Abstract
Targeted imaging requires contrast agents that remain in the vasculature for extended periods of time. A new contrast agent is described in which gadolinium is encapsulated within an extremely stable carbon sphere, thus allowing for safe extended residence. Water solubility and small particle size is achieved with novel fullerene chemistry, attaching multiple oligoethylene glycol groups through nitrogen chemistry. These new compounds can be used to visualize tissue architecture in vivo with standard MRI techniques.
Discoveries in molecular biology have produced an explosive growth in efforts to identify new targets for diagnostic medicine. However, imaging the distribution of validated targets in patients has lagged for want of safe, high-resolution imaging tools. Some nuclear medicine agents are targeted, but the relatively low resolution of PET/SPECT requires that these methods be combined with CT scans for interpretation, and there is concern about the long-term effects of exposure to ionizing radiation.1 Magnetic resonance imaging (MRI) is widely available, highly translatable, and can provide high contrast, especially for the study of soft tissue. However, recent concerns over the role of current Gd-based contrast agents in the development of nephrogenic systemic fibrosis (NSF) in patients with impaired renal function2,3 highlight the need for safer imaging agents with enhanced imaging capabilities. In this report, we describe a new technology for improving MRI contrast based on endohedral metallofullerenes containing Gd. Surface modification of the fullerene provides much higher spin coupling with water protons than other forms of Gd despite separation by the fullerene cage. These nanomaterials are remarkably stable and hence are unlikely to release the Gd. These new nanomaterials injected intravenously demonstrate enhanced contrast in a mouse MRI, with circulation of at least 30 min. This technology could serve as a platform for developing agents that can specifically target molecular entities uniquely expressed on certain cells, tissues, or organs, thus providing caregivers improved ways to diagnose, treat, and monitor progression of a wide range of diseases.
The fullerene-based nanomaterials described here may provide a unique platform for developing such technologies based on their enhanced relaxivity, stability, and ability to be derivatized with molecular targeting moieties. Endohedral metallofullerenes containing one Gd atom,4,5 derivatized by polyhydroxylation or through addition of carboxyl groups to improve water solubility, exhibit high relaxivity, though the relaxivity has been attributed primarily to the formation of aggregates.6,7 This problem was overcome through chemistry that adds groups onto the fullerene cage that optimize magnetic coupling between the unpaired electrons on the enclosed paramagnetic Gd and water protons outside the cage. The cage is extremely stable; extreme oxidizing conditions are required to release gadolinium from the cage.
The first trimetallic nitride MRI contrast agent, based on Gd3N@C80, was hydroxylated and PEGylated with long-chain glycols.8 In the present work, we have synthesized9 a series of novel derivatives of Gd3N@C80 termed Hydrochalarones (derived from χαλαρω = relax). These Hydrochalarones are not hydroxylated but are made water soluble by addition of a series of glycol methyl ethers, ranging from monoethylene glycol to hexaethylene glycol (Figure 1A). Importantly, the polar ethylene glycols are extremely close to the fullerene cage which increases its water solubility. The paramagnetic Gd atoms preclude using standard NMR to confirm the structure of these nanomaterials, but the number of attached groups can be inferred from thermogravimetric (TGA) analysis, where oxidation of the side chains takes place at a lower temperature than the cage. The TGA measurements on Gd3N@C80 and Hydrochalarones revealed the number of attachments on the cage to be 20–22, 12–14, and 10–12 for Hydrochalarone-1, -3, and -6, respectively. The connectivity between fullerene and glycol is not clear but likely includes RNOH and isoxazoline-like rings as inferred from their IR stretches (Figure 1B).
Figure 1.
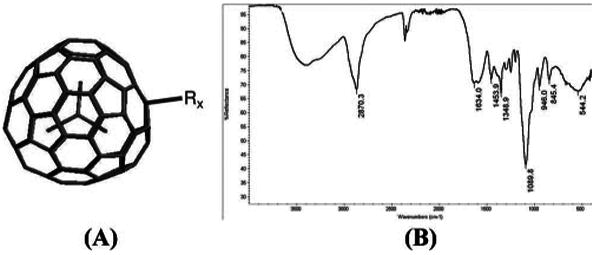
(A) General structure of Hydrochalarones is Gd3N@C80-Rx, where R = −[N(OH)(CH2CH2O)nCH3]x where n = 1,3,6 and x is 10–22. (B) Infrared spectra of Hydrochalarone-6.
It is known that a very stable species Gd3N@C80 is formed by combining two unstable species, Gd3N and C80. The Hydrochalarone derivatives are even more stable. For example, TGA shows Gd3N@C80 begins to oxidize at 180 °C and disintegrates very rapidly thereafter. By contrast, the Hydrochalarone cages start to decompose at ∼500 °C. Apparently the addition of polyethylene glycol groups stabilizes the carbon cage, presumably by relieving stress on the C=C bonds. Similarly, even heating in concentrated nitric acid at 80 °C for 4 h does not release the Gd atoms from the cage. Hydrochalarones are stable at room temperature for at least 6 months, monitored by relaxivity.
The particle size of these nanomaterials was measured using DLS and found to be 10–15 nm, which is small enough to circulate through capillaries. Measurements of T1 relaxivity, r1, the standard metric of contrast enhancement, were performed on these nanomaterials in water using a 20 MHz Maran Ultra relaxometer (Oxford Instruments). The optimal member of this series, Hydrochalarone-6, has r1 of 205 mM−1 s−1 (68 mM−1 s−1 Gd, compared with 3.8 mM−1 s−1 for Magnevist, a commercial product containing Gd DTPA). Interestingly, the ratio of T2 relaxivity to T1 relaxivity, r2/r1, is comparable to those of the small Gd chelates, which is a critical factor in controlling signal intensity during T1-weighted MRI.
Chelate-based MRI contrast agents, with one or more coordinated water molecules that exchange with bulk water molecules, exhibit a relaxivity described by the Solomon–Bloembergen–Morgan (SBM) equation.10 In Hydrochalarone, the Gd atoms are entrapped in the cage and there is no possibility of water being coordinated to the paramagnetic metal centers. Thus, a different mechanism of spin transfer between Gd and water must be at work.
Previous work on Gd@C60(OH)x and Gd@C60-[C(COOHyNa1−y)2]10 implicated proton exchange from bulk water with hydroxyl and protonated malonate groups as integral to magnetic coupling.4 Application of this hypothesis to Hydrochalarones can be tested by looking for −OH/−OD exchange in the FTIR spectrum. During deuterium exchange experiments no −OD stretches were observed, indicating that proton exchange cannot account for the relaxivity observed.
The X-ray crystal structure shows that the Gd3N@C80 cage is slightly distorted by the entrapped Gd3N species and the Gd atoms are closer to the carbon atoms of the cage.11 The unpaired f-orbital electrons of the Gd atoms may interact with electrons in the molecular orbitals on the six-carbon rings near the distortions. Water forms weak hydrogen bonds on benzene rings.12 Gel electrophoresis shows that Hydrochalarones bear a negative charge that could enhance hydrogen bonding. Unpaired f-orbital electrons of Gd may couple their spins through the electrons on the carbon molecular orbitals to water protons that are hydrogen-bonded to the cage surface. This represents another example of “spin leakage”13,14 and supports the hypothesis that metallofullerenes behave as “superatoms.”15 Hydrogen-bonded water molecules may act like inner-sphere water that rapidly exchange with many surrounding water molecules. Different length polyethylene glycol attachments may alter relaxivity by changing water diffusion rates through the hydration shells of the polyethylene glycol moieties.
In vivo MRI studies were performed at 4.7 T using a small-animal scanner interfaced to a Varian INOVA console. Hydochalarone-6 was administered intravenously to a BALB/c mouse at a dose of 0.01 mmol/kg. (A typical clinical dose of commercial Gd-based MRI contrast agent is 0.1 mmol/kg.) Figure 2 shows a coronal slice from a T1-weighted whole-body image of a mouse before and 30 min after Hydrochalarone administration. The persistent image enhancement of the aorta (arrow) indicates Hydrochalarone-6 remains in the vasculature, consistent with the observation that polyethylene glycol modifications increase the circulation time of proteins.16
Figure 2.
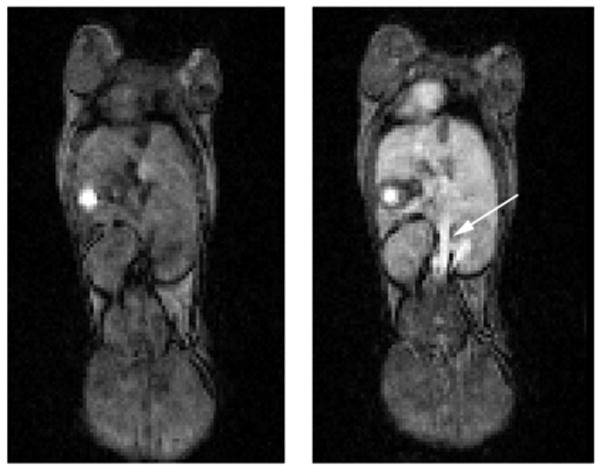
Coronal slice from a whole-body MRI of a mouse before (left) and 30 min after (right) administration of Hydrochalarone-6. The unidentified bright spot in both the pre- and postcontrast images is likely an artifact.
In summary, Hydrochalarones are stable, appear well-tolerated in vivo, provide excellent MRI enhancement, and have “stealth” properties, meaning that rapid clearance mechanisms seem not to be triggered. These are desirable properties for the development of Hydrochalarones as imaging agent platforms upon which can be attached a broad range of targeting species for improved clinical diagnosis and management of a number of diseases.
Acknowledgments
We thank Professor J. J. H. Ackerman for helpful discussions and R. Sadler for obtaining analytical data. The Biomedical MR Laboratory acknowledges support from NIH/NCI Small Animal Imaging Resource Program (SAIRP) (Grant R24 CA83060), NIH/NHLBI Program for Excellence in Nanotechnology (Grant U01 HL080729), and the Alvin J. Siteman Cancer Center at Washington University in St. Louis, an NCI Comprehensive Cancer Center (Grant P30 CA91842). C.L.K. acknowledges NIH Grants 1R01GM083274-01 and 1R43HL087578-01A1.
Footnotes
Supporting Information Available: Synthetic procedure for Hydrochalarones, IR spectra, TGA trace, and r1 plot. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the U.K. and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 2.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 1996;17:2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 3.Grobner T. Gadolinium. A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol, Dial, Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 4.Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, Wallace S, Wilson LJ, Alford JM. First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J Am Chem Soc. 2003;125:5471–5478. doi: 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- 5.Mikawa M, Kato H, Okumura M, Narazaki Y, Kanazawa Y, Miwa N, Shinohara H. Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjugate Chem. 2001;12:510–514. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- 6.Laus S, Sitharaman B, Toth E, Bolskar RD, Helm L, Asokan S, Wong MS, Wilson LJ, Merbach AE. Destroying gadofullerene aggregates by salt addition in aqueous solution of Gd@C60(OH)x and Gd@C60[C(COOH)2]10. J Am Chem Soc. 2005;127:9368–9369. doi: 10.1021/ja052388+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laus S, Sitharaman B, Toth E, Bolskar RD, Helm L, Wilson LJ, Merbach AE. Understanding paramagnetic relaxation phenomenon for water soluble gadofullerenes. J Phys Chem C. 2007;111:5633–5639. [Google Scholar]
- 8.Fatouros PP, Corwin FD, Chen Z, Broaddus WC, Taturn JL, Kettenmann B, Ge Z, Gibson HW, Russ JL, Leonard AP, Duchamp JC, Dorn HC. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology. 2006;240:756–764. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 9.MacFarland DK, Walker KP. Manuscript in preparation. [Google Scholar]
- 10.Bloembergen N, Morgan LO. Proton relaxation times in paramagnetic solutions. Effects of electron spin relaxation. J Chem Phys. 1961;34:842–850. [Google Scholar]
- 11.Stevenson S, Philips JP, Reid JE, Olmstead MM, Rath SP, Balch AL. Pyramidalization of Gd3N inside a C80 cage. The synthesis and structure of Gd3N@C80. Chem Commun (Cambridge) 2004:2814–2815. doi: 10.1039/b412338g. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Green PG, Bumgarner RE, Dasgupta S, Goddard WA, Blake GE. Benzene forms hydrogen bonds with water. Science. 1992;257:942–945. doi: 10.1126/science.257.5072.942. [DOI] [PubMed] [Google Scholar]
- 13.Koltover VK, Bubnov VP, Estrin YI, Lodygina VP, Davydov RM, Subramoni M, Manoharan PT. Spin-transfer complexes of endohedral metallofullerenes: ENDOR and NMR evidences. Phys Chem Chem Phys. 2003;5:2774–2777. [Google Scholar]
- 14.Koltover VK, Logan JW, Heise H, Bubnov VP, Estrin YI, Kareev IE, Lodygina VP, Pines A. Diamagnetic clusters of paramagnetic endometallofullerenes: a solid-state NMR study. J Phys Chem B. 2004;108:12450–12455. [Google Scholar]
- 15.Shinohara H. Endohedral metallofullerenes. Rep Prog Phys. 1998;63:843–892. [Google Scholar]
- 16.Beauchamp CO, Gonias SL, Menapace DP, Pizzo SV. A new procedure for the synthesis of polyethylene glycol–protein adducts; effects on function, receptor recognition and clearance of superoxide dismutase, lactoferrin and alpha 2-macroglobulin. Anal Biochem. 1983;131:25–33. doi: 10.1016/0003-2697(83)90131-8. [DOI] [PubMed] [Google Scholar]


