Abstract
Background
Many psychiatric and neurodevelopmental disorders are associated with mild enlargement of the lateral ventricles thought to have origins in prenatal brain development. Little is known about development of the lateral ventricles and the relationship of prenatal lateral ventricle enlargement with postnatal brain development.
Methods
We performed a neonatal MRI on 34 children with isolated mild ventriculomegaly (MVM, width of the atrium of the lateral ventricle ≥ 1.0 cm) on prenatal ultrasound and 34 age and gender matched controls with normal prenatal ventricle size. Lateral ventricle and cortical gray and white matter volumes were assessed. Fractional anisotropy (FA) and mean diffusivity (MD) in corpus callosum and cortico-spinal white matter tracts were determined obtained using quantitative tractography.
Results
Neonates with prenatal MVM had significantly larger lateral ventricle volumes than matched controls (286.4%; p < 0.0001). Neonates with MVM also had significantly larger intracranial volumes (ICV; 7.1%, p = 0.0063) and cortical gray matter volumes (10.9%, p = 0.0004) compared to controls. DTI tractography revealed a significantly greater MD in the corpus callosum and cortico-spinal tracts, while FA was significantly smaller in several white matter tract regions.
Conclusions
Prenatal enlargement of the lateral ventricle is associated with enlargement of the lateral ventricles after birth, as well as greater gray matter volumes and delayed or abnormal maturation of white matter. It is suggested that prenatal ventricle volume is an early structural marker of altered development of the cerebral cortex and may be marker of risk for neuropsychiatric disorders associated with ventricle enlargement.
Keywords: Magnetic resonance imaging, ultrasound, diffusion tensor imaging, lateral ventricle, autism, schizophrenia, cortex
Introduction
Mild enlargement of the lateral ventricles is a structural brain abnormality observed in neuropsychiatric disorders that are thought to be the result of abnormal prenatal brain development, including schizophrenia (1, 2), autism (3), and attention-deficit/hyperactivity disorder(4, 5). We have proposed that prenatal ultrasound can be used to detect structural brain abnormalities in the developing brain, including enlarged lateral ventricles, associated with these neurodevelopmental disorders (6). A recent retrospective study has found some evidence of prenatal lateral ventricle enlargement in children at high risk for schizophrenia (7); another retrospective study found no enlargement of fetal head circumference on prenatal ultrasound in children with autism (8).
Routine clinical prenatal ultrasound typically assesses the width of the atrium of the lateral ventricle. The average normal width for the atrium of the fetal lateral ventricle is approximately 0.76 cm, and most studies find that while brain size increases, ventricle width is stable during the second and third trimesters (9–13). A magnetic resonance imaging (MRI) study of postmortem fetal brains found that lateral ventricle volume increased until 20 weeks gestational age and was stable or declined thereafter (14), consistent with ultrasound studies.
Enlargement of the lateral cerebral ventricles in the fetus has been associated with a variety of pediatric brain disorders including progressive hydrocephalus, gray matter migration abnormalities, agenesis of the corpus collosum, trisomies, and microcephaly (15–19). Mildly enlarged ventricles in the fetus in the absence of other central nervous system abnormalities is designated isolated mild ventriculomegaly (MVM), and is typically defined as a width of the atrium of the lateral ventricle of 1.0–1.5 cm (20–22). Isolated MVM is observed in up to 0.7% of pregnancies (21, 22), and is associated with developmental delays and other abnormal neurodevelopmental outcomes (16–19, 23–27). One longer-term follow-up of several cases of prenatal MVM at 6–9 years of age found autism, ADHD and learning disabilities (28). Little is known about the causes of isolated MVM, though it is associated with older maternal age and lower gestational age at birth (6), and high levels of interferon-α in the cord blood indicating prenatal exposure to infection (29), each of which in turn is associated with higher risk for neurodevelopmental disorders.
We hypothesize that fetal isolated MVM observed on prenatal ultrasound is a structural marker of altered brain development that is ultimately associated with high risk for neuropsychiatric and neurodevelopmental disorders associated with enlargement of the lateral ventricles. Little is known about postnatal brain development in children with prenatal MVM. Using MRI, including diffusion tensor imaging (DTI), we compared neonatal brain structure in children with prenatal MVM and age and gender matched controls. We hypothesized that children with MVM would continue to have enlargement of the lateral ventricles as neonates, as well as associated alterations of cortical gray and white matter development.
Methods
Subjects
This study was approved by the Institutional Review Board of the University of North Carolina (UNC) School of Medicine and Duke University Medical Center (DUMC). Subjects with isolated MVM (atrial width 1.0 cm or greater) or normal lateral ventricle size (less than 1.0 cm) identified on routine clinical prenatal screening ultrasound were recruited in the second trimester of pregnancy from the prenatal diagnostic clinics of UNC Hospitals and DUMC. Recruitment exclusion criteria for controls included abnormalities on ultrasound, major medical illness or pregnancy complication, or psychotic disorder in mother. Singleton and twin subjects with MVM and matched controls who had T1, T2/PD scans that were free of major motion were included in this analysis (n=37/group). 20 of these subject/control pairs also had motion free DTI scans. Controls were matched pairwise on the basis of gender, status as a singleton or twin, maternal age, gestational age at birth, age at MRI, and maternal ethnicity. Exclusion criteria for this analysis included premature birth (gestational age < 33 weeks), major perinatal complications (asphyxia, seizure, sepsis, pneumonia, stay in neonatal ICU > 24 hours) or abnormality on MRI other than small subdural hematoma. 15 subjects (7 MVM, 8 controls) had incidental intracranial bleeds on MRI, which are present in about 25% of neonates after vaginal delivery (30).
Prenatal Ultrasound
Ultrasound scans obtained at approximately 22 and 32 weeks gestational age were performed by trained study ultrasonographers. At UNC prenatal ultrasounds were done on an ATL 5000 (Philips, Andover MA) and Voluson Expert (General Electric, Waukesha, WI), on a GE Voluson Expert at DUMC. Ventricle width was determined at the atrium of the lateral ventricle at the tail of the choroid, measuring from inner wall to inner wall (31). The width was measured twice and averaged for each visit; both the ventricle nearest and farthest from the transducer were measured when possible. Prior studies have shown good inter-rater reliability for atrial width measures (32). In our study, the intraclass correlation (Shourt-Fleiss) of the first and second measures of width of the far atrium was 0.9820 at 22 week ultrasound and 0.9905 at 32 weeks ultrasound.
MR Image Acquisition
Images were acquired on a Siemens head-only 3 T scanner (Allegra, Siemens Medical System, Erlangen, Germany). Neonates were scanned unsedated while asleep and were fed prior to scanning, swaddled, fitted with ear protection and had their heads secured in a vacuum-fixation device. A physician or nurse was present during each scan; a pulse oximeter was used to monitor heart rate and oxygen saturation. T1-weighted structural pulse sequences were a 3D T1-weighted FLASH (TR/TE/Flip Angle 15/7msec/25°) in the initial phase of the study (n=11), then a 3D magnetization prepared rapid gradient echo (MP-RAGE TR/TI/TE/Flip Angle 1820/400/4.38ms/7°, n=57). Proton density and T2 weighted images were obtained with a turbo spin echo sequence (TSE TR/TE1/TE2/Flip Angle 6200/20/119ms/150°). Spatial resolution was 1 × 1 × 1mm voxel for T1 weighted images, 1.25 × 1.25 × 1.5mm voxel with 0.5 mm interslice gap for proton density/T2 weighted images. We have previously confirmed that the scan sequences did not cause significant temperature increases with a phantom (33).
A single shot echo planar SE DTI imaging sequence was used with the following parameters: repetition time (TR) = 5200 ms; echo time (TE) = 73 ms; TH = 2 mm; inplane resolution = 2×2 mm2, and 45 slices. Seven images are acquired for each slice, one without diffusion gradient (b=0) while the remaining six with b=1000s/mm2 and diffusion gradients along , separately. In order to improve signal-to-noise ratio (SNR) for the DTI images, five separate sets of images with 2 averages in each set were acquired. This approach shortens data acquisition time (1.18 minutes/set) and minimizes motion artifacts.
Segmentation and Lobe Parcellation Analysis
Brain tissue was automatically classified as gray matter, non-myelinated white matter, myelinated white matter and cerebrospinal fluid using an atlas-moderated iterative expectation maximization segmentation algorithm as previously described (34,35). Parcellation of the cortex into anatomical regions (frontal, prefrontal, parietal, and occipital) was achieved by non-linear warping of a parcellation atlas template as previously described (35). Left and right hemispheres were subdivided into four regions along the anterior-posterior axis (pre-frontal, frontal, parietal, occipital) and into infra- and supratentorial regions. The cerebellum, brainstem and combined sets of subcortical structures are represented separately.
The volume of the lateral ventricles was segmented with a user-supervised, highly automated level-set evolution tool, itkSNAP (36). Left and right lateral ventricles are separated by a 3D cutting tool. Intracranial volume (ICV) was the sum of the automatic full brain segmentation results for gray, white and CSF (ventricles and subarachnoid space) volumes.
Diffusion Tensor Analysis
Each individual directional gradient image was screened offline for motion artifacts using an automatic DTI quality control tool (DTIchecker) that calculates an average of the 5 repeated sequences after correction for motion and removal of outliers (37). Quantitative analysis of fiber tracts was accomplished using a set of tools for computation of fractional anisotropy (FA) and mean diffusivity (MD) maps, quantitative tractography, fiber clustering and parametrization as previously described (37, 38). Tracts are initialized by drawing source and target regions of interest on FA images. The fiber tracking tool (FiberViewer; software download at http://www.ia.unc.edu/dev) reads the set of diffusion image channels, calculates the tensor field, reads the region of interest image, and performs the tracking. The resulting sets of streamlines are stored as list of polylines which carry the full tensor information at each location. Cross-sectional regions of interest were defined in the cortical and central regions of the genu and splenium of the corpus callosum and in the left and right cortico-spinal tracts as previously described (37). Supplemental Figure 1 presents a representative FA map in an MVM subject and regions of analysis in the specific white mater tracts.
Statistical Analysis
For cross-sectional analysis of demographic characteristics between two groups, two sample t-tests for continuous variables and Fisher Exact tests for categorical variables were used. All statistical hypothesis tests were conducted for all children with MVM and age and gender matched controls, and repeated separately for each subgroup of MVM with matched controls for secondary analysis. For all comparisons between children with MVM and age and gender matched controls, analysis of variance (ANOVA) or analysis of covariance (ANCOVA) with matched-pair as blocks was used to control the effect of between-pair variation. To examine the relationship of maximal and oldest lateral prenatal ventricle widths to the postnatal ventricle volume, we correlated linearly maximal and oldest lateral prenatal ventricle width with postnatal ventricle volume. Analyses of correlation are repeated separately for the MVM and matched normal control groups using Pearson correlation coefficients. For general differences in location along genu, splenium, and left and right corticospinal tract, we fit mixed models with region as the within-subject variable of interest without any other covariates. ANOVA with matched-pair as blocks were used for comparison in specific locations between two groups. For postnatal segmentation, we use ANOVA with matched-pair as blocks, and ANCOVA with matched-pair as blocks and ICV as a continuous predictor. In this model, we examined differences in slopes between children with MVM and matched controls. All statistical hypothesis tests are conducted at a significance level of 0.05. There were no corrections for multiple comparisons and as such, all findings should be considered exploratory, hypothesis generating, and in need of replication.
Results
Demographic information and other descriptive statistics are presented in Table 1. There were no significant differences in birth weight, gestational age at birth, or age at MRI between the MVM and control groups. As expected, children in the MVM group had significantly larger prenatal maximum lateral ventricle width (maximum width on any prenatal ultrasound scan; p < 0.0001; Figure 1). 2 MVM subjects and 2 controls had a positive family history of schizophrenia. None of the subjects or controls had a family history of autism.
Table 1.
Sample Descriptive Statistics
| Control | MVM | p Value | ||
|---|---|---|---|---|
| Gender | Male | 26 | 26 | 1.00 |
| Female | 8 | 8 | ||
| Singleton/Twin | Singleton | 30 | 30 | 1.00 |
| Twin | 4 | 4 | ||
| Ethnicity | Caucasian | 27 | 25 | 0.5588 |
| African-American | 7 | 7 | ||
| Asian | 0 | 2 | ||
| Gestational age at Birth (weeks) | 38.9 (1.6) | 38.74 (1.6) | 0.7300 | |
| Birthweight (kg) | 3.384 (0.55) | 3.521 (0.57) | 0.3224 | |
| Age at MRI (days) | 22.7 (8.7) | 22.2 (12.5) | 0.8314 | |
| Maternal Age (years) | 30.09 (4.73 ) | 29.76 (5.93 ) | 0.8044 | |
| Maxiumum Prenatal Ventricle Width | 0.59 (0.10) | 1.15 (0.16) | < 0.0001 |
Values are mean (SD).
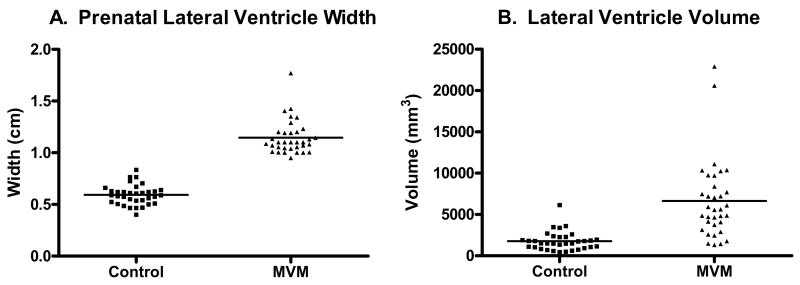
Figure 1.
A. Maximum lateral ventricle width in controls and MVM cases (n= 34/group; p < 0.0001). B. Neonates with prenatal MVM have significantly larger lateral ventricle volumes than matched controls (n= 34/group; p < 0.0001). This difference remained significant even when excluding the two outliers in the MVM group (p < 0.0001).
Children with prenatal MVM had significantly larger lateral ventricle volumes on neonatal MRI (295% larger, p < 0.0001; Figure 1), even when controlling for ICV (p < 0.0001). The difference was remained significant even when excluding the two outliers (greater than 3 SD above the mean) in the MVM group (p<0.0001). Figure 2 presents representative scans of the lateral ventricles MVM cases. Children with prenatal MVM had significantly larger intracranial volumes (ICV) than controls (7.1%, p = 0.0063; Table 2) and subcortical gray matter volumes (8.3%, p = 0.0298: Table 2). There was no significant difference in the volume of the cerebellum (p = 0.8184) between groups.
Figure 2.
Lateral ventricle size and configuration from representative MVM cases, ranging from largest volume (upper left) to normal volume (lower right).
Table 2.
Neonatal Brain Volumes
| Control LS mean* (SE) | MVM LS mean* (SE) | % difference | F-value (DF) | P value | |
|---|---|---|---|---|---|
| Intracranial Volume (mm3) | 475,757 (8,207) | 509,615 (8,207) | 7.1% | 8.51 (1,33) | 0.0063 |
| Lateral Ventricle (mm3) | 1,701 (585) | 6,572 (585) | 286.4% | 34.64 (1,33) | < 0.0001 |
| Cortical Gray Matter (mm3) | 197,625 (3,839) | 219,156 (3,839) | 10.9% | 15.72 (1,33) | 0.0004 |
| Cortical White Matter (mm3) | 152,426 (2,962) | 158,680 (2,962) | 4.1% | 2.23 (1,33) | 0.1449 |
| Subcortical Gray Matter (mm3) | 20351 (521) | 22025 (521) | 8.2% | 5.15 (1,33) | 0.0298 |
| Cerebellum (mm3) | 27,361 (547) | 27,181 (547) | −0.06% | 0.05 (1,33) | 0.8184 |
Blocking for matching pairs was used to diminish the effect of variation within matching pairs, so MVM subjects have the same standard errors for the LSmeans as the matched control subjects when the two groups have same number of blocks and there is no missing data.
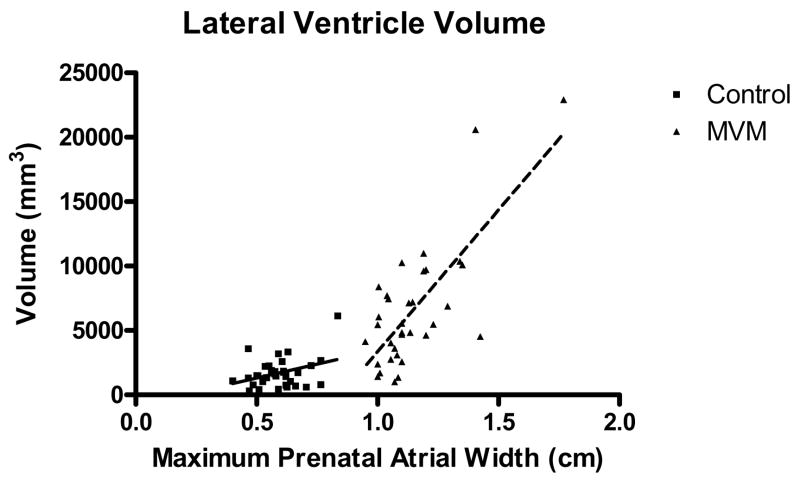
There was a significant correlation between the prenatal maximum lateral ventricle width on ultrasound and neonatal lateral ventricle volume on MRI for both the normal control (Pearson r = 0.3563; p = 0.0386) and the MVM groups (Pearson r = 0.7482, p < 0.0001; Figure 3), with larger prenatal ventricle widths predicting larger neonatal ventricle volumes.
Figure 3.
There was a significant correlation between the prenatal maximum lateral ventricle width on ultrasound and neonatal lateral ventricle volume on MRI for both the normal control (Pearson r = 0.3563; p = 0.0386) and the MVM groups (Pearson r = 0.7482, p < 0.0001).
Children with prenatal MVM had significantly larger cortical gray matter volumes than controls (10.89%, p = 0.0004; Table 2), even excluding the two outliers with very large lateral ventricle volumes (p = 0.0015). This greater gray matter volume was observed in each sub-region of the cerebral cortex – prefrontal (12.3%), frontal (9.1%), parietal (9.1%) and occipital (12.8%). This enlargement was observed in both males [LS means squared ± SE; MVM: 221,209 (4374); Control: 199,432 (4374); p = 0.0017] and females [MVM: 212,480 (8552); Control: 191,751 (8552); p = 0.1303]. Finally, the MVM group had a significantly larger cortical gray matter/white ratio compared to controls [LS means squared ± SE; MVM: 1.39 (0.02); Control: 1.30 (0.02.); p = 0.0098]. This pattern was observed in both males [MVM: 1.38 (0.2); Control: 1.29 (0.02.); p = 0.0015] and females [MVM: 1.40 (0.07); Control: 1.33 (0.07.); p = 0.5460].
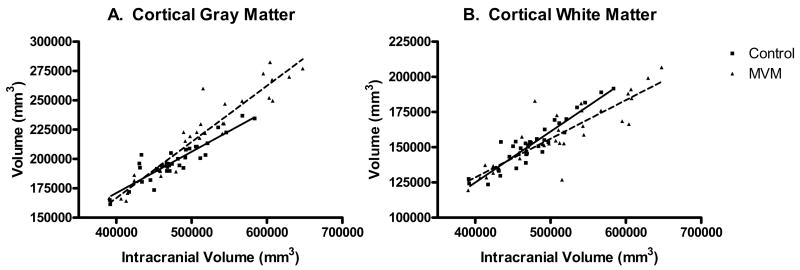
A comparison of the relationship between ICV and cortical gray matter volume revealed a significant difference in slopes (homogeneity of slopes, F = 13.15 (1, 31); p = 0.0010) with MVM cases overall having larger gray matter volumes than controls at larger ICVs (Figure 4). Cortical white matter volumes were not significantly different between the MVM and control groups (p = 0.1449; Table 2); there were however, significant differences in the relationship between ICV and hemispheric white matter were present (homogeneity of slopes, F = 7.04 (1, 31); p = 0.0125), with MVM cases overall having smaller white matter volumes than controls at higher ICVs (Figure 4).
Figure 4.
A. There is a significant difference in the relationship between ICV and cortical gray matter volume in MVM cases compared to controls (homogeneity of slope F=13.15 (1,31); p=0.0010), with MVM cases having larger gray matter volumes at larger ICV values. B. There is a significant difference in the relationship between ICV and cortical white matter volume in MVM cases compared to controls (homogeneity of slope F= 7.04 (1,31); p=0.0125) with MVM cases smaller white matter volumes than controls at larger ICVs.
Quantitative tractography revealed significantly larger mean diffusivity (MD) values in all but one region of interest in the MVM cases (Table 3). Fractional anisotropy (FA) was significantly smaller in several regions of interest, including the central region of the splenium (Table 4). Analysis of axial (λ1) and radial ([λ2 +λ3]/2) diffusivities, where the λ’s represent tensor eigenvalues sorted by magnitude, better explains the general pattern as seen in MD ([λ1+λ2+λ3]/3). MVM subjects generally had higher values than controls for diffusions along and also perpendicular to the axis of fiber bundles (Supplemental Tables 1 and 2).
Table 3.
White Matter Tract Mean Diffusivity
| Mean Diffusivity | LSMean (SE) | F-Test for difference: Normal Control vs MVM | ||
|---|---|---|---|---|
| Location/track | Normal Control | MVM | F-value (DF) | P-Value |
| Right Cortical Genu (−21) | 14.58 (0.16) | 15.63 (0.17) | 20.46 (1,24) | 0.0001 |
| Central Genu (0) | 13.71 (0.28) | 14.28 (0.30) | 1.92 (1,24) | 0.1782 |
| Left Cortical Genu (21) | 13.61 (0.44) | 15.31 (0.48) | 6.79 (1,24) | 0.0155 |
| Right Cortical Splenium (−24) | 15.01 (0.33) | 16.70 (0.35) | 12.12 (1,24) | 0.0019 |
| Central Splenium (0) | 14.23 (0.21) | 14.91 (0.22) | 4.88 (1,24) | 0.0370 |
| Left Cortical Splenium (24) | 14.59 (0.24) | 16.26 (0.26) | 22.47 (1,24) | <0.0001 |
| Left Central Cortico-spinal (−12) | 10.08 (0.08) | 10.41 (0.08) | 8.55 (1,23) | 0.0076 |
| Left Cortical Cortico-spinal (9) | 12.43 (0.18) | 13.22 (0.20) | 8.92 (1,23) | 0.0066 |
| Right Central Cortico-spinal (−12) | 10.06 (0.07) | 10.54 (0.08) | 20.37 (1,23) | 0.0002 |
| Right Cortical Cortico-spinal (9) | 12.45 (0.21) | 13.10 (0.23) | 4.42 (1,23) | 0.0466 |
Table 4.
White Matter Tract Fractional Anisotropy
| Fractional Anisotropy | LSMean (SE) | F-Test for difference: Normal Control vs MVM | ||
|---|---|---|---|---|
| Location/track | Normal Control | MVM | F-value (DF) | P-Value |
| Right Cortical Genu (−21) | 0.23 (0.01) | 0.21 (0.01) | 3.08 (1,24) | 0.0920 |
| Central Genu (0) | 0.50 (0.01) | 0.47 (0.01) | 2.22 (1,24) | 0.1493 |
| Left Cortical Genu (21) | 0.23 (0.01) | 0.22 (0.01) | 0.74 (1,24) | 0.3972 |
| Right Cortical Splenium (−24) | 0.29 (0.01) | 0.29 (0.01) | 0.04 (1,24) | 0.8448 |
| Central Splenium (0) | 0.56 (0.01) | 0.49 (0.02) | 10.59 (1,24) | 0.0034 |
| Left Cortical Splenium (24) | 0.28 (0.01) | 0.25 (0.01) | 4.27 (1,24) | 0.0498 |
| Left Central Cortico-spinal (−12) | 0.51 (0.01) | 0.50 (0.01) | 1.24 (1,23) | 0.2763 |
| Left Cortical Cortico-spinal (9) | 0.31 (0.01) | 0.28 (0.01) | 4.29 (1,23) | 0.0497 |
| Right Central Cortico-spinal (−12) | 0.54 (0.01) | 0.50 (0.01) | 5.20 (1,23) | 0.0322 |
| Right Cortical Cortico-spinal (9) | 0.28 (0.01) | 0.28 (0.01) | 0.03 (1,23) | 0.8629 |
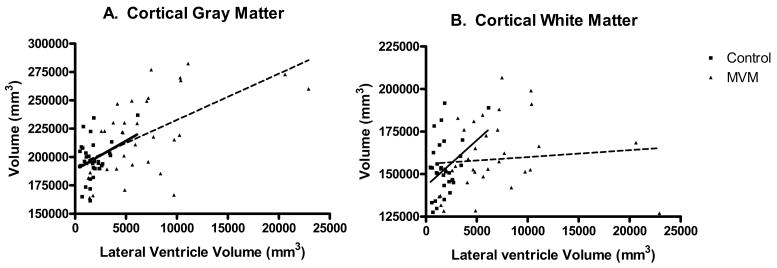
Because neonates with prenatal MVM had significantly more cortical gray matter than controls, we explored the relationship between lateral ventricle volume and cortical gray and white matter. There was a significant correlation between the neonatal lateral ventricle volume and larger cortical gray matter volume in both the normal control (Pearson r = 0.3539; p = 0.0400) and the MVM groups (Pearson r = 0.5617, p = 0.0005; Figure 5). There was a significant correlation between lateral ventricle volume and cortical white matter volume in the normal controls (Pearson r = 0.3794; p = 0.0269), but not in the MVM group (Pearson r = 0.0892, p = 0.6160; Figure 5).
Figure 5.
A. There was a significant correlation between the lateral ventricle volume and cortical gray matter volume in both the normal control (Pearson r = 0.3539; p = 0.0400) and the MVM groups (Pearson r = 0.5617, p = 0.0005). B. There was a significant correlation between lateral ventricle volume and cortical white matter volume in the normal controls (Pearson 0.3794; p = 0.0269), but not in the MVM group (Pearson 0.0892, p = 0.6160).
In a secondary analysis, the MVM group was divided into a group with persistent MVM on the last ultrasound (n = 15) and a group that “normalized” or had ventricle widths below 1.0 cm on the last ultrasound (n = 15). 4 MVM subjects had only one prenatal ultrasound and were excluded from this analysis. For the persistent MVM group, the atrial width of the far ventricle on the last ultrasound was significantly greater than matched controls [LS means squared ± SE; 0.97 ± 0.07 cm vs. 0.48 ± 0.07 cm; 24.92 (1, 14), p = 0.0002]. The normalized group also had prenatal ventricle widths greater than the matched controls, though within the normal range [0.71 ± 0.04 cm vs. 0.45 ± 0.04 cm; 18.75 (1, 13), p = 0.0008]. Lateral ventricle volumes were significantly larger than matched controls in both the persistent (8707 ± 1122 mm3 vs. 1924 ± 1122 mm3; p = 0.0008) and normalized (4032 ± 401 mm3 vs. 1532 ± 401 mm3; p = 0.0006) MVM subgroups. The normalized MVM group also had larger ICV and cortical gray matter volumes than matched controls, though this was not statistically significant (data not shown). Finally, the normalized MVM group demonstrated the same overall pattern of greater MD and smaller FA in most of the white matter tract regions of interest; this difference was statistically significant in several regions (data not shown).
Discussion
We found that prenatal isolated MVM is associated with significantly enlarged lateral ventricles on postnatal MRI, indicating enlargement of the lateral ventricles detected in the second trimester persists at least through neonatal brain development. In addition to enlarged lateral ventricles, neonates with prenatal MVM had greater cortical gray matter volumes and evidence of reduced cortical white matter volumes. Finally, quantitative DTI tractography revealed larger MD and radial diffusivity, and reduced FA in developing corpus callosum and cortico-spinal tracts. These findings indicate that prenatal MVM is a marker of altered cortical development in the neonatal period.
Neonatal lateral ventricle volume was highly correlated with prenatal lateral ventricle width, indicating that relative lateral ventricle size is conserved throughout the second half of prenatal brain development into the neonatal period. Even cases in which the lateral ventricle width “normalized” or fell below 1.0 cm width on later ultrasounds had significantly enlarged lateral ventricles compared to controls. In a case series, we have previously found that fetal lateral ventricle enlargement can persist until ages 6–9 years in some cases (6). Therefore, it appears that prenatal lateral ventricle structure is conserved well into childhood and may serve as a prenatal marker of altered childhood brain development. Bloom et al. (27) found a significant linear relationship between prenatal ventricle width and decreased mental development index of the Bayley Scales of Infant Development at a mean age of 21 months, evidence that prenatal ventricle structure can be predictive of postnatal neurocognitive development.
Interestingly, prenatal isolated MVM was associated with greater cortical gray matter volume in the neonatal period and larger lateral ventricle volume was associated with larger cortical gray matter volumes in both the control and MVM groups. Cortical neurogenesis occurs in the ventricular and subventricular zones of the developing human brain (39, 40), and it has been proposed that the evolutionary enlargement of the cortex in humans is related in part to expanded lateral ventricle size and increased numbers of neuronal progenitor cells (41). It is possible that a larger lateral ventricle would have a greater volume of ventricular and subventricular zone proliferating cells associated with it, giving rise to more gray matter, as the lateral ventricle enlargement observed in MVM is present before many of the neurons of the cortex are formed (42). Because the skull is developing and not closed during this period, any enlargement of the lateral ventricle and cortical gray matter would be accommodated by an enlarging skull. There was no difference in cerebellum volume between groups, indicating that enlargement in the MVM cases was not generalized throughout the brain. There is also evidence that the flow of cerebrospinal fluid within the lateral ventricle influences migration of neuroblasts to the olfactory bulb in mice (43). An abnormally enlarged lateral ventricle could potentially have abnormal CSF flow altering migration of neurons and cortical development.
Autism has been associated with increased cortical gray and white matter volumes; this increased growth appears to occur in the first year or two of life (44, 45). Increased lateral ventricle volumes have been observed in autism (3), and it is temping to speculate that enlarged lateral ventricles could possibly give rise to enlarged gray matter volumes in some forms of autism. There is enormous growth of gray matter, and a less robust growth of white matter in the first two years of life in normal children (46); it will be important to determine if increased lateral ventricle size is associated with increased cortical gray matter development in the first two years of life. We are obtaining follow-up MRIs at ages one and two years in this cohort and hope to address this question. Other disorders of neurodevelopment have been also associated with increased regional or global cortical gray matter volumes, including neurofibromatosis (47), prematurity (48), ADHD (49–51) and cleft lip/palate (52).
There was a difference in the correlations between lateral ventricle volume and cortical white matter volume in the MVM and control groups, to the extent the correlation in controls reflects normal development, this difference suggests MVM cases have altered cortical white matter development. In general, mean diffusivity decreases with age and fractional anisotropy increases with age in the neonatal period (37, 53, 54), presumably reflecting increasing organization and myelination of white matter tracts. Radial diffusivity decreases with increasing myelin development in the rodent corpus callosum(55). In the human neonatal period, MD appears to be more sensitive to changes in myelination than FA (37). The overall smaller white matter volume, along with greater MD, greater radial diffusivity, and smaller FA observed in infants with MVM suggests either that there is a temporal lag in white matter development, or that white matter is abnormal. Longer-term follow-up studies will need to be done to differentiate these possibilities.
The definition of mild ventriculomegaly has been debated in the literature (56, 57). An atrial width of 1.0 cm on prenatal ultrasound is 3–4 standard deviations from the mean 20. Outcome studies indicate that atrial widths of 1.2 cm or greater are more often associated with an unfavorable outcome than widths of 1.0–1.2 cm (24–26), and it has been argued that widths between 1.0 and 1.2 cm should be considered normal (58). The mean maxiumum lateral ventricle width for our MVM cases was 1.15 cm, placing them in the lower range of MVM. It is important to note that prenatal atrial widths in the 1.0–1.2 cm range in this study are associated with significant enlargement of the lateral ventricle in the neonatal period (Figure 2), as well as alterations of cortical gray and white matter.
In our sample, boys were more likely to have MVM than girls, with a gender ratio of 3.25. This male bias is consistent with previous studies of MVM, with gender ratios ranging from 1.3 to 3.0 (6, 19, 25, 26). There is evidence that males have larger prenatal atrial widths than girls (13, 59) though we did not find gender differences in lateral ventricle volume on neonatal MRI (46). The predominance of males with MVM is consistent with higher rates of many neurodevelopmental disorders in males, and provides evidence that males are more vulnerable to alterations of brain development.
Ultimately, the value of lateral ventricle enlargement as a prenatal or neonatal structural marker of risk will need to be determined in long-term outcome studies. Most outcome studies of MVM are limited to the first few years of childhood, have not followed the children into the age of risk and involve interviews of parents and review of clinical records. Only Bloom et al. (27) has conducted a prospective, standardized follow-up study and found that a third of children had evidence of developmental delays. In a case series we previously found that MVM can be associated with autism, attention deficit-hyperactivity disorder, and learning disabilities (6), suggesting that prenatal MVM may be a marker of risk for these disorders. We are obtaining follow-up MRIs and neurodevelopmental assessments on this cohort and hope to follow these children into later childhood. A present, prenatal MVM is, at best, a non-specific marker of risk for a variety of poor neurodevelopmental and neuropsychiatric outcomes. In addition, it should be noted that there is overlap between the MVM subjects and controls in cortical gray and white matter and lateral ventricle volumes, making the predictive value less certain. Future studies may provide the basis for more specificity.
In summary, mild enlargement of the lateral ventricles in the fetal brain is associated with enlarged lateral ventricles after birth, as well as evidence of altered cortical gray and white matter development. The overall relative size of the fetal lateral ventricle appears to be conserved into the early postnatal periods and beyond, suggesting that it may have value as an early structural marker of risk for poor neurodevelopmental outcome and neuropsychiatric disorders associated with enlarged lateral ventricles. The study of prenatal and neonatal brain development with ultrasound offers the potential for an improved understanding of neurodevelopmental mechanisms that contribute to neuropsychiatric disorders as well as very early identification of children at risk.
Supplementary Material
Acknowledgments
Funded by NIMH Silvio O. Conte Center MH064065 and the Foundation of Hope, Raleigh, NC.
Footnotes
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Presented at the Annual Meeting of the American College of Neuropsychopharmacology, Boca Raton, FL, December 11, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–20. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 2.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- 4.Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biol Psychiatry. 1996;40:1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Jiang T, Cao Q, Wang Y. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR Am J Neuroradiol. 2007;28:543–547. [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmore JH, van Tol J, Kliewer MA, Silva SG, Cohen SB, Hertzberg BS, Chescheir NC. Mild ventriculomegaly detected in utero with ultrasound: clinical associations and implications for schizophrenia. Schizophr Res. 1998;33:133–140. doi: 10.1016/s0920-9964(98)00073-5. [DOI] [PubMed] [Google Scholar]
- 7.Clarke MC, Cannon M, Hogg MW, Marks MN, Conroy S, Pawlby SJ, et al. Foetal brain development in offspring of women with psychosis. Br J Psychiatry. 2007;190:445–446. doi: 10.1192/bjp.bp.106.023747. [DOI] [PubMed] [Google Scholar]
- 8.Hobbs K, Kennedy A, Dubray M, Bigler ED, Petersen PB, McMahon W, Lainhart JE. A retrospective fetal ultrasound study of brain size in autism. Biol Psychiatry. 2007;62:1048–1055. doi: 10.1016/j.biopsych.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Pilu G, Reece EA, Goldstein I, Hobbins JC, Bovicelli L. Sonographic evaluation of the normal developmental anatomy of the fetal cerebral ventricles: II. The atria. Obstet Gynecol. 1989;73:250–256. [PubMed] [Google Scholar]
- 10.Farrell TA, Hertzberg BS, Kliewer MA, Harris L, Paine SS. Fetal lateral ventricles: reassessment of normal values for atrial diameter at US. Radiology. 1994;193:409–411. doi: 10.1148/radiology.193.2.7972754. [DOI] [PubMed] [Google Scholar]
- 11.Alagappan R, Browning PD, Laorr A, McGahan JP. Distal lateral ventricular atrium: reevaluation of normal range. Radiology. 1994;193:405–408. doi: 10.1148/radiology.193.2.7972753. [DOI] [PubMed] [Google Scholar]
- 12.Hilpert PL, Hall BE, Kurtz AB. The atria of the fetal lateral ventricles: a sonographic study of normal atrial size and choroid plexus volume. AJR Am J Roentgenol. 1995;164:731–734. doi: 10.2214/ajr.164.3.7863903. [DOI] [PubMed] [Google Scholar]
- 13.Patel MD, Goldstein RB, Tung S, Filly RA. Fetal cerebral ventricular atrium: difference in size according to sex. Radiology. 1995;194:713–715. doi: 10.1148/radiology.194.3.7862967. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita Y, Okudera T, Tsuru E, Yokota A. Volumetric analysis of the germinal matrix and lateral ventricles performed using MR images of postmortem fetuses. AJNR Am J Neuroradiol. 2001;22:382–388. [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson RD, Hitchman D, Wittman BK. Clinical follow-up of prenatally diagnosed isolated ventriculomegaly, microcephaly and encephalocele. Fetal Ther. 1989;4:49–57. doi: 10.1159/000263390. [DOI] [PubMed] [Google Scholar]
- 16.Mahony BS, Nyberg DA, Hirsch JH, Petty CN, Hendricks SK, Mack LA. Mild idiopathic lateral cerebral ventricular dilatation in utero: sonographic evaluation. Radiology. 1988;169:715–721. doi: 10.1148/radiology.169.3.3055035. [DOI] [PubMed] [Google Scholar]
- 17.Bromley B, Frigoletto FD, Jr, Benacerraf BR. Mild fetal lateral cerebral ventriculomegaly: clinical course and outcome. Am J Obstet Gynecol. 1991;164:863–867. doi: 10.1016/0002-9378(91)90530-5. [DOI] [PubMed] [Google Scholar]
- 18.Hertzberg BS, Lile R, Foosaner DE, Kliewer MA, Paine SS, Paulson EK, et al. Choroid plexus-ventricular wall separation in fetuses with normal-sized cerebral ventricles at sonography: postnatal outcome. AJR Am J Roentgenol. 1994;163:405–410. doi: 10.2214/ajr.163.2.7518643. [DOI] [PubMed] [Google Scholar]
- 19.Patel MD, Filly AL, Hersh DR, Goldstein RB. Isolated mild fetal cerebral ventriculomegaly: clinical course and outcome. Radiology. 1994;192:759–764. doi: 10.1148/radiology.192.3.7520183. [DOI] [PubMed] [Google Scholar]
- 20.Wax JR, Bookman L, Cartin A, Pinette MG, Blackstone J. Mild fetal cerebral ventriculomegaly: diagnosis, clinical associations, and outcomes. Obstet Gynecol Surv. 2003;58:407–414. doi: 10.1097/01.OGX.0000070069.43569.D7. [DOI] [PubMed] [Google Scholar]
- 21.Wyldes M, Watkinson M. Isolated mild fetal ventriculomegaly. Arch Dis Child Fetal Neonatal Ed. 2004;89:F9–13. doi: 10.1136/fn.89.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laskin MD, Kingdom J, Toi A, Chitayat D, Ohlsson A. Perinatal and neurodevelopmental outcome with isolated fetal ventriculomegaly: a systematic review. J Matern Fetal Neonatal Med. 2005;18:289–298. doi: 10.1080/14767050500329775. [DOI] [PubMed] [Google Scholar]
- 23.Vergani P, Locatelli A, Strobelt N, Cavallone M, Ceruti P, Paterlini G, Ghidini A. Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol. 1998;178:218–222. doi: 10.1016/s0002-9378(98)80003-3. [DOI] [PubMed] [Google Scholar]
- 24.Gaglioti P, Danelon D, Bontempo S, Mombro M, Cardaropoli S, Todros T. Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol. 2005;25:372–377. doi: 10.1002/uog.1857. [DOI] [PubMed] [Google Scholar]
- 25.Ouahba J, Luton D, Vuillard E, Garel C, Gressens P, Blanc N, et al. Prenatal isolated mild ventriculomegaly: outcome in 167 cases. Br J Obstet Gynecol. 2006;113:1072–1079. doi: 10.1111/j.1471-0528.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- 26.Falip C, Blanc N, Maes E, Zaccaria I, Oury JF, Sebag G, Garel C. Postnatal clinical and imaging follow-up of infants with prenatal isolated mild ventriculomegaly: a series of 101 cases. Pediatr Radiol. 2007;37:981–989. doi: 10.1007/s00247-007-0582-2. [DOI] [PubMed] [Google Scholar]
- 27.Bloom SL, Bloom DD, DellaNebbia C, Martin LB, Lucas MJ, Twickler DM. The developmental outcome of children with antenatal mild isolated ventriculomegaly. Obstet Gynecol. 1997;90:93–97. doi: 10.1016/S0029-7844(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 28.Gilmore JH, van Tol JJ, Lewis Streicher H, Williamson K, Cohen SB, Greenwood RS, et al. Outcome in children with fetal mild ventriculomegaly: a case series. Schizophr Res. 2001;48:219–226. doi: 10.1016/s0920-9964(00)00140-7. [DOI] [PubMed] [Google Scholar]
- 29.Dommergues M, Mahieu-Caputo D, Fallet-Bianco C, Mirlesse V, Aubry MC, Delezoide AL, et al. Fetal serum interferon-alpha suggests viral infection as the aetiology of unexplained lateral cerebral ventriculomegaly. Prenat Diagn. 1996;16:883–892. doi: 10.1002/(SICI)1097-0223(199610)16:10<883::AID-PD959>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, Gilmore JH. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242:535–541. doi: 10.1148/radiol.2422060133. [DOI] [PubMed] [Google Scholar]
- 31.Monteagudo A, Haratz-Rubinstein N, Timor-Tritsch IE. Biometry of the Fetal Brain. Ultrasonography of the Prenatal and Neonatal. In: Timor-Tritsch IE, Monteagudo A, Cohen HL, editors. Brain. Stamford, CT: Appleton & Lange; 1996. pp. 113–117. 1996. [Google Scholar]
- 32.Heiserman J, Filly RA, Goldstein RB. Effect of measurement errors on sonographic evaluation of ventriculomegaly. J Ultrasound Med. 1991;10:121–124. doi: 10.7863/jum.1991.10.3.121. [DOI] [PubMed] [Google Scholar]
- 33.Gilmore JH, Zhai G, Wilber K, Smith JK, Lin W, Gerig G. 3 Tesla magnetic resonance imaging of the brain in newborns. Psychiatry Res. 2004;132:81–85. doi: 10.1016/j.pscychresns.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Prastawa M, Gilmore JH, Lin W, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 2005;9:457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Gilmore JH, Lin W, Corouge I, Vetsa YS, Smith JK, Kang C, et al. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR Am J Neuroradiol. 2007;28:1789–1795. doi: 10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corouge I, Fletcher PT, Joshi S, Gilmore JH, Gerig G. Fiber tract-oriented statistics for quantitative diffusion tensor MRI analysis. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2005;8:131–139. doi: 10.1007/11566465_17. [DOI] [PubMed] [Google Scholar]
- 39.Chan WY, Lorke DE, Tiu SC, Yew DT. Proliferation and apoptosis in the developing human neocortex. Anat Rec. 2002;267:261–276. doi: 10.1002/ar.10100. [DOI] [PubMed] [Google Scholar]
- 40.Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 42.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 44.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 45.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Knickmeyer RC, Vetsa YSK, Gouttard S, Lin W, Evans D, Wilber K, et al. A structural MRI study of human brain development from birth to age 2 (submitted) 2008 doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore BD, Slopis JM, Jackson EF, DeWinter AE, Leeds NE. Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status. Neurology. 2000;54:914–920. doi: 10.1212/wnl.54.4.914. [DOI] [PubMed] [Google Scholar]
- 48.Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Jiang T, Cao Q, Wang Y. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR Am J Neuroradiol. 2007;28:543–7. [PMC free article] [PubMed] [Google Scholar]
- 51.Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, McMillan A, Seidenberg M. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130(Pt 12):3135–48. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- 52.Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161:753–758. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- 53.Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- 55.Bockhorst KH, Narayana PA, Liu R, Ahobila-Vijjula P, Ramu J, Kamel M, Wosik J, Bockhorst T, Hahn K, Hasan KM, Perez-Polo JR. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J Neurosci Res. 2008;86:1520–1528. doi: 10.1002/jnr.21607. [DOI] [PubMed] [Google Scholar]
- 56.Filly RA, Goldstein RB. The fetal ventricular atrium: fourth down and 10 mm to go. Radiology. 1994;193:315–317. doi: 10.1148/radiology.193.2.7972733. [DOI] [PubMed] [Google Scholar]
- 57.Pilu G. Borderline fetal cerebral ventriculomegaly - the Twilight Zone. Ultrasound Obstet Gynecol. 1993;3:85–87. doi: 10.1046/j.1469-0705.1993.03020085.x. [DOI] [PubMed] [Google Scholar]
- 58.Signorelli M, Tiberti A, Valseriati D, Molin E, Cerri V, Groli C, Bianchi UA. Width of the fetal lateral ventricular atrium between 10 and 12 mm: a simple variation of the norm? Ultrasound Obstet Gynecol. 2004;23:14–18. doi: 10.1002/uog.941. [DOI] [PubMed] [Google Scholar]
- 59.Kramer RL, Yaron Y, Johnson MP, Evans MI, Treadwell MC, Wolfe HM. Differences in measures of the artia of the lateral ventricle: does gender matter? Fetal Diagn Ther. 1997;12:304–305. doi: 10.1159/000264492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.