Abstract
Phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] occurs in the apical plasma membrane of growing pollen tubes. Because enzymes responsible for PtdIns(4,5)P2 production at that location are uncharacterized, functions of PtdIns(4,5)P2 in pollen tube tip growth are unresolved. Two candidate genes encoding pollen-expressed Arabidopsis thaliana phosphatidylinositol-4-phosphate 5-kinases (PI4P 5-kinases) of Arabidopsis subfamily B were identified (PIP5K4 and PIP5K5), and their recombinant proteins were characterized as being PI4P 5-kinases. Pollen of T-DNA insertion lines deficient in both PIP5K4 and PIP5K5 exhibited reduced pollen germination and defects in pollen tube elongation. Fluorescence-tagged PIP5K4 and PIP5K5 localized to an apical plasma membrane microdomain in Arabidopsis and tobacco (Nicotiana tabacum) pollen tubes, and overexpression of either PIP5K4 or PIP5K5 triggered multiple tip branching events. Further studies using the tobacco system revealed that overexpression caused massive apical pectin deposition accompanied by plasma membrane invaginations. By contrast, callose deposition and cytoskeletal structures were unaltered in the overexpressors. Morphological effects depended on PtdIns(4,5)P2 production, as an inactive enzyme variant did not produce any effects. The data indicate that excessive PtdIns(4,5)P2 production by type B PI4P 5-kinases disturbs the balance of membrane trafficking and apical pectin deposition. Polar tip growth of pollen tubes may thus be modulated by PtdIns(4,5)P2 via regulatory effects on membrane trafficking and/or apical pectin deposition.
INTRODUCTION
Pollen tubes grow by polar tip expansion and are an important model system for the study of directional tip growth of eukaryotic cells. Cellular elongation involves the biogenesis and targeted delivery of endomembrane vesicles transporting membrane patches and cell wall material to the growing tip (Franklin-Tong, 1999; Krichevsky et al., 2007). Vesicle fusion with the plasma membrane is restricted to a small target area in the tip of the growing pollen tube, which defines the zone of expansion for unidirectional tip growth (Ryan et al., 2001; Moscatelli et al., 2007; Zonia and Munnik, 2008). The transport of exocytotic vesicles from the endoplasmic reticulum and Golgi to the plasma membrane occurs along F-actin strands that form along the longitudinal axis of the growing cell (Voigt et al., 2005; Lee et al., 2008). Vesicles move in a reverse-fountain pattern (Hepler et al., 2001), enabling peripheral delivery of exocytotic vesicles to the tip and central retrieval of endocytotic vesicles that are being recycled to endosomal compartments after deposition of their cargo (Picton and Steer, 1983; Hepler et al., 2001). The presence of a ring-like arrangement of cortical actin, termed the actin fringe, near the growing pollen tube tip has been demonstrated to be essential for reverse-fountain streaming, and pharmacological perturbation of actin fringe assembly results in the loss of directional vesicle traffic and abolishes cell polarity (Cardenas et al., 2008). Other elements participating in the regulation of tip growth in plant cells include a variety of essential regulatory GTPases (Kost et al., 1999; Preuss et al., 2004; Gu et al., 2005; Klahre and Kost, 2006; Song et al., 2006) as well as factors required for vesicle trafficking and targeted secretion of cargo vesicles (Yuen et al., 2005; Song et al., 2006).
The main vesicle cargo transported to the growing pollen tube apex is cell wall material, and the specific properties of the cell wall components deposited in the growing tip are important for directional growth of pollen tubes (Krichevsky et al., 2007). The apical cell wall of pollen tubes consists of a single layer of pectin (Ferguson et al., 1998), granting elasticity to the growing tip while maintaining structural integrity of the pollen tube cell (Steer and Steer, 1989; Bosch and Hepler, 2005). Cell wall–modifying enzymes delivered to the growing tip progressively promote rigidification of the pectin layer toward the basal end of the apical region, whereas secreted pectin in the advancing apex maintains an elastic state (Catoire et al., 1998; Wen et al., 1999; Ren and Kermode, 2000; Jiang et al., 2005). Regulating the balance between apical cell wall stiffening and loosening is crucial for directional pollen tube growth (Moustacas et al., 1991; Bordenave et al., 1996; Catoire et al., 1998), and pollen tube growth was slowed or irregular in plants deficient in some of the various cell wall–modifying enzymes (Jiang et al., 2005; Tian et al., 2006). While the cell wall of the growing tip secretes pectin, the cell wall of the shank of plant pollen tubes ∼15 μm or more away from the tip is additionally rigidified by incorporation of callose and cellulose (Taylor and Hepler, 1997; Cheung and Wu, 2008). In pollen tubes of Nicotiana alata, callose represents the most prominent structural cell wall component, accounting for ∼86% of the cell wall mass, whereas cellulose is only a minor constituent, occupying up to 5% of the cell wall mass (Li et al., 1999). Pectin is more prominent, constituting ∼15% of the cell wall mass (Li et al., 1999). It is important to note that neither callose nor cellulose are present in the growing apical region of tobacco (Nicotiana tabacum) pollen tubes, nor are they detected in secretory vesicles in the clear zone (Ferguson et al., 1998), indicating that pectin secretion via targeted vesicle delivery is a key process important for pollen tube growth (Franklin-Tong, 1999).
Although the mechanics of pollen tube growth have been extensively studied, the factors coordinating exocytosis or pectin deposition are not well understood. In this study, we aimed to elucidate the roles of the membrane lipid, phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2], in polar tip growth of pollen tubes. PtdIns(4,5)P2 has been visualized in apical plasma membrane microdomains of pollen tube and root hair tips using immunofluorescence (Braun et al., 1999) or expression of the fluorescence-tagged Pleckstrin homology (PH) domain of human phospholipase C (PLC) δ1 (Kost et al., 1999; Vincent et al., 2005; Dowd et al., 2006; Preuss et al., 2006; Stenzel et al., 2008), which can serve as a PtdIns(4,5)P2-specific reporter (Varnai and Balla, 1998). Because of its occurrence in plasma membrane microdomains of the apical region of these tip-growing cells, PtdIns(4,5)P2 has been proposed to play a role in the regulation of directional tip growth of plant cells, including pollen tubes and root hairs (Braun et al., 1999; Kost et al., 1999; Vincent et al., 2005). Microinjection of caged PtdIns(4,5)P2 and photorelease has been shown to alter directional growth in pollen tubes (Monteiro et al., 2005); however, the mode of action of PtdIns(4,5)P2 in the regulation of polar tip growth is, so far, not clear.
PtdIns(4,5)P2 can regulate multiple physiological processes in all eukaryotic cell types studied so far by interacting with various protein partners that are regulated in their biochemical activity or localization (Mueller-Roeber and Pical, 2002; Meijer and Munnik, 2003; Balla, 2006). Relevant examples for proteins regulated by PtdIns(4,5)P2 include the actin-modifying enzymes profilin, cofilin, and gelsolin (Drobak et al., 1994; Lemmon et al., 2002; Doughman et al., 2003; Wasteneys and Galway, 2003; Wenk and De Camilli, 2004) as well as factors implicating PtdIns(4,5)P2 in the regulation of vesicle fusion (Cremona and De Camilli, 2001; Di Paolo et al., 2004; Gong et al., 2005; Milosevic et al., 2005; He et al., 2007; Liu et al., 2007). While little information is available about the functions of PtdIns(4,5)P2 in exocytosis, we have recently shown that PtdIns(4,5)P2 associates with endocytotic vesicles in plant cells (König et al., 2008b), suggesting a role in membrane trafficking. In addition to its functions as an intact lipid, PtdIns(4,5)P2 can be hydrolyzed by PLC, yielding diacylglycerol and the soluble second messenger, inositol 1,4,5-trisphosphate (Berridge, 1983; Meijer and Munnik, 2003; Dowd et al., 2006; Helling et al., 2006).
Because the enzymes and corresponding genes responsible for generating PtdIns(4,5)P2 in pollen tubes have not been identified, the exact physiological roles of PtdIns(4,5)P2 in the context of polar tip growth have so far eluded functional investigation. In plants, PtdIns(4,5)P2 is formed by phosphorylation of the more abundant (König et al., 2008a) precursor lipid, phosphatidylinositol-4-phosphate (PtdIns4P), which is catalyzed by phosphatidylinositol-4-phosphate 5-kinases (PI4P 5-kinases) (Drobak et al., 1999; Mueller-Roeber and Pical, 2002). The Arabidopsis thaliana genome contains 11 genes with similarity to animal PI4P 5-kinase genes (Mueller-Roeber and Pical, 2002), some of which have been experimentally characterized in detail and been shown to encode enzymes with PI4P 5-kinase activity (Elge et al., 2001; Perera et al., 2005; Lee et al., 2007; Kusano et al., 2008; Stenzel et al., 2008). Plant PI4P 5-kinase sequences can be classified as subfamilies A and B (Mueller-Roeber and Pical, 2002). Type A enzymes (isoforms 10 and 11) exhibit a domain structure similar to animal and human PI4P 5-kinases. Type B kinases (isoforms 1 to 9) contain a large N-terminal extension characterized by membrane occupation and recognition nexus (MORN) repeats (Mueller-Roeber and Pical, 2002), which are also found in proteins of animal or plant origin that mediate protein-to-membrane contacts, such as junctophilins (Takeshima et al., 2000); the Toxoplasma gondii protein, MORN1, involved in cell-division (Gubbels et al., 2006); or the Arabidopsis accumulation and replication of chloroplasts 3 (ARC3) protein, involved in plastidial fission (Maple et al., 2007). PI4P 5-kinases are soluble proteins that can be dynamically recruited to membranes (Rao et al., 1998). Membrane recruitment of PI4P 5-kinases in mammalian cells involves the action of small Rac-type GTPases (Santarius et al., 2006). In plants, it has been shown that the monomeric GTPase, Rac2, associates with an unspecified PI4P 5-kinase activity in immuno-pull-down assays (Kost et al., 1999). So far, however, membrane recruitment of a particular PI4P 5-kinase isoform by direct interaction with a small GTPase has not been reported in plants.
In this study, two pollen-expressed type B PI4P 5-kinase genes, PIP5K4 and PIP5K5, were identified in the Arabidopsis genome. Pollen of T-DNA insertion lines lacking transcripts of both these genes exhibited reduced pollen germination and defects in pollen tube elongation. The subcellular distribution of fluorescence-tagged enzymes indicates association of both PIP5K4 and PIP5K5 with the plasma membrane PtdIns(4,5)P2 microdomain of the pollen tube tip. Heterologous overexpression of PIP5K4 or PIP5K5 resulted in increased apical pectin deposition, multiple tube branching events, and some other severe morphological changes. The data indicate that PtdIns(4,5)P2 may contribute to the regulation of pollen tube growth by affecting membrane trafficking and apical secretion of pectin.
RESULTS
Arabidopsis PI4P 5-Kinase Isoforms, PIP5K4 and PIP5K5, Are Expressed in Pollen and Catalyze the Conversion of PtdIns4P to PtdIns(4,5)P2
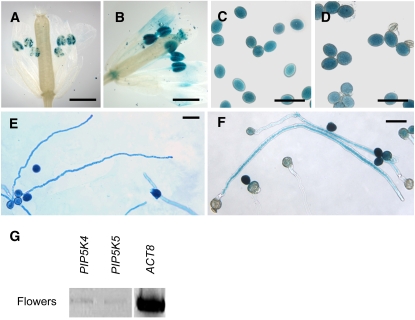
Arabidopsis PI4P 5-kinase isoforms with potential roles in the regulation of pollen tube growth were identified using transcript array information accessible through the Genevestigator portal (Zimmermann et al., 2004). In addition to the uncharacterized PI4P 5-kinase gene, PIP5K5, the gene PIP5K4 was included in our analysis, which was previously shown to encode an active PI4P 5-kinase with a role in guard cell function (Lee et al., 2007). Expression of these selected genes in pollen was verified by expression of 1500-bp promoter fragments of these genes driving expression of a β-glucuronidase (GUS) reporter in transgenic plants, followed by histochemical staining (Figures 1A to 1F) and independently by RT-PCR (Figure 1G). The promoter-GUS experiments indicate that PI4P 5-kinase isoforms 4 and 5 are both expressed in pollen grains (Figures 1A to 1D) and that promoter activity is also present in growing pollen tubes of Arabidopsis (Figures 1E and 1F). RT-PCR analysis of expression in Arabidopsis flowers (Figure 1G) indicates low expression for both PI4P 5-kinase isoforms tested. Note that the expression patterns do not exclude expression of PIP5K4 or PIP5K5 in organs other than pollen. It is also possible that additional PI4P 5-kinases may contribute to PtdIns(4,5)P2 production in pollen tubes.
Figure 1.
Type B-PI4P 5-Kinase Genes, PIP5K4 and PIP5K5, Are Expressed in Pollen.
Histochemical staining for GUS activity in transgenic Arabidopsis plants expressing the GUSplus reporter gene under the putative (1500-bp 5′ untranslated region) promoters of PIP5K4 and PIP5K5. Samples were stained for 24 h at room temperature. Images are representative of at least three independent transgenic lines. For each line, at least 20 flowers, 100 pollen grains, and 100 pollen tubes were examined. Bars = 300 μm in (A) and (B) and 50 μm in (C) to (F).
(A), (C), and (E) PIP5K4.
(B), (D), and (F) PIP5K5.
(A) and (B) GUS activity in flowers.
(C) and (D) GUS activity in pollen grains.
(E) and (F) GUS activity in pollen tubes.
(G) Transcript abundance in Arabidopsis flowers according to RT-PCR analysis. The ACT8 gene was used as a control. RT-PCR detection of PIP5K4 and PIP5K5 transcripts was performed three times with similar results.
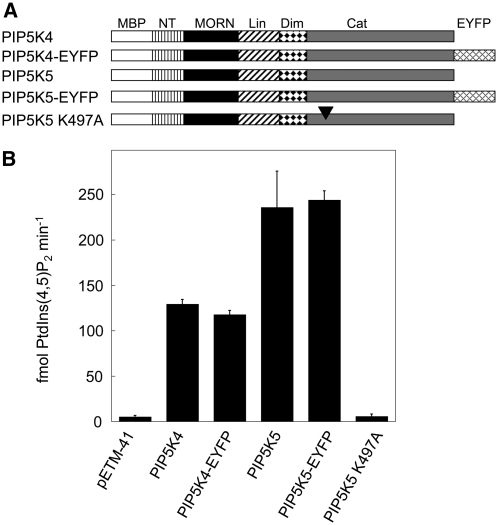
To characterize the functions of PIP5K4 and PIP5K5 gene products, the corresponding cDNAs were cloned and heterologously expressed in Escherichia coli as fusions to maltose binding protein (MBP) tags, and the recombinant proteins were tested for activity in vitro. Catalytic activities of the recombinant enzymes with the preferred substrate, PtdIns4P, are presented in Figure 2. To determine whether enhanced yellow fluorescent protein (EYFP) tags of fluorescently labeled fusion proteins would obstruct catalytic activity, relevant protein fusions (Figure 2A) were recombinantly expressed and tested for activity. Note that the presence of a fluorescence tag did not affect catalytic activity of the recombinant enzymes (Figure 2B). A variant of PIP5K5 mutated in its ATP binding site (Ishihara et al., 1998) was also tested, and the catalytic activity of the mutant protein PIP5K5 K497A against PtdIns4P was reduced to background levels (Figure 2B). When the catalytic activity of recombinant enzymes not carrying fluorescence tags was tested against different potential lipid substrates, the preferred substrate was PtdIns4P (Table 1). Minor phosphorylation activity against PtdIns3P was also observed, whereas no activity was detected with PtdIns5P as a substrate (Table 1). The in vitro activity assays confirm that PIP5K4 and PIP5K5 represent active PI4P 5-kinases.
Figure 2.
Arabidopsis PI4P 5-Kinase Isoforms PIP5K4 and PIP5K5 Catalyze the Conversion of PtdIns4P to PtdIns(4,5)P2.
The gene products PIP5K4 and PIP5K5 were heterologously expressed in E. coli as fusion proteins to N-terminal MBP tags, and the recombinant extracts were tested in vitro for enzymatic activity against PtdIns4P.
(A) Graphical representation of constructs tested. In addition to PIP5K4 and PIP5K5, constructs encoding these proteins as fusions with C-terminal EYFP tags were also created and tested. A mutated variant of PIP5K5, PIP5K5 K497A with a dysfunctional ATP binding site (arrowhead), was also included. NT, N-terminal domain; Lin, linker domain; Dim, dimerization domain; Cat, catalytic domain; EYFP, EYFP tag.
(B) Catalytic activity of recombinant enzymes expressed in E. coli. Concentrations of recombinant proteins were equalized according to the results of a protein gel blot analysis. Data indicate the mean formation of PtdIns(4,5)P2 (in fmol min−1) from three to five independent experiments ± sd. pETM-41, vector control.
Table 1.
Relative Activities of Arabidopsis PI4P 5-Kinase Isoforms Expressed in Pollen
| Protein | PtdIns3P | PtdIns4P | PtdIns5P | PtdIns4P/PtdIns3P Activity Ratio |
|---|---|---|---|---|
| MBP | 4.0 ± 0.3 | 5 ± 1 | 1 ± 0.1 | – |
| PIP5K4 | 12.3 ± 2.0 | 129 ± 6 | 1 ± 0.3 | 10 |
| PIP5K5 | 19.7 ± 27.0 | 236 ± 40 | 1 ± 0.2 | 12 |
Activities were tested in vitro against different phosphatidylinositol-monophosphate substrates and indicate the rate of product formation in fmol min−1. Recombinant protein concentrations were balanced according to protein gel blot analysis. Data are the means of two or four (PtdIns4P) independent experiments ± sd. No PtdIns4P/PtdIns3P ratio was determined for the inactive MBP control, as indicated by the dash.
The T-DNA pip5k4 pip5k5 Double Mutant Is Impaired in in Vitro Pollen Germination and in Pollen Tube Growth
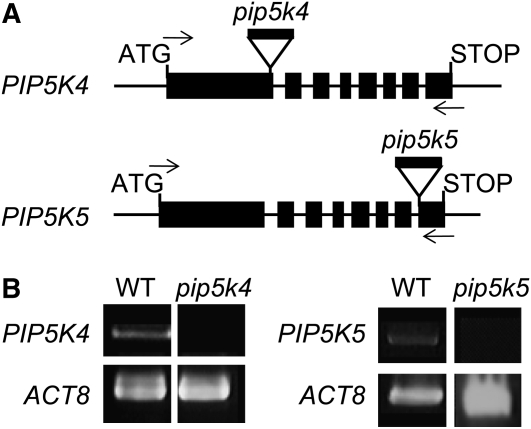
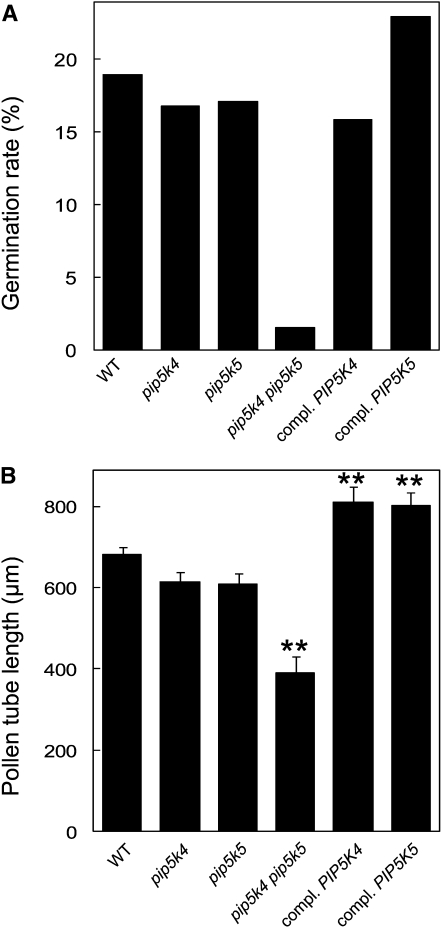
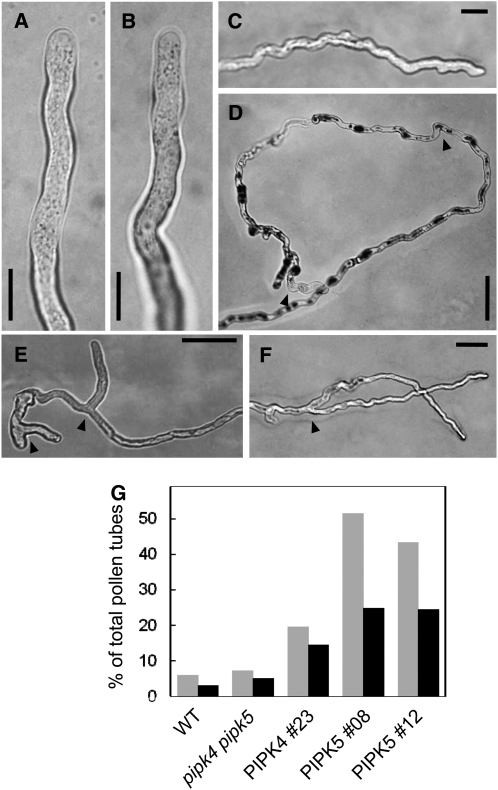
Homozygous Arabidopsis T-DNA mutant lines with exon insertions in the genes for PIP5K4 (SALK_001138) and PIP5K5 (SALK_147475) (Figure 3A) were isolated. Homozygosity was inferred by the inability to amplify wild-type alleles concomitant with positive amplification of T-DNA–tagged alleles (see Supplemental Figure 1 online). The exact sites of the T-DNA insertions were determined by sequencing, and the T-DNA insertions were found to be located in the first exon of pip5k4, 1186 bp downstream from the ATG start codon, and in the eighth exon of pip5k5, 2811 bp downstream from the ATG. Reduction in transcript levels was tested by RT-PCR, indicating that the respective transcripts were lacking or reduced to levels below detection (Figure 3B). Single homozygous mutants showed no obvious defects in pollen germination or pollen tube growth (Figure 4); therefore, pip5k4 pip5k5 double mutants were obtained by crossing homozygous pip5k4 and pip5k5 mutant lines. When pollen of the double mutant was germinated in vitro, the rate of germination was substantially reduced (Figure 4A) and emerging pollen tubes showed significantly less tube growth (Figure 4B), indicating that PIP5K4 and PIP5K5 contributed to the functionality of the pollen tube. Pollen tubes emerging from pip5k4 pip5k5 double mutant pollen were not obviously altered in their morphology, aside from their reduced length, and no wavy growth, tip swelling, or other morphological changes were observed compared with wild-type controls (Figures 5A and 5B). To verify that the pip5k4 pip5k5 double mutant phenotype was attributable to the disruption of the PIP5K4 and PIP5K5 genes, double mutant plants were transformed with PIP5K4:EYFP or PIP5K5:EYFP under the control of intrinsic PIP5K4 or PIP5K5 1500-bp promoter fragments (Figure 4). Both ProPIP5K4:PIP5K4:EYFP and ProPIP5K5:PIP5K5:EYFP independently rescued the germination and growth phenotypes of pip5k4 pip5k5 double mutant pollen, and germination (Figure 4A) and pollen tube growth (Figure 4B) even exceeded those of wild-type pollen. In one example, 47% (n = 200) of the pollen grains from the same T1 flower showed EYFP fluorescence, indicating only one PIP5K5:EYFP insertion site. Close to all (96%; 144 out of 150) of the pollen tubes counted emerging from pollen grains of this plant showed EYFP fluorescence, indicating successful complementation of the pip5k4 pip5k5 double mutant by a single insertion of the ectopic construct encoding PIP5K5-EYFP. In some transgenic lines likely containing multiple copies of the PIP5K4:EYFP or the PIP5K5:EYFP transgene and exhibiting high levels of fluorescence, aberrant pollen tube morphology was observed (Figures 5C to 5F). Morphologies observed in strong overexpressors were characterized by a zigzag or wavy growth pattern (Figures 5C and 5D) or by pollen tube branching (Figures 5E and 5F). Even though zigzag growth and/or tube branching also occasionally occurs in Arabidopsis wild-type pollen tubes, the frequency of tube branching was roughly eight times higher for both phenotypes of transgenic type B PI4P 5-kinase overexpressors than for nontransformed controls (Figure 5G). The data indicate a role for PIP5K4 and PIP5K5 in pollen germination and pollen tube growth.
Figure 3.
Isolation of T-DNA Insertion Mutants pip5k4 and pip5k5.
T-DNA insertion lines for PIP5K4 (SALK_001138) and PIP5K5 (SALK_147475) were isolated.
(A) Graphical representation of the positions of the insertions. The T-DNA insertion in pip5k4 is located in the first exon and that in pip5k5 in the 8th exon. Arrows indicate the approximate positions of PCR primers used for genotyping and for transcript detection in cDNA preparations. Black boxes indicate exons; lines between boxes indicate introns.
(B) Reduction in transcript levels according to RT-PCR analysis. ACT8 was used as a loading control. The experiment was performed three times with similar results.
Figure 4.
Germination and Growth of Pollen Tubes of T-DNA Insertion Mutants Deficient in PIP5K4 and/or PIP5K5.
Pollen from different Arabidopsis lines were germinated in vitro, and the germination rate and pollen tube length were determined. Complementation of mutant phenotypes was accomplished by expression of the transgenes under their respective intrinsic promoters.
(A) Scoring of pollen germination of the lines shown. Data represent the distribution observed in >1300 scored pollen.
(B) Quantification of pollen tube length 24 h after germination according to digital image analysis. Data represent mean values ± se of at least 89 pollen tubes and were determined using pollen pooled from at least 30 flowers from five different plants for each line shown. Double asterisks indicate significant differences from the wild type according to a Student's t test (P < 0.01).
Figure 5.
Morphological Changes of Arabidopsis Pollen Tubes Overexpressing Type B PI4P 5-Kinases.
Complemented pip5k4 pip5k5 double mutants showing high fluorescence intensity of EYFP-tagged PIP5K4 or PIP5K5 were imaged by light microscopy.
(A) Wild-type control.
(B) pip5k4 pip5k5 double mutant control. Bars = 10 μm.
(C) and (D) Zigzag growth pattern observed with overexpression of PIP5K4 (C) or PIP5K5 (D); arrowheads indicate characteristic zigzag turns of >90°. Bars = 20 μm in (C) and 50 μm in (D).
(E) and (F) Pollen tube branching observed with overexpression of PIP5K4 (E) or PIP5K5 (F). Arrowheads indicate sites of branching. Bars = 50 μm.
(G) As morphological changes also occasionally occurred in wild-type controls, the incidence of altered morphology was calculated for controls and transgenic lines. Gray bars, zigzag growth; black bars, branching. Data presented are from three independent transgenic lines overexpressing PIP5K4 (#23) or PIP5K5 (#08 and #12) and from wild-type and pip5k4 pip5k5 double mutant controls. For each line, between 120 and 200 pollen tubes were scored.
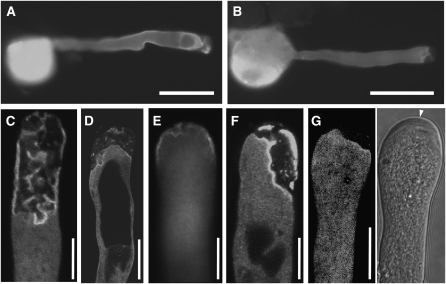
PIP5K4 and PIP5K5 Localize to a Distinct Domain of the Apical Plasma Membrane of Arabidopsis and Tobacco Pollen Tubes
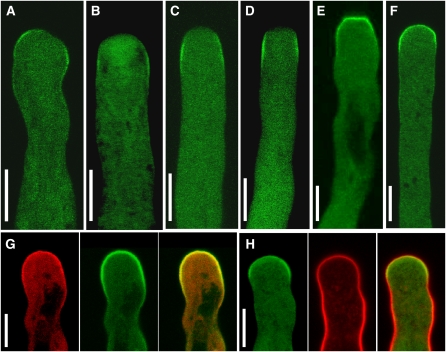
To test whether the PI4P 5-kinases under study were candidate enzymes responsible for the generation of the PtdIns(4,5)P2 domain in the apical plasma membrane of pollen tube cells, we tested whether PIP5K4 and PIP5K5 localized to this subcellular region. PIP5K4 and PIP5K5 were expressed in Arabidopsis plants as fusions to C-terminal EYFP tags driven by 1500-bp intrinsic promoter fragments. The structure of the fluorescence-tagged constructs corresponds to those shown in Figure 2A, with the exception that fusion proteins expressed in pollen tubes did not contain the MBP tag present in the recombinant proteins expressed in E. coli. Pollen of the transgenic Arabidopsis progeny was germinated in vitro, and the fluorescence distribution was monitored in the emerging pollen tubes (Figures 6A and 6B). Both PIP5K4 and PIP5K5 localized in a peripheral ring-like pattern close to the apex of Arabidopsis pollen tubes, moving forward as the pollen tubes grew. For both enzymes, a substantial degree of diffuse cytosolic fluorescence that was restricted to the apical region of the pollen tubes was also observed. From the localization of PIP5K4 and PIP5K5, it can be concluded that both enzymes could play roles in the generation of the tip-localized PtdIns(4,5)P2 domain. PIP5K4 and PIP5K5 were also expressed as fusions to C-terminal EYFP tags in Arabidopsis driven by the pollen-specific Lat52 promoter (Twell et al., 1990), and similar levels of expressed fluorescence and similar localization patterns were observed as with the intrinsic promoters.
Figure 6.
Type B PI4P 5-Kinases Localize to a Distinct Plasma Membrane Microdomain of the Pollen Tube Tip.
Fluorescence distribution of EYFP-tagged PIP5K4 or PIP5K5 was monitored by recording 0.9-μm confocal midplane sections in Arabidopsis ([A] and [B]) or tobacco pollen tubes ([C] to [H]) during growth ([A] to [D]) or after cessation of growth ([E] to [H]). Images are representative for >20 ([A] and [B]; [G] and [H]) or >50 ([C] to [F]) recorded pollen tubes.
(A) PIP5K4.
(B) PIP5K5.
(C) PIP5K4.
(D) PIP5K5.
(E) PIP5K4.
(F) PIP5K5.
(G) Coexpression of EYFP-tagged PIP5K4 with CFP-tagged PIP5K5. Left, PIP5K5-CFP; middle, PIP5K4-EYFP; right, merge.
(H) Plasma membrane association of EYFP-tagged PIP5K5 was analyzed in reference to the steryl-dye FM 4-64. Left, PIP5K5-EYFP; middle, FM 4-64; right, merge. Yellow color indicates plasma membrane localization of EYFP-tagged PIP5K5. Bars = 10 μm.
In parallel to experiments on Arabidopsis plants, PIP5K4 and PIP5K5 were transiently expressed in tobacco pollen tubes to study the effects of perturbing PtdIns(4,5)P2 production. The tobacco system provides a number of experimental advantages over Arabidopsis. Arabidopsis pollen tubes do not grow as reliably as pollen tubes from some other species (Boavida and McCormick, 2007). Furthermore, transformation of Arabidopsis pollen by particle bombardment has not been reported and was also not successful in our hands, limiting localization experiments in Arabidopsis pollen tubes to stable expression approaches. The tobacco pollen tube system has long been established, is reliable in its growth and germination characteristics, and makes efficient transient expression possible (Kost et al., 1998). Transient expression levels of proteins reached in tobacco were also higher than those obtained using the same promoters for stable expression in Arabidopsis, opening additional avenues of investigation that were exploited in the experiments described below. To test whether or not Arabidopsis PIP5K4 or PIP5K5 would mislocalize in tobacco pollen tubes, the Arabidopsis enzymes were transiently expressed under the Lat52 promoter in tobacco pollen grains as in-frame fusions with C-terminal EYFP tags. The tobacco pollen were germinated, and pollen tube growth was monitored. Transient gene expression by particle bombardment yields a range of expression levels, indicated by variations in the resulting fluorescence intensities of the expressed fusion proteins. To allow for the documentation of meaningful subcellular distribution patterns of fluorescence-tagged PI4P 5-kinases, tobacco pollen tubes were selected that exhibited low fluorescence intensities of the expressed fusion proteins comparable to those achieved with stable expression using intrinsic promoter fragments in Arabidopsis (Figures 6C to 6F). In growing tobacco pollen tubes, PIP5K4 and PIP5K5 localized laterally in a ring-like plasma membrane domain close to the growing tip (Figures 6C and 6D, respectively). Dynamic localization of PIP5K5 in tobacco pollen tubes can be assessed from Supplemental Movie 1 online. With cessation of growth, PIP5K4 and PIP5K5 both localized to plasma membrane areas that included the apex of tobacco pollen tubes (Figures 6E and 6F, respectively). To document overlapping localization patterns of PIP5P4 and PIP5K5 in tobacco pollen tubes, the subcellular distribution of PIP5K4 C-terminally tagged with cyan-fluorescent protein (CFP) was directly compared with that of PIP5K5 C-terminally tagged with EYFP in a coexpression experiment (Figure 6G). Because the coexpression of PIP5K4-CFP and PIP5K5-EYFP resulted in cessation of growth, no coexpression was observed in growing pollen tubes; in nongrowing tobacco pollen tubes, the localization of PIP5K4 and PIP5K5 was similar. Plasma membrane localization of PIP5K5 in tobacco pollen tubes was compared with the distribution of the steryl-dye, FM 4-64 (Figure 6H), and PIP5K5 was found to decorate a distinct plasma membrane–associated domain in addition to a diffuse pattern in the cytosol. Using both Arabidopsis and tobacco pollen, plasma membrane association was always accompanied by diffuse cytosolic localization. Note that to limit FM 4-64 staining to the plasma membrane, the dye was allowed to perfuse cells for only a few minutes. Overall, the localization data indicate that the localization of PIP5K4 and PIP5K5 in growing tobacco pollen tubes (Figures 6C and 6D) closely reflected that observed in growing Arabidopsis pollen tubes (Figures 6A and 6B), and no mislocalization was observed.
Overexpression of PIP5K4 or PIP5K5 Results in Altered Pollen Tube Morphology
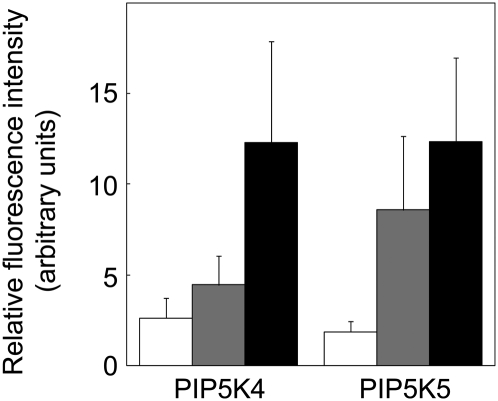
To test possible physiological roles of PtdIns(4,5)P2 in pollen tube growth, tobacco pollen tubes were analyzed in which PIP5K4 or PIP5K5 were overexpressed to different levels, thus perturbing PtdIns(4,5)P2 production. PI4P 5-kinase overexpression drastically altered pollen tube morphology, giving rise to pollen tube branching and a protoplast trapping phenotype likely resulting from a loss of flexibility of the apical cell wall and subsequent restriction of cell expansion, as will be described in more detail below. Altered pollen tube morphology was restricted to tubes with increased expression of the transgenes in comparison to expressors exhibiting no morphological changes (Figure 7). Tobacco pollen tubes with low expression levels exhibited normal growth (cf. Figure 6 and Supplemental Movie 1 online), tubes with intermediate expression levels exhibited tip branching (cf. Figure 8), and tubes with the highest expression levels showed the protoplast trapping phenotype described in more detail below (cf. Figure 9), as summarized in Figure 7.
Figure 7.
Phenotypes Resulting from Overexpression of Type B PI4P 5-Kinases Can Be Categorized According to Expression Levels.
Phenotypes observed in tobacco pollen tubes expressing PIP5K4 or PIP5K5 were categorized and the associated mean fluorescence intensities determined in the apical cytoplasm. White bars, no morphological alteration; gray bars, pollen tube branching; black bars, protoplast trapping phenotype. Intensity data represent means from 10 pollen tubes for each category ± sd. The intensity measurements were repeated twice with similar results.
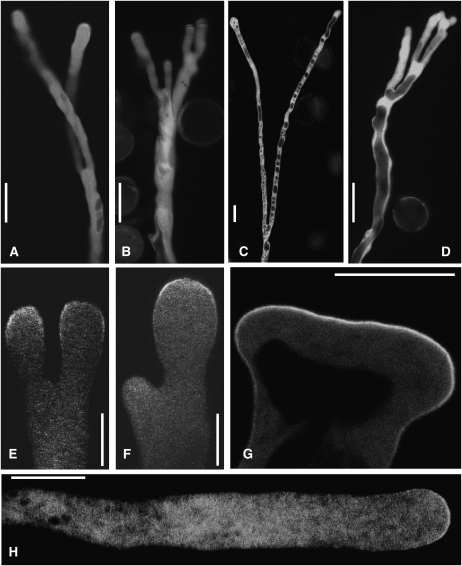
Figure 8.
Branching of Pollen Tubes Expressing Intermediate Levels of Type B PI4P 5-Kinases.
Phenotypes of pollen tubes expressing EYFP-tagged PIP5K4 or PIP5K5 were monitored by epifluorescence ([A] to [D]) or imaging of 0.9-μm confocal midplane sections ([E] to [H]). Bars = 25 μm in (A) to (D) and 10 μm in (E) to (H). Branching was observed in 42 out of 247 pollen tubes expressing PIP5K4 and in 65 out of 253 pollen tubes expressing PIP5K5. The pattern shown in (G) was observed once. Absence of morphological effects with expression of the inactive PIP5K5 K497A was observed in 75 out of 75 cases.
(A) and (B) Dichotomous (A) and multiple (B) branching with expression of PIP5K4.
(C) and (D) Dichotomous (C) and multiple (D) branching with expression of PIP5K5.
(E) Symmetrical branching (PIP5K4).
(F) Asymmetrical branching (PIP5K4).
(G) Expanded PIP5K5 microdomain in a broadened tip, possibly prior to branching.
(H) Unaltered morphology of a tobacco pollen tube upon expression of the inactive PIP5K5 K497A protein. To rule out possible future branching or protoplast trapping, the image was taken after pollen tube growth had ceased.
Figure 9.
Growth Inhibition and Apical Membrane Invaginations of Pollen Tubes Strongly Expressing Type B PI4P 5-Kinases.
Phenotypes of pollen tubes strongly expressing EYFP-tagged PIP5K4 or PIP5K5 were monitored by epifluorescence ([A] and [B]) or confocal microscopy ([C] to [G]).
(A) PIP5K4.
(B) PIP5K5. Note the morphologically altered tip region. Tips of strong overexpressors showed varying degrees of membrane invaginations. Bars = 50 μm.
(C) and (D) PIP5K4.
(E) and (F) PIP5K5.
(G) Strong expression of PIP5K5 (left panel) resulted in apical deposition of cell wall material (right panel, arrowhead). Reduced growth and protoplast trapping were observed in 93 out of 247 pollen tubes expressing PIP5K4 and in 103 out of 253 pollen tubes expressing PIP5K5. Bars = 10 μm.
Pollen Tube Branching with Intermediate Overexpression of PIP5K4 or PIP5K5
Overexpression of either PIP5K4 or PIP5K5 induced a branching pattern of tobacco pollen tube growth at time points beyond 5 h after transformation (Figure 8), similar to that observed with high expression of PIP5K4 or PIP5K5 in Arabidopsis pollen tubes (Figure 5). Branching of pollen tubes was equal (Figures 8A, 8C, and 8E) or unequal (Figure 8F) and could also occur at multiple sites (Figures 8B and 8D), giving rise to pollen tubes with multiple tips. Separate branches showed substantial growth beyond the site of branching, as can be observed in Supplemental Movie 2 online. With expression of PIP5K4, formation of an extended apical microdomain was observed (Figure 8G), indicating an enlarged apical site of PtdIns(4,5)P2 production. Note that expression of the inactive PIP5K5 K497A (cf. Figure 2) did not alter the morphology of tobacco pollen tubes (Figure 8H), and zero tubes expressing the inactive PIP5K5 K497A exhibited morphological changes (n = 75). The branching phenotypes shown were recorded after 14 h of pollen tube growth and were obvious in 42 out of 247 (17%) and 65 out of 253 (26%) of scored tobacco pollen tubes overexpressing PIP5K4 or PIP5K5, respectively, at intermediate levels (cf. Figure 7).
Reduced Pollen Tube Length and Apical Membrane Invaginations with Strong Overexpression of PIP5K4 or PIP5K5
While 17 to 26% of tobacco pollen tubes overexpressing PIP5K4 or PIP5K5 at intermediate levels showed the tip-branching phenotype (Figure 8), tobacco pollen tubes with higher levels of overexpression were shortened and morphologically altered in the apical region (Figure 9). A characteristic effect accompanying very strong overexpression of PIP5K4 or PIP5K5 and the growth inhibition was the formation of membrane invaginations in the apical region of the pollen tubes (Figures 9C to 9G). The observed growth arrest of tobacco pollen tubes strongly overexpressing PIP5K4 or PIP5K5 was always accompanied by thick amorphous deposits of cell wall, as indicated in Figure 9G, which were subsequently studied in more detail. Because the thickened cell wall appeared to limit cell expansion and excess membrane area folded inwards, the observed pattern was designated as “protoplast trapping.” After 14 h of pollen tube growth, this phenotype was obvious in 38% (93 out of 247) and 41% (103 out of 253) of scored tobacco pollen tubes expressing PIP5K4 (Figure 9A) or PIP5K5 (Figure 9B), respectively. Note that the distribution of percentages of phenotypic categories depend on the particular expression levels attained in a particular experiment and will differ between experiments.
PtdIns(4,5)P2 Distribution in the Plasma Membrane
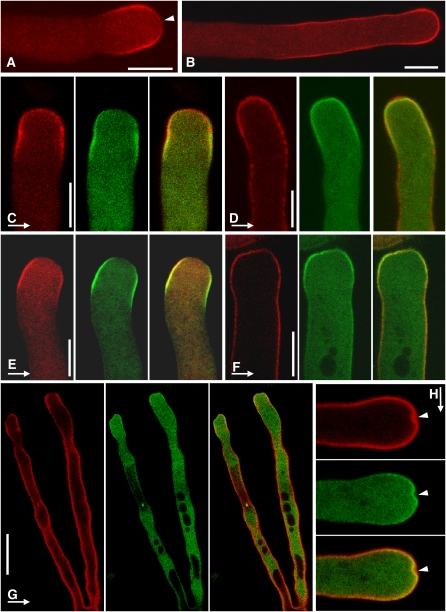
Because the results shown in Figure 8 indicated that catalytic activity and formation of PtdIns(4,5)P2 were responsible for the morphological changes observed, the distribution of PtdIns(4,5)P2 was visualized in tobacco pollen tubes. In vivo visualization was achieved using the PtdIns(4,5)P2-specific PH domain of human PLCδ1 (Varnai and Balla, 1998) expressed as a fusion to the N-terminally attached red fluorescent protein, RedStar (Janke et al., 2004). The RedStar-PLCδ1-PH-reporter decorated a plasma membrane microdomain at the tips of growing tobacco pollen tubes (Figure 10A) or a larger plasma membrane area of nongrowing tobacco pollen tubes (Figure 10B), as was previously reported for PtdIns(4,5)P2 (Kost et al., 1999; Dowd et al., 2006). Note that the distribution of PtdIns(4,5)P2 in growing tobacco pollen tubes closely resembled that of PIP5K4 or PIP5K5 (Figures 6C and 6D). In addition, PtdIns(4,5)P2 was decorated with somewhat less intensity at the extreme apex of growing pollen tubes (Figures 10A and 10C). When coexpressed with PIP5K4 or PIP5K5 each C-terminally tagged with EYFP (Figures 10C and 10D, and 10E and 10F, respectively), the localization of PIP5K4 or PIP5K5 was found to largely overlap that of PtdIns(4,5)P2 in growing tobacco pollen tubes; however, PI4P 5-kinases appeared to be excluded from the extreme apical region. In nongrowing tobacco pollen tubes, however, the localization of RedStar-PLCδ1-PH extended beyond that of PIP5K4 or PIP5K5 with overlap only at the extreme apex, indicating a PtdIns(4,5)P2-rich plasma membrane region further back from the tip that was not associated with PIP5K5 (Figures 10D and 10F). When the spatial distribution of PtdIns(4,5)P2 was analyzed in tobacco pollen tubes overexpressing PIP5K5, extended regions of PtdIns(4,5)P2 were observed in branched tubes (Figure 10G) compared with the distribution observed with low expression (Figure 10B). Emerging membrane invaginations (Figure 10H) were marked by a localized high intensity of the RedStar-PLCδ1-PH-reporter, suggesting localized accumulation of PtdIns(4,5)P2 at these sites. Note that it has been demonstrated that the expression of the human PLCδ1-PH-domain influences phosphoinositide levels of the host cells (Balla et al., 2000; Balla and Varnai, 2002), and such effects cannot be ruled out for PtdIns(4,5)P2 visualizations shown here. Because we observed decreased growth of tobacco pollen tubes when the RedStar-PLCδ1-PH-reporter was expressed at high levels, in the experiments shown, the subcellular distribution of the PtdIns(4,5)P2 reporter was analyzed only in pollen tubes exhibiting as low as possible expression, indicated by low fluorescence intensity, to ensure that effects on PtdIns(4,5)P2 functionality remained small.
Figure 10.
Distribution of Plasma Membrane PtdIns(4,5)P2 Relative to Type B PI4P 5-Kinases.
(A) and (B) Distribution of PtdIns(4,5)P2 decorated by the RedStar-PLCδ1-PH reporter in a growing (A) and a nongrowing tobacco pollen tube (B). Arrowhead indicates weak reporter fluorescence at the extreme apex in a growing pollen tube.
(C) to (H) PtdIns(4,5)P2 was visualized in combination with PIP5K4 ([C] and [D]) or PIP5K5 ([E] to [H]) by coexpression of the RedStar-PLCδ1-PH domain (red) with PIP5K4 or PIP5K5 carrying C-terminal EYFP tags (green). Yellow indicates colocalization. Bars = 10 μm. Images are representative of 20 ([A] and [B]), 10 ([C] to [F]), or 5 (G) recorded pollen tubes, respectively. The stage illustrated in (H) was observed once.
(C) and (E) Growing pollen tubes at low expression levels of the transgenes.
(D) and (F) Nongrowing pollen tubes at low expression levels of the transgenes.
(G) Branched pollen tube with intermediate expression of PIP5K5.
(H) Onset of membrane invagination with high expression of PIP5K5. Top panel, RedStar-PLCδ1-PH-domain; middle panel, PIP5K5-EYFP; bottom panel, merge.
Actin Cytoskeletal Structures in Pollen Tubes Overexpressing PI4P 5-Kinases
Because the actin cytoskeleton is a known target for PtdIns(4,5)P2 regulation in mammalian cells (Yin and Janmey, 2003) and has been demonstrated to contribute to pollen tube morphology (Vidali and Hepler, 2001), we tested whether the morphological alterations observed in pollen tubes overexpressing PI4P 5-kinases would also manifest as regulatory effects on the actin cytoskeleton. These experiments did not reveal an obvious relationship between the actin cytoskeleton and the phenotypes observed with overexpression of PIP5K4 or PIP5K5 (see Supplemental Figure 1 online). Further experiments focused on the pollen tube cell wall.
Overexpression of PIP5K4 or PIP5K5 Results in Increased Apical Secretion of Pectin
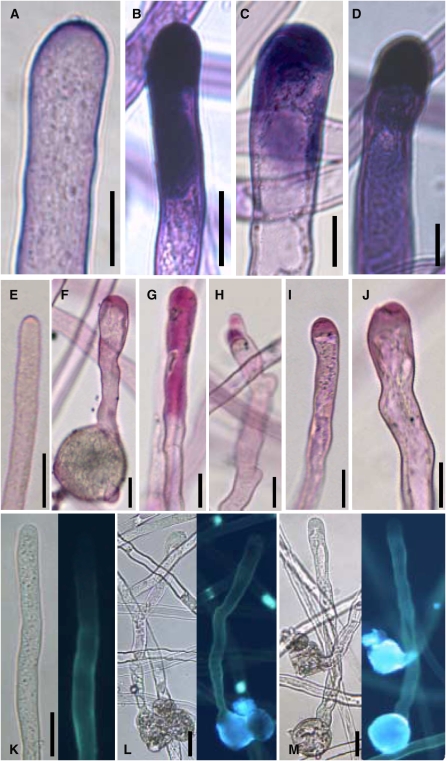
The cell walls of pollen tubes contain various components, such as callose and pectin, only the latter of which is deposited by apical vesicle fusion, as described above. To elucidate the nature of the increased cell wall deposition in the PIP5K4 or PIP5K5 overexpressors, different dyes were employed that stain specific cell wall components (Figure 11). When nontransformed tobacco pollen tubes (Figure 11A) and tubes overexpressing PIP5K4 (Figure 11B) or PIP5K5 (Figures 11C and 11D) were stained for pectin with toluidine blue O (O'Brien et al., 1964), the resulting patterns indicated that the material deposited in great amounts in the overexpressors was pectin. Independent staining with ruthenium red (Iwai et al., 2006), another dye specific for pectin, supported these results (Figures 11E to 11J). As toluidine blue and ruthenium red stain pectin but not callose (O'Brien et al., 1964; Iwai et al., 2006), tobacco pollen tubes overexpressing PIP5K5 were stained with aniline blue, a dye that specifically stains callose (Fukumoto et al., 2005). The tobacco pollen tubes presented in Figures 11A to 11J show the different degrees of pectin deposition observed. For nontransformed tobacco pollen tubes (Figure 11K) or tubes overexpressing PIP5K5 (Figures 11L and 11M) aniline blue fluorescence was observed in the cell wall of the shank but not in that of the tip of the pollen tube. Importantly, the callose patterns observed indicated no excess incorporation of callose into the cell wall of PIP5K4 or PIP5K5 overexpressors. The combined data indicate that overexpression of PIP5K4 or PIP5K5 resulted in increased apical deposition of pectin in tobacco pollen tubes, which may alter the elastic properties of the cell wall and underlie pollen tube branching and the protoplast trapping phenotype.
Figure 11.
Apical Accumulation of Cell Wall Material in Pollen Tubes Strongly Expressing Type B PI4P 5-Kinases.
Pollen tubes strongly expressing either PIP5K4 or PIP5K5 were stained with toluidine blue O ([A] to [D]) or with ruthenium red ([E] to [J]) for pectin or with aniline blue for callose ([K] to [M]). All tubes were imaged after cessation of growth but showed vigorous cytoplasmic streaming. Bars = 10 μm in (A) to (D) and 20 μm in (E) to (M). Images are representative of 50 ([A] to [D]) or 20 ([E] to [M]) recorded pollen tubes, respectively.
(A) Nontransformed control.
(B) PIP5K4 overexpressor.
(C) and (D) PIP5K5 overexpressor.
(E) Nontransformed control.
(F) to (H) PIP5K4 overexpressor.
(I) and (J) PIP5K5 overexpressor.
(K) Nontransformed control.
(L) PIP5K4 overexpressor.
(M) PIP5K5 overexpressor.
DISCUSSION
The directional growth of pollen tubes results from the interplay of multiple processes that require tight spatial and temporal coordination (Franklin-Tong, 1999; Krichevsky et al., 2007). Because the enzymes producing PtdIns(4,5)P2 in pollen tubes have not previously been identified, the roles of PtdIns(4,5)P2 in the regulation of pollen tube growth have remained unresolved. Here, the identification of pollen-expressed genes encoding PI4P 5-kinases (Figure 1) has provided candidate enzymes to investigate the roles of PtdIns(4,5)P2 in pollen tube growth by both underexpression and overexpression approaches. The promoter GUS data must be interpreted with some caution because the GUS protein is very stable and may linger for a considerable amount of time after promoter activity has ceased. The data presented here indicate that promoter activity of the PIP5K4 and PIP5K5 genes can generate proteins with a spatio-temporal distribution suitable for a role in pollen tube germination and growth.
The reported effects of PI4P 5-kinases on pollen tube morphology must be reviewed in light of the catalytic activity of the PI4P 5-kinases tested (Figure 2). The observed substrate preference for PtdIns4P (Table 1) is consistent with a conserved Glu residue being implicated in determining PI4P 5-kinase specificity (Kunz et al., 2000), as this residue is found in the active sites of both PIP5K4 and PIP5K5 (E742 and E735, respectively). Importantly, effects of PI4P 5-kinase overexpression on pollen tube morphology depended on enzyme activity, and the inactive variant PIP5K5 K497A did not alter pollen tube growth upon overexpression (Figure 8H). It follows that the observed effects on pollen tube growth are exerted by PtdIns(4,5)P2 production, rather than by structural properties of the overexpressed proteins.
Production of PtdIns(4,5)P2 by the type B PI4P 5-kinase, PIP5K3, has previously been demonstrated by mutant analysis to be essential for polar tip growth of root hairs (Kusano et al., 2008; Stenzel et al., 2008), a tip-growing cell type similar in many respects to pollen tubes. Data presented here demonstrate that pollen grains from pip5k4 pip5k5 double mutant plants had greatly reduced in vitro germination rates (Figure 4A) and that pollen tubes protruding from those grains that did germinate were significantly shorter than those of wild-type controls (Figure 4B). The observation of a small number of viable pollen in the pip5k4 pip5k5 double mutant suggests that PI4P 5-kinases other than PIP5K4 or PIP5K5 may provide residual functionality to the ensuing pollen tubes. The germination and growth defects in pip5k4 pip5k5 double mutants were reversed by the ectopic introduction of either PIP5K4:EYFP or PIP5K5:EYFP into the double mutant plants (Figure 4), demonstrating that the observed phenotypes were due to the combined disruption of the PIP5K4 and PIP5K5 genes rather than to unrelated T-DNA insertions elsewhere in the genome. The results also demonstrate that the fluorescence tag is not obstructive and that PI4P 5-kinase-EYFP fusion proteins are fully functional in vivo, as had been previously reported for PIP5K3 in root hairs (Stenzel et al., 2008). Interestingly, pollen germination frequencies and the length of pollen tubes were actually increased in pip5k4 pip5k5 double mutants overexpressing PIP5K4-EYFP or PIP5K5-EYFP under intrinsic promoter fragments in comparison to nontransformed controls (Figure 4B). Morphological changes and branching of Arabidopsis pollen tubes observed with strong expression of PIP5K4 and PIP5K5 in the double mutant background (Figure 5) was later found also in the heterologous tobacco system (Figures 8 and 9), indicating close functional congruence of PI4P 5-kinases in pollen tubes of Arabidopsis and tobacco. Importantly, the observation of pollen tube branching in pollen tubes of Arabidopsis and tobacco indicates that this phenotype was not an artifact of the heterologous tobacco expression.
PIP5K4 and PIP5K5 exhibited very similar localization patterns in growing pollen tubes of Arabidopsis and tobacco (Figure 6). The observation of substantial cytosolic fluorescence of the fusion proteins is consistent with their soluble nature and the notion of transient peripheral recruitment to the plasma membrane (Rao et al., 1998). Membrane association is likely dynamic and at an equilibrium with cytosolic localization, as has been reported for mammalian PI4P 5-kinases (Rao et al., 1998). The distribution of PIP5K4 and PIP5K5 in pollen tubes of Arabidopsis and tobacco was similar to that of PtdIns(4,5)P2 in tobacco pollen tubes (Figures 10A and 10B), suggesting a role for PIP5K4 and PIP5K5 in the generation of the apical PtdIns(4,5)P2 microdomain. In coexpression experiments of PIP5K4-EYFP or PIP5K5-EYFP with RFP-PLCδ1-PH, the localization of PI4P 5-kinases in growing pollen tubes largely overlapped with that of PtdIns(4,5)P2 (Figures 10C and 10E), which showed additional accumulation at the extreme apex of the cells. The ring-like PI4P 5-kinase microdomain moved forward with the growing tip (see Supplemental Movie 1 online). The observation that the domain of PtdIns(4,5)P2 production moved only in the forward direction indicates that movement was not due to random diffusion. Data previously published on the effects of pollen-expressed PLCs on polar tip growth (Dowd et al., 2006; Helling et al., 2006) indicate that a balance between synthesis and degradation of PtdIns(4,5)P2 must be maintained for formation of the dynamic PtdIns(4,5)P2 microdomain and for proper pollen tube elongation. In addition, data from in vivo labeling experiments indicate that PtdIns(4,5)P2 is turned over very rapidly (Perera et al., 2002; Im et al., 2007; Krinke et al., 2007), and that increased abundance of PI4P 5-kinases should, thus, unbalance the system and have immediate effects on pollen tube growth.
In contrast with the situation in growing pollen tubes, the pattern of PIP5K4 and PIP5K5 localization in nongrowing tubes was slightly different from the distribution of PtdIns(4,5)P2 (Figures 10D and 10E; cf. Figures 6E and 6F). When coexpressed with the PtdIns(4,5)P2 reporter, PIP5K4 and PIP5K5 overlapped with PtdIns(4,5)P2 only in the apical region of the plasma membrane but not with PtdIns(4,5)P2-rich regions located further back from the pollen tube tip (Figures 10D and 10F). These observations suggest that the apical type B PI4P 5-kinases may not be the sole source of PtdIns(4,5)P2 in pollen tubes. It can be assumed that PtdIns(4,5)P2 has only a very short lifespan in plant cells due to the limiting action of PLC (Dowd et al., 2006; Helling et al., 2006) or PI phosphatases (Ercetin and Gillaspy, 2004). Because of the abundance of PtdIns(4,5)P2-degrading enzymes (Mueller-Roeber and Pical, 2002; Dowd et al., 2006; Helling et al., 2006), the lifespan is likely even shorter for PtdIns(4,5)P2 freely diffusing in the plane of the membrane, and it may be concluded that PtdIns(4,5)P2 can act only in close proximity of the PI4P 5-kinase by which it was generated. In consequence, PtdIns(4,5)P2 detected at a distance from PIP5K4 or PIP5K5 may either be the result of its decreased degradation or of additional PI4P 5-kinases acting in a region further back from the pollen tube apex. So far, the nature and origin of PtdIns(4,5)P2 not spatially coinciding with PIP5P4 or PIP5K5 is unclear.
In root hairs, overproduction of PtdIns(4,5)P2 has been shown to have profound effects on cell polarity and polar tip growth (Stenzel et al., 2008), and the effects of PI4P 5-kinase overexpression reported here indicate that overproduction of PtdIns(4,5)P2 also alters polar tip growth of pollen tubes (Figures 8 and 9). One factor regulating the polarity of the pollen tube tip is the actin cytoskeleton (Cardenas et al., 2008). When the actin cytoskeleton was visualized in nontransformed pollen tubes (see Supplemental Figure 1A online) and in tubes overexpressing PIP5K4 or PIP5K5 and exhibiting branching (see Supplemental Figure 1B online), emerging membrane infolding (see Supplemental Figure 1C online), or full protoplast trapping (see Supplemental Figures 1D and 1E online), no alterations in actin cytoskeletal structure were observed. Importantly, distinct actin fringes were observed in cells showing the reported phenotypes (see Supplemental Figures 1B and 1C online). Only in tubes showing the most severe morphological alterations was the actin cytoskeletal fine structure altered (see Supplemental Figures 1D and 1E online), along with overall abolishment of the apical morphology. The data suggest that changes in actin organization of the pollen tube tip with overexpression of PIP5K4 or PIP5K5 became manifest only in cases of the most severe morphological changes. The contribution of the actin cytoskeleton to the morphological changes observed thus appeared small, and further attention was given to the influence of overexpression of PIP5K4 or PIP5K5 on the cell wall, specifically on apical pectin deposition.
In the tips of pollen tubes strongly overexpressing PIP5K4 or PIP5K5, we regularly observed increased cell wall deposition accompanied by plasma membrane invaginations in the tube apex (Figures 7, 9, and 11). Note that the increased cell wall components positively stained for pectin with toluidine blue O and ruthenium red (Figures 11B to 11J), whereas aniline blue staining for callose indicated no change in callose deposition with the overexpression of PIP5K4 or PIP5K5 (Figures 11L and 11M). Trafficking of pectin-containing vesicles and the secretion of pectin at the pollen tube tip have to be tightly regulated to preserve the plasticity of the apical cell wall, which is crucial for pollen tube elongation (Krichevsky et al., 2007). Pectin is secreted by exocytosis at the pollen tube tip (Bosch and Hepler, 2005; Tian et al., 2006), and the observation of increased pectin deposition in PI4P 5-kinase overexpressors suggests that increased production of PtdIns(4,5)P2 may have altered the balance of vesicle trafficking and apical pectin deposition. Importantly, phenotypic changes, such as pollen tube branching (Figures 5 and 8) and membrane invaginations (Figure 9), may be explained by an increased rate of pectin delivery to the apex and/or increased secretion. Similarly, the reduced germination and growth of the pip5k4 pip5k5 double mutant pollen (Figure 4) may be explained through effects on apical trafficking or secretion of pectin. When pectin deposition is increased due to strong overexpression of type B PI4P5-kinases, the apical cell wall will become thicker and less flexible. In consequence, the turgor pressure may no longer be strong enough to expand the cell wall, and pollen tube elongation will cease (Figure 9). Continuing delivery of vesicles containing pectin and membrane area to the apex may lead to excess pectin accumulation in the apical cell wall (Figures 11B to 11J). Apical fusion of cargo vesicles leads not only to cell wall deposition, but also increases the incorporated area of the apical plasma membrane. If the protoplast inside a rigidified cell wall cannot be expanded anymore, the membrane area continuously incorporated at the apical plasma membrane may fold inward, possibly explaining the observed protoplast trapping phenotype (Figure 9). Branching of tobacco pollen tubes (Figure 8) was observed with type B PI4P 5-kinase expression levels that were weaker than those in pollen tubes showing the protoplast trapping phenotype (cf. Figure 7). A possible explanation for the branching phenotype is that increased delivery of cargo vesicles and/or increased pectin deposition may not be strong enough to terminate growth, but may be sufficiently altered to cause aberrant plasticity of the apical cell wall. Such localized changes in pectin deposition in the apical cell wall may lead to two zones of flexible cell wall divided by a more rigid region, which would ultimately lead to a splitting of the pollen tube apex and the observed branching pattern. Both arms of a branched pollen tube could continue growing independently for long distances (Figures 8A and 8C), indicating that pollen tube elongation is a self-sustainable process, as was previously hypothesized (Cole and Fowler, 2006), and not dependent on the close vicinity of the vegetative nucleus, which was only present in one branch.
The question of how increased abundance of PI4P 5-kinases may unbalance vesicle trafficking and pectin deposition at the growing tip must be discussed on a somewhat speculative basis because information about the effects of PtdIns(4,5)P2 on the secretory machinery of plants is limited. From the animal and yeast fields, it is known that PtdIns(4,5)P2 recruits subunits of the exocyst complex, which is required for secretion at the plasma membrane (He et al., 2007; Liu et al., 2007). Even though interactions with phosphoinositides have not been studied using subunits of the plant exocyst complex, it has been shown that in plants some exocyst components are important for polar growth (Cole et al., 2005; Wen et al., 2005; Synek et al., 2006; Hala et al., 2008). Another possibility that has been previously discussed (Stenzel et al., 2008) is that type B PI4P 5-kinases could produce PtdIns(4,5)P2 not only at the plasma membrane but also on secretory vesicles. In this context, it is also relevant to consider that the phosphatidylinositol 4-kinase β1 and its product, PtdIns4P, have been found associated with tip-localized vesicles in root hairs (Preuss et al., 2006). Support for a role of PI4P 5-kinases acting on lipid substrates associated with vesicles rather than the plasma membrane comes from reports from the mammalian field, demonstrating that vesicle to plasma membrane fusion is promoted by increased levels of PtdIns(4,5)P2 in the membranes of secretory vesicles, rather than by PtdIns(4,5)P2 at the plasma membrane (Vicogne et al., 2006). Also, the function of synaptotagmin, a protein of the exocytotic machinery associated with exocytotic vesicles, is dependent on the presence of Ptdins(4,5)P2 (Sugita, 2008).
In summary, the data presented indicate that the type B PI4P 5-kinases, PIP5K4 and PIP5K5, have important functions in pollen germination and in pollen tube growth. The enzymes are associated with a PtdIns(4,5)P2 microdomain in the plasma membrane of the growing pollen tube tip and may regulate polar tip growth by generating PtdIns(4,5)P2 required for trafficking and/or secretion of pectin at the growing apex. Future experiments will be aimed at the identification of other important players of the secretory machinery that may be regulated by PtdIns(4,5)P2.
METHODS
cDNA Constructs
Cloning of PI4P 5-Kinase cDNA Sequences
The cDNA sequences encoding PIP5K4 and PIP5K5 were isolated by RT-PCR from RNA of young inflorescences of wild-type Arabidopsis thaliana (ecotype Columbia-0) plants using the following oligonucleotide combinations: PIP5K4, 5′-GATCCATGGGCAAGGAACAAAGCTGTGTTCTAAAGG-3′/5′-GATCCATGGCATTATCCTCAGTGAAGACCTTGA-3′; PIP5K5, 5′-GATTCATGAGCAAGGACCAAAGCTATGTTCTAAAAG-3′/5′-GATTCATGACATTGTCATCTGTGAAGACCTTGA-3′. PCR fragments were subcloned into the vector pGEM-Teasy (Promega) and sequenced. The resulting plasmids were designated as PIP5K4-pGEM-Teasy and PIP5K5-pGEM-Teasy, respectively. The coding sequence for the human PLCδ1-PH-domain was amplified from plasmid-DNA provided by Tamas Balla (Varnai and Balla, 1998) and modified to encode a seven–amino acid linker (gly-gly-ala-gly-ala-ala-gly) between the PH domain and RedStar as previously described (Dowd et al., 2006) using the primer combination 5′-GATCGCGGCCGCCGGTGGAGCTGGAGCTGCAGGAATGAGGATCTACAGGCGC-3′/5′-GATCGATATCTTAGATCTTGTGCAGCCCCAGCA-3′.
Cloning of Constructs for Bacterial Activity Tests
EYFP was amplified using primer combination 5′-GATCGCGGCCGCCCATGGTGAGCAAGGGCGAG-3′/5′-GCGGCCGCTTACTTGTACAGCTCGTCCATGCC-3′, which introduced a new NcoI restriction site in front of the coding sequence for EYFP and ligated as a NotI/NotI fragment into the pETM-41 vector that was then termed pETM-41-EYFP. The cDNA sequences encoding PIP5K4 and PIP5K5 were moved as NcoI/NcoI fragments from PIP5K4-pGEM-Teasy or as BspHI/BspHI fragments from PIP5K5-pGEM-Teasy, respectively, into the expression plasmids pETM-41 (EMBL Protein Expression and Purification Facility) and into pETM-41-EYFP digested with NcoI. All clones were introduced in frame with the cDNA encoding the N-terminal MBP and polyhistidine (His) tags of pETM-41.
Fusion Constructs for Particle Bombardment
cDNA fragments for +2-CFP (encoding CFP plus two additional nucleotides), +2-EYFP (encoding EYFP plus two additional nucleotides), and RedStar (Janke et al., 2004) were amplified from plasmids carrying the authentic clones provided by Martin Fulda (Göttingen University, Germany) using the following primer combinations: +2-CFP and +2-EYFP, 5′-GATCGCGGCCGCATGGTGAGCAAGGGCGAG-3′/5′-GATCGATATCTTACTTGTACAGCTCGTCCATG-3′; RedStar, 5′-GATCGTCGACATGAGTGCTTCTTCTGAAGATGTC-3′/5′-GATCGCGGCCGCCAAGAACAAGTGGTGTCTACCTT-3′. Amplicons were subcloned into pGemTeasy and moved into pENTR2b as NotI/EcoRV fragments, except in the case of RedStar, which was cloned as a SalI/NotI fragment, creating the plasmids pENTR-+2-CFP, pENTR-+2-EYFP, and pENTR-RedStar. Prior to ligation, the control of cell death b (ccdb) gene was eliminated from pENTR2b by EcoRI digestion and religation. cDNA fragments encoding PIP5K4 and PIP5K5 were each moved as NotI/NotI fragments from the plasmids PIP5K4-pGEM-Teasy or PIP5K5-pGEM-Teasy into pENTR-+2CFP and pENTR-+2EYFP, respectively. The cDNA encoding the PLCδ1-PH domain was moved as a NotI/EcoRV fragment into pENTR-RedStar. All constructs were transferred by Gateway technology (Invitrogen) from pENTR2b to the pLatGW plasmid, an expression vector containing the tomato Lat52 promotor (Twell et al., 1990), an attR gateway cassette, and a cauliflower mosaic virus 35S terminator. The pLatGW vector was a gift from Wolfgang Dröge-Laser (Göttingen University).
Cloning of PI4P 5-Kinase Promoter Sequences
For generation of promoter-GUS fusions, the GUSPlus gene was amplified from the vector pCAMBIA1305.1 (accession number AF354045) using the primer combination 5′-GATCGCGGCCGCCATGGTAGATCTGAGGGTAAATTTCTAGTTTTTCTC-3′/5′-GATCGAGCTCCTGTCAAACACTGATAGTTTAATTCCCGATC-3′ and the PCR product was introduced as a NotI/SacI fragment into the vector pgreen0029 (http://www.pgreen.ac.uk/) (Hellens et al., 2000), yielding the plasmid pgreenGUSPlus. Amplification of 1500-bp genomic sequences upstream of coding sequences for use as promoters was achieved with different Arabidopsis BAC clone templates as indicated, using the following primer combinations: PromPIP5K4, 5′-GATCGTCGACCCACCGGAGACTCCAGCAGCCAAAGCGGT-3′/5′-GATCGCGGCCGCCTTCTTAAACTAATAAAACTTTTCTCTAAGATAC-3′ from BAC-clone F24J3; PromPIP5K5, 5′-GATCGTCGACGACTCGAGCCATATAGTCGGCACCATG-3′/5′-GATCGCGGCCGCTATGTTAAACTAAGGAAACGTCTATCTAAAAAC-3′ from BAC-clone T3K9. The PCR products representing PromPIP5K4 and PromPIP5K5 were moved directionally as SalI/NotI fragments into the vector pgreenGUSPlus. The resulting plasmids were transformed into Agrobacterium tumefaciens strain EHA105 and used for stable Arabidopsis transformation.
Fusion Constructs for Stable Expression in Arabidopsis
For constructs for particle bombardment, +2EYFP amplicons were moved into the plasmid pUC18Entry (Hornung et al., 2005) as NotI/EcoRV fragments, creating the plasmid pUC18Entry-EYFP. Prior to ligation, the ccdb gene was eliminated from pUC18Entry by EcoRI digestion and religation. The PCR products representing PromPIP5K4 and PromPIP5K5 were moved as SalI/NotI fragments into pUC18Entry-EYFP, creating the plasmids PromPIP5K4-EYFP and PromPIP5K5-EYFP, respectively. The coding sequences for PIP5K4 or PIP5K5 were moved as NotI/NotI fragments from PIP5K4-pGEM-Teasy or PIP5K5-pGEM-Teasy into PromPIP5K4-EYFP and PromPIP5K5-EYFP, respectively. Finally, the fragments consisting of the promoter sequence and the cDNA encoding the fluorescence-tagged PI4P 5-kinases were transferred to the expression plasmid pCAMBIA3300.0 GC using Gateway technology (Invitrogen) according to the manufacturer's instructions.
Heterologous Expression in Escherichia coli
Recombinant enzymes were expressed in E. coli strain BL21-AI (Invitrogen) at 25°C for 18 h after induction with 1 mM isopropylthio-β-galactoside and 0.2% (w/v) l-arabinose. Cell lysates were obtained by sonication in a lysis buffer containing 50 mM Tris-HCl, 300 mM NaCl, 1 mM EDTA, and 10% (v/v) glycerol at pH 8.0.
Lipid Kinase Assays
Lipid kinase activity was assayed by monitoring the incorporation of radiolabel from γ[32P]ATP into defined lipid substrates (Avanti Polar Lipids) as described (Cho and Boss, 1995) using total extracts of BL-21-AI expression cultures. Recombinant expression levels were adjusted between individual cultures according to immunodetection of expressed MBP-tagged proteins. Radiolabeled lipid reaction products were separated by thin-layer chromatography using silica S60 plates (Merck) and CHCl3:CH3OH:NH4OH:H2O (57:50:4:11) as a developing solvent (Perera et al., 2005) and visualized by autoradiography using Kodak X-Omat autoradiography film (Eastman Kodak). Reaction products were identified according to comigration with authentic standards (Avanti Polar Lipids) that were visualized as described (König et al., 2007). Phosphatidylinositol-bisphosphate bands were scraped from thin-layer chromatography plates according to autoradiography, and the radiolabel incorporated in lipids present in the scraped silica powder was quantified using liquid scintillation counting (Analyzer Tricarb 1900 TR; Canberra Packard). Enzyme activities against PtdIns4P or other PtdIns-monophosphate substrates represent the means of five and two independent experiments, respectively.
Tobacco Pollen Tube Growth and Transient Gene Expression
Mature pollen was collected from four to six tobacco (Nicotiana tabacum) flowers of 8-week-old plants. Pollen was resuspended in growth medium (Read et al., 1993), filtered onto cellulose acetate filters, and transferred to Whatman paper moistened with growth medium. Within 5 to 10 min of harvesting, pollen was transformed by bombardment with plasmid-coated 1-μm gold particles with a helium-driven particle accelerator (PDS-1000/He; Bio-Rad) using 1350 p.s.i. rupture discs and a vacuum of 28 inches of mercury. Gold particles (1.25 mg) were coated with 3 to 7 μg of plasmid DNA. After bombardment, pollen was resuspended in growth medium and grown for 5 to 14 h in small droplets of media directly on microscope slides. Digital images were taken at room temperature as described below.
Arabidopsis Pollen Tube Growth
Newly opened Arabidopsis flowers were collected and prehydrated for 1 h. Pollen was transferred to solid growth medium slightly modified from the protocol described previously (Boavida and McCormick, 2007) containing 0.01% (w/w) boric acid, 5 mM CaCl2, 5 mM KCl, 1 mM MgSO4, 10% (w/w) sucrose, pH 7.5, and 0.5% (w/w) phytagel and incubated for 30 min at 30°C before transferring to 22°C for further growth.
Microscopy and Imaging
Images were recorded using either an Olympus BX51 epifluorescence microscope or a Zeiss LSM 510 confocal microscope. For BX51, images were obtained at ×200 or ×400 magnification using an F41-028 HQ-Filterset for Yellow GFP (Olympus) or a UMWU 2 UV filter set for aniline blue, an Olympus ColorView II camera, and analySIS Docu 3.2 software (Soft Imaging Systems). For LSM 510, EYFP was excited at 514 nm and imaged using an HFT 405/514/633-nm major beam splitter (MBS) and a 530- to 600-nm band-pass filter; CFP was excited at 405 nm and imaged using an HFT 405/514/633-nm MBS and a 470- to 500-nm band-pass filter; RedStar was excited at 561 nm and imaged using an HFT 405/488/561-nm MBS and a 583- to 604-nm band-pass filter; Phalloidin was excited at 561 nm and imaged using an HFT 458/561-nm MBS and a 575- to 615-nm band-pass filter. In coexpression experiments, CFP and EYFP were synchronously excited at 405 and 514 nm, respectively, and imaged using HFT 405/514/633-nm and NFT 515-nm MBSs and 470- to 500-nm and 530- to 600-nm band-pass filters, respectively; EYFP and RedStar were synchronously excited at 488 and 561 nm, respectively, and imaged using an HFT 405/488/561-nm MBS and a 518- to 550-nm band-pass filter and a 583- to 636-nm band-pass filter, respectively; EYFP and FM 4-64 were synchronously excited at 488 and 561 nm, respectively, and imaged using an HFT 405/488/561-nm MBS and a 518- to 550-nm band-pass filter and a 657- to 754-nm band-pass filter, respectively. If not specified otherwise, images of central optical plains were obtained by confocal microscopy at ×630 magnification using the Zeiss LSM510 image acquisition system and software (v 4.0; Zeiss). Fluorescence and transmitted light images were contrast-enhanced by adjusting brightness and γ-settings using image processing software (Photoshop; Adobe Systems). Video sequences were assembled using ImageReady software (Adobe Systems). Pollen tube widths, lengths, and fluorescence intensities were analyzed from digital images using analySIS Docu 3.2 software (Soft Imaging Systems). For the analysis of fluorescence intensities, pollen tubes were imaged at identical laser intensities and exposure times.
Staining of Cell Wall, Plasma Membrane, and F-Actin Structures
FM 4-64 (Molecular Imaging Products) was added to pollen tubes at a final concentration of 10 μM as described (Parton et al., 2001) and visualized after 5 to 15 min of incubation. Pollen tubes were stained according to O'Brien et al. (1964) with toluidine-blue O (Sigma-Aldrich) or according to Iwai et al. (2006) with Ruthenium red (Sigma-Aldrich) using final concentrations of 0.125% (w/v) or 0.01% (w/v), respectively, and imaged under the light microscope (Olympus BX51) within 5 to 15 min after addition of the dye. Callose was stained with aniline blue (Fukumoto et al., 2005) by addition of one-third volume of aniline blue staining solution to pollen tubes 5 min before imaging under the epifluorescence microscope. The aniline blue staining solution was prepared from equal volumes of aniline blue coloring fluid (Euromex) and double concentrated tobacco pollen tube medium. For actin staining with Alexa Fluor 568/phalloidin (Sigma-Aldrich), transformed pollen grains were spread onto 1-mm-thick growth medium solidified by the addition of 0.25% phytagel and grown for 10 h at room temperature. Fixation and staining were performed as described (Lovy-Wheeler et al., 2005).
Arabidopsis Transformation
Recombinant constructs were introduced into Arabidopsis plants through Agrobacterium-mediated transformation using the floral dip method (Clough and Bent, 1998). Independent transformants were subjected to selective conditions on Murashige and Skoog media containing 50 μg mL−1 kanamycin, 1% (w/v) sucrose, and 0.6% (w/v) agar. Resistant seedlings were transferred to soil after 2 to 3 weeks; homozygous T2 plants were used for the analysis.
Histochemical Staining for GUS Activity
Histochemical staining of plant tissue for GUS activity was performed as previously described (Jefferson et al., 1987). In brief, tissue samples were vacuum-infiltrated for 5 min in a GUS substrate solution of 100 mM sodium phosphate, pH 7.0, 2 mM 5-bromo-4-chloro-3-indolyl-glucuronide, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 10 mM EDTA, and 0.1% (v/v) Triton X-100 and then incubated at 37°C for 3 h. Subsequently, the samples were transferred to 70% ethanol to remove chlorophyll pigmentation. Pollen tubes were stained without vacuum infiltration for 14 h. GUS-positive samples were examined with a bright-field microscope (Olympus BX51) or a stereomicroscope (Olympus SZX12) at low magnification (×4 to ×10), and digital images were recorded. All GUS-stained samples shown represent typical results of at least three independent transgenic lines for each construct.
RT-PCR Analysis
RT-PCR analysis was performed to verify the expression of PIP5K4 and PIP5K5 in flower tissue of wild-type Arabidopsis plants. Total RNA was extracted using Plant RNA Purification Reagent (Invitrogen). RNA was incubated with RNase-free DNaseI for 30 min at 37°C to remove genomic DNA contamination, and RNA was ethanol-precipitated. Five micrograms of total RNA was used as a template for reverse transcription with RevertAid H Minus M-MuLV Reverse Transcriptase (Fermentas) in the presence of oligo(dT) primers. Equal amounts of first-strand cDNAs were used as templates for PCR amplification using the following primer combinations: PIP5K4, 5′-GATCCATGGGCAAGGAACAAAGCTGTGTTCTAAAGG-3′/5′-GATCCATGG CATTATCCTCAGTGAAGACCTTGA-3′; PIP5K5, 5′-GATTCATGAGCAAGGACCAAAGCTATGTTCTAAAAG-3′/5′-GATTCATGACATTGTCATCTGTGAAGACCTTGA-3′. The Arabidopsis actin gene, ACT8, was amplified using primer combination 5′-GCTGGATTCGCTGGAGATGATGCT-3′/5′-TCTCTCAGCACCGATCGTGATC-3′ and served as an internal positive control as described (Bustin, 2000). DNA bands were visualized after electrophoresis by incubating agarose gels in buffer containing 40 mM Tris, pH 8.5, 1 mM EDTA, 20 mM acetic acid, and 0.005% (w/v) ethidium bromide and subsequent exposure to UV light.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: PIP5K4, At3g56960; PIP5K5, At2g41210; ACT8, At1g49240.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Genotyping of T-DNA Insertion Lines pip5k4 and pip5k5.
Supplemental Figure 2. Actin Cytoskeletal Structures in Pollen Tubes Overexpressing PI4P 5-Kinases.
Supplemental Movie 1. Subcellular Localization of PIP5K5 in a Growing Pollen Tube.
Supplemental Movie 2. Pollen Tube Branching Resulting from Overexpression of PIP5K5.
Supplementary Material
Acknowledgments
We thank Tamas Balla (National Institute for Child Health and Human Development, Rockville, MD) for providing the HsPLCδ1-PH-domain construct. We thank Peter Hepler (University of Massachusetts, Amherst, MA), Ivo Feussner, Wolfgang Dröge-Laser, and Tim Iven (all Göttingen University, Germany), and Reinhard Jahn (Max-Planck-Institute for Biophysical Chemistry, Göttingen, Germany) for helpful discussion. We also thank the following individuals at Göttingen University: Martin Fulda and Wolfgang Dröge-Laser for plasmids; Andreas Wodarz and Michael Krahn for access to the confocal microscope and technical support, respectively; Liudmila Filonava, Jan Jäger, Xu Jin, Yun Ling, Anette Mähs, Alexander Meier, and Konstanze Steiner for technical assistance; Susanne Mesters for expert plant culture. We gratefully acknowledge financial support through an Emmy Noether grant from the German Research Foundation (to I.H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ingo Heilmann (iheilma@uni-goettingen.de).
Online version contains Web-only data.
References
- Balla, T. (2006). Phosphoinositide-derived messengers in endocrine signaling. J. Endocrinol. 188 135–153. [DOI] [PubMed] [Google Scholar]
- Balla, T., Bondeva, T., and Varnai, P. (2000). How accurately can we image inositol lipids in living cells? Trends Pharmacol. Sci. 21 238–241. [DOI] [PubMed] [Google Scholar]
- Balla, T., and Varnai, P. (2002). Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE 2002 PL3. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J. (1983). Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem. J. 212 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boavida, L.C., and McCormick, S. (2007). Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 52 570–582. [DOI] [PubMed] [Google Scholar]
- Bordenave, M., Breton, C., Goldberg, R., Huet, J.C., Perez, S., and Pernollet, J.C. (1996). Pectinmethylesterase isoforms from Vigna radiata hypocotyl cell walls: Kinetic properties and molecular cloning of a cDNA encoding the most alkaline isoform. Plant Mol. Biol. 31 1039–1049. [DOI] [PubMed] [Google Scholar]
- Bosch, M., and Hepler, P.K. (2005). Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, M., Baluska, F., von Witsch, M., and Menzel, D. (1999). Redistribution of actin, profilin and phosphatidylinositol-4,5-bisphosphate in growing and maturing root hairs. Planta 209 435–443. [DOI] [PubMed] [Google Scholar]
- Bustin, S.A. (2000). Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25 169–193. [DOI] [PubMed] [Google Scholar]
- Cardenas, L., Lovy-Wheeler, A., Kunkel, J.G., and Hepler, P.K. (2008). Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 146 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoire, L., Pierron, M., Morvan, C., du Penhoat, C.H., and Goldberg, R. (1998). Investigation of the action patterns of pectinmethylesterase isoforms through kinetic analyses and NMR spectroscopy. Implications in cell wall expansion. J. Biol. Chem. 273 33150–33156. [DOI] [PubMed] [Google Scholar]
- Cheung, A.Y., and Wu, H.M. (2008). Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 59 547–572. [DOI] [PubMed] [Google Scholar]
- Cho, M.H., and Boss, W.F. (1995). Transmembrane signaling and phosphoinositides. Methods Cell Biol. 49 543–554. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Cole, R.A., and Fowler, J.E. (2006). Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 9 579–588. [DOI] [PubMed] [Google Scholar]
- Cole, R.A., Synek, L., Zarsky, V., and Fowler, J.E. (2005). SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol. 138 2005–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona, O., and De Camilli, P. (2001). Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 114 1041–1052. [DOI] [PubMed] [Google Scholar]
- Di Paolo, G., Moskowitz, H.S., Gipson, K., Wenk, M.R., Voronov, S., Obayashi, M., Flavell, R., Fitzsimonds, R.M., Ryan, T.A., and De Camilli, P. (2004). Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431 415–422. [DOI] [PubMed] [Google Scholar]
- Doughman, R.L., Firestone, A.J., and Anderson, R.A. (2003). Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J. Membr. Biol. 194 77–89. [DOI] [PubMed] [Google Scholar]
- Dowd, P.E., Coursol, S., Skirpan, A.L., Kao, T.H., and Gilroy, S. (2006). Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell 18 1438–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobak, B.K., Dewey, R.E., and Boss, W.F. (1999). Phosphoinositide kinases and the synthesis of polyphosphoinositides in higher plant cells. Int. Rev. Cytol. 189 95–130. [DOI] [PubMed] [Google Scholar]
- Drobak, B.K., Watkins, P.A.C., Valenta, R., Dove, S.K., Lloyd, C.W., and Staiger, C.J. (1994). Inhibition of plant plasma membrane phosphoinositide phospholipase C by the actin-binding protein, profilin. Plant J. 6 389–400. [Google Scholar]
- Elge, S., Brearley, C., Xia, H.J., Kehr, J., Xue, H.W., and Mueller-Roeber, B. (2001). An Arabidopsis inositol phospholipid kinase strongly expressed in procambial cells: Synthesis of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in insect cells by 5-phosphorylation of precursors. Plant J. 26 561–571. [DOI] [PubMed] [Google Scholar]
- Ercetin, M.E., and Gillaspy, G.E. (2004). Molecular characterization of an Arabidopsis gene encoding a phospholipid-specific inositol polyphosphate 5-phosphatase. Plant Physiol. 135 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, C., Teeri, T.T., Siika, A.M., Read, S.M., and Bacic, A. (1998). Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206 452–460. [Google Scholar]
- Franklin-Tong, V.E. (1999). Signaling and the modulation of pollen tube growth. Plant Cell 11 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto, T., Hayashi, N., and Sasamoto, H. (2005). Atomic force microscopy and laser confocal scanning microscopy analysis of callose fibers developed from protoplasts of embryogenic cells of a conifer. Planta 223 40–45. [DOI] [PubMed] [Google Scholar]
- Gong, L.W., Di Paolo, G., Diaz, E., Cestra, G., Diaz, M.E., Lindau, M., De Camilli, P., and Toomre, D. (2005). Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc. Natl. Acad. Sci. USA 102 5204–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y., Fu, Y., Dowd, P., Li, S., Vernoud, V., Gilroy, S., and Yang, Z. (2005). A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels, M.J., Vaishnava, S., Boot, N., Dubremetz, J.F., and Striepen, B. (2006). A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J. Cell Sci. 119 2236–2245. [DOI] [PubMed] [Google Scholar]
- Hala, M., Cole, R., Synek, L., Drdova, E., Pecenkova, T., Nordheim, A., Lamkemeyer, T., Madlung, J., Hochholdinger, F., Fowler, J.E., and Zarsky, V. (2008). An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20 1330–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B., Xi, F., Zhang, X., Zhang, J., and Guo, W. (2007). Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 26 4053–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens, R.P., Edwards, E.A., Leyland, N.R., Bean, S., and Mullineaux, P.M. (2000). pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42 819–832. [DOI] [PubMed] [Google Scholar]
- Helling, D., Possart, A., Cottier, S., Klahre, U., and Kost, B. (2006). Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 18 3519–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler, P.K., Vidali, L., and Cheung, A.Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17 159–187. [DOI] [PubMed] [Google Scholar]
- Hornung, E., Krueger, C., Pernstich, C., Gipmans, M., Porzel, A., and Feussner, I. (2005). Production of (10E,12Z)-conjugated linoleic acid in yeast and tobacco seeds. Biochim. Biophys. Acta 1738 105–114. [DOI] [PubMed] [Google Scholar]
- Im, Y.J., Perera, I.Y., Brglez, I., Davis, A.J., Stevenson-Paulik, J., Phillippy, B.Q., Johannes, E., Allen, N.S., and Boss, W.F. (2007). Increasing plasma membrane phosphatidylinositol(4,5)bisphosphate biosynthesis increases phosphoinositide metabolism in Nicotiana tabacum. Plant Cell 19 1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara, H., Shibasaki, Y., Kizuki, N., Wada, T., Yazaki, Y., Asano, T., and Oka, Y. (1998). Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J. Biol. Chem. 273 8741–8748. [DOI] [PubMed] [Google Scholar]
- Iwai, H., Hokura, A., Oishi, M., Chida, H., Ishii, T., Sakai, S., and Satoh, S. (2006). The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proc. Natl. Acad. Sci. USA 103 16592–16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke, C., Magiera, M.M., Rathfelder, N., Taxis, C., Reber, S., Maekawa, H., Moreno-Borchart, A., Doenges, G., Schwob, E., Schiebel, E., and Knop, M. (2004). A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21 947–962. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L., Yang, S.L., Xie, L.F., Puah, C.S., Zhang, X.Q., Yang, W.C., Sundaresan, V., and Ye, D. (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre, U., and Kost, B. (2006). Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 18 3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König, S., Hoffmann, M., Mosblech, A., and Heilmann, I. (2008. a). Determination of content and fatty acid composition of unlabeled phosphoinositide species by thin layer chromatography and gas chromatography. Anal. Biochem. 378 197–201. [DOI] [PubMed] [Google Scholar]
- König, S., Ischebeck, T., Lerche, J., Stenzel, I., and Heilmann, I. (2008. b). Salt stress-induced association of phosphatidylinositol-4,5-bisphosphate with clathrin-coated vesicles in plants. Biochem. J. 415 387–399. [DOI] [PubMed] [Google Scholar]
- König, S., Mosblech, A., and Heilmann, I. (2007). Stress-inducible and constitutive phosphoinositide pools have distinct fatty acid patterns in Arabidopsis thaliana. FASEB J. 21 1958–1967. [DOI] [PubMed] [Google Scholar]
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C., and Chua, N.H. (1999). Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, B., Spielhofer, P., and Chua, N.H. (1998). A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16 393–401. [DOI] [PubMed] [Google Scholar]
- Krichevsky, A., Kozlovsky, S.V., Tian, G.W., Chen, M.H., Zaltsman, A., and Citovsky, V. (2007). How pollen tubes grow. Dev. Biol. 303 405–420. [DOI] [PubMed] [Google Scholar]
- Krinke, O., Ruelland, E., Valentova, O., Vergnolle, C., Renou, J.P., Taconnat, L., Flemr, M., Burketova, L., and Zachowski, A. (2007). Phosphatidylinositol 4-kinase activation is an early response to salicylic acid in Arabidopsis suspension cells. Plant Physiol. 144 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, J., Wilson, M.P., Kisseleva, M., Hurley, J.H., Majerus, P.W., and Anderson, R.A. (2000). The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell 5 1–11. [DOI] [PubMed] [Google Scholar]
- Kusano, H., Testerink, C., Vermeer, J.E.M., Tsuge, T., Shimada, H., Oka, A., Munnik, T., and Aoyama, T. (2008). The Arabidopsis phosphatidylinositol phosphate 5-kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 20 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y., Kim, Y.W., Jeon, B.W., Park, K.Y., Suh, S.J., Seo, J., Kwak, J.M., Martinoia, E., and Hwang, I. (2007). Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. Plant J. 52 803–816. [DOI] [PubMed] [Google Scholar]
- Lee, Y.J., Szumlanski, A., Nielsen, E., and Yang, Z. (2008). Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 181 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, M.A., Ferguson, K.M., and Abrams, C.S. (2002). Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 513 71–76. [DOI] [PubMed] [Google Scholar]
- Li, H., Bacic, A., and Read, S.M. (1999). Role of a callose synthase zymogen in regulating wall deposition in pollen tubes of Nicotiana alata. Planta 208 528–538. [Google Scholar]
- Liu, J., Zuo, X., Yue, P., and Guo, W. (2007). Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 18 4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler, A., Wilsen, K.L., Baskin, T.I., and Hepler, P.K. (2005). Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221 95–104. [DOI] [PubMed] [Google Scholar]
- Maple, J., Vojta, L., Soll, J., and Moller, S.G. (2007). ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Rep. 8 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer, H.J., and Munnik, T. (2003). Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 54 265–306. [DOI] [PubMed] [Google Scholar]
- Milosevic, I., Sorensen, J.B., Lang, T., Krauss, M., Nagy, G., Haucke, V., Jahn, R., and Neher, E. (2005). Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 25 2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, D., Castanho Coelho, P., Rodrigues, C., Camacho, L., Quader, H., and Malho, R. (2005). Modulation of endocytosis in pollen tube growth by phosphoinositides and phospholipids. Protoplasma 226 31–38. [DOI] [PubMed] [Google Scholar]
- Moscatelli, A., Ciampolini, F., Rodighiero, S., Onelli, E., Cresti, M., Santo, N., and Idilli, A. (2007). Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J. Cell Sci. 120 3804–3819. [DOI] [PubMed] [Google Scholar]
- Moustacas, A.M., Nari, J., Borel, M., Noat, G., and Ricard, J. (1991). Pectin methylesterase, metal ions and plant cell-wall extension. The role of metal ions in plant cell-wall extension. Biochem. J. 279 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Roeber, B., and Pical, C. (2002). Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 130 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, T.P., Feder, N., and McCully, M.E. (1964). Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59 368–373. [Google Scholar]
- Parton, R.M., Fischer-Parton, S., Watahiki, M.K., and Trewavas, A.J. (2001). Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J. Cell Sci. 114 2685–2695. [DOI] [PubMed] [Google Scholar]
- Perera, I.Y., Davis, A.J., Galanopoulou, D., Im, Y.J., and Boss, W.F. (2005). Characterization and comparative analysis of Arabidopsis phosphatidylinositol phosphate 5-kinase 10 reveals differences in Arabidopsis and human phosphatidylinositol phosphate kinases. FEBS Lett. 579 3427–3432. [DOI] [PubMed] [Google Scholar]
- Perera, I.Y., Love, J., Heilmann, I., Thompson, W.F., and Boss, W.F. (2002). Up-regulation of phosphoinositide metabolism in tobacco cells constitutively expressing the human type I inositol polyphosphate 5-phosphatase. Plant Physiol. 129 1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton, J.M., and Steer, M.W. (1983). Membrane recycling and the control of secretory activity in pollen tubes. J. Cell Sci. 63 303–310. [DOI] [PubMed] [Google Scholar]
- Preuss, M.L., Schmitz, A.J., Thole, J.M., Bonner, H.K., Otegui, M.S., and Nielsen, E. (2006). A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 172 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss, M.L., Serna, J., Falbel, T.G., Bednarek, S.Y., and Nielsen, E. (2004). The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16 1589–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, V.D., Misra, S., Boronenkov, I.V., Anderson, R.A., and Hurley, J.H. (1998). Structure of type IIbeta phosphatidylinositol phosphate kinase: a protein kinase fold flattened for interfacial phosphorylation. Cell 94 829–839. [DOI] [PubMed] [Google Scholar]
- Read, S.M., Clarke, A.E., and Bacic, A. (1993). Stimulation of growth of cultured Nicotiana tabacum W 38 pollen tubes by poly(ethylene glycol) and Cu(II) salts. Protoplasma 177 1–14. [Google Scholar]
- Ren, C., and Kermode, A.R. (2000). An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol. 124 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, E., Steer, M., and Dolan, L. (2001). Cell biology and genetics of root hair formation in Arabidopsis thaliana. Protoplasma 215 140–149. [DOI] [PubMed] [Google Scholar]
- Santarius, M., Lee, C.H., and Anderson, R.A. (2006). Supervised membrane swimming: Small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem. J. 398 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X.F., Yang, C.Y., Liu, J., and Yang, W.C. (2006). RPA, a class II ARFGAP protein, activates ARF1 and U5 and plays a role in root hair development in Arabidopsis. Plant Physiol. 141 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer, M.W., and Steer, J.M. (1989). Pollen tube tip growth. New Phytol. 111 323–358. [DOI] [PubMed] [Google Scholar]
- Stenzel, I., Ischebeck, T., König, S., Holubowska, A., Sporysz, M., Hause, B., and Heilmann, I. (2008). The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell 20 124–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita, S. (2008). Mechanisms of exocytosis. Acta Physiol. (Oxf.) 192 185–193. [DOI] [PubMed] [Google Scholar]
- Synek, L., Schlager, N., Elias, M., Quentin, M., Hauser, M.T., and Zarsky, V. (2006). AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 48 54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima, H., Komazaki, S., Nishi, M., Iino, M., and Kangawa, K. (2000). Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 6 11–22. [DOI] [PubMed] [Google Scholar]
- Taylor, L.P., and Hepler, P.K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 461–491. [DOI] [PubMed] [Google Scholar]
- Tian, G.W., Chen, M.H., Zaltsman, A., and Citovsky, V. (2006). Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 294 83–91. [DOI] [PubMed] [Google Scholar]
- Twell, D., Yamaguchi, J., and McCormick, S. (1990). Pollen-specific gene expression in transgenic plants: Coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109 705–713. [DOI] [PubMed] [Google Scholar]
- Varnai, P., and Balla, T. (1998). Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicogne, J., Vollenweider, D., Smith, J.R., Huang, P., Frohman, M.A., and Pessin, J.E. (2006). Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc. Natl. Acad. Sci. USA 103 14761–14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali, L., and Hepler, P.K. (2001). Actin and pollen tube growth. Protoplasma 215 64–76. [DOI] [PubMed] [Google Scholar]
- Vincent, P., Chua, M., Nogue, F., Fairbrother, A., Mekeel, H., Xu, Y., Allen, N., Bibikova, T.N., Gilroy, S., and Bankaitis, V.A. (2005). A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J. Cell Biol. 168 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt, B., et al. (2005). Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur. J. Cell Biol. 84 609–621. [DOI] [PubMed] [Google Scholar]
- Wasteneys, G.O., and Galway, M.E. (2003). Remodeling the cytoskeleton for growth and form: An overview with some new views. Annu. Rev. Plant Biol. 54 691–722. [DOI] [PubMed] [Google Scholar]
- Wen, F., Zhu, Y., and Hawes, M.C. (1999). Effect of pectin methylesterase gene expression on pea root development. Plant Cell 11 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, T.J., Hochholdinger, F., Sauer, M., Bruce, W., and Schnable, P.S. (2005). The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol. 138 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk, M.R., and De Camilli, P. (2004). Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: Insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. USA 101 8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H.L., and Janmey, P.A. (2003). Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65 761–789. [DOI] [PubMed] [Google Scholar]
- Yuen, C.Y., Sedbrook, J.C., Perrin, R.M., Carroll, K.L., and Masson, P.H. (2005). Loss-of-function mutations of ROOT HAIR DEFECTIVE3 suppress root waving, skewing, and epidermal cell file rotation in Arabidopsis. Plant Physiol. 138 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia, L., and Munnik, T. (2008). Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J. Exp. Bot. 59 861–873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.