Abstract
The risk of developing neurodegenerative disorders such as Alzheimer's (AD) and Parkinson's (PD) diseases increases with age. AD and PD are the two most common neurodegenerative diseases that currently affect millions of persons within the United States population. While many clues about the mechanisms of these disorders have been uncovered, to date, the molecular mechanisms associated with the cause of these diseases are not completely understood. Furthermore, there are no available cures or preventative treatments for either disorder. Animal models of AD and PD, though not perfect, offer a means to gain knowledge of the basic biochemistry associated with these disorders and with drug efficacy. The field of proteomics which focuses on identifying the dynamic nature of the protein content expressed within a particular cell, tissue, or organism, has provided many insights into these disturbing disorders. Proteomic studies have revealed many pathways that are associated with disease pathogenesis and that may lead to the development of potential therapeutic targets. This review provides a discussion of key findings from AD and PD proteomics-based studies in various animal models of disease.
Keywords: Alzheimer's disease, Parkinson's disease, proteomics, animal models
1. Introduction
1.1 Alzheimer's Disease
Alzheimer's disease (AD) is a neurodegenerative disorder that currently plagues five million Americans. AD can be broadly classified into either familial or sporadic forms of the disease. Familial cases result from genetic mutations in amyloid precursor protein (APP) and proteins involved in APP processing, including presenilin 1 (PS1) and presenilin 2 (PS2) (Rocchi et al. 2003) (Goate et al. 1991; Levy-Lahad et al. 1995; Sherrington et al. 1995). However, the vast majority (>90%) of AD cases are of the sporadic variety. A well-established genetic risk factor for sporadic AD is apolipoprotein E (ApoE), specifically its ApoE4 isoform. ApoE4 has been the subject of extensive research because individuals that are homozygous for the ApoE4 allele have a high incidence of developing sporadic AD (Corder et al. 1993; Strittmatter and Roses 1996). Clinical AD diagnosis occurs when multiple symptoms including memory impairment, aphasia, apraxia, agnosia, and general loss of executive functions, are manifested in the individual and collectively contribute to dementia not due to other causes (Association 2000). It is interesting to note that these symptoms also arise in the reverse order of which they are acquired throughout childhood development (Reisberg et al. 1986).
On a molecular level AD is characterized by the accumulation of senile plaques (SP), which are predominately composed of the short amyloid β-peptide (Aβ), and neurofibrillary tangles (NFT), which are composed largely of hyperphosphorylated tau protein. The 40 and 42 amino acid peptides of Aβ are prevalent in SP of AD patients. Both SPs and NFTs are formed in the hippocampus and neocortical regions of the brain. Molecular confirmation of the clinical diagnosis of AD is made post-mortem based upon criteria such as the number of senile plaques and neurofibrillary tangles (Mirra et al. 1991) and by Braak-stage scoring (Braak and Braak 1991).
SPs and NFTs, together with synapse loss, are the classical pathological hallmarks of AD. The exact mechanisms accounting for these pathological hallmarks and their contribution to the clinical symptoms associated with the disease are not yet fully understood. Using the knowledge of genetic mutations that result in familial AD and the association of sporadic AD with the ApoE4 allele (see Table I), several rodent models have been developed to aid in the elucidation of AD pathogenesis.
Table I. Genetic mutations in Alzheimer's Disease (AD) and Parkinson's Disease (PD).
| Gene/protein | Pathology | Function | Form | Refs. |
|---|---|---|---|---|
| APP | Senile plaques | unknown | familial AD | Goate et al. 1991 |
| PS1 | Senile plaques | Notch signaling | familial AD | Sherrington et al. 1995 |
| PS2 | Senile plaques | Notch signaling | familial AD | Levy-Lahad et al. 1995 |
| ApoE | Senile plaques & neurofibrillary tangles | cholesterol regulation and triglyceride metabolism | familial & sporadic AD | Corder et al. 1993; Strittmatter and Roses 1996 |
| α-synuclein | Lewy bodies | synaptic vesicle processing | familial & sporadic PD | Polymeropoulos et al. 1997 |
| DJ-1 | unknown | cellular stress response | familial PD | Kruger et al. 1998; Zarranz et al. 2004 |
| LRRK2 | Lewy bodies | mitochondrial-associated protein kinase | familial & sporadic PD | Zimprich et al. 2004; Paisan-Ruiz et al. 2004 |
| Parkin | Lewy bodies, rarely | ubiquitination of proteins | familial PD | Kitada et al. 1998 |
| PINK-1 | unknown | mitochondrial kinase | familial PD | Valente et al. 2004 |
| UCH-L1 | Lewy bodies | de-ubiquitination of proteins | familial & sporadic PD | Leroy et al. 1998 |
1.2 Parkinson's Disease
Parkinson's disease (PD) follows AD as the second most common age-related neurodegenerative disorder amongst the elderly, occurring in 1-2% of the population over the age of 60 years (de Rijk et al. 2000; Lang and Lozano 1998; Martin 1999; Nutt and Wooten 2005). Disruptions to the central motor system in PD patients result in symptoms such as bradykinesia, resting tremors, postural instability, and muscular rigidity (Lotharius and Brundin 2002; Moore et al. 2005). Thus, unlike AD patients, PD patients have normal access to learning and memory brain functions, however, PD patients have severe physical impairments. Pathologically, PD is similar to other tauopathies and neurodegenerative disorders, in which protein aggregates are associated with disease pathogenesis. Specifically, Lewy-body (LB) inclusions found in the substania nigra (SN) of PD patients consist of aggregates of α-synuclein protein. In addition to LBs, the SN also exhibits substantial dopaminergic loss such that the disease clinically manifests when approximately 70% neuronal death has occurred in the SN and striatum brain regions (Fearnley and Lees 1991; Lees 1992). Because dopamine is the primary neurotransmitter involved in motor functions, its loss directly impacts physical movements and contributes to the clinical symptoms.
More than 90% of PD cases are sporadic and associated with unknown causes, while the remaining 10% of cases represent familial inherited forms of the disease resulting from genetic mutations to genes listed in Table I. These genes are parkin, ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), DJ-1, α-synuclein, PTEN-induced putative kinase 1 (PINK-1) and leucine rich repeat kinase (LRRK2) (Bonifati et al. 2003; Farrer et al. 2005; Kitada et al. 1998; Leroy et al. 1998; Lesage et al. 2005; Polymeropoulos et al. 1997; Recchia et al. 2004; Valente et al. 2004; von Coelln et al. 2004) (Paisan-Ruiz et al. 2004; Savitt et al. 2006). The most common mutations in the α-synuclein protein associated with PD are A30P, A53T, and E46K (Kruger et al. 1998; Spira et al. 2001; Zarranz et al. 2004). In addition to being present in LBs, α-synuclein is believed to be involved in synaptic vesicle formation (Abeliovich et al. 2000). Parkin and UCH-L1 are proteins involved in ubiquination and de-ubiquination, respectively, of misfolded or damaged proteins that become targets for proteasome degradation (Moore et al. 2005; Zhang et al. 2000), although UCH-L1 also has synaptic functions associated with memory (Gong et al. 2006). The functions of DJ-1 are still not clear, although this protein is believed to be involved in cellular stress responses by acting as an antioxidant, redox-sensitive chaperone, and protease (Moore et al. 2005). PINK-1 is a mitochondrial protein kinase, and its mutations may contribute to mitochondrial dysfunction in PD (Valente et al. 2004). Lastly, the LRRK2 protein, which is also associated with mitochondria, has been found to bind to parkin and is believed to be involved in membrane and protein trafficking (Savitt et al. 2006).
Key factors believed to contribute to the development of PD are oxidative stress, mitochondrial dysfunction, proteasome dysfunction, inflammation, protein aggregation and exposure to various environmental toxins (Dawson and Dawson 2002; Dawson and Dawson 2003; Hunot and Hirsch 2003; Paolini et al. 2004; Savitt et al. 2006; Vila et al. 2000). Because the exact mechanisms governing dopamine neuron loss are not fully understood, proteomics studies in PD model systems may provide valuable insight to disease pathogenesis. These animal models have been established based on genetic mutations of PD and PD induction by exposure to toxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or rotenone. Current treatment for PD involves L-DOPA administration and MAO inhibitors, which at best reduce associated disease symptoms (Savitt et al. 2006).
To-date, there are no cures or preventive therapeutic targets available for AD or PD. Thereby, novel strategies and clues that assist in drug development for aid in the prevention and cure of these diseases are necessary. A brief summary of proteomic methods and a discussion of key findings from proteomics studies of various AD and PD animal models is provided in this review.
2. Proteomics Methods
The field of proteomics involves the determination of the protein identities of a cell, tissue, or organism under a given set of conditions. Primarily, proteomic techniques are used to examine differences in protein expression in a normal versus diseased state (e.g., control vs. AD), although they are also used to determine the structures and functions of proteins. The most traditional and widely used proteomic method is two-dimensional polyacrylamide gel electrophoresis (2D PAGE) (Rabilloud 2002). In the first dimension of this approach, proteins are separated on an immobilized pH gradient strip with isoelectric focusing and migrate to the point on the strip at which their net charge is zero (i.e., isoelectric point). In the second dimension, charge-separated proteins are exposed to sodium dodecyl sulfate (SDS) PAGE and are separated according to their molecular migration distance through the gel–a separation that is approximately proportional to the molecular weight of the protein (Klose and Kobalz 1995).
A typical high-resolution gel can contain hundreds to thousands of protein spots in which spot intensities can be used to calculate differences in expression between various samples. Another method for gel quantitation is differential gel electrophoresis (DIGE), in which different fluorophores (e.g., Cy2, Cy3 and Cy5) are used to derivatize individual samples. Derivatized samples are then combined into a single mixture that is separated with 2D PAGE, and the gels are scanned at excitation and emission wavelengths corresponding to the individual fluorophores (Tonge et al. 2001). Quantitation of protein expression differences from multiple gel replicates of control and experimental samples is performed with sophisticated image analysis software (e.g., PDQuest, BioRad). Limitations of 2D PAGE include a limited protein pI range (i.e., pH 3-10), poor solubilization of highly acidic, basic proteins and membrane-associated proteins, and poor detection of low-abundance proteins.
The protein map obtained from a 2D PAGE gel can also be used to identify post-translational modifications such as glycosylation, phosphorylation, and/or carbonylation. The identification of carbonylated proteins is one aspect of the field of redox proteomics (Butterfield 2004). In this instance, carbonylated proteins can be derivatized with 2,4-dinitrophenylhydrazine (DNPH) prior to 2D PAGE separation. After separation, proteins in gels are transferred onto a nitrocellulose or polyvinylidene fluoride membrane and probed with an anti-DNP antibody for immunoreactivity. The level of carbonylation of individual proteins in 2D Oxyblots is normalized to their total protein content in 2D gels and the specific carbonylation levels compared between control and diseased samples.
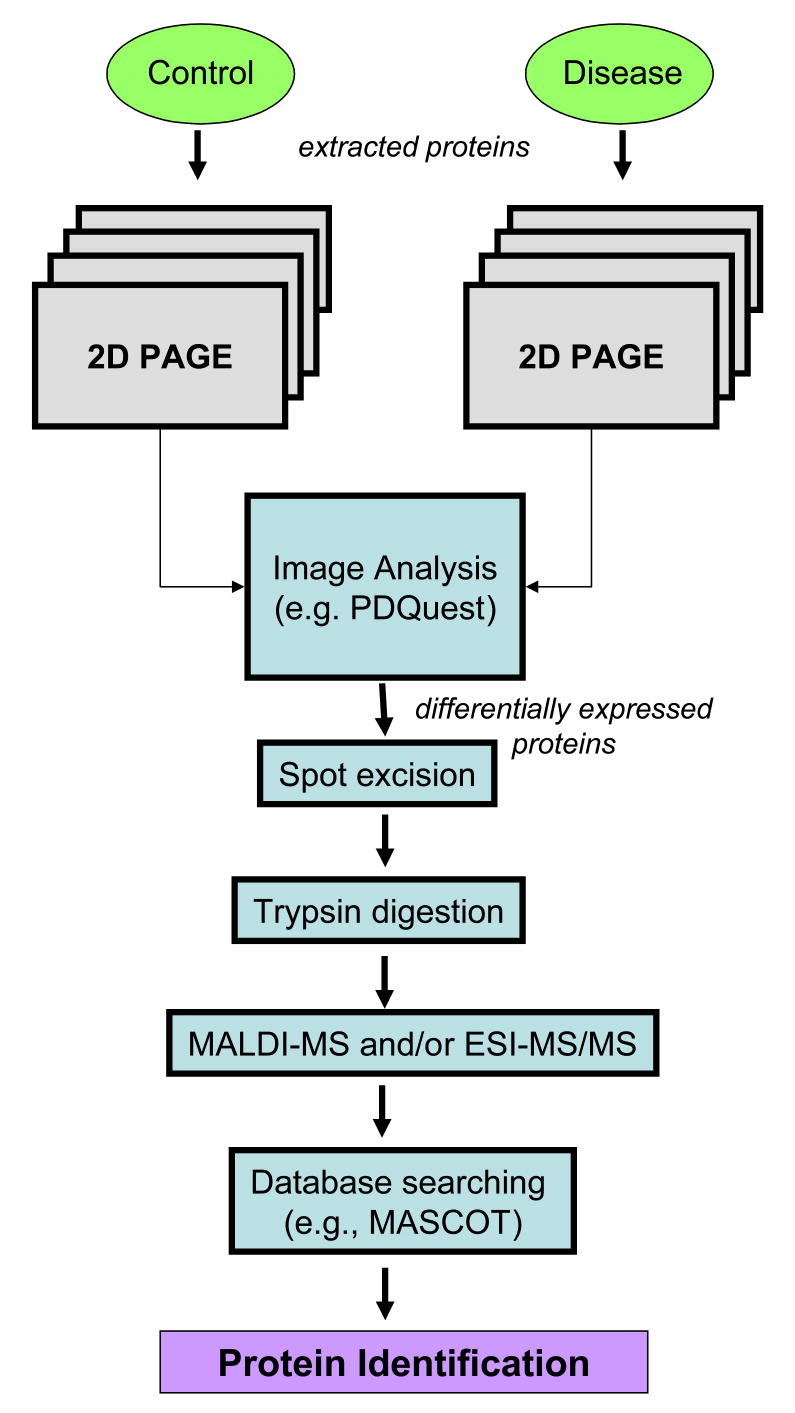
Individual protein spots of interest (e.g., up-regulated in disease, increased carbonylation) from 2D gels or 2D Oxyblots are excised, digested with trypsin, and the resultant peptides are analyzed by matrix assisted laser desportion ionization (MALDI)-MS or electrospray ionization (ESI)-MS to generate a peptide fingerprint. The list of peptide masses is then submitted to a database search engine, such as MASCOT, and searched against a species-specific database for protein identification. MASCOT is a probability-based scoring algorithm in which a returned protein hit with a score greater than the defined cutoff, has a 1 in 20 chance of being a random identification (p < 0.05) (Perkins et al. 1999). An overview of the 2D PAGE approach is shown in Figure 1.
Figure 1.
Schematic diagram of 2D PAGE proteomics analysis in control and diseases tissues.
Non-gel proteomic approaches generally involve the separation of proteins or peptides (resulting from enzymatic digestion with trypsin) with high-performance liquid chromatography (HPLC). Multiple dimensions of LC based on different separation principles (e.g., strong cation exchange, reversed-phase chromatography) can be coupled to increase overall protein resolution and peak capacity prior to MS and tandem MS (MS/MS) detection. The most noted of these approaches is multidimensional protein identification (MUDPIT) technology, which is capable of identifying low-abundance proteins (Wolters et al. 2001). A drawback of MUDPIT is the extensive data collection and analysis necessary for protein identification (e.g., terabytes of storage are often necessary). Quantitation of proteins with LC-MS/MS methods can be performed by the incorporation of isotopic labels at different stages of sample preparation. For example, in the isotopically coded affinity tags (ICAT) method, amino acids in individual samples are derivatized with a light or heavy label after protein extraction before tryptic digestion (Smolka et al. 2001). The relative intensities of light and heavy labeled peptides (and/or proteins) in the ICAT generated mass spectra can be used to examine relative amounts of up- or down-regulation of proteins in the mixture. A drawback of ICAT is that cysteine residues must be present and reactive in a protein in order for it to be detected.
3. Proteomics Findings from Animal Models of Alzheimer's Disease
3.1 Amyloid Precursor Protein Models: Swedish Double Mutant-based Mouse Models
The APP gene is located on chromosome 21 and genetic mutations that occur in APP in familial AD have been well characterized. To-date, there are 18 missense mutations reported, which occur in amyloid-beta (Aβ) peptide encoded exon sequences 16 or 17 of the APP gene (Papassotiropoulos et al. 2006). Approximately 40 mouse models have been raised with both single and multiple combinations of these genetic alterations, with the most commonly used being the Hsiao Tg-2576 mice (Hsiao et al. 1996), the Swedish double mutant (K670M/N671L) and the London mutant (V717I) (http://www.alzforum.org/res/com/tra/app/appsw.asp). For the purpose of this review only models used in proteomics studies will be covered.
The APP Swedish (APPSw) transgenic mutant experiences amyloidosis in the second year of life, with Aβ (1-40) levels as high as 200 ng·mg-1 of tissue in the hippocampus and neocortex occurring at 24 months of age (http://www.alzforum.org/res/com/tra/app/appsw.asp). Proteomics studies of APPSw mice show differential expression of several proteins with varying physiological functions. Pyruvate kinase (PK), aconitase, α-enolase, glial fibrillary acidic protein (GFAP), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), dihydropyrimidase-like 2 (DRP2), similar to zinc finger 1111 protein, and dynamin-1 have shown increased levels (Shin et al. 2004). Conversely, proteasome complex activator subunit 2, malate dehydrogenase, γ-enolase, and ATP synthase α chain have decreased levels in APPSw mutants (Shin et al. 2004). The functions of these proteins encompass a wide range of physiological roles, including metabolism (e.g., PK, GAPDH, and malate dehydrogenase) the ubiquitin/proteasome system, cellular transport (e.g., dynamin), synaptic and axonal integrity (e.g., DRP2), and inflammatory markers (e.g., GFAP). Of the aforementioned proteins, GAPDH, DRP2, ATP synthase α chain, similar to zinc finger 1111, and dynamin-1 have also been shown to be differentially expressed in human AD brain (Butterfield et al. 2007) and thus may be key pathways important for AD pathogenesis.
Proteomics studies in the Swedish/London (Swe/Lon) combination mutant mice have revealed eight proteins with increased levels compared to age-matched wild-type controls (Sizova et al. 2007) that overlap with human AD (Castegna et al. 2002a; Castegna et al. 2002b; Schonberger et al. 2001; Tsuji et al. 2002). These proteins are GFAP, ApoE precursor, peroxiredoxin 6 (Prdx6), DRP2, synaptotagamin I, N-ethylmaleimide sensitive fusion protein, serum albumin precursor, and PK. Like APPSw mutants, the Swe/Lon mice also express changes in metabolic, inflammatory marker, and synaptic and axonal integrity proteins relative to controls. Prdx6 and ApoE are also up-regulated in this AD model. Prdx6 is an antioxidant enzyme that is critical for clearance of hydrogen peroxide, a reactive oxygen species (Sarafian et al. 1999). ApoE allele type has a link to AD, and cholesterol metabolism is altered in AD (Casserly and Topol 2004). It is important to note that in this particular mouse model neuronal loss is limited even though plaque deposition begins at six months of age (Sizova et al. 2007). Up-regulation of these proteins supports the notion that oxidative stress and disruption to metabolic processes occur in AD brain.
3.2 Presenilin 1, Presenilin 2, and APP/PS Mouse Models
Mutations in the presenilins, PS1 and PS2, account for the majority of familial AD cases (Papassotiropoulos et al. 2006). It is widely accepted that the presenilins are a part of the γ-secretase complex (Scheuner et al. 1996) and mutations in the transmembrane domain lead to aberrant APP processing. PS1 and PS2 are highly homologous proteins; however, PS2 warrants its own classification because it has been traced to a single German family (Bird et al. 1988). PS1 and PS2 mouse models experience varying degrees of altered PS1 and PS2 expression; however, none of the models develops AD pathology at a quicker rate than the wild-type counterpart. Therefore, models with mutations in both APP and PS1 and/or PS2 were developed. It should be noted that proteomic studies of PS1 or PS2 models are limited (Wood et al. 2005).
The coupling of APP and PS mutations allow for reduced phenotypic variance of APP mutants and more rapid senile plaque deposition. APP/PS mice overcome the shortcomings of single gene mutations of APP or PS models of AD because they experience cognitive defects and senile plaque deposition. While, proteomics studies also are limited in this model, APP/PS models may be useful in applications testing efficacy of therapeutic approaches. Proteomics analysis of oxidatively modified APP/PS-1 mice as a function of age has recently been completed in our laboratory and the results will be published soon.
3.3 Human P301L and GSKβ Tau Mouse Models
The mouse models discussed above have been associated with mutations in APP and PS, which are related to SP formation. None of these models have hyperphosphorylated tau and NFTs present, in spite of the importance of NFTs as pathological hallmarks of AD. To date, proteomics studies have been conducted on only two of 26 tau mutant/transgenic mouse models (http://www.alzforum.org/res/com/tra/tau/default.asp). One of the models expresses the longest mutant human tau isoform P301L, while the other has a constitutively active glycogen synthase kinase β protein present (David et al. 2006; Tilleman et al. 2002).
A proteomics study conducted by David et al. used P301L tau (P301) mice that were injected with Aβ (1-42) into the amygdala (David et al. 2006). As a control, the reverse sequence of Aβ (e.g., 42-1) was used. The researchers observed a wide range of differentially expressed proteins in this AD model. Up-regulated proteins include: actin, carbonic anhydrase II, isocitrate dehydrogenase NADH cytoplasm (ICDH), DRP2, proteasome subunit α-3, transferrin, phosphoglycerate mutase 1, synapsin-2, stress protein 70 (grp75), and DRP5. Proteins that were down-regulated include: Prdx5, Prdx6, tubulin α-4 chain, ribose-phosphate pyrophosphate kinase II, NADH ubiquinone oxidoreductase B15 subunit, and NADH dehydrogenase. Differentially expressed proteins identified in this model were numerous and vary in function; in addition, there is overlap with proteins identified in other AD models. For example, DRP2 is important for synaptic/axonal maintenance and is affected in AD (Castegna et al. 2002b) and the aforementioned transgenic models.
Tilleman et al. conducted proteomics experiments on an aberrant GSKβ protein that abnormally phosphorylates tau, leading to the development of NFTs (Tilleman et al. 2002). The study identified a number of proteins with varying expression and functionalities that were also present in aforementioned models (e.g., DRP2, creatine kinase BB, heat shock protein 90, α-enolase, isocitrate dehydrogenase, succinate dehydrogenase, and glutathione-s-transferase).
3.4 ApoE4 Mouse Model
The ApoE4 allele is a well-established risk factor for sporadic AD (Papassotiropoulos et al. 2006). ApoE is important in the regulation of cholesterol and triglyceride metabolism (Breslow et al. 1982) and has three major isoforms: ApoE2, ApoE3, and ApoE4. The only differences present in the isoforms occur in the amino acid residues at positions 112 and 158.
Specifically, ApoE2 has a cysteine at both positions, ApoE3 has a cysteine residue at 112 and an arginine at 158, and ApoE4 has two arginine residues at both positions (Osorio et al. 2007).
Osorio et al. conducted a study using ApoE3 and ApoE4 targeted replacement mice. In this study, the endogenous mouse ApoE was replaced by either human ApoE3 (control) or ApoE4 (Osorio et al. 2007). The only protein that was found to be differentially expressed in ApoE4 mice hippocampus relative to controls was the mitochondrial protein mortalin, also known as mtHSP70 and GRP75. The mortalin-c isoform was shown to have a 14-fold increase in expression in ApoE4 mouse, while the mortalin-d isoform was shown to have a 3.4-fold increase. Both mortalin isoforms were also found to be differentially phosphorylated and mortalin-d is was identified as differently expressed in human AD hippocampus (Osorio et al. 2007). Mortalin plays a role in diverse processes, including cell survival, stress response, mitochondrial biogenesis, intracellular trafficking, and cell differentiation (Kaul et al. 2002; Wadhwa et al. 2005). The authors hypothesize that mortalin plays a protective role in a yet to be defined mechanism and may be useful as a biomarker for AD.
3.5 Senescence-accelerated Prone Mouse Model
The senescence-accelerated prone mouse (SAMP8) mouse strain has a shorter lifespan and experiences problems with learning and memory in an age-dependent manner compared to wild-type mice (Butterfield and Poon 2005). SAMP8 mice experience impaired immunity and have Aβ deposition at an advanced rate (Flood and Morley 1998). Since the single greatest risk factor for sporadic AD is age, studying the SAMP8 mouse provides a useful platform for studying the effects of aging. Redox proteomics studies have been conducted in SAMP8 mice using APP-directed antisense oligonucleotide (AO) and α-lipoic acid (LA) as treatments to decrease Aβ (1-42) levels (Poon et al. 2005b; Poon et al. 2004b). Aβ (1-42) is believed to be the most toxic form of Aβ in human AD (Butterfield et al. 2007).
LA is a potent endogenous molecule that induces antioxidant proteins that have the capacity to scavenge reactive oxygen species (ROS), chelate metals, and recycle other endogenous enzymes (Kagan et al. 1992; Ou et al. 1995; Sen et al. 1997). SAMP8 mice given subcutaneous injection of LA (100 mg/kg dose) had lower levels of carbonyls and performed better in cognitive tests (Poon et al. 2005b). A proteomics study of brain proteins found neurofilament triplet L protein, mitochondrial creatine kinase, and α-enolase to have increased expression. Neurofilament triplet L (NF-L) is a component of neurofilaments (NFT), which are responsible for axonal structural integrity (Brady 1993; Hoffman et al. 1987). NF-L protein levels were found to be lower in AD brains relative to age-matched controls, thus suggesting that NF-L protein is important for proper brain activity (Bajo et al. 2001) and that LA may be helpful in maintaining axonal integrity. Mitochondrial creatine kinase (mCK) and its cytosolic counterpart are implicated in regulating ATP concentration in cerebral gray matter (Kekelidze et al. 2001). Cytosolic creatine kinase (cCK) has been previously shown to be oxidized in brains of aged SAMP8 mice (Poon et al. 2004a). The upregulation of mCK by LA treatment may compensate for aberrant cCK, in order to maintain normal neuronal ATP concentrations. α-enolase has also been shown to be oxidized in aged SAMP8 brain (Poon et al. 2004a). LA treatment reduces the level of α-enolase oxidation and increases its expression in SAMP8 mouse brain; this suggests that altered glucose metabolism, characteristic of the SAMP8 mouse model, is improved with LA treatment (Ikegami et al. 1992; Poon et al. 2005b).
Redox proteomics showed that lactate dehydrogenase, DRP2, and α-enolase were significantly less carbonylated (Poon et al. 2005b) in LA-treated SAMP8 mice. Redox proteomics on the AO-treated-mice showed that aldolase 3 (Aldo3), Coronin 1a (Coro1a), and Prdx2 were significantly less carbonylated (Poon et al. 2005a). Both AO and LA treatments resulted in changes to protein expression and levels of carbonyl modification. In addition to being a glycolytic enzyme, Aldo3 interacts with DRP2 during times of oxidative stress, aiding in the guidance of synaptic vesicles (Bulliard et al. 1997). Coro1a is important for cytoskeletal integrity and was shown to be impaired in Down's syndrome, a disorder characterized by an extra copy of the APP bearing chromosome 21 (de Hostos et al. 1991). Less oxidative modification of these proteins from AO treatment may provide some protection for neurotransmission through restoration of cytoskeletal repair, antioxidant capability, and increased metabolism and may be associated with improved learning and memory in SAMP8 mice treated with LA or AO (Farr et al. 2003; Morley et al. 2002).
3.6 Aβ Injected-Rat Models
Aβ (1-42) has been proposed to play a critical role in oxidative stress observed in AD (Butterfield et al. 2001). To test this hypothesis, Aβ (1-42) was injected into rat nucleus basalis magnocellularis (NBM) and compared to saline injected controls for an in vivo redox proteomics study (Boyd-Kimball et al. 2005). Glutamine synthetase (GS) and tubulin chain 15/α were found to be oxidized in the cortex, 14-3-3ζ and HSP60 were oxidized in the NBM, and 14-3-3ζ, β-synuclein, pyruvate dehydrogenase, GAPDH, and phosphoglycerate mutase-1 were oxidized in the hippocampus. Metabolic enzymes, chaperones, and GS, tubulin chain 15/α, and 14-3-3ζ were perturbed in this model. GS catalyzes the conversion of glutamate to glutamine, preventing the build-up of the potentially excitotoxic amino acid glutamate and keeping ammonia levels in balance (Casamenti et al. 1999). Tubulin chain 15/α is part of the microtuble assembly core and 14-3-3ζ has multiple roles, including protein trafficking and metabolism (Dougherty and Morrison 2004). In vivo Aβ (1-42) affects in GS are similar to changes observed in AD (Castegna et al. 2002a) which support the notion that Aβ (1-42) plays a key role in the oxidative stress present in AD brain. It is noteworthy that although Aβ (1-42) was injected in the NBM, oxidative modification of hippocampal and cortical proteins was observed. This observation may relate to cholinergic innervation of the outer molecular layer of the hippocampus in the NBM. The NBM is extensively affected in AD as is the hippocampus.
3.7 Transgenic Rats Expressing Human Mutant APP Model
A proteomics study of transgenic rats expressing Swedish mutant human APP found 12 proteins to be differentially expressed in hippocampal CA1 region pyramidal cells (Wilson et al. 2005). The transgenic rats were developed to express the Swedish mutant of APP at levels slightly higher than basal endogenous rat APP levels (Wilson et al. 2005). Proteins identified as upregulated were adenine phosphoribosyltransferase, annexin V, peroxiredoxin 2A, peroxiredoxin 2B, phosphoglycerate mutase, and phosphoglycerate mutase B. Down-regulated proteins included ATP synthase subunit D, tubulin β chain A, tubulin β chain B, heat shock protein 70, NEDD-4, and vasopressin activated calcium mobilizing receptor (CUL5). NEDD4 is a protein involved in neddylation, a process that stabilizes/destabilizes cullin proteins. Cullin proteins have been shown to promote E3 ubiquitin ligase activity in vitro (Wu et al. 2005) and therefore are indirectly involved in the ubiquitin/proteosome pathway. CUL5 is a cullin protein stabilized by neddylation, such that the observed decreased expression of NEDD-4 and CUL5 is consistent with the biological functions of these proteins.
3.8 Caenorhabditis elegans Expressing Human Aβ (1-42) Model
Caenorhabditis elegans expressing human Aβ (1-42) is a non-mammalian model used to test the in vivo effects of Aβ (1-42). In a redox proteomics study, 16 proteins were found to be oxidatively modified in this model (Boyd-Kimball et al. 2006). Interestingly even in this non-mammalian model, the oxidatively modified proteins are associated with similar pathways as in the mammalian models including energy metabolism, antioxidant defense, and cytoskeletal structural integrity.
4. Proteomic Findings from Animal Models of Parkinson's Disease
4.1 Parkin Knock-out and A30P Transgenic Mouse Models
Proteomic findings from animal models of PD have recently provided insights into several pathways that may be related to disease pathogenesis. Models that are designed to mimic human familial PD include parkin knock-out (KO) and A30P α-synuclein transgenic mice. Utilizing DIGE, Periquet et al. identified a total of 87 proteins that are differentially expressed in parkin KO mice relative to controls (Periquet et al. 2005). These proteins were associated with pathways involving energy metabolism, ubiquitin/proteasome degradation, detoxification, stress-related chaperones, and synaptic scaffolding. Interestingly, several of these pathways have also been implicated in proteomic studies of AD as discussed above.
Our laboratory has used redox proteomics to determine proteins that undergo oxidative modification in the brains of A30P α-synuclein transgenic mice relative to controls (Poon et al. 2005c). In these studies, lactate dehydrogenase 2, carbonic anhydrase 2, and α-enolase had significantly higher levels of carbonylation in A30P mice relative to controls. Oxidative modification of proteins has been shown to lead to loss of function (Butterfield 2004; Butterfield et al. 1997; Hensley et al. 1995; Lauderback et al. 2001). The activities of these three proteins were shown to be significantly lower in the A30P mice than in controls, highlighting the importance of oxidative stress in PD.
4.2 MPTP-treated Mouse Models
One of the commonly used mouse models of PD is MPTP-treated rodents, which results in mitochondrial toxicity through inhibition of Complex I (Heikkila and Sonsalla 1987; Ogawa et al. 1987). Jin et al. used ICAT proteomics to identify 110 mitochondrial-isolated proteins that were differentially expressed in SN from MPTP- and probenecid-treated animals relative to controls (Jin et al. 2005). Probenecid reduces clearance of MPTP and its metabolites. Of these proteins, particular attention was focused on DJ-1, whose significant increase in expression in PD mice, was validated by Western blot analysis. In addition, immunohistochemical studies revealed that DJ-1 is localized in granular inclusions in dopaminergic neurons with the α-synuclein protein (Jin et al. 2005). While the function of DJ-1 is still not clear, this protein may be involved in mitochondrial function (Dawson and Dawson 2003). This observation is particularly noteworthy since PD mitochondrial function is altered (Jin et al. 2005).
The MPTP-treated mouse also has decreased expression of Purkinje cell protein 4 (PEP-19), as assessed by nanoflow LC-ESI-MS (Skold et al. 2006). PEP-19 was found to be localized to the striatum from imaging MALDI MS analyses (Skold et al. 2006). PEP-19 is a calmodulin-binding protein that is involved in neuronal signal transduction through Ca2+-independent mechanisms (Putkey et al. 2003). In other proteomic studies of MPTP-induced parkinsonism changes in the expression levels of over 400 microglial-associated proteins have been identified in a variety of mouse strains stimulated with lipopolysaccharide (McLaughlin et al. 2006). Due to the large number of proteins that were identified in these studies, the authors limited their preliminary validation of proteomics results to inducible nitric oxide synthase (iNOS). Elevation of the iNOS protein in the lipopolysaccharide-treated strains studied (i.e., C57BL6 and SWR/J) relative to controls provided consistent evidence that iNOS is increased during inflammatory processes (McLaughlin et al. 2006).
A more recent proteomic study of the MPTP-treated mouse model focused on kinetic proteome changes in the ventral midbrains of a wild-type mice strain (i.e., C57BL/6) and a transgenic mice strain that overexpresses L1 cell adhesion molecule (L1cam) in astrocytes (Diedrich et al. 2008). L1cam has been shown to counteract dopaminergic loss resulting from MPTP treatment by enhancing neurite growth and survival in dopaminergic neurons (Diedrich et al. 2008). Traditional MPTP methods to induce PD only result in a four day period of reduced locomotor activity, these proteomic studies focused on changes that occur at acute (i.e., 1 day post injection) and recovery (i.e., 7 days post injection) phases after MPTP injection. Overall, MPTP treatment in both wild-type mice and L1cam transgenic mice resulted in alterations to proteins involved in mitochondrial dysfunction, glycolysis, neurogenesis and the cytoskeleton and ubiquitin pathways (Diedrich et al. 2008). These altered pathways are consistent with those aforementioned in AD and/or PD animal models.
Our laboratory has used the MPTP-treated mouse model to examine drug treatments that cross the blood brain barrier and that may reduce dopamine loss (Chinta et al. 2006). In particular in vivo (and in vitro) treatment with the glutathione precursor, γ-glutamylcysteinyl ethyl ester (GCEE), was shown to reduce dopamine-associated striatal neuron loss in MPTP-treated mice (Chinta et al. 2006). Although proteomic studies have not been performed to-date, studies of this nature are in progress and are expected to provide insight into approaches which might reduce dopamine loss in PD.
4.3 “Hemiparkinsonian” Rat Model
The most common rat model of PD is the “hemiparkinsonian rat”, which develops PD pathology by intracerebral injection of 6-hydroxydopamine, a neurotoxin that causes dopaminergic loss in the SN. Proteomic studies by DeIuliis et al. identified increased levels of α-enolase and β-actin in hemiparkinsonian rats relative to controls within the SN and striatum brain regions (De Iuliis et al. 2005). These two proteins have previously been implicated in AD where they were found to be oxidatively modified (Butterfield et al. 2006; Reed et al. 2008). α-Enolase is a metabolic enzyme involved in glycolysis, while β-actin is a structural cytoskeletal protein. It is possible that in addition to increases of these two proteins in PD, oxidation levels may also change.
Proteomic studies can also be used to identify proteins that change in expression after treatment of subjects with potential drug candidate compounds. For example, Valastro et al. treated hemiparkinsonian rats with L-DOPA or bromocriptine, a dopamine receptor agonist, and assessed the corresponding protein changes relative to controls (Valastro et al. 2007). Overall, striatal proteins from animals treated with either L-DOPA or bromocriptine that were changed were primarily involved in energy metabolism, structural synaptic plasticity, oxidative stress, and protein degradation (Valastro et al. 2007). These observations support the possibility that oxidative stress and impairments in the proteasome are key to PD pathogenesis. Subsequent detailed analysis identified five proteins with significant changes in L-DOPA-induced dyskinesia (LID)-associated animals; αβ-crystallin, a heat shock protein, and guandiacetate methyltransferase, a protein involved in creatine synthesis, were down-regulated in LID rats relative to non-dyskinetic and bromocriptine-treated rats (Valastro et al. 2007). γ-Enolase, a glycolytic enzyme, proteasome α-2 subunit, and vinculin, a protein involved in endothelial adherent junctions, were up-regulated in LID rats (Valastro et al. 2007). These findings provide insights into pathways that have not previously been associated with motor activity and may be useful in the development of new therapies for PD.
4.4 Axotomized Rat Models
Axotomy of the medial forebrain has been used to induce PD in rats (Venero et al. 1997), resulting in an ∼50% loss of dopaminergic neurons. 2D-PAGE MALDI-MS analyses identified increased expression in haptoglobin and transthyretin proteins and decreased ApoE in the cerebrospinal fluid (CSF) of axotomized rats (Rite et al. 2007). Haptoglobin is a hemoglobin-binding protein that also has antioxidant, antibacterial, and acute-phase response activity. Transthyretin, a thyroxine hormone-delivering protein, while shown to increase in this PD model (Rite et al. 2007) and in aging, was decreased in AD (Serot et al. 1997). ApoE is a major protein found in CSF and astrocytes in brain that is involved in lipid metabolism. Decreases in ApoE, as revealed by this PD proteomic study, suggest the possible role of ApoE in other neurodegenerative diseases in addition to AD.
4.5 In Vitro Dopamine Quinone-treated Mitochondria Isolated from Rat Brain Model
Dopamine oxidation products, such as dopamine quinone, cause mitochondrial dysfunction in rat brain or liver and are associated with dopaminergic neuronal loss(Berman and Hastings 1999); thus, this system provides another model with which to investigate PD pathogenesis. Recently, Van Laar et al. utilized 2D-DIGE proteomics to identify altered proteins isolated from rat brain mitochondria following in vitro treatment with dopamine quinone (Van Laar et al. 2008). Proteins that exhibited greater than 50% reduced expression level in dopamine quinone-treated mitochondria relative to controls, in both a Cys- and Lys-CyDye DIGE labeling scheme, include mitochondrial creatine kinase (MtCK), mitofilin, fumarylacetoacetate hydrolase domain containing 2A, voltage dependent anion channel 2, and glycerol-3-phosphate dehydrogenase (Van Laar et al. 2008). Western blot analysis validated the changes associated with MtCK and mitofilin in dopamine quinone-treated mitochondria. Reductions in the levels of other proteins included mortalin, the 75 kDa subunit of NADH dehydrogenase, and superoxide dismutase 2 (Van Laar et al. 2008). These proteins are involved in various mitochondrial functions including structural integrity, energy metabolism, antioxidant defense, and cellular transport. These perturbations to the mitochondria are consistent with other reports presented in this review and support the possibility that mitochondrial dysfunction is a key contributor to PD pathogenesis.
4.6 A30P and A53T α-synuclein Transgenic Drosophila Models
Drosophila melanogaster has recently been exploited in proteomics as a useful model for PD. In particular, Drosophila expressing the human A30P mutant α-synuclein have provided further evidence that mitochondrial- and cytoskeletal-related pathways are important in PD pathogenesis (Xun et al. 2007b). Of 1727 proteins that were identified across different disease stages (i.e., presymptomatic, early and advanced stages), altered expression levels of 49 proteins observed in an A30P transgenic α-synuclein Drosophila model (Xun et al. 2007b). Changes unique to presymptomatic and early disease stages in PD Drosophila were primarily associated with the cytoskeleton and mitochondria. Additional evidence for perturbations to these pathways also was obtained in proteomic studies that sampled seven ages spanning the entire lifespan of PD Drosophila (Xun et al. 2007a). Xun et al. also examined protein changes in the A53T Drosophila PD model that are specific to the presymptomatic stage (Xun et al. 2008). The expression levels of twenty-four proteins changed in A53T transgenic α-synuclein Drosophila and represented proteins with a variety of biological functions. Proteins such as heat shock protein 70 cognate 3, Mn superoxide dismutase and ATP-synthase were up-regulated in A53T Drosophila (Xun et al. 2008), supporting the possibility that oxidative stress, mitochondrial, energy metabolism and protein folding/degradation pathways are important in PD pathogenesis.
4.7 Wild-type α-synuclein Transgenic Caenorhabditis elegans Models
The importance of actin (or cytoskeletal-associated proteins) as a target in PD pathogenesis from the models discussed above was recently demonstrated by proteomic studies of Caenorhabditis elegans that overexpressed wild-type human α-synuclein protein (Ichibangase et al. 2008). In these studies employing fluorogenic derivatization LC-MS/MS, five proteins including, actin, ribosomal protein large subunit (rpl) 13, rpl 23, rpl 30, and rpl 43 had decreased expression in PD worms (Ichibangase et al. 2008). Moreover these findings support the utility of various animal models of PD and their ability to provide clues to commonality of molecular pathways across a variety of models of disease etiology.
5. Conclusions
There are several commonalities from the proteomic studies of models of AD and PD discussed in this review. Primarily, it important to note that proteomics can provide insightful and powerful information that can be used to further exploit specific pathways. For example, proteomics studies have identified a number of common proteins and/or functional categories that change in AD and/or PD model systems (Table 2). Table 2 provides a more comprehensive view of key biological pathways altered in AD and PD that extends upon a recent review by Zabel et al. that compared protein expression overlap in AD, PD, Huntington's disease, and amyotrophic lateral sclerosis (Zabel et al. 2008). Thus, these results have revealed specific pathways relevant to neurodegeneration and that should be further investigated to fully understand their role in human disease pathogenesis. A recurring theme of synaptic/axonal maintenance, metabolic, chaperone, and antioxidant protein variability occurs in the AD models described in this review. Similarly, metabolic, transport, stress response, synaptic integrity, and ubiquitin/proteasome pathways consistently were perturbed in PD models at the protein level.
Table 2. Compilation of key biological pathways and proteins that are altered in animal models of Alzheimer's and Parkinson's Diseases determined by proteomicsa.
| Biological Function/Protein(s) | AD | PD | ||
|---|---|---|---|---|
| Model(s) | References | Model(s) | References | |
| Amino Acid Synthesis | ||||
| D-3-phosphoglycerate dehydrogenase | GSKβ | Tilleman et al. 2002 | --- | --- |
| Aspartate aminotransferase | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Dimethylarginine dimethylaminohydrolase 1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Glutamate oxaloacetate transaminase 2 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Glutamine synthetase | Aβ (1-42) injected rat, GSKβ | Boyd-Kimball et al. 2005, Tilleman et al. 2002 | parkin KO mice, MPTP mice | Periquet et al. 2005, Jin et al. 2005, Diedrich et al. 2008 |
| Henna | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Serine racemase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Blood pH Regulation | ||||
| Carbonic anhydrase II | P301L tau | David et al. 2007 | A30P α-synuclein mice, parkin KO mice | Poon et al. 2005c, Periquet et al. 2005 |
| Cellular Transport | ||||
| ADP, ATP carrier protein | --- | --- | MPTP mice | Jin et al. 2005 |
| ATPase, H+ transporting, V1 subunit 5 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Calcium ATPase at 60A | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Complexin 1 | GSKβ | Tilleman et al. 2002 | MPTP mice | Diedrich et al. 2008 |
| Complexin 2 | --- | --- | MPTP mice | Jin et al. 2005, Diedrich et al. 2008 |
| Dynamin-1 | APP/Sw | Shin et al. 2004 | parkin KO mice | Periquet et al. 2005 |
| Ferritin heavy chain | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Ferritin 1 heavy chain homologue | --- | --- | A30P Drosophila | Xun et al. 2007a |
| G protein β-subunit 13F | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Globin 1 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Larval serum protein 2b | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Na+/K+ transporting ATPase α-1 chain | --- | --- | MPTP mice | Jin et al. 2005 |
| Na+/K+ transporting ATPase α-2 chain | --- | --- | MPTP mice | Jin et al. 2005 |
| Na+/K+ transporting ATPase α-3 chain | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Jin et al. 2005 |
| Neutral amino acid transporter A | --- | --- | MPTP mice | Jin et al. 2005 |
| Phosphate carrier protein | --- | --- | MPTP mice | Jin et al. 2005 |
| Rho GDP-dissociation inhibitor 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Septin 2 or 6 homologue | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Septin 5 | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2005 |
| Septin 7 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Septin 11 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Septin-like protein KIAA0202 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Serum albumin precursor | Swe/Lon | Sizova et al. 2007 | --- | --- |
| Sorting nexin 5 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Transferrin | P301L tau | David et al. 2007 | --- | --- |
| Vesicular fusion protein | GSKβ | Tilleman et al. 2002 | --- | --- |
| Cytoskeletal Structural Integrity | ||||
| 14-3-3ε | --- | --- | MPTP mice | Jin et al. 2005 |
| 14-3-3γ | --- | --- | MPTP mice | Diedrich et al. 2008 |
| 14-3-3ζ | Aβ (1-42) injected rat | Boyd-Kimball et al. 2005 | parkin KO mice, MPTP mice | Periquet et al. 2005, Jin et al. 2005 |
| α-internexin | GSKβ | Tilleman et al. 2002 | MPTP mice | Diedrich et al. 2008 |
| β-actin | P301L tau | David et al. 2007 | hemiparkinsonian rat, α-synuclein C. elegans, parkin KO mice, MPTP mice | Deluliius et al. 2005, Ichibangase et al. 2008, Periquet et al. 2005, Diedrich et al. 2008 |
| Actin related protein 2/3 complex, subunit 5-lik | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Adenyl cyclase-associated protein 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| ARP2/3 complex 20 kDa subunit | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Capping protein of actin filament | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Cofilin | --- | --- | MPTP mice | Jin et al. 2005, Diedrich et al. 2008 |
| Fascin | GSKβ | Tilleman et al. 2002 | --- | --- |
| Glial fibrillary acidic protein | APP/Sw, Swe/Lon | Shin et al. 2004, Sizova et al. 2007 | --- | --- |
| Histone H4 replacement | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Kinesin heavy chain | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Microtubule associated protein 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Muscle LIM protein at 60A | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Muscle specific protein 300b | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Myosin alkali light chain 1 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Paramyosin | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Profilin | SAMP8 | Poon et al. 2005a | --- | --- |
| Profilin II splice isoform IIB | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Similar to ARP1 actin-related protein 1 | --- | --- | MPTP mice | Jin et al. 2005 |
| Similar to myosin Vb | --- | --- | MPTP mice | Jin et al. 2005 |
| Spectrin α-chain | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Spectrin β-chain | --- | --- | MPTP mice | Jin et al. 2005 |
| Stathmin 1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Transketolase | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Troponin C | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Troponin T | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Tubulin chain 15/α | Aβ (1-42) injected rat | Boyd-Kimball et al. 2005 | --- | --- |
| Tubulin α-1 chain | GSKβ | Tilleman et al. 2002 | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Tubulin α-1a chain | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Tubulin α-2 chain | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Tubulin α-4 chain | P301L tau | David et al. 2007 | MPTP mice | Jin et al. 2005, Diedrich et al. 2008 |
| Tubulin β chain 1 | GSKβ | Tilleman et al. 2002 | --- | --- |
| Tubulin β chain A | Swedish human mutant transgenic rat | Wilson et al. 2005 | MPTP mice | Diedrich et al. 2008 |
| Tubulin β chain B | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Tubulin β-4 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Tubulin polymerization-promoting protein | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Upheld | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Vinculin | --- | --- | L-DOPA mice | Valastro et al. 2007 |
| Energy Metabolism | ||||
| α-enolase | APP/Sw, GSKβ, SAMP8 | Shin et al. 2004, Tilleman et al. 2002, Poon et al. 2005b | A30P α-synuclein mice; hermiparkinsonia n rat, MPTP mice | Poon et al. 2005c, DeIuliius et al. 2005, Diedrich et al. 2008 |
| γ-enolase | APP/Sw | Shin et al. 2004 | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Aconitase | APP/Sw | Shin et al. 2004 | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Aconitate hydratase | MPTP mice | Diedrich et al. 2008 | ||
| Aldolase 3 | SAMP8 | Poon et al. 2005a | --- | --- |
| Aldose reductase | GSKβ | Tilleman et al. 2002 | --- | --- |
| ATP synthase subunit D | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| ATP synthase α chain | APP/Sw | Shin et al. 2004 | in vitro dopamine quinone treated rats, parkin KO mice, A30P Drosophila, MPTP mice | Van Laar et al. 2008, Periquet et al. 2005, Xun et al. 2007a, Diedrich et al. 2008 |
| ATP synthase α chain, mitochondrial | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| ATP synthase β chain, mitochondrial | --- | --- | parkin KO mice, A53T Drosophila, A30P Drosophila | Periquet et al. 2005, Xun et al. 2008, Xun et al. 2007a |
| ATP synthase γ chain, mitochondrial | --- | --- | MPTP mice, A30P Drosophila | Jin et al. 2005, Xun et al. 2007a |
| ATP α | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Citrate synthase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Creatine kinase β chain | GSKβ | Tilleman et al. 2002 | MPTP mice | Jin et al. 2005 |
| Creatine kinase | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Cytochrome c, oxidase | --- | --- | MPTP mice | Jin et al. 2005 |
| Cytochrome c, oxidase subunit 5A | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Cytochrome c, oxidase subunit 6B1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Cytochrome c1 | --- | --- | MPTP mice | Jin et al. 2005 |
| Cytochrome P450 reductase | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Dihydrolipoadmide dehydrogenase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Fructose-bisphosphate aldolase | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Fructose-bisphosphate aldolase A | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Fructose-bisphosphate aldolase C | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Jin et al. 2005, Diedrich et al. 2008 |
| Glyceraldehyde-3-phosphate dehydrogenase | APP/Sw, Aβ (1-42) injected rat | Shin et al. 2004, Boyd-Kimball et al. 2005 | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Glycerol-3-phosphate dehydrogenase | --- | --- | L-DOPA mice, A53T Drosophila, MPTP mice | Valastro et al. 2007, Xun et al. 2008, Jin et al. 2005 |
| GTP:AMP phosphtransferase mitochondrial | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Hexokinase | --- | --- | MPTP mice | Jin et al. 2005 |
| Isocitrate dehydrogenase NADH cytoplasm | P301L tau, GSKβ | David et al. 2007, Tilleman et al. 2002 | --- | --- |
| Lactate dehydrogenase | SAMP8 | Poon et al. 2005b | parkin KO mice, A53T Drosphila | Periquet et al. 2005, Xun et al. 2008 |
| Malate dehydrogenase | APP/Sw | Shin et al. 2004 | parkin KO mice | Periquet et al. 2005 |
| Mitochondrial creatine kinase | SAMP8 | Poon et al. 2005b | --- | --- |
| NADH dehydrogenase | P301L tau | David et al. 2007 | --- | --- |
| NADH dehydrogenase 1 α subcomplex 8 | --- | --- | MPTP mice | Jin et al. 2005 |
| NADH dehydrogenase, 75 kDa subunit | --- | --- | in vitro dopamine quinone treated rats | Van Laar et al. 2008 |
| NADH dehydrogenase Fe-S protein 8 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| NADH ubiquinone oxidoreductase 23 kDa subunit | GSKβ | Tilleman et al. 2002 | --- | --- |
| NADH ubiquinone oxidoreductase 24 kDa subunit | GSKβ | Tilleman et al. 2002 | --- | --- |
| NADH ubiquinone oxidoreductase 39 kDa subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| NADH ubiquinone oxidoreductase 49 kDa subunit | GSKβ | Tilleman et al. 2002 | parkin KO mice | Periquet et al. 2005 |
| NADH ubiquinone oxidoreductase 51 kDa subunit | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| NADH ubiquinone oxidoreductase 75 kDa subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| NADH ubiquinone oxidoreductase B15 subunit | P301L tau | David et al. 2007 | ||
| Phosphofructokinase | --- | --- | A30P Drosophila, MPTP mice | Xun et al. 2007a, Diedrich et al. 2008 |
| Phosphoglucomutase | GSKβ | Tilleman et al. 2002 | --- | --- |
| Phosphoglycerate kinase 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Phosphoglycerate mutase 1 | P301L tau, Aβ (1-42) injected rat, Swedish mutant human transgenic rat | David et al. 2007, Boyd-Kimball et al. 2005, Wilson et al. 2005 | in vitro dopamine quinone treated rats, MPTP mice | Van Laar et al. 2008, Diedrich et al. 2008 |
| Phosphoglycerate mutase B | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Phospoglycerate kinase 1 | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Pyruvate dehydrogenase | Aβ (1-42) injected rat | Boyd-Kimball et al. 2005 | A30P α-synuclein mice | Poon et al. 2005c |
| Pyruvate dehydrogenase E1 component α subunit | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Jin et al. 2005, Diedrich et al. 2008 |
| Pyruvate dehydrogenase E1 component β subunit | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Pyruvate kinase | APP/Sw, Swe/Lon | Shin et al. 2004, Sizova et al. 2007 | parkin KO mice, A30P Drosophila, MPTP mice | Periquet et al. 2005, Xun et al. 2007a, Diedrich et al. 2008 |
| Pyruvate kinase 3 | --- | --- | MPTP mice | Jin et al. 2005 |
| Succinate dehydrogenase | GSKβ | Tilleman et al. 2002 | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Triosephosphate isomerase | --- | --- | MPTP mice | Jin et al. 2005 |
| Vacuolar ATP synthase subunit G1 | --- | --- | MPTP mice | Jin et al. 2005 |
| Vacuolar ATP synthase subunit β-brain isoform | GSKβ | Tilleman et al. 2002 | --- | --- |
| Vacuolar H+ ATPase E1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Energy Metabolism/Mitochondrial Function | ||||
| Acetyl-CoA acetyltransferase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Acetyl-CoA acetyltransferase, mitochondrial | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Mitochondrial import inner membrange translocase subunit TIM13 A | --- | --- | MPTP mice | Jin et al. 2005 |
| Succinyl-CoA ligase β-chain, mitochondrial | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Ubiquinol-cytochrone c reductase complex 11kDa protein, mitochondrial precursor | --- | --- | MPTP mice | Jin et al. 2005 |
| Ubiquinol-cytochrone c reductase complex core protein 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Ubiquinol-cytochrone c reductase complex core protein 2 | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Ubiquitin thiolesterase L1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Inflammation/Immune Response | ||||
| CD166 antigen precursor | --- | --- | MPTP mice | Jin et al. 2005 |
| Complement c1 q | Swe/Lon | Sizova et al. 2007 | --- | --- |
| Inducible nitric oxide synthase | --- | --- | MPTP mice | McLaughlin et al. 2006 |
| Low-affinity immunoglobulin ε FC receptor | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Macrophage migration inhibitory factor | --- | --- | MPTP mice | Jin et al. 2005 |
| Lipid Metabolism | ||||
| ACAT | Swe/Lon | Sizova et al. 2007 | --- | --- |
| Acyl carrier protein, mitochondrial precursor | --- | --- | MPTP mice | Jin et al. 2005 |
| Acyl-CoA dehydrogenase, very long chain specific | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Acyl-CoA oxidase 2, peroxisomal | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Acyl-CoA thioester hydrolase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Acyl-protein thioesterase 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Annexin 5 | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| ApoE precursor | Swe/Lon | Sizova et al. 2007 | axotomized rats | Rite et al. 2007 |
| Lysophospholipase 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Phosphatidylethanolamine-binding protein | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Propionyl CoA carboxylase α-chain | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Yippee interacting protein 2 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Mitochondrial Function | ||||
| Atp5b protein | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Dihydrolipoyl dehydrogenase, mitochondrial | --- | --- | MPTP mice | Jin et al. 2005 |
| DJ-1 | --- | --- | MPTP mice | Jin et al. 2005 |
| Mitochondrial matrix protein P1 precursor | GSKβ | Tilleman et al. 2002 | --- | --- |
| Mitochondrial precursor proteins import receptor | --- | --- | MPTP mice | Jin et al. 2005 |
| Mitofilin | --- | --- | in vitro dopamine quinone treated rats | Van Laar et al. 2008 |
| Voltage dependent anion channel 2 | --- | --- | in vitro dopamine quinone treated rats, MPTP mice | Van Laar et al. 2008, Jin et al. 2005, Diedrich et al. 2008 |
| Voltage dependent anion-selective channel protein 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Neurotransmission Related | ||||
| 4-Aminobutyrate aminotransferase, mitochondrial | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Comatose | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Excitatory amino acid transporter 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Gamma-aminobutyric acid | --- | --- | MPTP mice | Jin et al. 2005 |
| n-Synaptobrevin | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Ras opposite | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Sodium- and chloride-dependent | ||||
| GABA transporter 3 homolog | --- | --- | MPTP mice | Jin et al. 2005 |
| Tyrosine 3-hydroxylase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Oxidative Stress/Antioxidant Defense | ||||
| Antioxidant protein 2 | GSKβ | Tilleman et al. 2002 | --- | --- |
| Carbonyl reductase 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Glutathione-s-transferase | GSKβ | Tilleman et al. 2002 | parkin KO mice | Periquet et al. 2005 |
| Glutathione-s-transferase Mu 3 | --- | --- | MPTP mice | Jin et al. 2005 |
| Glyoxalase 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Haptoglobin | --- | --- | axotomized rats | Rite et al. 2007 |
| Peroxidase | MPTP mice | Diedrich et al. 2008 | ||
| Peroxiredoxin 1 | MPTP mice | Diedrich et al. 2008 | ||
| Peroxiredoxin 2 | SAMP8 | Poon et al. 2005a | MPTP mice | Diedrich et al. 2008 |
| Peroxiredoxin 2A | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Peroxiredoxin 2B | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Peroxiredoxin 3 | MPTP mice | Diedrich et al. 2008 | ||
| Peroxiredoxin 5 | P301L tau | David et al. 2007 | MPTP mice | Diedrich et al. 2008 |
| Peroxiredoxin 6 | Swe/Lon | Sizova et al. 2007 | MPTP mice | Diedrich et al. 2008 |
| PHGPx | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Superoxide dismutase 1 (Cu-Zn) | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Superoxide dismutase 2 (Mn) | --- | --- | in vitro dopamine quinone treated rats, A53T Drosphila | Van Laar et al. 2008, Xun et al. 2008 |
| Thioredoxin reductase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Thioredoxin-like protein 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Protein Biosynthesis | ||||
| 40S ribosomal protein SA | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Ribosomal protein S12 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Ribobsomal protein S17 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Ribosomal protein large subunits 13, 23, 30, and 43 | --- | --- | α-synuclein C. elegans | Ichibangase et al. 2008 |
| Ribosomal protein S3A | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Ribosomal protein small subunit 8 | --- | --- | A53T Drosophila | Xun et al. 2008 |
| Ribosomal proteins large subunits 6, 14, and 23A | --- | --- | A53T Drosophila | Xun et al. 2008 |
| Similar to 60S ribosomal protein L18a | --- | --- | MPTP mice | Jin et al. 2005 |
| Stab | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Threonyl tRNA synthetase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Tyrosyl tTNA synthetase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| RNA Processing/Transcription Related | ||||
| ATP-dependent RNA helicase DDX19, dead box protein | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Elongation factor 1 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Elongation factor 1 R48D | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Elongation factor 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Poly(rC)binding protein | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Probable ATP-dependent RNA helicase p47 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Similar to transcription elongation factor B polypeptide 3 | --- | --- | MPTP mice | Jin et al. 2005 |
| Transcriptional associated protein purine rich single stranded DNA binding protein α | GSKβ | Tilleman et al. 2002 | --- | --- |
| Zinc finger protein TZF-L | --- | --- | MPTP mice | Jin et al. 2005 |
| Signal Transduction | ||||
| 2′,3′-Cyclic nucleotide 3′-phosphodiesterase | --- | --- | MPTP mice | Jin et al. 2005 |
| Calbindin | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Calcium/calmodulin-dependent 3′,5′-cyclic nucleotide phosphodiesterase | --- | --- | MPTP mice | Jin et al. 2005 |
| Calcium/calmodulin-dependent protein kinase type II β chain | --- | --- | MPTP mice | Jin et al. 2005 |
| Calretinin | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| CaMK-II alpha subunit | Swe/Lon | Sizova et al. 2007 | --- | --- |
| CaMK-II of P11798 calcium/calmodulin dependent kinase type II α chain | --- | --- | MPTP mice | Jin et al. 2005 |
| cAMP/cGMP diesterase | --- | --- | MPTP mice | Jin et al. 2005 |
| Clathrin coat assembly protein AP50 | --- | --- | MPTP mice | Jin et al. 2005 |
| Dual specificity mitogen-actived protein kinase kinase 1 | GSKβ | Tilleman et al. 2002 | --- | --- |
| G protein, γ 2 subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| G protein, γ 12 subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| MAGUK p55 subfamily member 2 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| MAGUK p55 subfamily member 3 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| MAGUK p55 subfamily member 6 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Mitogen-activated protein kinase 1 | GSKβ | Tilleman et al. 2002 | --- | --- |
| Peptidylprolyl cis-trans isomerase | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Phosphatidyl inotisol transferase α isoform | GSKβ | Tilleman et al. 2002 | --- | --- |
| Protein kinase C and casine kinase substrate in neurons protein 1 | --- | --- | MPTP mice | Jin et al. 2005 |
| Protein kinase C, γ type | --- | --- | MPTP mice | Jin et al. 2005 |
| Protein phosphatase 2 regulatory subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| Protein TYPROTEIN tyrosine kinase 9-like | --- | --- | MPTP mice | Jin et al. 2005 |
| Protein tyrosine phosphatase, non receptor type 1-1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Proto-oncogene C-crk | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Purkinje cell protein 4 | --- | --- | MPTP mice | Skold et al. 2006 |
| Ras-related C3 botulinum toxin substrate 1 | --- | --- | NADH ubiquinone oxidoreductase 49 kDa subunit | |
| Receptor of activated protein kinase C1 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Ser/Thr protein phosphatase 1 catalytic γ subunit | GSKβ | Tilleman et al. 2002 | --- | --- |
| Ser/Thr protein phosphatase 2B catalytic subunit, α isoform | GSKβ | Tilleman et al. 2002 | parkin KO mice | Periquet et al. 2005 |
| Ser/Thr protein phosphatase PP1-β catalytic subunit | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Ser/Thr protein phosphatase PP1-γ catalytic subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| Similar to peptidyl-prolyl cis-trans isomerase | --- | --- | MPTP mice | Jin et al. 2005 |
| Sumo-1 activating enzyme subunit 2 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| β-Adrenergic receptor kinase 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Stress Related Proteins/Chaperones | ||||
| αβ-crystallin | --- | --- | L-DOPA mice | Valastro et al. 2007 |
| Aconitate hydratase, mitochondrial | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Calnexin 99A | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Glucose-regulated protein 78 kDa | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Heat shock 70-related protein APG-1 | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Heat shock cognate 71 kDa protein | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Heat shock cognate 73 | --- | --- | MPTP mice | Jin et al. 2005 |
| Heat shock protein 1 | MPTP mice | Diedrich et al. 2008 | ||
| Heat shock protein 60 | Aβ (1-42) injected rat | Boyd-Kimball et al. 2005 | MPTP mice | Diedrich et al. 2008 |
| Heat shock protein 70 | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Heat shock protein 90b | GSKβ | Tilleman et al. 2002 | --- | --- |
| Heat shock protein cognate 3 | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Heat shock related 70 kDa protein 2 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Mortalin (mtHSP70, GRP75) | ApoE4 | Osorio et al. 2007 | in vitro dopamine quinone treated rats | Van Laar et al. 2008 |
| Rtn11 | A53T Drosphila | Xun et al. 2008 | ||
| Stress protein 70 | P301L tau | David et al. 2007 | parkin KO mice | Periquet et al. 2005 |
| T-complex protein 1 ε subunit | GSKβ | Tilleman et al. 2002 | MPTP mice | Jin et al. 2005 |
| T-complex protein 1 θ and β subunits | GSKβ | Tilleman et al. 2002 | --- | --- |
| T-complex protein 1, α-subunit B | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Transgelin-3 | P301L tau | David et al. 2007 | MPTP mice | Diedrich et al. 2008 |
| Transthyretin | --- | --- | axotomized rat | Rite et al. 2007 |
| Synaptic/Axonal Integrity | ||||
| Clipin E/coronin 6 type C | --- | --- | MPTP mice | Jin et al. 2005 |
| Coronin 1a | SAMP8 | Poon et al. 2005a | --- | --- |
| Coronin like protein P57 fragment | GSKβ | Tilleman et al. 2002 | MPTP mice | Jin et al. 2005 |
| Dihydropyrimidase-like 2 | APP/Sw, Swe/Lon, P301L tau, GSKβ, SAMP8 | Shin et al. 2004, Sizova et al. 2007, David et al. 2007, Tilleman et al., 2002, Poon et al. 2005b | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Dihydropyrimidase-like 5 | P301L tau | David et al. 2007 | MPTP mice | Jin et al. 2005, Diedrich et al. 2008 |
| Micotubule-associated protein 1B | MPTP mice | Diedrich et al. 2008 | ||
| Myelin basic protein | P301L tau | David et al. 2007 | MPTP mice | Jin et al. 2005 |
| N-ethylmaleimide sensitive fusion protein | Swe/Lon | Sizova et al. 2007 | parkin KO mice | Periquet et al. 2005 |
| Neurofascin precursor | --- | --- | MPTP mice | Jin et al. 2005 |
| Neurofilament triplet L protein | SAMP8, GSKβ | Poon et al. 2005b, Tilleman et al. 2002 | MPTP mice | Diedrich et al. 2008 |
| Neurofilament M | MPTP mice | Diedrich et al. 2008 | ||
| Stoned | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Synapsin-2 | P301L tau | David et al. 2007 | MPTP mice | Diedrich et al. 2008 |
| Synaptonal associated protein | GSKβ | Tilleman et al. 2002 | --- | --- |
| Synaptophysin | --- | --- | MPTP mice | Jin et al. 2005 |
| Synaptotagamin I | Swe/Lon | Sizova et al. 2007 | ||
| Syntaxin 1B | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Syntaxin-binding protein 1 | --- | --- | parkin KO mice, MPTP mice | Periquet et al. 2005, Jin et al. 2005 |
| Ubiquitin/Proteaosome Degradation | --- | --- | ||
| 26S protease regulatory subunit 4 | --- | --- | MPTP mice | Jin et al. 2005 |
| α-synuclein | MPTP mice | Diedrich et al. 2008 | ||
| Deubiquitinating enzyme OTUB1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| NEDD-4 | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Proteasome complex activator subunit 2 | APP/Sw | Shin et al. 2004 | --- | --- |
| Proteasome subunit α-2 | --- | --- | L-DOPA mice | Valastro et al. 2007 |
| Proteasome subunit α-3 | P301L tau | David et al. 2007 | --- | --- |
| Proteasome subunit β type 5 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Ubiquitin activating enzyme 1 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Ubiquitin carboxyterminal hydrolase L1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Ubiquitin-like protein Nedd8 | MPTP mice | Diedrich et al. 2008 | ||
| Vasopressin activated calcium mobilizing receptor (CUL5) | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Others | ||||
| 3-oxoacid CoA transferase 1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| 11 days embryo cDNA | --- | --- | MPTP mice | Jin et al. 2005 |
| α-1-antitrypsin 1-5 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| β-synuclein | Aβ (1-42) injected rat | Boyd-Kimball et al. 2005 | MPTP mice | Diedrich et al. 2008 |
| ADAM22 | --- | --- | MPTP mice | Jin et al. 2005 |
| ADAM23 | --- | --- | MPTP mice | Jin et al. 2005 |
| Adapter-related protein complex 2 α1 subunit | --- | --- | --- | --- |
| Adenine phosphoribosyltransferase | Swedish human mutant transgenic rat | Wilson et al. 2005 | --- | --- |
| Adenosine | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Adenosylhomocysteinase at 13 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Adenylosuccinate synthetase | --- | --- | MPTP mice | Jin et al. 2005 |
| Adenyl cyclase-associated protein 1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| ADP-ribosylarigine hydrolase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Alpha adducing | --- | --- | MPTP mice | Jin et al. 2005 |
| Amyloid β A4 protein precursor | --- | --- | MPTP mice | Jin et al. 2005 |
| Astrocytic phosphoprotein PEA-15 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Bent | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Chaoptic | --- | --- | A53T Drosphila, A30P Drosophila | Xun et al. 2008, Xun et al. 2007a |
| Cheerio | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Chickadee | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Claudin-11 | --- | --- | MPTP mice | Jin et al. 2005 |
| Cleavage and polyadenylation specificity factor, 100 kDa subunit | --- | --- | MPTP mice | Jin et al. 2005 |
| D-dopachrome tautomerase | --- | --- | MPTP mice | Jin et al. 2005 |
| Diphenol oxidase 2 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Discs large homolog 1 | --- | --- | MPTP mice | Jin et al. 2005 |
| DnaJ homolog subfamily A member 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Failed axon connections | --- | --- | A53T Drosophila | Xun et al. 2008 |
| Fat body protein 1 | --- | --- | A53T Drosophila, A30P Drosophila | Xun et al. 2008, Xun et al. 2007a |
| Fatty acid binding protein | MPTP mice | Diedrich et al. 2008 | ||
| Frizzled 7 precursor | --- | --- | MPTP mice | Jin et al. 2005 |
| Glutactin | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Growth factor receptor bound protein 2 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Guanine nucleotide-binding protein β-subunit 1 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Hemoglobin α | GSKβ | Tilleman et al. 2002 | --- | --- |
| Heparin sulfate N-deacetylase/N-sulfotransferase | --- | --- | MPTP mice | Jin et al. 2005 |
| HLA-B-asssociated transcript 1a | --- | --- | MPTP mice | Jin et al. 2005 |
| Hypothetical serine-rich containing protein | --- | --- | MPTP mice | Jin et al. 2005 |
| Hypoxanthine guanine phosphoribosyl transferase | GSKβ | Tilleman et al. 2002 | --- | --- |
| Karst | --- | --- | A53T Drosophila | Xun et al. 2008 |
| Lactoylglutathione lyase | --- | --- | MPTP mice | Jin et al. 2005 |
| MKIAA0968 protein, splice isoform α | --- | --- | MPTP mice | Jin et al. 2005 |
| Methylcrotonyl-CoA carboxlase β-chain | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Nectin-like protein 1 | --- | --- | MPTP mice | Jin et al. 2005 |
| Nucleoside diphosphate kinase A | GSKβ | Tilleman et al. 2002 | --- | --- |
| Nucleoside diphosphate kinase 2 | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Odorant binding protein 44a | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Odorant binding protein 99 | --- | --- | A53T Drosophila | Xun et al. 2008 |
| Peanut-like protein | GSKβ | Tilleman et al. 2002 | --- | --- |
| Protein CGI-51 homolog | --- | --- | MPTP mice | Jin et al. 2005 |
| Pugilista | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Punch | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Purple | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Q8R3V5 SH3 domain GRB2-like protein B2 | --- | --- | MPTP mice | Jin et al. 2005 |
| Retinal degeneration A | --- | --- | A53T Drosphila | Xun et al. 2008 |
| Retinin | --- | --- | A53T Drosophila, A30P Drosophila | Xun et al. 2008, Xun et al. 2007a |
| Ribose-phosphate pyrophosokinase 1 | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Rpt1 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Serine (or cysteine) proteinase inhibitor, clade A, member 1e | MPTP mice | Diedrich et al. 2008 | ||
| Serum albumin | GSKβ | Tilleman et al. 2002 | parkin KO mice, MPTP mice | Periquet et al. 2005, Diedrich et al. 2008 |
| Similar to CGI-49 | --- | --- | MPTP mice | Jin et al. 2005 |
| Small nuclear ribonucleoprotein polypeptide F | --- | --- | MPTP mice | Diedrich et al. 2008 |
| Spermidine synthase | --- | --- | parkin KO mice | Periquet et al. 2005 |
| Splice isoform 1 of P60202 myelin proteolipid protein | --- | --- | MPTP mice | Jin et al. 2005 |
| Splice isoform 3 of Q9EPR4 solute carrier family 23, member 2 | --- | --- | MPTP mice | Jin et al. 2005 |
| TER94 | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Transformation sensitive protein IEF SSP 3521 | GSKβ | Tilleman et al. 2002 | --- | --- |
| Trehalose-6-phosphate synthase 1b | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Tropomyosin | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Uncharacterized hematopoietic stem/progenitor cells protein MDS029 homolog | --- | --- | MPTP mice | Jin et al. 2005 |
| Walrus | --- | --- | A30P Drosophila | Xun et al. 2007a |
| Valosin-containing protein | --- | --- | MPTP mice | Diedrich et al. 2008 |
It should be noted that proteins listed in this table were grouped according to the text and tables found within the corresponding references (including Zabel et al. 2008). Bolded proteins were identified in proteomics studies of both AD and PD models. Refer to references for a complete list of proteins identified in individual studies.
Overall, these findings are consistent with hypotheses that mechanisms for energy production, protection from oxidative damage and improper protein clearance, and synapse integrity are disrupted and contribute to AD and PD pathogenesis. Animal models that take advantage of known genetic mutations in both familial and sporadic forms of AD and PD have shed light on important physiological processes that may be implicated in disease pathogenesis. Additional studies of this nature, that utilize proteomics as a tool to understand disease etiology, will help in the development of treatments to slow or prevent these devasting neurodegenerative disorders.
Acknowledgments
This work was supported in part by NIH grants to D.A.B. [AG-10836-AG-05119].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual for Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bajo M, Yoo BC, Cairns N, Gratzer M, Lubec G. Neurofilament proteins NF-L, NF-M and NF-H in brain of patients with Down syndrome and Alzheimer's disease. Amino Acids. 2001;21(3):293–301. doi: 10.1007/s007260170015. [DOI] [PubMed] [Google Scholar]
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J Neurochem. 1999;73(3):1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Bird TD, Lampe TH, Nemens EJ, Miner GW, Sumi SM, Schellenberg GD. Familial Alzheimer's disease in American descendants of the Volga Germans: probable genetic founder effect. Ann Neurol. 1988;23(1):25–31. doi: 10.1002/ana.410230106. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM, Jr, Klein JB, Ferguson J, Link CD, Butterfield DA. Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Abeta(1-42): implications for Alzheimer's disease. Neurobiol Aging. 2006;27(9):1239–1249. doi: 10.1016/j.neurobiolaging.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB, Butterfield DA. Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid beta-peptide (1-42) into rat brain: implications for Alzheimer's disease. Neuroscience. 2005;132(2):313–324. doi: 10.1016/j.neuroscience.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brady ST. Motor neurons and neurofilaments in sickness and in health. Cell. 1993;73(1):1–3. doi: 10.1016/0092-8674(93)90151-f. [DOI] [PubMed] [Google Scholar]
- Breslow JL, Zannis VI, SanGiacomo TR, Third JL, Tracy T, Glueck CJ. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J Lipid Res. 1982;23(8):1224–1235. [PubMed] [Google Scholar]
- Bulliard C, Zurbriggen R, Tornare J, Faty M, Dastoor Z, Dreyer JL. Purification of a dichlorophenol-indophenol oxidoreductase from rat and bovine synaptic membranes: tight complex association of a glyceraldehyde-3-phosphate dehydrogenase isoform, TOAD64, enolase-gamma and aldolase C. Biochem J. 1997;324(Pt 2):555–563. doi: 10.1042/bj3240555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA. Proteomics: a new approach to investigate oxidative stress in Alzheimer's disease brain. Brain Res. 2004;1000(12):1–7. doi: 10.1016/j.brainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7(12):548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, Martins RN. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer's disease: an initial assessment. J Alzheimers Dis. 2006;10(4):391–397. doi: 10.3233/jad-2006-10407. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, Bummer PM, Haley BE, Carney JM. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer's disease. J Neurochem. 1997;68(6):2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer's disease. Exp Gerontol. 2005;40(10):774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43(5):658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer's disease. Neuroscience. 1999;91(3):831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363(9415):1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002a;33(4):562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002b;82(6):1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Rajagopalan S, Butterfield DA, Andersen JK. In vitro and in vivo neuroprotection by gamma-glutamylcysteine ethyl ester against MPTP: relevance to the role of glutathione in Parkinson's disease. Neurosci Lett. 2006;402(12):137–141. doi: 10.1016/j.neulet.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- David DC, Ittner LM, Gehrig P, Nergenau D, Shepherd C, Halliday G, Gotz J. Beta-amyloid treatment of two complementary P301L tau-expressing Alzheimer's disease models reveals similar deregulated cellular processes. Proteomics. 2006;6(24):6566–6577. doi: 10.1002/pmic.200600634. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Neuroprotective and neurorestorative strategies for Parkinson's disease. Nat Neurosci. 2002;5 Suppl:1058–1061. doi: 10.1038/nn941. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991;10(13):4097–4104. doi: 10.1002/j.1460-2075.1991.tb04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Iuliis A, Grigoletto J, Recchia A, Giusti P, Arslan P. A proteomic approach in the study of an animal model of Parkinson's disease. Clin Chim Acta. 2005;357(2):202–209. doi: 10.1016/j.cccn.2005.03.028. [DOI] [PubMed] [Google Scholar]
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21–23. [PubMed] [Google Scholar]
- Diedrich M, Mao L, Bernreuther C, Zabel C, Nebrich G, Kleene R, Klose J. Proteome analysis of ventral midbrain in MPTP-treated normal and L1cam transgenic mice. Proteomics. 2008;8(6):1266–1275. doi: 10.1002/pmic.200700754. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci. 2004;117(Pt 10):1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84(5):1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- Farrer M, Stone J, Mata IF, Lincoln S, Kachergus J, Hulihan M, Strain KJ, Maraganore DM. LRRK2 mutations in Parkinson disease. Neurology. 2005;65(5):738–740. doi: 10.1212/01.wnl.0000169023.51764.b0. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22(1):1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126(4):775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Sonsalla PK. The use of the MPTP-treated mouse as an animal model of parkinsonism. Can J Neurol Sci. 1987;14(3 Suppl):436–440. doi: 10.1017/s0317167100037860. [DOI] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65(5):2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987;84(10):3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S49–58. doi: 10.1002/ana.10481. discussion S58-60. [DOI] [PubMed] [Google Scholar]
- Ichibangase T, Saimaru H, Takamura N, Kuwahara T, Koyama A, Iwatsubo T, Imai K. Proteomics of Caenorhabditis elegans over-expressing human alpha-synuclein analyzed by fluorogenic derivatization-liquid chromatography/tandem mass spectrometry: identification of actin and several ribosomal proteins as negative markers at early Parkinson's disease stages. Biomed Chromatogr. 2008;22(3):232–234. doi: 10.1002/bmc.931. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Shumiya S, Kawamura H. Age-related changes in radial-arm maze learning and basal forebrain cholinergic systems in senescence accelerated mice (SAM) Behav Brain Res. 1992;51(1):15–22. doi: 10.1016/s0166-4328(05)80307-9. [DOI] [PubMed] [Google Scholar]
- Jin J, Meredith GE, Chen L, Zhou Y, Xu J, Shie FS, Lockhart P, Zhang J. Quantitative proteomic analysis of mitochondrial proteins: relevance to Lewy body formation and Parkinson's disease. Brain Res Mol Brain Res. 2005;134(1):119–138. doi: 10.1016/j.molbrainres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Shvedova A, Serbinova E, Khan S, Swanson C, Powell R, Packer L. Dihydrolipoic acid--a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem Pharmacol. 1992;44(8):1637–1649. doi: 10.1016/0006-2952(92)90482-x. [DOI] [PubMed] [Google Scholar]
- Kaul SC, Taira K, Pereira-Smith OM, Wadhwa R. Mortalin: present and prospective. Exp Gerontol. 2002;37(1011):1157–1164. doi: 10.1016/s0531-5565(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Kekelidze T, Khait I, Togliatti A, Benzecry JM, Wieringa B, Holtzman D. Altered brain phosphocreatine and ATP regulation when mitochondrial creatine kinase is absent. J Neurosci Res. 2001;66(5):866–872. doi: 10.1002/jnr.10060. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Klose J, Kobalz U. Two-dimensional electrophoresis of proteins: an updated protocol and implications for a functional analysis of the genome. Electrophoresis. 1995;16(6):1034–1059. doi: 10.1002/elps.11501601175. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1-42. J Neurochem. 2001;78(2):413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lees AJ. When did Ray Kennedy's Parkinson's disease begin? Mov Disord. 1992;7(2):110–116. doi: 10.1002/mds.870070203. [DOI] [PubMed] [Google Scholar]
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson's disease. Nature. 1998;395(6701):451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Lesage S, Leutenegger AL, Ibanez P, Janin S, Lohmann E, Durr A, Brice A. LRRK2 haplotype analyses in European and North African families with Parkinson disease: a common founder for the G2019S mutation dating from the 13th century. Am J Hum Genet. 2005;77(2):330–332. doi: 10.1086/432422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340(25):1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- McLaughlin P, Zhou Y, Ma T, Liu J, Zhang W, Hong JS, Kovacs M, Zhang J. Proteomic analysis of microglial contribution to mouse strain-dependent dopaminergic neurotoxicity. Glia. 2006;53(6):567–582. doi: 10.1002/glia.20294. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Flood JF. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiol Learn Mem. 2002;78(1):125–138. doi: 10.1006/nlme.2001.4047. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson's disease. N Engl J Med. 2005;353(10):1021–1027. doi: 10.1056/NEJMcp043908. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Mizukawa K, Hirose Y, Kajita S, Ohara S, Watanabe Y. MPTP-induced parkinsonian model in mice: biochemistry, pharmacology and behavior. Eur Neurol. 1987;26 Suppl 1:16–23. doi: 10.1159/000116351. [DOI] [PubMed] [Google Scholar]
- Osorio C, Sullivan PM, He DN, Mace BE, Ervin JF, Strittmatter WJ, Alzate O. Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiol Aging. 2007;28(12):1853–1862. doi: 10.1016/j.neurobiolaging.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol. 1995;50(1):123–126. doi: 10.1016/0006-2952(95)00116-h. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44(4):595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Paolini M, Sapone A, Gonzalez FJ. Parkinson's disease, pesticides and individual vulnerability. Trends Pharmacol Sci. 2004;25(3):124–129. doi: 10.1016/j.tips.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Fountoulakis M, Dunckley T, Stephan DA, Reiman EM. Genetics, transcriptomics, and proteomics of Alzheimer's disease. J Clin Psychiatry. 2006;67(4):652–670. doi: 10.4088/jcp.v67n0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periquet M, Corti O, Jacquier S, Brice A. Proteomic analysis of parkin knockout mice: alterations in energy metabolism, protein handling and synaptic function. J Neurochem. 2005;95(5):1259–1276. doi: 10.1111/j.1471-4159.2005.03442.x. [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20(18):3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience. 2004a;126(4):915–926. doi: 10.1016/j.neuroscience.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Poon HF, Farr SA, Banks WA, Pierce WM, Klein JB, Morley JE, Butterfield DA. Proteomic identification of less oxidized brain proteins in aged senescence-accelerated mice following administration of antisense oligonucleotide directed at the Abeta region of amyloid precursor protein. Brain Res Mol Brain Res. 2005a;138(1):8–16. doi: 10.1016/j.molbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Poon HF, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Proteomic analysis of specific brain proteins in aged SAMP8 mice treated with alpha-lipoic acid: implications for aging and age-related neurodegenerative disorders. Neurochem Int. 2005b;46(2):159–168. doi: 10.1016/j.neuint.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice--a model of familial Parkinson's disease. Neurobiol Dis. 2005c;18(3):492–498. doi: 10.1016/j.nbd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, Butterfield DA. Antisense directed at the Abeta region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res. 2004b;1018(1):86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Putkey JA, Kleerekoper Q, Gaertner TR, Waxham MN. A new role for IQ motif proteins in regulating calmodulin function. J Biol Chem. 2003;278(50):49667–49670. doi: 10.1074/jbc.C300372200. [DOI] [PubMed] [Google Scholar]
- Rabilloud T. Two-dimensional gel electrophoresis in proteomics: old, old fashioned, but it still climbs up the mountains. Proteomics. 2002;2(1):3–10. [PubMed] [Google Scholar]
- Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson's disease. FASEB J. 2004;18(6):617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol Dis. 2008;30(1):107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, Shulman E, Steinberg G, Buttinger C, Sinaiko E, Borenstein J, de Leon MJ, Cohen J. Longitudinal course of normal aging and progressive dementia of the Alzheimer's type: a prospective study of 106 subjects over a 3.6 year mean interval. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10(35):571–578. doi: 10.1016/0278-5846(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rite I, Arguelles S, Venero JL, Garcia-Rodriguez S, Ayala A, Cano J, Machado A. Proteomic identification of biomarkers in the cerebrospinal fluid in a rat model of nigrostriatal dopaminergic degeneration. J Neurosci Res. 2007;85(16):3607–3618. doi: 10.1002/jnr.21452. [DOI] [PubMed] [Google Scholar]
- Rocchi A, Pellegrini S, Siciliano G, Murri L. Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res Bull. 2003;61(1):1–24. doi: 10.1016/s0361-9230(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Verity MA, Vinters HV, Shih CC, Shi L, Ji XD, Dong L, Shau H. Differential expression of peroxiredoxin subtypes in human brain cell types. J Neurosci Res. 1999;56(2):206–212. [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116(7):1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schonberger SJ, Edgar PF, Kydd R, Faull RL, Cooper GJ. Proteomic analysis of the brain in Alzheimer's disease: molecular phenotype of a complex disease process. Proteomics. 2001;1(12):1519–1528. doi: 10.1002/1615-9861(200111)1:12<1519::aid-prot1519>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sen CK, Roy S, Han D, Packer L. Regulation of cellular thiols in human lymphocytes by alpha-lipoic acid: a flow cytometric analysis. Free Radic Biol Med. 1997;22(7):1241–1257. doi: 10.1016/s0891-5849(96)00552-7. [DOI] [PubMed] [Google Scholar]
- Serot JM, Christmann D, Dubost T, Couturier M. Cerebrospinal fluid transthyretin: aging and late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63(4):506–508. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shin SJ, Lee SE, Boo JH, Kim M, Yoon YD, Kim SI, Mook-Jung I. Profiling proteins related to amyloid deposited brain of Tg2576 mice. Proteomics. 2004;4(11):3359–3368. doi: 10.1002/pmic.200400961. [DOI] [PubMed] [Google Scholar]
- Sizova D, Charbaut E, Delalande F, Poirier F, High AA, Parker F, Van Dorsselaer A, Duchesne M, Diu-Hercend A. Proteomic analysis of brain tissue from an Alzheimer's disease mouse model by two-dimensional difference gel electrophoresis. Neurobiol Aging. 2007;28(3):357–370. doi: 10.1016/j.neurobiolaging.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Skold K, Svensson M, Nilsson A, Zhang X, Nydahl K, Caprioli RM, Svenningsson P, Andren PE. Decreased striatal levels of PEP-19 following MPTP lesion in the mouse. J Proteome Res. 2006;5(2):262–269. doi: 10.1021/pr050281f. [DOI] [PubMed] [Google Scholar]
- Smolka MB, Zhou H, Purkayastha S, Aebersold R. Optimization of the isotope-coded affinity tag-labeling procedure for quantitative proteome analysis. Anal Biochem. 2001;297(1):25–31. doi: 10.1006/abio.2001.5318. [DOI] [PubMed] [Google Scholar]
- Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49(3):313–319. [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer's disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Tilleman K, Stevens I, Spittaels K, Haute CV, Clerens S, Van Den Bergh G, Geerts H, Van Leuven F, Vandesande F, Moens L. Differential expression of brain proteins in glycogen synthase kinase-3 transgenic mice: a proteomics point of view. Proteomics. 2002;2(1):94–104. [PubMed] [Google Scholar]
- Tonge R, Shaw J, Middleton B, Rowlinson R, Rayner S, Young J, Pognan F, Hawkins E, Currie I, Davison M. Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics. 2001;1(3):377–396. doi: 10.1002/1615-9861(200103)1:3<377::AID-PROT377>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Shiozaki A, Kohno R, Yoshizato K, Shimohama S. Proteomic profiling and neurodegeneration in Alzheimer's disease. Neurochem Res. 2002;27(10):1245–1253. doi: 10.1023/a:1020941929414. [DOI] [PubMed] [Google Scholar]
- Valastro B, Dekundy A, Krogh M, Lundblad M, James P, Danysz W, Quack G, Cenci MA. Proteomic analysis of striatal proteins in the rat model of L-DOPA-induced dyskinesia. J Neurochem. 2007;102(4):1395–1409. doi: 10.1111/j.1471-4159.2007.04655.x. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Laar VS, Dukes AA, Cascio M, Hastings TG. Proteomic analysis of rat brain mitochondria following exposure to dopamine quinone: implications for Parkinson disease. Neurobiol Dis. 2008;29(3):477–489. doi: 10.1016/j.nbd.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero JL, Revuelta M, Cano J, Machado A. Time course changes in the dopaminergic nigrostriatal system following transection of the medial forebrain bundle: detection of oxidatively modified proteins in substantia nigra. J Neurochem. 1997;68(6):2458–2468. doi: 10.1046/j.1471-4159.1997.68062458.x. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74(2):721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- von Coelln R, Dawson VL, Dawson TM. Parkin-associated Parkinson's disease. Cell Tissue Res. 2004;318(1):175–184. doi: 10.1007/s00441-004-0924-4. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Takano S, Kaur K, Aida S, Yaguchi T, Kaul Z, Hirano T, Taira K, Kaul SC. Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60. Biochem J. 2005;391(Pt 2):185–190. doi: 10.1042/BJ20050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KE, Marouga R, Prime JE, Pashby DP, Orange PR, Crosier S, Keith AB, Lathe R, Mullins J, Estibeiro P, Bergling H, Hawkins E, Morris CM. Comparative proteomic analysis using samples obtained with laser microdissection and saturation dye labelling. Proteomics. 2005;5(15):3851–3858. doi: 10.1002/pmic.200401255. [DOI] [PubMed] [Google Scholar]
- Wolters DA, Washburn MP, Yates JR., 3rd An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem. 2001;73(23):5683–5690. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- Wood DR, Nye JS, Lamb NJ, Fernandez A, Kitzmann M. Intracellular retention of caveolin 1 in presenilin-deficient cells. J Biol Chem. 2005;280(8):6663–6668. doi: 10.1074/jbc.M410332200. [DOI] [PubMed] [Google Scholar]
- Wu JT, Lin HC, Hu YC, Chien CT. Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat Cell Biol. 2005;7(10):1014–1020. doi: 10.1038/ncb1301. [DOI] [PubMed] [Google Scholar]
- Xun Z, Sowell RA, Kaufman TC, Clemmer DE. Lifetime proteomic profiling of an A30P alpha-synuclein Drosophila model of Parkinson's disease. J Proteome Res. 2007a;6(9):3729–3738. doi: 10.1021/pr0700504. [DOI] [PubMed] [Google Scholar]
- Xun Z, Sowell RA, Kaufman TC, Clemmer DE. Protein expression in a Drosophila model of Parkinson's disease. J Proteome Res. 2007b;6(1):348–357. doi: 10.1021/pr060488o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun Z, Sowell RA, Kaufman TC, Clemmer DE. Quantitative proteomics of a presymptomatic A53T a-synuclein drosophila model of Parkinson's disease. Mol Cell Proteomics. 2008 doi: 10.1074/mcp.M700467-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel C, Andreew A, Mao L, Hartl D. Protein expression overlap: more important than which proteins change in expression? Expert Rev Proteomics. 2008;5(2):187–205. doi: 10.1586/14789450.5.2.187. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97(24):13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]