Abstract
We have identified the novel protein Glycine max PEROXISOMAL ADENINE NUCLEOTIDE CARRIER (Gm PNC1) by proteomic analyses of peroxisomal membrane proteins using a blue native/SDS-PAGE technique combined with peptide mass fingerprinting. Gm PNC1, and the Arabidopsis thaliana orthologs At PNC1 and At PNC2, were targeted to peroxisomes. Functional integration of Gm PNC1 and At PNC2 into the cytoplasmic membranes of intact Escherichia coli cells revealed ATP and ADP import activities. The amount of Gm PNC1 in cotyledons increased until 5 d after germination under constant darkness and then decreased very rapidly in response to illumination. We investigated the physiological functions of PNC1 in peroxisomal metabolism by analyzing a transgenic Arabidopsis plant in which At PNC1 and At PNC2 expression was suppressed using RNA interference. The pnc1/2i mutant required sucrose for germination and suppressed the degradation of storage lipids during postgerminative growth. These results suggest that PNC1 contributes to the transport of adenine nucleotides that are consumed by reactions that generate acyl-CoA for peroxisomal fatty acid β-oxidation during postgerminative growth.
INTRODUCTION
Peroxisomes are single membrane-bound organelles that are ubiquitously found in eukaryotic cells. Plant peroxisomes play important roles in a variety of metabolic reactions (Baker et al., 2006; Hayashi and Nishimura, 2006). During postgerminative growth of seedlings, fatty acids released from triacylglycerols that are stored in the lipid bodies of seeds are metabolized to produce sucrose. Conversion of these fatty acids to succinate takes place in peroxisomes, namely glyoxysomes, via fatty acid β-oxidation and the glyoxylate cycle. In addition to fatty acids, peroxisomal β-oxidation plays a role in a number of pathways, including part of jasmonic acid biosynthesis, and the degradation of branched amino acids (Koo et al., 2006; Zolman et al., 2001a). Peroxisomes therefore need to exchange a variety of metabolites with other organelles through their membrane.
Adenine nucleotide transport activity though peroxisomal membranes has been demonstrated by the reconstitution of Sc Ant1p (for Saccharomyces cerevisiae adenine nucleotide transporter 1) in liposomes by Palimeri et al. (2001), who suggested that the physiological role of Sc Ant1p is probably to transport cytoplasmic ATP into the peroxisomal lumen in exchange for the AMP generated in the activation of fatty acids. Homo sapiens PMP34 is a functional ortholog of Sc Ant1p (Visser et al., 2002). Candida boidinii PMP47 is involved in the transport of a small molecule (possibly ATP) required for the conversion of lauric acid to its CoA form in peroxisomes (Nakagawa et al., 2000). In plants, At PMP38 has been suggested in Arabidopsis thaliana to act as a putative ATP/ADP carrier protein that shows similarities to Hs PMP34 and Cb PMP47, which are known homologs of mitochondrial ATP/ADP carrier proteins (Fukao et al., 2001). However, the transport activities of At PMP38 have not been examined.
Only two proteins, the voltage-dependent anion-selective channel and Arabidopsis PED3, have been reported to act as potential peroxisomal membrane transporters. The activities of voltage-dependent anion-selective channels were detected in boundary peroxisomal membranes using isolated peroxisomes from spinach (Spinacia oleracea) leaves and castor seed (Ricinus communis) endosperms (Reumann et al., 1995, 1997, 1998). We have also identified voltage-dependent anion-selective channel proteins in peroxisomal membranes from soybean (Glycine max) cotyledons (Arai et al., 2008). At PED3, also known as PXA1 or CTS, has the typical characteristic of a full-size ATP binding cassette transporter (Zolman et al., 2001b; Footitt et al., 2002; Hayashi et al., 2002a). At PED3 may contribute to the transport of fatty acids and their derivatives (Hayashi et al., 2002a). Fatty acids imported into peroxisomes are esterified by acyl-CoA synthetases inside peroxisomes where they are then catabolized by fatty acid β-oxidation. Therefore, the ped3 mutant requires sucrose for postgerminative growth (Zolman et al., 2001b; Footitt et al., 2002; Hayashi et al., 2002a). It is likely that metabolites are transported through the peroxisomal membrane not only by these two proteins but also by unknown proteins. Identification of these unknown peroxisomal membrane proteins is important to understand the physiological functions of peroxisomes.
Peroxisomal membrane proteins are synthesized in the cytosol before being targeted directly to peroxisomes or traveling to the peroxisomes via the endoplasmic reticulum (ER) (Tabak et al., 2003; McCartney et al., 2005; Sparkes et al. 2005). The mechanisms responsible for the targeting of these proteins to the peroxisomal membrane are still poorly understood. Trafficking of peroxisomal membrane proteins to peroxisomes depends on the presence of cis-acting targeting signals, called mPTS (Dyer et al., 1996). The mPTS show great variability both in the identity and the number of requisite residues (Van Ael and Fransen, 2006). This makes predicting peroxisomal membrane proteins by searching for mPTS sequences in the Arabidopsis genome very difficult.
We used a proteomics approach to characterize peroxisomal proteins using etiolated Arabidopsis and soybean cotyledons and explored the unidentified functions of peroxisomes (Fukao et al., 2002, 2003; Arai et al., 2008). Proteins in the prepared peroxisomes were separated by two-dimensional (2-D) gel electrophoresis using isoelectric focusing and SDS-PAGE, before being identified by peptide mass fingerprinting (PMF) using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Soybean plants have large cotyledons that are suitable for isolating large amounts of highly purified peroxisomes. Moreover, the Dana Farber Cancer Institute (DFCI) Soybean Gene Index is available as a resource of nonredundant cDNA sequences consisting of ESTs from soybean. Using a combination of large cotyledons and the availability of rich sequence information, we succeeded in characterizing many matrix proteins from the soybean peroxisomes (Arai et al., 2008). However, we did not identify any membrane transporters except voltage-dependent anion-selective channel proteins. This limitation was mainly due to the difficulty in separating hydrophobic proteins, such as membrane proteins, by isoelectric focusing. Isoelectric focusing can be replaced with other separation techniques, such as blue native PAGE (BN-PAGE). BN-PAGE was first employed for the investigation of molecular mass, subunit composition, and the stoichiometry of protein complexes from bovine heart mitochondria (Schagger and von Jagow, 1991; Schagger et al., 1994). BN-PAGE, which appears to be a promising solution for the investigation of membrane proteins, was also used to separate membrane proteins in plants (Eubel et al., 2004).
Here, we report the identification of a G. max peroxisomal adenine nucleotide transporter by the proteomic analysis of peroxisomal membrane proteins. We measured the transport activity of the identified plant peroxisomal adenine nucleotide transporter and characterized an Arabidopsis knockdown mutant of this novel transporter. We show evidence that plant peroxisomal adenine nucleotide transporters are involved in transporting ATP and in reactions that generate acyl-CoA for peroxisomal fatty acid β-oxidation during postgerminative growth.
RESULTS
Separation of Peroxisomal Proteins
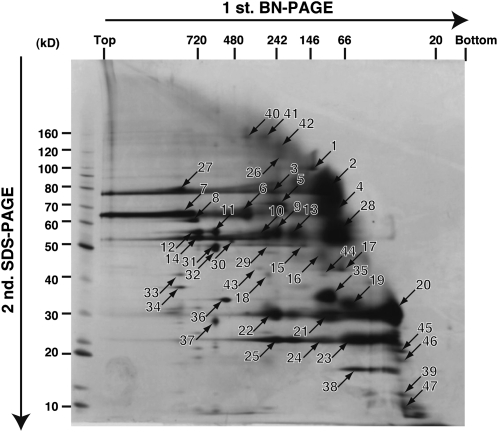
Peroxisomes were purified from etiolated soybean cotyledons by iodixanol density gradient centrifugation. Peroxisomal membrane proteins contained in the purified peroxisomes were enriched by dissolving the hydrophilic proteins in a sample buffer containing 50 mM NaCl. Hydrophobic proteins that were insoluble in this buffer were then resuspended in a sample buffer containing 1% digitonin. After centrifugation, the supernatant was used as a digitonin-soluble fraction containing peroxisomal membrane proteins. Following electrophoresis of the supernatant on a BN-PAGE gel, the BN gel lane was cut out and placed horizontally on an SDS-PAGE gel. After electrophoresis, the protein spots in the gel were detected by silver staining (Figure 1).
Figure 1.
2-D Map of Proteins in the Digitonin-Soluble Fraction of Purified Peroxisomes.
A 2-D gel in which the first dimension was BN-PAGE and the second was SDS-PAGE is shown. Protein spots (numbers 1 to 47) were detected by silver staining. The protein spots numbered 1 to 25 correspond to the numbers in Table 1. The numbers above and on the left of the 2-D gel indicate the molecular mass markers.
Assignment of Spots to Tentative Consensus Sequences Using PMF Analysis
We recovered 47 spots from the gel (Figure 1, spot numbers 1 to 47) and digested them with lysyl endopeptidase. A PMF of the digested peptides was obtained by MALDI-TOF MS. We were able to obtain PMFs for 39 proteins (Figure 1, spot numbers 1 to 39). We used the MASCOT program to search the DFCI Soybean Gene Index with these PMFs. This database consists of nonredundant soybean tentative consensus (TC) sequences that were created by assembling soybean ESTs (Quackenbush et al., 2001; Tsai et al., 2001; Lee et al., 2002; Pertea et al., 2003). Twenty-five (spot numbers 1 to 25) of the 39 proteins were assigned to 16 TC sequences by PMF analysis (Table 1). In some cases, more than one protein was assigned to a single TC sequence (Table 1). The remaining 14 proteins were not assigned to any TC sequence in the database (Figure 1, spot numbers 26 to 39).
Table 1.
Assignment of Spots to TC Sequences Using PMF Analysis
| MASCOTc
|
|||
|---|---|---|---|
| TC No.a | Spot No.b | Number of Matched Peptides | Score |
| TC205692 | 19 | 4 | 62 |
| TC225656 | 9, 13, 15, 16, 23, 24, 25 | 3, 3, 3, 3, 5, 5, 5 | 51, 54, 46, 48, 102, 83, 95 |
| TC208346 | 17 | 5 | 89 |
| TC205158 | 5, 9, 10, 13, 15, 20, 21, 22 | 3, 3, 3, 3, 3, 5, 4, 4 | 53, 62 46, 54, 45, 86, 72, 72 |
| TC210966 | 2 | 3 | 50 |
| TC230020 | 3 | 3 | 46 |
| TC216734 | 18 | 5 | 103 |
| TC216121 | 2 | 8 | 124 |
| TC204772 | 6 | 5 | 82 |
| TC204771 | 6 | 5 | 83 |
| TC225586 | 4, 7 | 7, 7 | 104, 120 |
| TC204238 | 14 | 3 | 39 |
| TC231525 | 12 | 4 | 70 |
| TC225495 | 1 | 10 | 178 |
| TC204724 | 11 | 4 | 77 |
| TC205132 | 8 | 6 | 87 |
Accession number in the DFCI Soybean Gene Index.
Spot number shown in Figure 1.
Number of matched peptides and score given by MASCOT software.
Characterization of Polypeptide Sequences Encoded by Assigned TC Sequences
To characterize the polypeptides encoded by the assigned 16 TC sequences, we searched for their orthologs in GenBank, Protein Information Resource, and Swiss-Prot databases. The polypeptide encoded by TC205692 was most similar to two Arabidopsis proteins of unknown function, At3g05290.1 (73.0% identity and 94.4% similarity) (Table 2) and At5g27520.1 (71.0% identity and 93.1% similarity). Four polypeptides were similar to peroxisomal membrane proteins previously characterized in plants other than soybean: peroxisome biogenesis factor 11 (PEX11), monodehydroascorbate reductase (MDAR), ascorbate peroxidase (pAPX), and long-chain acyl-CoA synthetase (LACS) (Table 2). Six polypeptides were identical to previously reported soybean peroxisomal matrix proteins (Table 2). One polypeptide was similar to a peroxisomal matrix protein previously characterized in castor bean (Table 2). The other polypeptides, TC231525, TC225495, TC204724, and TC205132, were assigned to soybean mitochondrial H(+)-transporting ATPase subunit 1, soybean cytosolic lipoxygenase-3, potato (Solanum tuberosum) mitochondrial processing peptidase, and pumpkin (Cucurbita sp) chaperonin CPN60-2 protein, respectively (Yenofsky et al., 1988; Tsugeki et al., 1992; Chanut et al., 1993; Emmermann and Schmitz, 1995). These polypeptides could be contaminants of the final peroxisomal membrane fraction.
Table 2.
Characterization of Polypeptides Encoded by TC Sequences by BLAST Searching
| Previously Characterized Soybean Protein or Closest Orthologc
|
||||
|---|---|---|---|---|
| TC No.a | Accession No.b | Protein Name | Taxonomy | Identity (%)d |
| Unknown protein | ||||
| TC205692 | AB442083 | At3g05290.1 | Arabidopsis | 73 |
| Peroxisomal protein | ||||
| Membrane | ||||
| TC225656 | AB442084 | Peroxisome biogenesis factor 11 | Arabidopsis | 78 |
| TC208346 | AB442087 | Monodehydroascorbate reductase | Arabidopsis | 68 |
| TC205158 | AB331961 | Ascorbate peroxidase | Soybean | 100 |
| TC210966 | AB442086 | Long chain acyl-CoA synthetase | Arabidopsis | 81 |
| Matrix | ||||
| TC230020 | Q945U3 | Acyl-CoA oxidase 1; 2 | Soybean | 100 |
| TC216734 | AB333786 | Acyl-CoA oxidase 4 | Soybean | 100 |
| TC216121 | AB333787 | Fatty acid multifunctional protein | Soybean | 100 |
| TC204772 | AB333789 | Isocitrate lyase 1 | Soybean | 100 |
| TC204771 | AB442085 | Isocitrate lyase 2 | Castor bean | 83 |
| TC225586 | L01629 | Malate synthase | Soybean | 99 |
| TC204238 | AB333792 | Catalase 4 | Soybean | 100 |
Accession number in the DFCI Soybean Gene Index.
Accession number in the National Center for Biotechnology Information database.
The previously characterized soybean protein or the closest ortholog of the corresponding TC sequence was identified in the GenBank, Protein Information Resource, and Swiss-Prot protein databases.
The percentage of identity between the polypeptide encoded by the TC sequence and the closest homolog was calculated by the BLAST algorithm.
Cloning of Full-Length cDNAs for TC205692 Protein, At3g05290.1 and At5g27520.1
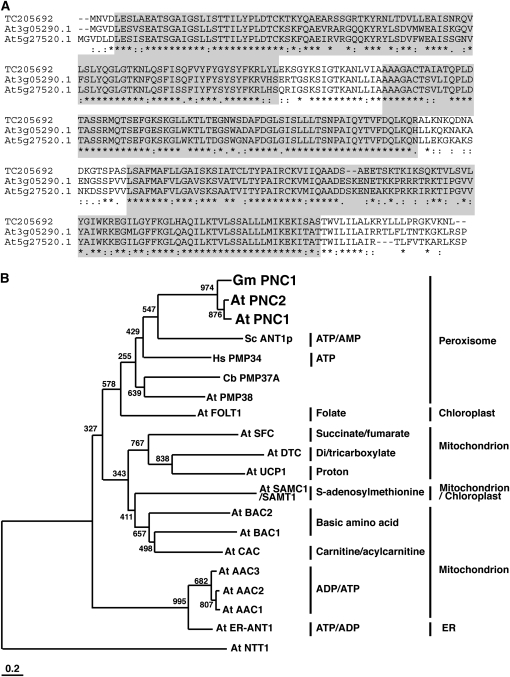
We cloned a full-length cDNA of the open reading frame region of TC205692 by RT-PCR using mRNA isolated from etiolated soybean cotyledons. The open reading frame of the full-length cDNA consisted of 957 nucleotides encoding a protein of 318 amino acid residues with a calculated molecular mass of 34.8 kD (Figure 2A; TC205692, accession number AB442083). We also cloned two homologous cDNAs (At3g05290 and At5g27520) using mRNA isolated from Arabidopsis seedlings. Open reading frames of the full-length At3g05290 and At5g27520 cDNAs consisted of 969 and 966 nucleotides encoding proteins of 322 and 321 amino acid residues with calculated molecular masses of 35.6 and 35.2 kD, respectively (Figure 2A). The amino acid sequences of the TC205692 protein, At3g05290.1, and At5g27520.1 contained the conserved solute carrier repeat profile (accession number of PROSITE: PS50920) (Figure 2A). The solute carrier repeat profile is conserved in the amino acid sequences of proteins that belong to the mitochondrial carrier family. This family contains not only mitochondrial proteins but also ER, chloroplastic, and peroxisomal proteins (Picault et al., 2004; Bedhomme et al., 2005; Leroch et al., 2008). We compared the amino acid sequences of TC205692, At3g05290.1, and At5g27520.1 with mitochondrial carrier family proteins and previously reported peroxisomal adenine nucleotide transporters (Figure 2B). A phylogenetic tree of these proteins showed that they belong to a cluster of two families. TC205692, At3g05290.1, and At5g27520.1 belong to the same clade as adenine nucleotide transporters in peroxisomes. We designated TC205692, At3g05290.1, and At5g27520.1, as Gm PNC1 (for G. max PEROXISOMAL ADENINE NUCLEOTIDE CARRIER1), At PNC1, and At PNC2, respectively.
Figure 2.
Identification of Novel Adenine Nucleotide Transporters.
(A) Alignment of amino acid sequences of polypeptides encoded by the TC205692 sequence and Arabidopsis orthologs, At3g05290.1 and At5g27520.1. Areas of gray background show the residues in the solute carrier repeat profile of the mitochondrial carrier family. Asterisks indicate identical amino acid residues, and colons or dots below the alignment denote similar amino acid replacements.
(B) Amino acid sequences encoded by TC205692, At3g05290.1, At5g27520.1, peroxisomal transporters, and Arabidopsis mitochondrial carrier family proteins were compared. The peroxisomal proteins were At PMP38, Hs PMP34, Cb PMP37A, and Sc ANT1p. The Arabidopsis mitochondrial carrier family proteins were At ER-ANT1, At AAC1-3, At FOLT1, At SFC, At UCP1, At DTC, At CAC, At BAC1-2, and At SAMC1/SAMT1. We designated the polypeptide encoded by TC205692, At3g05290.1, and At5g27520.1 as Gm PNC1, At PNC1, and At PNC2, respectively. At NTT1 is used as an outgroup. A text file of the alignment used to generate this tree is available as Supplemental Data Set 1 online.
Gm PNC1, At PNC1, and At PNC2 Are Targeted to Peroxisomes
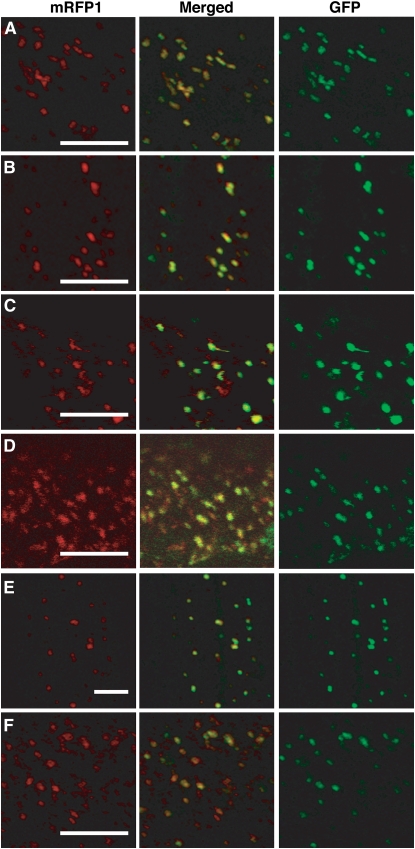
To determine the subcellular localization of Gm PNC1, At PNC1, and At PNC2, we fused the genes for monomeric red fluorescent protein 1 (mRFP1) in frame to the N-terminal or C-terminal sequences of Gm PNC1, At PNC1, and At PNC2. The fused genes were transiently expressed in onion epidermal cells under the control of the cauliflower mosaic virus 35S promoter. We then analyzed the subcellular localization of the fusion proteins by confocal fluorescence microscopy. The fusion proteins mRFP1-Gm PNC1 and Gm PNC1-mRFP1 were targeted to small particles (Figures 3A and 3B) that moved quickly in living cells. These particles completely coincided with peroxisomes labeled with a control fusion protein containing green fluorescent protein and tripeptides for peroxisomal targeting signal 1 (GFP-PTS1) (Figure 3). By contrast, the localization of mitochondria labeled with a fusion protein comprising sequences of the mitochondrial targeting signal (Mt) and GFP differed from that of the organelles labeled with mRFP1-Gm PNC1 and Gm PNC1-mRFP1 (see Supplemental Figure 1 online). Fusion proteins of mRFP1 and At PNC1 or At PNC2 were also targeted to small particles labeled with GFP-PTS1 but not with Mt-GFP (Figures 3C to 3F; see Supplemental Figure 1 online). These results suggest that Gm PNC1, At PNC1, and At PNC2 were targeted to peroxisomes in plant cells.
Figure 3.
Gm PNC1, At PNC1, and At PNC2 Are Targeted to Peroxisomes.
The polypeptides encoding Gm PNC1, At PNC1, and At PNC2 were fused at the N terminus or C terminus of mRFP1 under the control of the cauliflower mosaic virus 35S promoter. GFP-PTS1 was used as a marker of peroxisomes. Onion epidermal cells were used for the transient expression of combinations of GFP-PTS1 with mRFP1-Gm PNC1 (A), Gm PNC1-mRFP1 (B), mRFP1-At PNC1 (C), At PNC1-mRFP1 (D), mRFP1-At PNC2 (E), or At PNC2-mRFP1 (F). Confocal laser microscopy observation of the transiently expressed fluorescent proteins (RFP, red; GFP, green) was performed in epidermal cells. Center panels showed merged images. Bars = 10 μm.
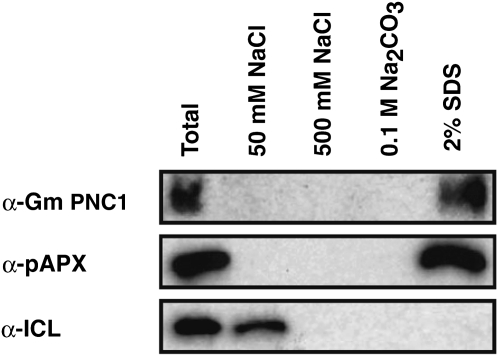
Gm PNC1 Is a Peroxisomal Integral Membrane Protein
To confirm that Gm PNC1 is a membrane protein, peroxisomes purified from etiolated soybean cotyledons were subject to subfractionation analyses performed by successive treatment with 50 and 500 mM NaCl, 0.1 M Na2CO3, and 2% SDS buffers. Gm PNC1 was recognized as a 35-kD protein by immunoblot analysis of total protein extracts of purified peroxisomes with an antibody raised against a fusion protein containing a partial amino acid sequence (2Asn-72Thr) and a six-histidine (His) tag (Figure 4). After subfractionation of the purified peroxisomes, Gm PNC1 was found only in the SDS-soluble fraction (Figure 4). pAPX, a marker protein for peroxisomal membranes, was also only detected in the SDS-soluble fraction (Figure 4; pAPX). By contrast, isocitrate lyase (ICL), a marker protein of the peroxisomal matrix, was completely dissolved in the low salt fraction (Figure 4). This result suggests that Gm PNC1 is a peroxisomal integral membrane protein.
Figure 4.
Gm PNC1 Is a Peroxisomal Integral Membrane Protein.
Purified peroxisomes prepared from etiolated soybean cotyledons were subjected to subfractionation studies performed by successive treatment with 50 mM NaCl, 500 mM NaCl, 0.1M Na2CO3, pH 11, and 2% SDS buffers. Proteins were suspended in each buffer and then centrifuged. The supernatants were subjected to SDS-PAGE and immunoblotting. Gm PNC1, ascorbate peroxidase (pAPX), and ICL were detected by immunoblotting using the antibodies indicated on the left. pAPX and ICL are markers for membrane and matrix proteins of peroxisomes, respectively.
Gm PNC1 Is Abundant during Postgerminative Growth
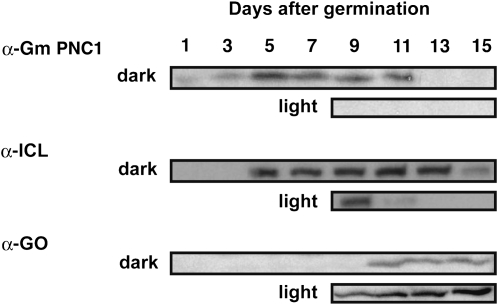
Immunoblot analyses revealed that the amount of Gm PNC1 in etiolated soybean cotyledons increased until 5 d after germination and then declined during seedling growth under constant darkness (Figure 5; Gm PNC1, dark). The amount of PNC1 in seedlings germinated in the dark decreased rapidly in response to illumination from the eighth day after germination (Figure 5; Gm PNC1, light). The appearance and disappearance of PNC1 during postgerminative growth is similar to that of glyoxysomal matrix proteins such as ICL (Figure 5) but is different from that of leaf peroxisomal proteins such as glycolate oxidase (GO) (Figure 5). This result suggests that Gm PNC1 functions in glyoxysomes that degrade fatty acids through a combination of fatty acid β-oxidation and the glyoxylate cycle, but not in leaf peroxisomes during postgerminative growth.
Figure 5.
Gm PNC1 Is Abundant during Postgerminative Growth.
The homogenates of etiolated soybean cotyledons grown for 1, 3, 5, 7, 9, 11, 13, and 15 d in the dark were prepared. Green cotyledons were obtained from seedlings grown for 8 d in the dark followed by 1, 3, 5, and 7 d in light were also analyzed. Gm PNC1, ICL, and GO were detected by immunoblotting using the antibodies indicated on the left. ICL and GO are markers for glyoxysomal and leaf peroxisomal proteins, respectively. Total protein obtained from an equal number of cotyledons was contained in the lanes of the gel.
Functional Expression of Gm PNC1 and At PNC2 in Escherichia coli Cells
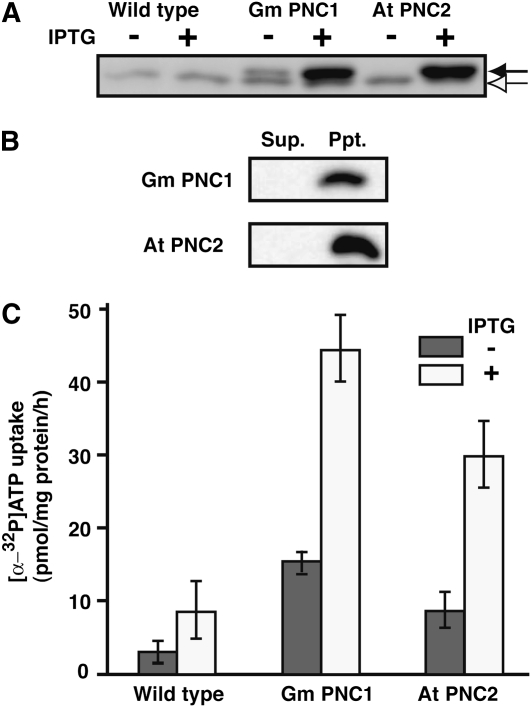
To confirm the biochemical functions of Gm PNC1, At PNC1, and At PNC2, we measured the adenine nucleotide transport activities of these proteins by expressing them in E. coli. This was expected to lead to their functional integration into E. coli cellular membranes with transport properties similar to those of peroxisomal membranes. The synthesis of His-tagged derivatives of Gm PNC1, At PNC1, and At PNC2 was induced by adding isopropyl-β-d-thiogalactopyramoside (IPTG) to the E. coli cultures. Recombinant Gm PNC1 and At PNC2 were detected as 36.8-kD proteins by immunoblot analysis using an antibody raised against the His tag (Figure 6A).The low-abundance recombinant Gm PNC1 was synthesized even without induction by IPTG (Figure 6A). A protein of 36.1 kD that nonspecifically reacted with the antibody raised against the His tag was detected both in the transformed and untransformed cells. By contrast, recombinant At PNC1 protein was not expressed, since no signal for a 36.8-kD protein was detected by immunoblot analysis (see Supplemental Figure 2 online). The Gm PNC1 and At PNC2 recombinant proteins were insoluble in 0.1 M Na2CO3 (Figure 6B), thus behaving as integral membrane proteins in the E. coli transformants.
Figure 6.
Analyses of Heterologously Expressed Gm PNC1 and At PNC2.
(A) Expression of His-tagged Gm PNC1 and At PNC2 following IPTG induction in E. coli cells. Untransformed cells (wild type) were used as a negative control. For each sample, total protein prepared from E. coli cells was subjected to SDS-PAGE. Recombinant proteins were detected by immunoblotting using anti-His-tag antibodies. Black arrow, recombinant protein; white arrow, nonspecific band.
(B) Recombinant Gm PNC1 and At PNC2 localize to E. coli cellular integral membranes. Total proteins from E. coli cells were fractionated into soluble (Sup.) and membrane (Ppt.) proteins with 0.1 M Na2CO3, pH 11. The proteins in each fraction were then subjected to SDS-PAGE. Recombinant proteins were detected by immunoblotting using anti-His tag antibodies.
(C) Uptake studies with radioactively labeled [α-32P]ATP into intact E. coli cells harboring Gm PNC1 and At PNC2. Gray bars, noninduced; white bars, induced. Error bars indicate se values of three independent experiments.
Uptake studies using radioactively labeled [α-32P]ATP with intact E. coli cells harboring Gm PNC1 or At PNC2 clearly revealed that this molecule was imported (Figure 6C). By contrast, wild-type E. coli cells had a relatively lower [α-32P]ATP uptake rate. Cells containing Gm PNC1 with no induction by IPTG imported slightly more [α-32P]ATP than the wild type (Figure 6C; without IPTG of Gm PNC1); this was probably due to low-level expression of Gm PNC1 in the absence of IPTG (Figure 6A; without IPTG of Gm PNC1). To investigate the substrate specificity of Gm PNC1 and At PNC2, we measured the effect of nonlabeled ATP, ADP, AMP, GTP, and GDP on the rate of [α-32P]ATP uptake (Table 3). Inhibition of [α-32P]ATP uptake catalyzed by Gm PNC1 or At PNC2 was observed in the presence of nonlabeled ATP and ADP; these molecules reduced the rate to below 30% of the control values (without effectors). Unlabeled AMP exhibited no inhibitory effects on [α-32P]ATP uptake catalyzed by Gm PNC1 or At PNC2. Unlabeled GTP and GDP had relatively lower inhibitory effects on the [α-32P]ATP uptake than nonlabeled ATP and ADP. These results show that recombinant Gm PNC1 and At PNC2 have the ability to import ATP and ADP through cellular membranes in E. coli.
Table 3.
Effects of Nucleotides on [α-32P]ATP Transport Rates of E. coli Cells Expressing Gm PNC1 or At PNC2
| Rate of Transport (%)
|
||
|---|---|---|
| Effectora | Gm PNC1 | At PNC2 |
| None | 100.0 ± 6.5 | 100.0 ± 22.5 |
| ATP | 27.3 ± 12.9 | 28.9 ± 18.8 |
| ADP | 19.8 ± 3.5 | 11.8 ± 0.9 |
| AMP | 99.1 ± 13.2 | 92.3 ± 18.3 |
| GTP | 78.9 ± 12.0 | 62.8 ± 12.6 |
| GDP | 59.5 ± 9.6 | 64.4 ± 12.5 |
Effectors were added at a concentration of 12.5 μM. [α-32P]ATP was present at a concentration of 2.5 μM. Data represent the means ± se of three or four independent experiments.
Expression of At PNC1 and At PNC2 in Arabidopsis Seedlings
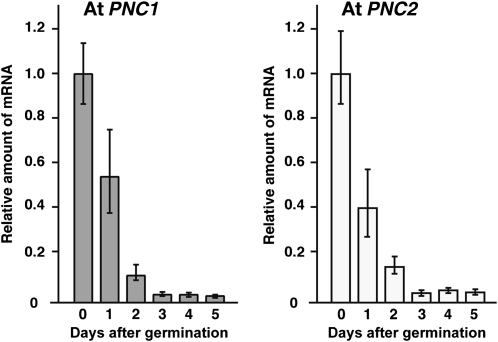
We analyzed the expression of At PNC1 and PNC2 using quantitative RT-PCR, which revealed that the amount of PNC1 and PNC2 mRNA in Arabidopsis plants declined during postgerminative growth under constant darkness (Figure 7). These results suggested that At PNC1 and PNC2 are expressed in the early seedling stage of postgerminative growth.
Figure 7.
Expression of At PNC1 and At PNC2 during Postgerminative Growth.
The amounts of At PNC1 (gray bars) and At PNC2 (white bars) mRNA were determined by quantitative RT-PCR using seedlings of wild-type plants grown for 0 to 5 d in the dark. The data were normalized with respect to β-actin, and the amount of mRNA in seedlings on day 0 was normalized to 1.0. Error bars indicate se values of three independent experiments.
Generation of At PNC1 and PNC2 Knockdown Mutants
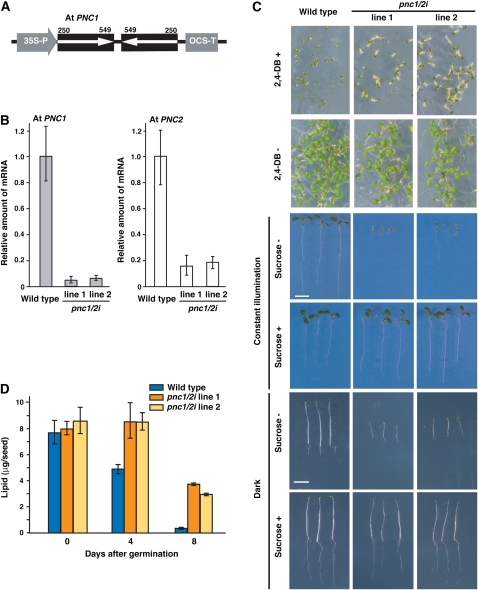
To elucidate the biological functions of At PNC1 and PNC2, we attempted to generate a double-knockdown mutant by inducing posttranscriptional gene silencing using double-stranded RNA interference, as At PNC1 and PNC2 were expected to be functional homologs. To induce double-stranded RNA interference (dsRNAi) in Arabidopsis plants, we constructed an artificial gene that encodes RNA capable of double strand formation at a gene-specific sequence (Figure 8A). We selected a fragment containing base pairs 250 to 549 of the At PNC1 cDNA to be the artificial gene. This fragment contained a 36-bp region with complete identity to part of At PNC2. Therefore, the dsRNAi of the 250- to 549-bp cDNA fragment was expected to decrease the expression of both At PNC1 and PNC2. The effect of the dsRNAi in T2 progeny selected by kanamycin resistance was determined by quantitative RT-PCR. We selected two independent T2 progenies that exhibited the greatest dsRNAi effect (line 1 and line 2). The expression of At PNC1 in the line 1 and line 2 T2 progenies was 6 and 8% of that of the wild type, respectively (Figure 8B). The expression of At PNC2 in the line 1 and line 2 T2 progenies was 16 and 19% of that of the wild type, respectively (Figure 8B). The T2 progenies, pnc1/2i line 1 and line 2, were then used for physiological analyses.
Figure 8.
Generation and Phenotypes of the pnc1/2i Knockdown Mutants.
(A) Transgene construction for generation of the pnc1/2i knockdown mutants in Arabidopsis. A partial cDNA fragment (250 to 549 bp) was derived from At PNC1. 35S-P is the cauliflower mosaic virus 35S promoter. OCS-T is octopin synthase terminator.
(B) The amounts of At PNC1 (gray bars) and At PNC2 (white bars) mRNA were determined by quantitative RT-PCR using seedlings grown for 3 d in the dark. The seedlings used were individual pnc1/2i mutants, line 1 and line 2, and the wild type. The data were normalized with respect to β-actin, and the amount of mRNA in wild-type seedlings on day 3 was normalized to 1.0. Error bars indicate se values of three independent experiments.
(C) Effects of 2,4-DB and sucrose on the growth of the knockdown mutant. Wild-type and pnc1/2i were grown for 7 d on growth medium with (+) or without (−) 0.35 μg/mL 2,4-DB under constant illumination. Wild-type and pnc1/2i were grown for 7 d on growth medium with (+) or without (−) sucrose under constant illumination or in the dark. Bars = 5 mm.
(D) The knockdown mutant exhibits defective degradation of seed lipid reserves during postgerminative growth. The amounts of reserved lipids in wild-type (blue bars) and pnc1/2i mutants (orange and yellow bars) were determined during postgerminative growth. Samples were prepared dry seeds (zero time) and etiolated seedlings. Error bars indicate se values of three or four independent experiments.
Physiological Analyses of the pnc1/2i Mutants
To determine the activity of fatty acid β-oxidation in the pnc1/2i mutants, we examined the effect of 2,4-dichlorophenoxybutyric acid (2,4-DB) and sucrose deficiency on the growth of the mutant seedlings (Figure 8C). Seedlings lacking peroxisomal fatty acid β-oxidation became resistant to 2,4-DB because 2,4-D is not produced from 2,4-DB in the peroxisome. Seedlings of the pnc1/2i mutants were still bigger than those of the wild type on the medium containing 0.35 μg/mL 2,4-BD (Figure 8C; 2,4-DB+), whereas those of pnc1/2i mutants and the wild type were grown normally in the absence of 2,4-DB (Figure 8C; 2,4-DB−). This result shows that the pnc1/2i mutants have defects in peroxisomal fatty acid β-oxidation. Defects in peroxisomal fatty acid β-oxidation appear to inhibit the conversion of seed lipid reserves into sucrose that is required for heterotrophic growth. The seedlings of the pnc1/2i mutants exhibited growth defects, while the wild type grew normally in the absence of sucrose (Figure 8C; sucrose −). The growth defects were observed for seedlings grown both in the dark and under light. The growth defect could be recovered when these mutant seedlings were grown on medium containing sucrose (Figure 8C; sucrose +). The growth inhibition that occurred in the absence of sucrose may be due to the inhibition of gluconeogenesis from seed lipid reserves during postgerminative growth. Mature seeds from the wild type and pnc1/2i mutants contained ∼8 μg of triacylglycerol as seed lipid reserves (Figure 8D). Ninety-eight percent of the seed lipid reserves in the wild-type seeds were rapidly degraded within 8 d of germination, presumably because they had been used for gluconeogenesis. By contrast, 8-d-old seedlings of the pnc1/2i mutants still contained ∼50% of their seed lipid reserves (Figure 8D). The requirement of sucrose for postgerminative growth and the reduced lipid degradation clearly demonstrated that the pnc1/2i mutants had defects in peroxisomal fatty acid β-oxidation and in subsequent gluconeogenesis. These results show that At PNC1 and PNC2 function in peroxisomal fatty acid β-oxidation at the early stage of postgerminative growth.
DISCUSSION
Identification of an Adenine Nucleotide Transporter Localized to the Plant Peroxisomal Membrane
This study has identified peroxisomal membrane proteins by proteomic analyses employing BN-PAGE to separate hydrophobic proteins. Three polypeptides were identified as soybean orthologs of peroxisomal membrane proteins, including PEX11, MDAR, andLACS, which were previously characterized in plants other than soybean (Hayashi et al., 2002b; Lisenbee et al., 2005; Orth et al., 2007). Moreover, we succeeded in identifying Gm PNC1 as a plant peroxisomal adenine nucleotide transporter in etiolated soybean cotyledons. Gm PNC1 and the Arabidopsis ortholog At PNC2 exhibit [α-32P]ATP transport activity when they are expressed in E. coli membranes (Figure 6). Moreover, ADP inhibited [α-32P]ATP transport in E. coli membranes heterologously expressing Gm PNC1 and At PNC2 (Table 3). By contrast, AMP did not affect [α-32P]ATP transport in these membranes (Table 3). Thus, ATP and ADP have been experimentally established as substrates of this plant peroxisomal transporter.
PNC1, a member of the mitochondrial carrier family, is related to plant mitochondrial ADP/ATP carriers (AACs) and ER-ANT1 (Figure 2B). Mitochondrial AACs and ER-ANT1 function in ATP and ADP exchange across membranes (Haferkamp et al., 2002; Leroch et al., 2008). Mitochondrial AACs export ATP that is synthesized in mitochondria and import cytosolic ADP (Haferkamp et al., 2002). ER-ANT1 catalyzes ATP transport into the ER (Leroch et al., 2008). Transport of ATP, ADP, and AMP through peroxisomal membranes was demonstrated by the reconstitution of S. cerevisiae Ant1p in liposomes (Palmieri et al., 2001). Sc Ant1p is thought to catalyze ATP/AMP exchange across the peroxisomal membrane (Palmieri et al., 2001). However, PNC1 may catalyze ATP/ADP exchange across the plant peroxisomal membrane. The mechanism of adenine nucleotide transport through the plant peroxisomal membrane seems to be more similar to that in mitochondria and the ER than to that in yeast peroxisomes.
Function of PNC1 during Postgerminative Growth
The expression of Gm PNC1 was similar to that of glyoxysomal proteins in seedlings. Glyoxysomes contain enzymes responsible for β-oxidation and the glyoxylate cycle and play a role in the conversion of seed-reserved triacylglycerols into sucrose during postgerminative growth. The sucrose provides a carbon source that is necessary for growth before plants begin photosynthesis. Fatty acids produced from seed-reserved triacylglycerols are imported into peroxisomes where they are esterified to acyl-CoAs by LACSs using ATP inside the peroxisome. In Arabidopsis, two genes encoding peroxisomal LACS have been identified and designated At LACS6 and At LACS7 (Fulda et al., 2002). Transport of ATP through peroxisomal membranes is required to perform this reaction; however, the mechanism underlying the transport of ATP through the peroxisomal membrane has not been identified in higher plants. In this report, we identified Gm PNC1, At PNC1, and At PNC2 as adenine nucleotide transporters of plant peroxisomes.
At the amino acid level, the closest ortholog of Gm PNC1 is At PNC1 (73.0% identity and 94.4% similarity) in Arabidopsis (Table 2). The amino acid sequences of At PNC1 and At PNC2 are 87.1% identical and 99.4% similar to each other. Both At PNC1 and At PNC2 were highly expressed in early stages of germination (Figure 7). The double knockdown mutants showed defective lipid degradation and a requirement for sucrose during postgerminative growth (Figures 8C and 8D). These results suggest that At PNC1 and PNC2 are functional homologs involved in peroxisomal fatty acid β-oxidation during postgerminative growth.
Physiological Role of PNC1 in Plant Metabolism
AtGenExpress analysis revealed that At PNC1 and PNC2 are expressed in other tissues besides seedlings (Schmid et al., 2005). Moreover, AtGenExpress analysis revealed that At PNC2 is expressed in response to stress (Schmid et al., 2005). At LACS6 and At LACS7 have been characterized as glyoxysomal genes that exhibit high expression in seedlings and low expression in other organs, such as leaves, stems, flowers, siliques, and roots (Kamada et al., 2003). At LACS6 and At LACS7 are acyl-activating enzyme superfamily proteins that consume ATP. This superfamily contains 63 Arabidopsis genes encoding LACSs, 4-coumarate-CoA ligases, and proteins that are closely related to 4-coumarate-CoA ligases with unknown activities (Shockey et al., 2003). Among these, 16 acyl-activating enzymes have conserved peroxisomal targeting signals in their amino acid sequences. At PNC1 and At PNC2 might provide ATP for these peroxisomal acyl-activating enzymes in peroxisomes. At PNC1 and At PNC2 can therefore be used as indicators of peroxisomal β-oxidation in plant metabolism.
The presence of protein kinases and corresponding phosphatases in peroxisomes has been previously predicted (Dammann et al., 2003; Fukao et al., 2003; Reumann et al., 2004). At PNC1 and PNC2 might provide ATP for protein kinases in Arabidopsis peroxisomes. Further analyses of At PNC1 and PNC2 might enhance our understanding of regulatory systems that are mediated by protein phosphorylation in peroxisomes.
Identification of Peroxisomal Membrane Proteins
We speculated that the physiological role of these proteins is probably to transport cytoplasmic ATP into the peroxisomal lumen where it is consumed by the LACSs for the activation of fatty acids. The LACSs also produce AMP and pyrophosphate, which need to be exported from the peroxisome. A pyrophosphate transporter has not been identified in peroxisomes, although phosphate transporter activity has been detected in Bos taurus kidney peroxisomal membranes using reconstituted proteoliposomes (Visser et al., 2005). Moreover, the peroxisome needs to import/export a variety of metabolites, such as citrate, isocitrate, succinate, phosphoglycolate, CoA, NAD+, NADH, and jasmonic acid (Hayashi and Nishimura, 2006). In this study, we used digitonin to solubilize peroxisomal membrane proteins (Figure 1) because it was more efficient in this respect than Triton X-100, CHAPS, or dodecylmaltoside. However, digitonin failed to solubilize all peroxisomal proteins, although a marker protein of the peroxisomal membrane was detected as a digitonin-soluble protein. We could not detect any other protein involved in metabolite transport other than Gm PNC1 in the digitonin-soluble fraction, although four other peroxisomal membrane proteins were clearly present (Table 2). It is possible that the digitonin-insoluble fraction may contain yet unknown peroxisomal proteins. Transport of metabolites through peroxisomal membranes is required to perform essential reactions for plant growth. Further identification and characterization of peroxisomal membrane proteins would contribute to our understanding of the regulation of peroxisomal metabolism in plant cells.
METHODS
Plant Materials
Soybean seeds (Glycine max cv Bansei Shirodaizu) were soaked in moist rock fiber (66R; Nitto Bouseki) and allowed to germinate in the dark for 7 d at 25°C. Arabidopsis thaliana ecotype Columbia was used as the wild-type plant. All of the Arabidopsis seeds were surface sterilized in 2% antiformin and 0.02% Tween 20 and germinated on growth media containing 2.3 g/L Murashige and Skoog salt (Wako), 1% sucrose, 100 mg/L myoinositol, 0.5 mg/L nicotinic acid, 0.1 mg/L thiamine-HCl, 0.5 mg/L pyridoxine-HCl, 2 mg/L glycine, 0.5 g/L MES-KOH, pH 5.7, and 0.8% agar. Arabidopsis germination was induced by a 48-h incubation in the dark at 4°C followed by exposure to white light at 22°C. To determine the effect of 2,4-DB, Arabidopsis plants were grown for 7 d on the growth media with or without 0.35 μg/mL 2,4-DB under constant illumination. To determine sucrose deficiency, Arabidopsis plants were grown for 7 d on growth media with or without 1% sucrose under constant illumination or in the dark.
Protein Preparation for BN-PAGE and 2-D Gel Electrophoresis
Etiolated soybean cotyledons (100 g fresh weight) from 7-d-old seedlings were used to prepare peroxisomes. The isolation of peroxisomes by iodixanol density gradient centrifugation has been reported (Arai et al., 2008). The isolated peroxisomes were suspended in sample buffer (10 mM HEPES-KOH, pH 7.0) with 50 mM NaCl for 1 h at 4°C and then centrifuged at 100,000g for 30 min. The resulting pellets were resuspended in a sample buffer (10 mM HEPES-KOH, pH 7.0) containing 1% digitonin for 16 h at 4°C and then centrifuged at 100,000g for 30 min. Proteins in these supernatants were subjected to BN-PAGE using a 3 to 12% gradient gel. BN-PAGE was performed according to the manufacturer's instructions (BN-PAGE system; Invitrogen). A lane of the BN gel was cut out and placed horizontally on a 4 to 12% SDS-PAGE gradient gel. Proteins that were separated on the 2D gel were detected by silver staining.
MALDI-TOF MS Analysis and Protein Identification
MS analysis was conducted by MALDI-TOF MS (Reflex III, Bruker Daltonik). We used MASCOT software (Matrix Science) to search a database for PMFs using the parameters of missed cleavage = 0 and mass tolerance 150 ppm (Perkins et al., 1999). We considered proteins with three matched peptides in their sequences or probability-based scores that exceeded a certain threshold of MASCOT software to have significant homology (P < 0.05). The database was downloaded from the DFCI Soybean Gene Index (version 12.0) (http://compbio.dfci.harvard.edu/tgi/plant.html). Translate+ software of SeqWebv3.1 was used for translation of the TC sequences. The translated sequences were used to identify identical or similar clones in the GenBank (http://www.ncbi.nlm.nih.gov/), Protein Information Resource (http://pir.georgetown.edu/), and Swiss-Prot (http://www.expasy.org/sprot/) protein databases.
Cloning of Full-Length cDNAs
cDNA fragments encoding the amino acid sequences of TC205692, At3g05290, and At5g27520 conjugated to attB1 and attB2 sites at their 5′ and 3′ termini were amplified by RT-PCR using RNA isolated from etiolated cotyledons of soybeans and Arabidopsis using the following primers: 5′-AAAAAGCAGGCTTAATGAACGTGGATCTG and 3′-AGAAAGCTGGGTATTACAAGTTCTTAACC; 5′-AAAAAGCAGGCTTAATGGGTGTCGATTTG and 3′-AGAAAGCTGGGTACTAAGGACTTCTTAAC; 5′-AAAAAGCAGGCTTAATGGGTGTTGATTTG and 3′-AGAAAGCTGGGTATTATGGACTTTTCAAT. The PCR-amplified fragments were cloned into the entry vector pDONR221 (Gateway technology; Invitrogen) using BP recombination (Invitrogen).
Phylogenetic Analysis
A multiple sequence alignment was generated with ClustalX 2.0 (Larkin et al., 2007) and then manually adjusted to optimize alignment (available in Supplemental Data Set 1 online). At NTT1, chloroplast ADP/ATP carrier protein 1 (Neuhaus et al., 1997), was used as an outgroup. Protein distances were calculated using the program Protdist from the PHYLIP suite of programs (Felsenstein, 1989) with the JTT matrix on 1000 bootstraps (Felsenstein, 1985) and then used to generate phylogenetic trees based on neighbor joining. The resulting trees were then joined to a consensus tree using the Consense program (Felsenstein, 1989) and plotted with NJplot (Perrière and Gouy, 1996). The peroxisomal proteins were At PMP38, putative ATP/ADP carrier (Fukao et al., 2001); Hs PMP34, Homo sapiens ATP transporter (Visser et al., 2002); Cb PMP37A, Candida boidinii mitochondrial ATP/ADP exchanger homolog (Nakagawa et al., 2000); and Sc ANT1p, Saccharomyces cerevisiae adenine nucleotide transporter 1 (Palmieri et al., 2001). The Arabidopsis mitochondrial carrier family proteins were At ER-ANT1, ER ATP/ADP carrier 1 (Leroch et al., 2008); At AAC 1-3, mitochondrial ADP/ATP carrier 1-3 (Haferkamp et al., 2002); At FOLT1, chloroplastic folate transporter 1 (Bedhomme et al., 2005); At SFC, mitochondrial succinate/fumarate carrier (Catoni et al., 2003); At UCP1, mitochondrial uncoupling protein 1 (Maia et al., 1998); At DTC, mitochondrial di/tricarboxylate carrier (Picault et al., 2002); At CAC, mitochondrial carnitine/acylcarnitine carrier (Lawand et al., 2002); At BAC1-2, mitochondrial basic amino acid carrier 1-2 (Hoyos et al., 2003); and At SAMC1/SMAT1, mitochondrial/chloroplast S-adenosylmethionine transporter 1 (Bouvier et al., 2006; Palmieri et al., 2006).
Plasmid Construction and Plant Transformation
The Gm PNC1, At PNC1, and At PNC2 regions of the entry clones were inserted into pDEST17 and a transient expression vector containing the mRFP1 gene (either pUGW54 or pUGW55; Nakagawa et al., 2007) by LR recombination (Invitrogen). These vectors were used for the heterologous expression assay and analysis of the subcellular localization of the expressed proteins. DNA fragments encoding the region between the 2Asn-72Thr polypeptide of Gm PNC1 and the full-length amino acid sequence of Arabidopsis pAPX were conjugated to the attB1 and attB2 sites at their 5′ and 3′ ends and amplified by PCR using Gm PNC1 cDNA and the 5′ primer (AAAAAGCAGGCTTAAACGTGGATCTGGAA) and the 3′ primer (AGAAAGCTGGGTACTACTTTGTTCCAAGGCC), and Arabidopsis pAPX cDNA and 5′ primer (AAAAAGCAGGCTTAATGGCTGCACCGATTGTTGA) and the 3′ primer (AGAAAGCTGGGTACTTCATCCTCTTCCGGA), respectively. The PCR-amplified fragments were cloned into the pDONR221 vector by performing BP recombination and were then inserted into the pET32a vector again by LR recombination (Invitrogen), before being expressed and used for the protein purifications, and then preparation of specific antibodies against Gm PNC1 and pAPX as follows in the next section. A partial cDNA fragment (250 to 549 bp) derived from At PNC1 was amplified by PCR using the full-length cDNA of the gene and the 5′ primer (AAAAAGCAGGCTTATACTTTTATAGCTAT) and the 3′ primer (AGAAAGCTGGGTACTGTTTCAGCTGATC). It was then subcloned into pDONR221 and transferred to the Ti plasmid, pHELLSGATE8 (Wesley et al., 2001), and a plasmid that is suitable for performing dsRNAi. The recombinant pHELLSGATE8 clone was introduced into wild-type plants by infiltration using Agrobacterium tumefaciens strain C58 C1RifR. The primary transformant was designated as a T0 plant. Transformed Arabidopsis lines were selected on growth medium containing 50 μg/L kanamycin and designated as T1 transformants. T2 progeny showing kanamycin resistance were used for further analysis.
Heterologous Expression of PNC1 in Escherichia coli
The expression plasmids were transformed into the IPTG-inducible E. coli strain BL21 (DE3). Wild-type (untransformed) E. coli cells and those harboring the expression vectors were grown at 37°C in Lysogeny Broth Amp medium (5 g/liter yeast extract, 10 g/liter peptone, and 10 g/liter NaCl). ITPG (1 mM) was added to the cultures at an A600 value of 0.5 to 0.6. After 2 h of induction, the cells were collected by centrifugation at 5000g for 5 min at 8°C. The pellet was resuspended to an A600 value of 1.5 using sodium phosphate buffer (50 mM, pH 7.0) and used in the analyses of expression, localizations, and uptake experiments. Prepared total proteins from IPTG-induced and noninduced cultures were used to check the expression of PNC1s by immunoblotting analysis. IPTG-induced cultures were treated with alkaline buffer (0.1 M Na2CO3, pH 11) for 1 h at 4°C and centrifuged at 100,000g for 30 min. Expressed PNC1s in the supernatant and pellet fractions were detected by immunoblot analyses.
Uptake of Adenine Nucleotides into Intact E. coli Cells
The uptake of adenine nucleotides into intact E. coli cells was done as described by Leroch et al. (2005) with modifications. IPTG-induced E. coli cells (25 μL; either untransformed or harboring an expression vector) were added to 25 μL of sodium phosphate buffer (50 mM, pH 7.0) containing radioactively labeled ATP. [α-32P]ATP was used with a specific activity of 50 μCi/mol. Uptake of nucleotides was performed at 30°C and terminated after 1 h by transferring the cells to a 0.45-μm membrane filter (mixed cellulose ester, 25-μm diameter; Millipore) under vacuum. The cells were washed three times with 2 mL of sodium phosphate buffer (50 mM, pH 7.0) to remove unimported radioactivity. The filter was subsequently transferred into a 20-mL scintillation vessel and filled with 2 mL of water. Radioactivity in the samples was quantified in a scintillation counter LSC-5100 (Alokan). For effector assays, E. coli cells were incubated with sodium phosphate buffer (50 mM, pH 7.0) containing 2.5 μM [α-32P]ATP and 12.5 μM nonlabeled ATP, ADP, AMP, GTP, and GDP as effectors. After 10 min, uptake was measured by membrane filtration as described above.
Preparation of a Specific Antibody against Gm PNC1 and pAPX
The 2Asn-72Thr polypeptide Gm PNC1 and the full-length amino acid sequence of Arabidopsis pAPX fused to His tags on their N-terminal sides were synthesized in E. coli cells strain BL21 (DE3), purified as described previously (Hayashi et al., 1999), and then used for the production of rabbit antibodies. An immunoblot analysis of soybean crude homogenate using the Gm PNC1 antibodies detected a band with a molecular mass of 35 kD, in addition to some nonspecific bands with different molecular masses (see Supplemental Figure 3A online, lane 1). The recombinant Gm PNC1 immunodepleted the Gm PNC1 antibodies, showing that the antibodies cross-reacted with the antigen (see Supplemental Figure 3A online, lane 2). An immunoblot analysis of soybean crude homogenate using the pAPX antibodies detected one band with a molecular mass of 30 kD (see Supplemental Figure 3B online), showing that the antibody was specific for pAPX.
Immunoblot Analysis
Samples were prepared from soybean cotyledons and subjected to SDS-PAGE with sample buffer (125 mM Tris-HCl, pH 6.8, 5% 2-mercaptoethanol, and 2% SDS), and the proteins were electroblotted onto a polyvinylidine difluoride membrane. Immunodetection was performed on the membrane using rabbit antibodies raised against Gm PNC1 (in this study; diluted 1:2000), pAPX (in this study; diluted 1:2000), ICL (Maeshima et al., 1988; diluted 1:5000), GO (Tsugeki et al., 1993; diluted 1:5000), and His tag (Qiagen; diluted 1:2000). A horseradish peroxidase–conjugated anti-rabbit or anti-mouse secondary antibodies diluted 1:10,000 or 1:5000, respectively, were used; horseradish peroxidase activity was detected with the ECL Plus detection kit (GE Healthcare BioSciences).
Subcellular Localization Analyses
Particle bombardment was used to introduce the fluorescence protein fusion plasmids into onion epidermal cells with a Biolistic PDS-1000/He system (Bio-Rad Laboratories) according to the manufacturer's instructions. GFP-PTS1 was used as a marker of peroxisomes; PTS1 (peroxisome targeting signal 1) is five amino acids derived from the C terminus of Arabidopsis hydroxypyruvate reductase (Mano et al., 1999). Mt-GFP was used as a marker of mitochondria; the mitochondrial targeting signal (Mt) is 42 amino acids long and is derived from the N terminus of the Arabidopsis F1-ATPase γ-subunit. The expression of fusion proteins in onion epidermal cells was examined as described previously (Arai et al., 2008).
Purified peroxisomes (100 μg of total protein) from etiolated soybean cotyledons were successively treated with solutions including a low salt buffer (10 mM HEPES-KOH, pH 7.2, 1 mM EDTA, and 50 mM NaCl), a high salt buffer (10 mM HEPES-KOH, pH 7.2, 1 mM EDTA, and 500 mM NaCl), an alkaline buffer (0.1 M Na2CO3, pH 11), and an SDS buffer (10 mM HEPES-KOH and 2% SDS). Proteins were suspended in each buffer for 1 h at 4°C, and then the solution was centrifuged at 100,000g for 30 min. Peroxisomal proteins in the supernatants were detected by immunoblot analyses.
Quantitative RT-PCR Methods
Total RNA was extracted from etiolated seedlings of wild-type and pnc1/2i lines. cDNA was synthesized from 2 μg of the total RNA with Ready-to-Go RT-PCR beads (GE Healthcare). Quantitative RT-PCR was performed by the TaqMan Gene Expression assay (ID number of TaqMan probe: At02235405_g1 and At02310404_g1) using the 7500Fast Real-Time PCR system (Applied Biosystems). The relative quantity of the target mRNA was calculated using β-actin (At1g49240, ID number of TaqMan probe: At02270958_gH) as a standard.
Quantitative Analyses of Total Triacylglycerols
The amounts of seed lipid reserves contained in dry seeds and in 4- and 8-d-old etiolated seedlings were measured by determining the amount of total triacylglycerol using the assay kit TRIGLYCERIDE E-TEST (Wako). Either 20 dry seeds or 20 seedlings were homogenized in a mortar in 100 μL of water. Homogenates were mixed with 0.75 mL of reaction buffer provided in the kit. The concentration of triacylglycerols in the sample was determined according to the manufacturer's protocol.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL database under the following accession numbers: At AAC 1 (At3g08580), At AAC 2 (At5g13490), At AAC 3 (At4g28390), At BAC1 (At2g33820), At BAC2 (At1g79900), At CAC (At5g46800), At DTC (At5g19760), At FOLT1 (At5g66380), At PNC1 (At3g05290), At PNC2 (At5g27520), At NTT1 (At1g80300), At PMP38 (At2g39970), At SAMC1/SAMT1 (At4g39460), At SFC (At5g01340), At UCP1 (At3g54110), Cb PMP37A (P21245), Gm ICL2 (AB442085), Gm LACS (AB442086), Gm MDAR (AB442087), Gm PNC1 (AB442083), Gm PEX11 (AB442084), Hs PMP34 (O43808), and Sc ANT1p (Q06497).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Gm PNC1, At PNC1, and At PNC2 Do Not Colocalize with Mitochondria.
Supplemental Figure 2. At PNC1 Is Not Expressed in E. coli Cells.
Supplemental Figure 3. Preparation of Specific Antibodies against Gm PNC1 and pAPX.
Supplemental Data Set 1. Text File of the Alignment Used to Generate the Phylogenetic Tree in Figure 2B.
Supplementary Material
Acknowledgments
We thank Peter M. Waterhouse (CSIRO) for providing pHELLSGATE8, Tsuyoshi Nakagawa (Shimane University) for providing vectors, Roger Tsien (University of California, San Diego) for providing mRFP1, Shoji Mano (National Institute for Basic Biology) for providing GFP-PTS1 and Mt-GFP, Yumiko Makino and Tomoko Mori (National Institute for Basic Biology) for their technical support with MALDI-TOF MS analyses, Hiroyo Nishide (National Institute for Basic Biology) for her technical support with database searches, and Chieko Nanba (National Institute for Basic Biology) for her support with plant growth.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Mikio Nishimura (mikosome@nibb.ac.jp).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Arai, Y., Hayashi, M., and Nishimura, M. (2008). Proteomic analysis of highly purified peroxisomes from etiolated soybean cotyledons. Plant Cell Physiol. 49 526–539. [DOI] [PubMed] [Google Scholar]
- Baker, A., Graham, I.A., Holdsworth, M., Smith, S.M., and Theodoulou, F.L. (2006). Chewing the fat: Beta-oxidation in signalling and development. Trends Plant Sci. 11 124–132. [DOI] [PubMed] [Google Scholar]
- Bedhomme, M., Hoffmann, M., McCarthy, E.A., Gambonnet, B., Moran, R.G., Rébeillé, F., and Ravanel, S. (2005). Folate metabolism in plants: An Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J. Biol. Chem. 280 34823–34831. [DOI] [PubMed] [Google Scholar]
- Bouvier, F., Linka, N., Isner, J.C., Mutterer, J., Weber, A.P., and Camara, B. (2006). Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development. Plant Cell 18 3088–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoni, E., Schwab, R., Hilpert, M., Desimone, M., Schwacke, R., Flügge, U.I., Schumacher, K., and Frommer, W.B. (2003). Identification of an Arabidopsis mitochondrial succinate-fumarate translocator. FEBS Lett. 534 87–92. [DOI] [PubMed] [Google Scholar]
- Chanut, F.A., Grabau, E.A., and Gesteland, R.F. (1993). Complex organization of the soybean mitochondrial genome: recombination repeats and multiple transcripts at the atpA loci. Curr. Genet. 23 234–247. [DOI] [PubMed] [Google Scholar]
- Dammann, C., Ichida, A., Hong, B., Romanowsky, S.M., Hrabak, E.M., Harmon, A.C., Pickard, B.G., and Harper, J.F. (2003). Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 132 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, J.M., McNew, J.A., and Goodman, J.M. (1996). The sorting sequence of the peroxisomal integral membrane protein PMP47 is contained within a short hydrophilic loop. J. Cell Biol. 133 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmermann, M., and Schmitz, U.K. (1995). Two cDNA clones encoding isoforms of the beta-subunit of the general mitochondrial processing peptidase from potato. Plant Physiol. 107 1467–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubel, H., Heinemeyer, J., Sunderhaus, S., and Braun, H.P. (2004). Respiratory chain supercomplexes in plant mitochondria. Plant Physiol. Biochem. 42 937–942. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution Int. J. Org. Evolution 39 783–791. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1989). PHYLIP - Phylogeny Inference Package (Version 3.2). Cladistics 5 164–166. [Google Scholar]
- Footitt, S., Slocombe, S.P., Larner, V., Kurup, S., Wu, Y., Larson, T., Graham, I., Baker, A., and Holdsworth, M. (2002). Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 21 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao, Y., Hayashi, M., Hara-Nishimura, I., and Nishimura, M. (2003). Novel glyoxysomal protein kinase, GPK1, identified by proteomic analysis of glyoxysomes in etiolated cotyledons of Arabidopsis thaliana. Plant Cell Physiol. 44 1002–1012. [DOI] [PubMed] [Google Scholar]
- Fukao, Y., Hayashi, Y., Mano, S., Hayashi, M., and Nishimura, M. (2001). Developmental analysis of a putative ATP/ADP carrier protein localized on glyoxysomal membranes during the peroxisome transition in pumpkin cotyledons. Plant Cell Physiol. 42 835–841. [DOI] [PubMed] [Google Scholar]
- Fukao, Y., Hayashi, M., and Nishimura, M. (2002). Proteomic analysis of leaf peroxisomal proteins in greening cotyledons of Arabidopsis thaliana. Plant Cell Physiol. 43 689–696. [DOI] [PubMed] [Google Scholar]
- Fulda, M., Shockey, J., Werber, M., Wolter, F.P., and Heinz, E. (2002). Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J. 32 93–103. [DOI] [PubMed] [Google Scholar]
- Haferkamp, I., Hackstein, J.H., Voncken, F.G., Schmit, G., and Tjaden, J. (2002). Functional integration of mitochondrial and hydrogenosomal ADP/ATP carriers in the Escherichia coli membrane reveals different biochemical characteristics for plants, mammals and anaerobic chytrids. Eur. J. Biochem. 269 3172–3181. [DOI] [PubMed] [Google Scholar]
- Hayashi, H., De Bellis, L., Ciurli, A., Kondo, M., Hayashi, M., and Nishimura, M. (1999). A novel acyl-CoA oxidase that can oxidize short-chain acyl-CoA in plant peroxisomes. J. Biol. Chem. 274 12715–12721. [DOI] [PubMed] [Google Scholar]
- Hayashi, H., De Bellis, L., Hayashi, Y., Nito, K., Kato, A., Hayashi, M., Hara-Nishimura, I., and Nishimura, M. (2002. b). Molecular characterization of an Arabidopsis acyl-coenzyme a synthetase localized on glyoxysomal membranes. Plant Physiol. 130 2019–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, M., and Nishimura, M. (2006). Arabidopsis thaliana – A model organism to study plant peroxisomes. Biochim. Biophys. Acta 1763 1382–1391. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., Nito, K., Takei-Hoshi, R., Yagi, M., Kondo, M., Suenaga, A., Yamaya, T., and Nishimura, M. (2002. a). Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant Cell Physiol. 43 1–11. [DOI] [PubMed] [Google Scholar]
- Hoyos, M.E., Palmieri, L., Wertin, T., Arrigoni, R., Polacco, J.C., and Palmieri, F. (2003). Identification of a mitochondrial transporter for basic amino acids in Arabidopsis thaliana by functional reconstitution into liposomes and complementation in yeast. Plant J. 33 1027–1035. [DOI] [PubMed] [Google Scholar]
- Kamada, T., Nito, K., Hayashi, H., Mano, S., Hayashi, M., and Nishimura, M. (2003). Functional differentiation of peroxisomes revealed by expression profiles of peroxisomal genes in Arabidopsis thaliana. Plant Cell Physiol. 44 1275–1289. [DOI] [PubMed] [Google Scholar]
- Koo, A.J., Chung, H.S., Kobayashi, Y., and Howe, G.A. (2006). Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J. Biol. Chem. 281 33511–33520. [DOI] [PubMed] [Google Scholar]
- Larkin, M.A., et al. (2007). ClustalW and Clustal X version 2.0. Bioinformatics 23 2947–2948. [DOI] [PubMed] [Google Scholar]
- Lawand, S., Dorne, A.J., Long, D., Coupland, G., Mache, R., and Carol, P. (2002). Arabidopsis A BOUT DE SOUFFLE, which is homologous with mammalian carnitine acyl carrier, is required for postembryonic growth in the light. Plant Cell 14 2161–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y., et al. (2002). Cross-referencing eukaryotic genomes. Genome Res. 12 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroch, M., Kirchberger, S., Haferkamp, I., Wahl, M., Neuhaus, H.E., and Tjaden, J. (2005). Identification and characterization of a novel plastidic adenine nucleotide uniporter from Solanum tuberosum. J. Biol. Chem. 280 17992–18000. [DOI] [PubMed] [Google Scholar]
- Leroch, M., Neuhaus, H.E., Kirchberger, S., Zimmermann, S., Melzer, M., Gerhold, J., and Tjaden, J. (2008). Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of Arabidopsis. Plant Cell 20 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisenbee, C.S., Lingard, M.J., and Trelease, R.N. (2005). Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J. 43 900–914. [DOI] [PubMed] [Google Scholar]
- McCartney, A.W., Greenwood, J.S., Fabian, M.R., White, K.A., and Mullen, R.T. (2005). Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 17 3513–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima, M., Yokoi, H., and Asahi, T. (1988). Evidence for no proteolytic processing during transport of isocitrate lyase into glyoxysomes in castor bean endosperm. Plant Cell Physiol. 29 381–384. [Google Scholar]
- Maia, I.G., Benedetti, C.E., Leite, A., Turcinelli, S.R., Vercesi, A.E., and Arruda, P. (1998). AtPUMP: An Arabidopsis gene encoding a plant uncoupling mitochondrial protein. FEBS Lett. 429 403–406. [DOI] [PubMed] [Google Scholar]
- Mano, S., Hayashi, M., and Nishimura, M. (1999). Light regulates alternative splicing of hydroxypyruvate reductase in pumpkin. Plant J. 17 309–320. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T., Imanaka, T., Morita, M., Ishiguro, K., Yurimoto, H., Yamashita, A., Kato, N., and Sakai, Y. (2000). Peroxisomal membrane protein Pmp47 is essential in the metabolism of middle-chain fatty acid in yeast peroxisomes and is associated with peroxisome proliferation. J. Biol. Chem. 275 3455–3461. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T., et al. (2007). Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71 2095–2100. [DOI] [PubMed] [Google Scholar]
- Neuhaus, H.E., Thom, E., Möhlmann, T., Steup, M., and Kampfenkel, K. (1997). Characterization of a novel eukaryotic ATP/ADP translocator located in the plastid envelope of Arabidopsis thaliana L. Plant J. 11 73–82. [DOI] [PubMed] [Google Scholar]
- Orth, T., Reumann, S., Zhang, X., Fan, J., Wenzel, D., Quan, S., and Hu, J. (2007). The PEROXIN11 protein family controls peroxisome proliferation in Arabidopsis. Plant Cell 19 333–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, L., Arrigoni, R., Blanco, E., Carrari, F., Zanor, M.I., Studart-Guimaraes, C., Fernie, A.R., and Palmieri, F. (2006). Molecular identification of an Arabidopsis S-adenosylmethionine transporter. Analysis of organ distribution, bacterial expression, reconstitution into liposomes, and functional characterization. Plant Physiol. 142 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, L., Rottensteiner, H., Girzalsky, W., Scarcia, P., Palmieri, F., and Erdmann, R. (2001). Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 20 5049–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, D.N., Pappin, D.J., Creasy, D.M., and Cottrell, J.S. (1999). Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20 3551–3567. [DOI] [PubMed] [Google Scholar]
- Perrière, G., and Gouy, M. (1996). WWW-Query: An on-line retrieval system for biological sequence banks. Biochimie 78 364–369. [DOI] [PubMed] [Google Scholar]
- Pertea, G., Huang, X., Liang, F., Antonescu, V., Sultana, R., Karamycheva, S., Lee, Y., White, J., Cheung, F., Parvizi, B., Tsai, J., and Quackenbush, J. (2003). TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 19 651–652. [DOI] [PubMed] [Google Scholar]
- Picault, N., Hodges, M., Palmieri, L., and Palmieri, F. (2004). The growing family of mitochondrial carriers in Arabidopsis. Trends Plant Sci. 9 138–146. [DOI] [PubMed] [Google Scholar]
- Picault, N., Palmieri, L., Pisano, I., Hodges, M., and Palmieri, F. (2002). Identification of a novel transporter for dicarboxylates and tricarboxylates in plant mitochondria. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 277 24204–24211. [DOI] [PubMed] [Google Scholar]
- Quackenbush, J., Cho, J., Lee, D., Liang, F., Holt, I., Karamycheva, S., Parvizi, B., Pertea, G., Sultana, R., and White, J. (2001). The TIGR Gene Indices: Analysis of gene transcript sequences in highly sampled eukaryotic species. Nucleic Acids Res. 29 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann, S., Bettermann, M., Benz, R., and Heldt, H.W. (1997). Evidence for the presence of a porin in the membrane of glyoxysomes of castor bean. Plant Physiol. 115 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann, S., Ma, C., Lemke, S., and Babujee, L. (2004). AraPerox. A database of putative Arabidopsis proteins from plant peroxisomes. Plant Physiol. 136 2587–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann, S., Maier, E., Benz, R., and Heldt, H.W. (1995). The membrane of leaf peroxisomes contains a porin-like channel. J. Biol. Chem. 270 17559–17565. [DOI] [PubMed] [Google Scholar]
- Reumann, S., Maier, E., Heldt, H.W., and Benz, R. (1998). Permeability properties of the porin of spinach leaf peroxisomes. Eur. J. Biochem. 251 359–366. [DOI] [PubMed] [Google Scholar]
- Schagger, H., Cramer, W.A., and von Jagow, G. (1994). Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217 220–230. [DOI] [PubMed] [Google Scholar]
- Schagger, H., and von Jagow, G. (1991). Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199 223–231. [DOI] [PubMed] [Google Scholar]
- Schmid, M., Davison, T.S., Henz, S.R., Pape, U.J., Demar, M., Vingron, M., Schölkopf, B., Weigel, D., and Lohmann, J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37 501–506. [DOI] [PubMed] [Google Scholar]
- Shockey, J.M., Fulda, M.S., and Browse, J. (2003). Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme a synthetases. Plant Physiol. 132 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes, I.A., Hawes, C., and Baker, A. (2005). AtPEX2 and AtPEX10 are targeted to peroxisomes independently of known endoplasmic reticulum trafficking routes. Plant Physiol. 139 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak, H.F., Murk, J.L., Braakman, I., and Geuze, H.J. (2003). Peroxisomes start their life in the endoplasmic reticulum. Traffic 4 512–518. [DOI] [PubMed] [Google Scholar]
- Tsai, J., Sultana, R., Lee, Y., Pertea, G., Karamycheva, K., Antonescu, V., Cho, J., Parvizi, B., Cheung, F., and Quackenbush, J. (2001). RESOURCERER: A database for annotating and linking microarray resources within and across species. Genome Biol. 2 002.1–0002.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeki, R., Hara-Nishimura, I., Mori, H., and Nishimura, M. (1993). Cloning and sequencing of cDNA for glycolate oxidase from pumpkin cotyledons and northern blot analysis. Plant Cell Physiol. 34 51–57. [PubMed] [Google Scholar]
- Tsugeki, R., Mori, H., and Nishimura, M. (1992). Purification, cDNA cloning and Northern-blot analysis of mitochondrial chaperonin 60 from pumpkin cotyledons. Eur. J. Biochem. 209 453–458. [DOI] [PubMed] [Google Scholar]
- Van Ael, E., and Fransen, M. (2006). Targeting signals in peroxisomal membrane proteins. Biochim. Biophys. Acta 1763 1629–1638. [DOI] [PubMed] [Google Scholar]
- Visser, W.F., van Roermund, C.W., Ijlst, L., Hellingwerf, K.J., Wanders, R.J., and Waterham, H.R. (2005). Demonstration and characterization of phosphate transport in mammalian peroxisomes. Biochem. J. 389 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, W.F., van Roermund, C.W., Waterham, H.R., and Wanders, R.J. (2002). Identification of human PMP34 as a peroxisomal ATP transporter. Biochem. Biophys. Res. Commun. 299 494–497. [DOI] [PubMed] [Google Scholar]
- Wesley, S.V., et al. (2001). Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 27 581–590. [DOI] [PubMed] [Google Scholar]
- Yenofsky, R.L., Fine, M., and Liu, C. (1988). Isolation and characterization of a soybean (Glycine max) lipoxygenase-3 gene. Mol. Gen. Genet. 211 215–222. [Google Scholar]
- Zolman, B.K., Monroe-Augustus, M., Thompson, B., Hawes, J.W., Krukenberg, K.A., Matsuda, S.P., and Bartel, B. (2001. a). chy1, an Arabidopsis mutant with impaired beta-oxidation, is defective in a peroxisomal beta-hydroxyisobutyryl-CoA hydrolase. J. Biol. Chem. 276 31037–31046. [DOI] [PubMed] [Google Scholar]
- Zolman, B.K., Silva, I.D., and Bartel, B. (2001. b). The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 127 1266–1278. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.