ± 0.3, 141 ± 32, 7.2 ± 2.3 μM (n=3) and Vmax/Km values of 4.0 ± 0.6, 0.26 ± 0.06, 12.0 ± 11.5 μl min−1 107 cell−1 (n=3) for adenine, hypoxanthine, and xanthine respectively. Hence adenine is the highest affinity substrate but xanthine is the most efficient permeant.
Fig. 5.
Substrate saturation curves for uptake of [3H]nucleobases by TbNT11.1 expressed in the Δlmant3 null mutant. Parallel uptake measurements were performed with Δlmant3 and Δlmant3[pTbNT11.1] cells, and the uptake values of the former were subtracted from the latter to obtain TbNT11.1-specific uptake. Values represent the mean and standard deviation of triplicate measurements. Data were fitted to the Michaelis-Menten equation by non-linear regression. Similar results were obtained in 3 independent experiments.
3.3 TbNT11.1 is a pentamidine transporter
Since TbAT1 and TbNT11.1 both mediate the uptake of one or more nucleobases, we examined the possibility that TbNT11.1, like TbAT1, might be a pentamidine transporter. In the Δlmant3[pTbNT11.1] line, pentamidine was able to inhibit the uptake of 12.5 μM [3H]hypoxanthine with an EC50 value of 4.8 μM (Fig. 6A) and 12.5 μM [3H]adenine with an EC50 value of 9.6 μM (Fig. 6B). In contrast, pentamidine did not significantly inhibit transport of hypoxanthine by TbNT8.1 (Fig. 6C), another purine nucleobase transporter characterized in T. brucei. Since Leishmania promastigotes exhibit robust uptake of pentamidine that is not mediated by nucleoside or nucleobase transporters [36] and are thus not a useful heterologous system for monitoring uptake of this drug by TbNT11.1, we used Xenopus oocytes to monitor directly transport of radiolabeled pentamidine by TbNT11.1. Indeed, oocytes injected with TbNT11.1 cRNA took up 50 μM [14C]pentamidine much more rapidly than control water injected oocytes (Fig. 7A) revealing that pentamidine is a substrate as well as an inhibitor of TbNT11.1. Furthermore, pentamidine uptake was saturable (Fig. 7B) with an estimated Km value of 407±119 μM (n=3), consistent with transporter-mediated uptake of this drug by TbNT11.1. For unknown reasons, uptake measurements above 400 μM pentamidine produced large standard deviations between replicate samples, preventing accurate rate measurements at the upper end of the saturation curve. The apparent affinity of the permease for pentamidine is lower than that for the natural nucleobase substrates but is still within the micromolar range. Nonetheless, the Km value of TbNT11.1 for pentamidine is much higher than that measured for TbAT1 in oocytes, which is ~3 μM (data not shown), indicating that TbNT11.1 is a relatively low affinity pentamidine transporter.
Fig. 6.
Pentamidine inhibition of uptake of (A) 12.5 μM [3H]hypoxanthine by TbNT11.1, (B) 12.5 μM [3H]adenine by TbNT11.1, and (C) 12.5 μM [3H]hypoxanthine by TbNT8.1. In each case, the T. brucei nucleobase transporter was expressed from the pX63NEORI expression vector in Δlmant3 null mutant promastigotes, and uptake values from Δlmant3 cells were subtracted to obtain TbNT11.1-specific uptake. Data points represent the mean and standard deviation of triplicate measurements and are reported as percentage of uptake compared to uptake in the absence of inhibitor. Inhibition was quantitated as EC50 rather than Ki values because calculation of the latter value assumes competitive inhibition [28], which may not apply for pentamidine.
Fig. 7.
Uptake of [14C]pentamidine by TbNT11.1 expressed in Xenopus laevis oocytes. (A) Time course for uptake of 50 μM pentamidine by oocytes injected with water (■) or with TbNT11.1 cRNA (▲). (B) Substrate saturation curve for uptake of pentamidine by oocytes expressing TbNT11.1. Uptake values for water-injected oocytes were subtracted from each data point to yield TbNT11.1-specific uptake. Data were fitted to the Michaelis-Menten equation by non-linear regression. (C) Inhibition of uptake of 50 μM [14C]pentamidine in oocytes expressing TbNT11.1 by 1 mM unlabeled substrates. The bar marked ‘C’ refers to the control uptake of pentamidine without competing substrate and is set at 100%. All measurements were made with at least 3 oocytes. Similar results were obtained in 3 independent experiments.
We consider it unlikely that pentamidine uptake by TbNT11.1 is an artifact of expression in Xenopus oocytes. TbNT11.1 mediates the uptake of pentamidine in oocytes (Fig. 7) and is inhibited by pentamidine when expressed in another heterologous system, the Δlmant3 null mutant of L. major (Fig. 6A,B). In contrast, another nucleobase transporter TbNT8.1 is not inhibited by this drug when expressed in Δlmant3 null mutants. The consistent results obtained for TbNT11.1 in two heterologous systems support the designation of TbNT11.1 as a pentamidine transporter.
Because TbAT1 transports both pentamidine and the arsenical drug melarsoprol, we tested the ability of melarsoprol to inhibit uptake of nucleobases by TbNT11.1. Using 12.5 μM adenine as permeant, pentamidine inhibited TbNT11.1 mediated uptake with and EC50 of 9.6 μM and melarsoprol inhibited uptake with an EC50 of 12.9 μM, indicating that both drugs are efficient inhibitors of the permease. However, measurement of melarsoprol uptake by oocytes expressing TbNT11.1 by inductively coupled mass spectrometry [37] revealed no uptake of this drug over the background level measured in water injected oocytes (data not shown), suggesting that melarsoprol is an inhibitor but not a substrate for TbNT11.1.
The nucleobase substrates of TbNT11.1 exhibited different abilities to inhibit the uptake of pentamidine in TbNT11-expressing oocytes (Fig. 7C). Whereas 1 mM xanthine and hypoxanthine partially inhibited uptake of this drug, 1 mM adenine is a very poor inhibitor for uptake of 50 μM [14C]pentamidine. Thus, while pentamidine is an efficient inhibitor of adenine uptake (Fig. 6B), adenine is not an efficient inhibitor of pentamidine uptake (Fig. 7C). These results are different from those typically observed for multi-substrate transporters where multiple permeants cross-inhibit each other and presumably translocate through a shared permeation pathway. However, a notable exception to cross-inhibition has been reported recently for the C03H5.2 nucleotide sugar transporter from Caenorhabditis elegans in which both UDP-N-acetylglucosamine and UDP-N-acetylgalactosamine permeate the transporter without inhibiting each other [38]. Adenine and pentamidine transport by TbNT11.1 may be a less extreme example of such behavior in which the second substrate inhibits the first but the first does not inhibit the second. Although we do not know the molecular explanation for this phenomenon, one potential model is that adenine and pentamidine bind to non-overlapping sites on TbNT11.1, but pentamidine has preferential access to the translocation pathway.
3.4 TbNT12.1 and 12.2 are adenine and pentamidine transporters
We have also characterized the final member of the TbNT family in T. brucei, TbNT12 (Table 1). Amplification of the TbNT12 ORF from genomic DNA generated two closely related sequences in multiple independent PCRs: TbNT12.1 and TbNT12.2. The sequence of TbNT12.1 was the same as that obtained from the T. brucei genome (Tb927.3.590, Fig. 1), and TbNT12.2 differed from this sequence at 8 amino acids: V159A, I168V, R231K, A236T, G243D, A244T, S255P, I417V, where the first amino acid in each pair is from TbNT12.1 and the second is from TbNT12.2. In addition, the TbNT12.2 ORF does not encode M1 but does encode M2 of TbNT12.1, so the two proteins could differ in whether or not they contain two methionines at the amino-terminus.
Expression of TbNT12.1 and 12.2 in Xenopus oocytes revealed that they transport adenine (Fig. 8A, B). The transport rates were linear with respect to adenine concentration from 0–1 mM and thus the affinity for this substrate was low for both permeases. Both carriers also transport pentamidine (Fig. 8C), and a Km value of 161 ± 8 μM and a Vmax of 21.8 ± 6.5 pmol/h/oocyte (n=3) was measured for TbNT12.1 (Fig. 8D). These permeases did not mediate the uptake of hyp, gua, or xan above the level observed in water-injected oocytes, suggesting that they are adenine-specific transporters. Expression of TbNT12.1 and TbNT12.2 in Δlmant3 promastigotes also demonstrated uptake of 50 μM adenine (103 ± 2 and 86.5 ± 1.1 pmol/min/107 parasites, n = 3, for TbNT12.1 and TbNT12.2 respectively) significantly above the background level observed in Δlmant3 parasites (41.8 ± 1.4 pmol/min/107 parasites), and uptake increased linearly with concentration from 0–1 mM adenine.
Fig. 8.
Uptake of adenine and pentamidine by TbNT12.1 and TbNT12.2 expressed in Xenopus oocytes. Time course for uptake of 25 μM [3H]adenine (A) and 50 μM pentamidine (C) in oocytes injected with water (□) or with TbNT12.1 (■) or TbNT12.2 (●) cRNA. Substrate saturation curves for (B) adenine and (D) pentamidine in oocytes injected with TbNT12.1 and TbNT12.2 cRNA. In (C, D), uptake values for water-injected oocytes were subtracted from each data point to yield TbNT12.1- or TbNT12.2-specific uptake. Data were fitted to the Michaelis-Menten equation by non-linear regression. Measurements were made with at least 3 oocytes.
3.5 Localization of TbNT11.1 and TbNT12.1 in trypanosomes
To determine the subcellular localization of the two nucleobase/pentamidine transporters, we epitope tagged TbNT11.1 with a triple hemagglutinin epitope [30] at its COOH terminus (TbNT11.1-3HA) and TbNT12.1 with the Ty epitope [31] at its NH2-terminus (Ty-TbNT12.1) and expressed these tagged proteins in both PF and BF trypanosomes from inducible expression vectors (Fig. 9). Immunofluorescence microscopy revealed that both tagged proteins are expressed primarily in the plasma membrane of both life cycle stages and co-localize with β-tubulin present in the subpellicular microtubules that subtend the pellicular plasma membrane.
Fig. 9.
Localization of epitope tagged TbNT11 and TbNT12 in BF and PF trypanosomes. (A) Trypanosomes expressing TbNT11.1 fused to a triple hemagglutinin tag at its COOH-terminus (TbNT11-3HA) were immunostained with anti-HA tag antibody (green, left) and anti-β-tubulin antibody (red, middle); the merged images are shown at the right. (B) Trypanosomes expressing TbNT12.1 tagged at its NH2-terminus with the Ty epitope (Ty-TbNT12) were stained with anti-Ty antibody (green, left) and anti-β-tubulin antibody (red, middle); the merged images are shown at right. TbNT12.2 tagged with the Ty epitope at its NH2-terminus also showed equivalent localization (not shown).
3.6 Expression of TbNT11 and TbNT12 in trypanosomes
To monitor expression of TbNT11 and TbNT12 mRNAs in procyclic form (PF) and bloodstream form (BF) trypanosomes, we performed quantitative real time polymerase chain reactions (qRT-PCR) using total RNA from both life cycle stages and oligonucleotide primers representing unique regions of the TbNT11 and TbNT12 sequences (these primers do not distinguish between the two closely related alleles of each permease). Following normalization of the qRT-PCR results to those from rRNA, TbNT11 mRNA was present at a level of 1.0 in PF and 1.3 in BF trypanosomes, and TbNT12 was present at 1.0 in PF and 2.5 in BF trypanosomes. Hence, both mRNAs are somewhat upregulated in BF compared to PF trypanosomes but are present in both life cycle stages.
Previous studies on intact parasites indicated that there are 3 pentamidine transport activities in BF trypanosomes (TbAT1, HAPT1, and LAPT1) and 1 high affinity pentamidine transport activity (PPT1) in PF parasites [18]. Thus the question arises of whether TbNT11 or TbNT12 represent any of these transport activities detected in whole parasites. It is notable that the inhibition of pentamidine uptake by hypoxanthine measured for TbNT11.1 (Fig. 7C) or expected on the basis of substrate specificity for TbNT12.1 (adenine would be expected to inhibit pentamidine uptake, Fig. 8) does not correspond with the inhibition properties PPT1, HAPT1, or LAPT1 measured in parasites. Specifically, hypoxanthine, guanine, or adenine at 1 mM concentrations did not inhibit uptake of 25 μM [14C]pentamidine by PFs where PPT1 is functional (data not shown). Furthermore, previous studies [18] showed that neither purines nor pyrimidines inhibited uptake of pentamidine by either HAPT1 or LAPT1. Thus TbNT11.1 and TbNT12.1 could represent other routes for pentamidine uptake that have not been detected by whole cell uptake experiments that attempt to dissect multiple co-expressed import pathways. Alternatively, it is possible that TbNT11.1 or TbNT12.1 is equivalent to PPT1, HAPT1, or LAPT1, but that the observed differences in inhibition properties are due to some technical disparity between measurements made in intact trypanosomes compared to those made in oocytes that are expressing a single transporter. It may be necessary to perform multiple sequential knockouts of the TbAT1, TbNT11, and TbNT12 genes to dissect the role of the corresponding permeases in pentamidine uptake and sensitivity within the parasite.
It is notable that the 3 nucleobase/pentamidine transporters, TbAT1, TbNT11.1, and TbNT12.1, are more closely related to each other in a phylogenetic tree than they are to the TbNT8.1 nucleobase permease that does not transport pentamidine (Fig. 2B). The observation that these 3 permeases share the ability to transport pentamidine is consistent with the hypothesis that this phylogenetic clustering reflects functional similarities, including the ability to take up diamidine drugs.
The multiplicity of nucleobase permeases expressed by T. brucei is puzzling. Adenine is transported by the BF specific TbAT1, the four purine nucleobases are transported by members of the TbNT8 family, hypoxanthine is a substrate for TbNT5, TbNT6, TbNT7, TbNT11.1 transports hypoxanthine, adenine, and xanthine, and TbNT12.1 transports adenine. While it is not clear why the parasite has evolved this plethora of nucleobase carriers, multiple nucleobase transporters appear to be the rule in other organisms, including the related parasite L. major that expresses the NT3 and NT4 nucleobase permeases [19].
Acknowledgments
This work was supported by NIH grant AI44138 to SML. We thank Dr. Robert Perkins of Portland State University for performing inductively coupled mass spectrometry experiments, and Dr. Najib El-Sayed for discussions concerning ENT family members in GeneDB. We thank the Research Triangle Institute International (Research Triangle Park, NC) for a gift of [14C]pentamidine obtained under the support of the Drug Synthesis and Chemistry Branch, National Cancer Institute. We thank Cosmo Buffalo for studies on functional expression of TbNT3 and TbNT4 in Leishmania null mutants.
Abbreviations
- BF
bloodstream form
- PF
procyclic form
- TM
transmembrane domain
- ENT
equilibrative nucleoside transporter
- qRT-PCR
quantitative real time polymerase chain reaction
- GFP
green fluorescent protein
- 3HA
triple hemagglutinin epitope tag
- ORF
open reading frame
- PBS
phosphate buffered saline, pH 7.4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landfear SM, Ullman B, Carter N, Sanchez M. Nucleoside and nucleobase transporters in parasitic protozoa. Eukaryotic Cell. 2004;3:245–54. doi: 10.1128/EC.3.2.245-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter NS, Rager N, Ullman B. Purine and pyrimidine transport and metabolism. In: Marr JJ, Nilsen T, Komuniecki R, editors. Molecular and Medical Parasitology. London: Academic Press; 2003. pp. 197–223. [Google Scholar]
- 3.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Muller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O’Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schafer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, Myler PJ. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309 (5733):436–42. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci. 2006;27 (8):416–25. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Mäser P, Sütterlin C, Kralli A, Kaminsky R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285:242–4. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- 6.Carter NS, Fairlamb AH. Arsenical-resistant trypanosomes lack an unusual adenine/adenosine transporter. Nature. 1993;361:173–5. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez MA, Ullman B, Landfear SM, Carter NS. Cloning and functional expression of a gene encoding a P1 type nucleoside transporter from Trypanosoma brucei. J. Biol. Chem. 1999;274:30244–9. doi: 10.1074/jbc.274.42.30244. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez MA, Tryon R, Green J, Boor I, Landfear SM. Six related nucleoside/nucleobase transporters from Trypanosoma brucei exhibit distinct biochemical functions. J. Biol. Chem. 2002;277 (24):21499–504. doi: 10.1074/jbc.M202319200. [DOI] [PubMed] [Google Scholar]
- 9.Henriques C, Sanchez M, Tryon R, Landfear SM. Molecular and functional characterization of the first nucleobase transporter gene from African trypanosomes. Mol Biochem Parasitol. 2003;130:101–10. doi: 10.1016/s0166-6851(03)00167-1. [DOI] [PubMed] [Google Scholar]
- 10.Burchmore RJS, Wallace LJM, Candlish D, Al-Salabi MI, Beal PR, Barrett MP, Baldwin SA, de Koning HP. Cloning, heterologous expression, and in situ characterization of the first high affinity nucleobase transporter from a protozoan. J. Biol. Chem. 2003;278:23502–7. doi: 10.1074/jbc.M301252200. [DOI] [PubMed] [Google Scholar]
- 11.Al-Salabi MI, Wallace LJ, Luscher A, Maser P, Candlish D, Rodenko B, Gould MK, Jabeen I, Ajith SN, de Koning HP. Molecular interactions underlying the unusually high adenosine affinity of a novel Trypanosoma brucei nucleoside transporter. Mol Pharmacol. 2007;71 (3):921–9. doi: 10.1124/mol.106.031559. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez M, Drutman S, van Ampting M, Matthews KR, Landfear SM. A novel purine nucleoside transporter whose expression is up-regulated in the short stumpy form of the Trypanosoma brucei life cycle. Mol. Biochem. Parasitol. 2004;136:265–72. doi: 10.1016/j.molbiopara.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Carter NS, Berger BJ, Fairlamb AH. Uptake of diamidine drugs by the P2 transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 1995;270:28153–7. doi: 10.1074/jbc.270.47.28153. [DOI] [PubMed] [Google Scholar]
- 14.Matovu E, Stewart ML, Geiser F, Brun R, Maser P, Wallace LJM, Burchmore RJ, Enyaru JCK, Barrett MP, Kaminsky R, Seebeck T, de Koning HP. Mechanisms of Arsenical and Diamidine Uptake and Resistance in Trypanosoma brucei. Eukaryotic Cell. 2003;2 (5):1003–8. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanteri CA, Stewart ML, Brock JM, Alibu VP, Meshnick SR, Tidwell RR, Barrett MP. Roles for the Trypanosoma brucei P2 transporter in DB75 uptake and resistance. Mol Pharmacol. 2006;70 (5):1585–92. doi: 10.1124/mol.106.024653. [DOI] [PubMed] [Google Scholar]
- 16.Bray PG, Barrett MP, Ward SA, de Koning HP. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 2003;19 (5):232–9. doi: 10.1016/s1471-4922(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 17.Athri P, Wenzler T, Ruiz P, Brun R, Boykin DW, Tidwell R, Wilson WD. 3D QSAR on a library of heterocyclic diamidine derivatives with antiparasitic activity. Bioorg Med Chem. 2006;14 (9):3144–52. doi: 10.1016/j.bmc.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 18.de Koning HP. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 2001;59 (3):586–92. doi: 10.1124/mol.59.3.586. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz D, Sanchez MA, Pierce S, Herrmann T, Kimblin N, Bouwer HG, Landfear SM. Molecular genetic analysis of purine nucleobase transport in Leishmania major. Mol. Microbiol. 2007;64:1228–43. doi: 10.1111/j.1365-2958.2007.05730.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Boitz JM, Galazka J, Arendt CS, Carter NS, Ullman B. Functional characterization of nucleoside transporter gene replacements in Leishmania donovani. Mol Biochem Parasitol. 2006;150 (2):300–7. doi: 10.1016/j.molbiopara.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirtz E, Leal S, Ochatt C, Cross GAM. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 22.Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234 (2):187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 23.Valdes R, Vasudevan G, Conklin D, Landfear SM. Transmembrane domain 5 of the LdNT1.1 nucleoside transporter is an amphipathic helix that forms part of the nucleoside translocation pathway. Biochemistry. 2004;43:6793–802. doi: 10.1021/bi049873m. [DOI] [PubMed] [Google Scholar]
- 24.Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128 (2):217–28. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 25.Arriza JL, Kavanaugh MP, Fairman WA, Wu Y-N, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–32. [PubMed] [Google Scholar]
- 26.Sanchez M, Tryon R, Vasudevan G, Landfear SM. Functional expression and characterisation of a purine nucleobase transporter gene from Leishmania major. Mol. Membrane Biol. 2004;21:11–8. doi: 10.1080/0968768031000140845. [DOI] [PubMed] [Google Scholar]
- 27.Vasudevan G, Ullman B, Landfear SM. Point mutations in a nucleoside transporter gene from Leishmania donovani confer drug resistance and alter substrate selectivity. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6092–7. doi: 10.1073/pnas.101537298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22 (23):3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 (9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms MJ, Ambit A, Appleton P, Tetley L, Coombs GH, Mottram JC. Bloodstream form Trypanosoma brucei depend upon multiple metacaspases associated with RAB11-positive endosomes. J Cell Sci. 2006;119 (Pt 6):1105–17. doi: 10.1242/jcs.02809. [DOI] [PubMed] [Google Scholar]
- 31.Bastin P, Bagherzadeh A, Matthews KR, Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol. Biochem. Parasitol. 1996;77:235–9. doi: 10.1016/0166-6851(96)02598-4. [DOI] [PubMed] [Google Scholar]
- 32.Sundaram M, Yao SYM, Ingram JC, Berry ZA, Abidi F, Cass CE, Baldwin SA, Young JD. Topology of a human equilibrative, nitrobenzylthioinosine (NBMPR)-sensitive nucleoside transporter (hENT1) implicated in the cellular uptake of adenosine and anti-cancer drugs. J Biol Chem. 2001;276:45270–5. doi: 10.1074/jbc.M107169200. [DOI] [PubMed] [Google Scholar]
- 33.Marr JJ. Purine analogs as chemotherapeutic agents in leishmaniasis and American trypanosomiasis. J Lab Clin Med. 1991;118:111–9. [PubMed] [Google Scholar]
- 34.Martinez S, Marr JJ. Allopurinol in the treatment of American cutaneous leishmaniasis. N Engl J Med. 1992;326:741–4. doi: 10.1056/NEJM199203123261105. [DOI] [PubMed] [Google Scholar]
- 35.Natto MJ, Wallace LJ, Candlish D, Al-Salabi MI, Coutts SE, de Koning HP. Trypanosoma brucei: expression of multiple purine transporters prevents the development of allopurinol resistance. Exp Parasitol. 2005;109 (2):80–6. doi: 10.1016/j.exppara.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Basselin M, Lawrence F, Robert-Gero M. Pentamidine uptake in Leishmania donovani and Leishmania amazonensis promastigotes and axenic amastigotes. Biochem J. 1996;315 (Pt 2):631–4. doi: 10.1042/bj3150631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montaser A, editor. Inductively Coupled Plasma Mass Spectrometry. Hoboken: Wiley-VCH; 1998. [Google Scholar]
- 38.Caffaro CE, Hirschberg CB, Berninsone PM. Independent and simultaneous translocation of two substrates by a nucleotide sugar transporter. Proc Natl Acad Sci U S A. 2006;103 (44):16176–81. doi: 10.1073/pnas.0608159103. [DOI] [PMC free article] [PubMed] [Google Scholar]