Summary
At hippocampal mossy fiber (MF)-st. lucidum interneuron (SLIN) synapses mGluR7 serves as a metaplastic switch controlling bidirectional plasticity. mGluR7 activation during high-frequency stimulation (HFS) triggers presynaptic LTD due to persistent P/Q-type Ca2+ channel inhibition. However, following mGluR7 internalization HFS produces presynaptic LTP. Surprisingly, LTP is not a simple molecular reversal of Ca2+ channel depression. Rather mGluR7 activation/internalization controls plasticity polarity by gating cAMP sensitivity of release. While naïve surface mGluR7 expressing MF-SLIN synapses are insensitive to cAMP elevation, synapses that have internalized mGluR7 robustly potentiate following cAMP increases. Moreover, MF-SLIN LTP requires adenylate cyclase (AC) and protein kinase A (PKA) activities. We also discovered an association between mGluR7 and RIM1α, an active zone molecule required for AC/PKA-dependent presynaptic LTP. Importantly, the mGluR7-RIM1α interaction is regulated by mGluR7 activation, and mice lacking RIM1α are deficient in MF-SLIN LTP. We conclude that state-dependent cAMP sensitivity controlled by mGluR7-RIM1α interactions underlies MF-SLIN metaplasticity.
Introduction
Rapid persistent modifications of synaptic strength following brief periods of patterned neural activity are considered fundamental for information processing and storage within central circuits. Importantly, such activity-dependent synaptic plasticity is bidirectional, exemplified by the ability of individual connections to undergo both long-term potentiation (LTP) and depression (LTD), allowing synaptic efficacy to be tuned within a broad range to optimize computational power. Interestingly, threshold activity levels required to induce LTP or LTD are not static binary properties of synapses, but instead, vary dynamically according to the recent history of synaptic activation (Clem et al., 2008; Frey and Morris, 1997; Kirkwood et al., 1996; Lee et al., 2000; Montgomery and Madison, 2002; Raymond et al., 2000). In other words, synapses reside along a continuum of discrete plastic competency states dictated by their activation history, and these states in turn govern the polarity, magnitude, duration, and cell signaling cascades invoked during future plasticity as well as receptivity to a given plasticity-inducing paradigm (Bienenstock et al., 1982; Mayford et al., 1995; Montgomery and Madison, 2004). Such higher order regulation of synaptic plasticity is termed metaplasticity and may preserve homeostasis by preventing synapses from accumulating within saturated states of potentiation or depression, and additionally, enable synaptic integration across prolonged temporal domains (Abraham and Bear, 1996; Bear, 2003; Frey and Morris, 1997).
Metaplasticity is well documented for postsynaptically expressed NMDAR-dependent forms of long-term plasticity, relying on alterations in the properties of AMPA and NMDA receptors as well as signaling cascades initiated by postsynaptic mGluR activation (Bortolotto et al., 1994; Clem et al., 2008; Lee et al., 2000; Philpot et al., 2007; Philpot et al., 2003; Raymond et al., 2000). In contrast, metaplasticity of presynaptically expressed forms of long-term plasticity has received less attention, and thus, the ubiquity of, as well as cellular and molecular mechanisms underlying, presynaptic metaplasticity remain unknown. Here we investigated the mechanisms underlying presynaptic metaplasticity at Ca2+-permeable AMPAR containing MF-SLIN synapses of the hippocampus, focusing on the processes unmasked by mGluR7 internalization that convert these synapses from depressing to potentiating (Lei and McBain, 2002; Lei and McBain, 2004; Maccaferri et al., 1998; Pelkey et al., 2005; Toth et al., 2000).
Results and Discussion
In electrophysiological recordings the metaplastic switch from LTD to LTP is readily observed when MF-SLIN transmission first undergoes LTD by transient application of the mGluR7 agonist L–AP4 combined with basal stimulation (Fig. 1A–B; Pelkey et al., 2005). This chemical-induction protocol mimics HFS-induced LTD of naïve synapses and ensures robust mGluR7 internalization, priming MF-SLIN synapses to undergo dedepression/LTP in response to subsequent HFS (Pelkey et al., 2005, reviewed in Pelkey and McBain, 2008). Both L-AP4- and HFS-induced LTD of naïve synapses are expressed as a persistent inhibition of P/Q-type voltage-gated Ca2+ channels that can be directly observed as a reduction in stimulus–evoked presynaptic Ca2+ transients (CaTs) at MF release sites opposing SLINs (Pelkey et al., 2006). To determine whether MF-SLIN LTP proceeds as a mechanistic reversal of LTD we used two-photon Ca2+ imaging to monitor CaTs within anatomically defined SLIN-targeting MF terminals loaded with OGB1-AM in acute mouse hippocampal slices (see methods and Acsady et al., 1998; Pelkey et al., 2006). Consistent with our prior observations transient activation of mGluR7 by superfusion of L-AP4 coupled with basal presynaptic stimulation (0.33Hz) robustly and persistently depressed stimulus-evoked CaTs at MF-SLIN presynaptic elements (CaTs were 47±7.6% of control at 10 minutes after L-AP4, n = 5, p < 0.05; Fig 1C–D). However, subsequent HFS did not produce any lasting changes of the depressed CaTs (10 minutes after HFS CaTs remained at 40.4±10.1% of control or 90.1±10.1% of the responses obtained immediately preceding HFS, p > 0.05; Fig 1C–D). Thus, L-AP4-induced LTD of CaTs merely occludes HFS-induced CaT depression without unmasking any ability of HFS to potentiate MF-SLIN presynaptic CaTs, indicating that MF-SLIN LTP does not result from an increase or recovery of presynaptic VGCC function.
Figure 1. MF-SLIN dedepression/LTP is not a mechanistic reversal of LTD.
Time course plot of EPSC amplitude from a representative recording illustrating HFS induced LTP at MF-SLIN synapses that have undergone prior chemical LTD by L-AP4 (400 μM) treatment. Each point represents the peak amplitude of an individual EPSC obtained at the time indicated along the X axis. Traces above are averaged EPSCs (20 consecutive events) obtained at the times indicated (bars 25 pA/20ms). In this and all subsequent time course plots the period of drug application and/or HFS (arrow) are shown at the top of the plot. B. Decimated group data time course plot for experiments similar to that shown in A (n =11). EPSC amplitudes for each recording were binned and averaged (1min. segments) then normalized to the average EPSC amplitude obtained during the baseline period prior to drug treatment. The break in the X axis results from different wash periods (5–10 minutes) following L-AP4 treatment with all recordings being realigned to the 1 minute prior to HFS. C. Representative sample images (top) and records (bottom) illustrating CaT recordings from SLIN targeting MF terminals. Upper panels show wide field (left) and zoomed (right) confocal images of a parent MF bouton with SLIN targeting filipodial extensions loaded with the Ca2+ indicator OGB1-AM. The positioning of line scan to monitor presynaptic CaTs is indicated by a line in the right panel. Bottom panels show a single line scan image of a stimulus evoked fluorescence transient (left) and associated CaT (trace at right) plotted as % ΔF/F in time from the average of 4 individual line scan images (bars, 500ms/50% ΔF/F). D. Normalized group data time course plot showing that HFS does not reverse L-AP4-induced CaT LTD (n = 5). Traces above are CaTs obtained from a representative recording obtained at the times indicated (bars, 500ms/20% ΔF/F). DCGIV (1 μM) depression at the end of recordings is used to confirm MF origin of the stimulated axons. E,F Representative recording (E) and normalized group data (F, n = 5) time course plots showing that P/Q type VGCC blockade with AgTx (250 nM) occludes L-AP4 induced LTD of MF-SLIN synapses but does not prevent LTP by HFS given after L-AP4 treatment. Traces above in E are average EPSC pairs (20 HZ) obtained at the times indicated (bars, 25pA/25ms). Inset in E (gray) reveals the boxed region of the plot with expanded Y axis and traces.
To confirm that MF-SLIN LTP proceeds independently of changes in P/Q-type VGCCs we next examined whether LTP can be induced in slices treated with the selective P/Q-type VGCC antagonist omega-agatoxin IVA (AgTx, 250 nM). As previously reported (Pelkey et al., 2006), AgTx dramatically depressed MF evoked AMPAR-mediated excitatory postsynaptic currents (EPSCs) recorded in visually identified SLINs leaving a small component of transmission supported by N-type VGCCs: at the end of AgTx treatment EPSCs were 17±7.9% of control responses obtained during the baseline period (Fig 1E-F, n = 5, p < 0.01). Importantly, this inhibition fully occluded the ability of L-AP4 treatment to produce any further synaptic depression but did not prevent subsequent HFS-induced potentiation of MF-SLIN synapses (Fig. 1E–F). EPSCs remained at 18±3.7 % of control responses following L-AP4 treatment (p > 0.5 vs. AgTx treatment), but recovered to 49±24% of control at 10 minutes post HFS (p < 0.05 vs. period immediately preceding HFS). Considered together our complementary imaging and electrophysiological findings conclusively demonstrate that MF-SLIN LTP does not rely on increased P/Q-type VGCC function, importantly revealing that this LTP is not simply a mechanistic reversal of the processes responsible for LTD.
In addition to serving as a metaplastic switch, mGluR7 also imparts cell-target specificity to MF regulation as the receptor selectively localizes to release sites opposing interneurons, being largely excluded from neighboring PYR-targeting MF boutons of the same axon (Pelkey et al., 2005; Shigemoto et al., 1997). Intriguingly, mGluR7-lacking MF-PYR connections exhibit pronounced presynaptic LTP following the same HFS that induces LTD at naïve MF-SLIN terminals (Nicoll and Schmitz, 2005). MF-PYR LTP requires the activation of an AC-cAMP-PKA signaling cascade which facilitates vesicle fusion independent of changes in presynaptic Ca2+ dynamics (Castillo et al., 1997; Castillo et al., 1994; Kamiya et al., 2002; Regehr and Tank, 1991; Weisskopf et al., 1994; Weisskopf and Nicoll, 1995). As this form of potentiation is conserved at various central synapses and serves as the prototypic model of presynaptic LTP (Castro-Alamancos and Calcagnotto, 1999; Fourcaudot et al., 2008; Lonart et al., 2003; Salin et al., 1996), we next considered a potential role for cAMP-mediated enhancement of release in MF-SLIN dedepression/LTP.
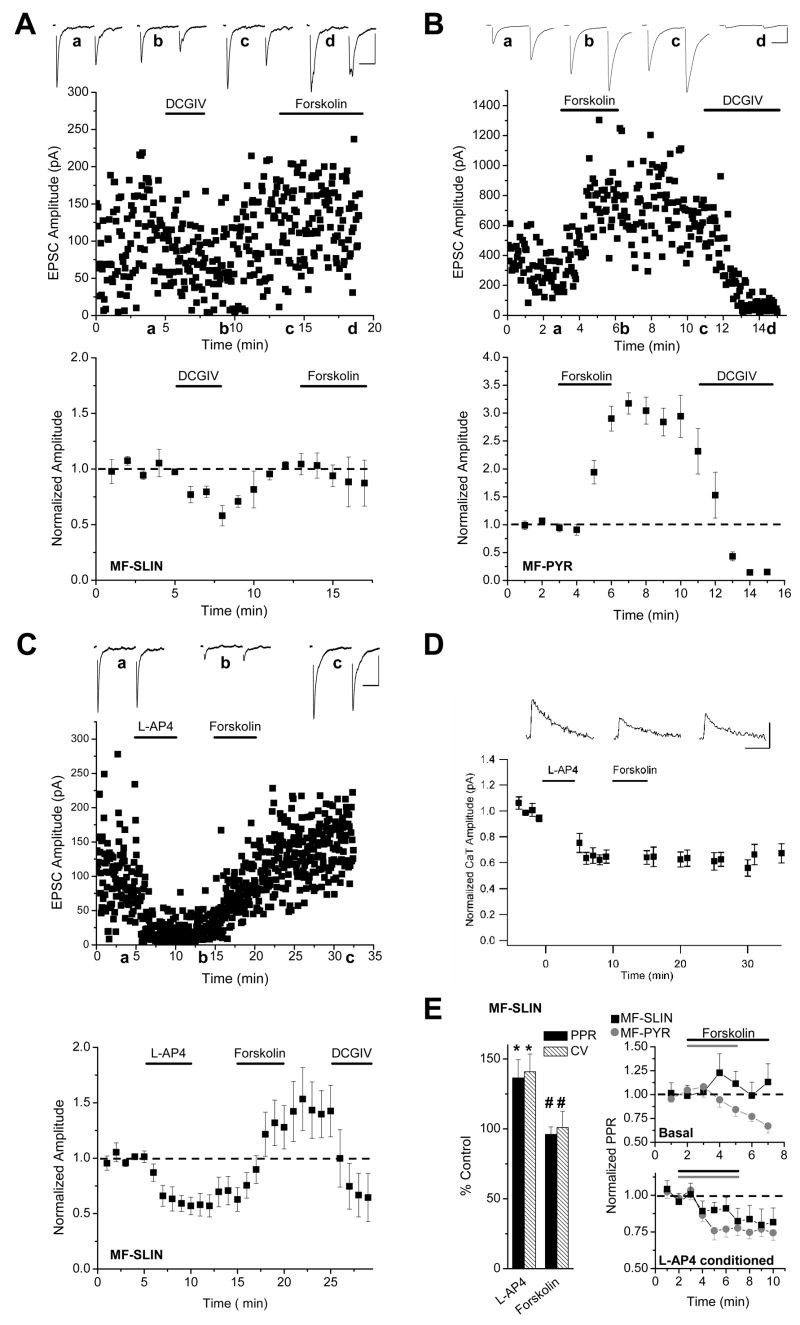
In mice, basal MF-SLIN transmission was insensitive to AC activation with forskolin (85±38% of control responses obtained preceding forskolin application, n = 4, p > 0.1), despite robust potentiation in interleaved control MF-PYR recordings (317±19 % of control, n = 6, p < 0.01; Fig. 2A,B). Forskolin also did not affect CaTs at naïve SLIN targeting MF terminals (CaTs were 108.5 ± 5.3% of control at 10 min after forskolin, n = 4, p > 0.05; Supplemental Fig. 1). This confirms and extends previous observations from rat hippocampal slices (Maccaferri et al., 1998) further highlighting the regulatory partitioning between neighboring PYR and SLIN release sites (Pelkey and McBain, 2007; Pelkey and McBain, 2008). Remarkably, in contrast to the naïve case, L-AP4 depressed MF-SLIN synapses readily potentiated in response to forskolin and this potentiation persisted following removal of the AC activator (Fig. 2C). Following L-AP4 treatment and immediately preceding forskolin application EPSCs were 63±11% of baseline control responses and potentiated to 142±27% of baseline control responses at the end of forskolin application (p < 0.05, n = 7) revealing more than 2 fold potentiation from the L-AP4 depressed levels. Importantly, this effect was not observed with 1,9-dideoxyforskolin (25 μM) a structurally related analog of forskolin that does not activate AC (EPSCs were 51±9% of control after L-AP4 treatment and remained at 49±14% of control following 1,9-dideoxyforskolin treatment, p>0.5, n=3; p<0.01 vs. forskolin). The unmasking of forskolin sensitivity appears specific to mGluR7 activation since group II mGluR activation with DCGIV did not confer forskolin sensitivity (Fig. 2A). Moreover, L-AP4 does not generally promote forskolin effects as L-AP4 did not uncover forskolin sensitivity of CA3 collateral inputs to SLINs or enhance forskolin-induced potentiation of MF-PYR synapses (Supplemental Figure 2). Like activity-induced MF-SLIN LTP (Pelkey et al., 2005), the forskolin stimulated enhancement of transmission proceeded independent of changes in presynaptic CaTs (CaTs remained at 95.1 ± 7.7% of the responses obtained 10 min after L-AP4, n = 5, p > 0.1, Fig 2D), but was accompanied by changes in the paired-pulse ratios, and CVs of synaptic events consistent with increased presynaptic function (Fig 2E). Indeed following L-AP4 treatment forskolin evoked parallel changes of similar magnitude in the PPRs at MF-SLIN and MF-PYR synapses, revealing a breakdown in the compartmentalization of forskolin sensitivity that exists between MF-SLIN and MF-PYR synapses under basal conditions (Fig. 2E). Thus, L-AP4 treatment unmasks forskolin sensitivity of MF-SLIN synapses, revealing a state-dependent cAMP sensitivity of the release machinery at SLIN-targeting MF terminals controlled by mGluR7 activation and surface expression.
Figure 2. State-dependent forskolin sensitivity of MF-SLIN synapses.
A,B. Representative recordings (upper panels) and normalized group data (lower panels) time course plots showing the effects of forskolin on basal MF-SLIN (A, n =4) and MF-PYR (B, n = 6) transmission respectively. A brief DCGIV (1 μM) treatment was used to confirm MF origin of EPSCs, however non-DCGIV treated naïve MF-SLIN synapses are also forskolin insensitive (see Maccaferri et al., 1998). Traces are averaged pairs of EPSCs (20Hz) obtained at the times indicated (bars, 50pA/20ms in A and 200pA/20ms in B). C. Representative recording (upper) and group data (lower, n = 7) showing the effects of forskolin on MF-SLIN synapses that have undergone L-AP4-induced LTD (bars for traces above, 50pA/20ms). DCGIV was applied at the end of experiments in a subset of these recordings (4/7) to confirm that the forskolin induced potentiation did not result from recruitment of non-MF inputs. D. Forskolin treatment does not recover L- AP4-induced LTD of CaTs evoked in SLIN targeting presynaptic terminals (n=4; bars, 200ms/20% ΔF/F). E. Summary bar graph (left) illustrating changes (expressed as percentage of baseline control) in paired-pulse ratios (PPR) and coefficients of variation (CV) of MF-SLIN synaptic currents following L-AP4 and forskolin treatments for recordings illustrated in C (*p<0.05 compared to baseline period; # p<0.05 compared to L-AP4 period). Time course plots at right show forskolin effects on PPRs for MF-PYR ( ) and MF-SLIN (■) synaptic events. PPRs are normalized to those obtained during the 2 minutes immediately preceding forskolin application. Upper plot (Basal) reveals effects of forskolin on basal MF-SLIN and MF-PYR synaptic PPRs and corresponds to the records summarized in A and B, while the lower plot shows the effects of forskolin on synapses previously conditioned with L-AP4 (corresponding to records summarized in C and supplemental figure 2B). Period of forskolin application is denoted by the horizontal lines above each plot.
) and MF-SLIN (■) synaptic events. PPRs are normalized to those obtained during the 2 minutes immediately preceding forskolin application. Upper plot (Basal) reveals effects of forskolin on basal MF-SLIN and MF-PYR synaptic PPRs and corresponds to the records summarized in A and B, while the lower plot shows the effects of forskolin on synapses previously conditioned with L-AP4 (corresponding to records summarized in C and supplemental figure 2B). Period of forskolin application is denoted by the horizontal lines above each plot.
To determine if HFS strengthens MF-SLIN connections by engaging the cAMP pathway uncovered with L-AP4 treatment, we investigated whether the metaplastic switch proceeds in the absence of AC activity (Fig. 3A–B). To accomplish this we incubated and constantly perfused slices with the AC inhibitor DDOA, a manipulation that reliably prevented MF-PYR LTP in interleaved positive control experiments (Fig 3A–B). In these DDOA treated slices L-AP4 treatment yielded typical MF-SLIN depression comparable to control slices: 5 minutes after L-AP4 washout EPSCs were 44±12% of baseline control responses (n = 6, p > 0.1 vs. untreated slices from Fig 1A). However, subsequent HFS in the continuing presence of DDOA failed to potentiate MF-SLIN synaptic efficacy from the L-AP4 depressed levels: 10 minutes after HFS EPSCs remained at 46±18% of baseline control responses which was not significantly different from responses obtained immediately preceding HFS (n = 6, p < 0.02 vs. untreated slices). Similarly, treatment of slices with the PKA inhibitor H-89 had no effect on L-AP4 induced depression of MF-SLIN synapses but prevented subsequent HFS-induced LTP: EPSCs were 55±15% of control 5 minutes after L-AP4 washout (n = 4, p > 0.1 vs. untreated slices, see also Pelkey et al., 2005) and remained at 59±9% of control after HFS (Fig 3B, p < 0.05 vs. untreated slices). These findings reveal requisite roles for AC and PKA function in the activity-induced enhancement of MF-SLIN synapses, indicating that MF-SLIN LTP proceeds through a cAMP/PKA-dependent pathway similar to presynaptic LTP observed at diverse synapses throughout the CNS. Moreover, the results provide further support for a state-dependent cAMP sensitivity of MF-SLIN release machinery controlled by mGluR7 activation and surface expression.
Figure 3. MF-SLIN LTP requires AC/PKA activities and can be revealed with multiple rounds of HFS.
A. Normalized group data time course plots (n = 6) showing that L-AP4-mediated unmasking of HFS-induced LTP does not occur in slices incubated and constantly perfused with the AC antagonist DDOA (8–16 μM). Inset shows normalized group data for MF-PYR recordings in DDOA treated slices performed as a positive control for DDOA efficacy (n=8). B. Bar chart summary of the effects of DDOA and H-89 (5 μM) on MF-SLIN and MF-PYR HFS-induced LTP (for MF-SLIN recordings, n = 7, 6, and 4 for control, DDOA, and H-89 respectively; for MF-PYR recordings n = 5, 8, and 3 for control, DDOA, and H-89 recordings respectively). C. Representative recording (upper panel) and group data summary histogram showing the effects of multiple rounds of HFS on MF-SLIN synapses (n = 5 to HFS3, and n = 4 to HFS4). The first two rounds of HFS produce saturating depression of MF-SLIN synapses, however subsequent HFS promotes dedepression/LTP. D. As in C but for recordings performed in slices incubated with and constantly perfused in DDOA (n=7). Traces throughout the figure are averaged (20 events) pairs of EPSCs (20 Hz) obtained at the times indicated from the representative recordings (bars, 25pA/20ms throughout). * p<0.05, **p<0.01 compared to baseline control or for the indicated comparison.
Although L-AP4 treatment reliably triggers mGluR7 endocytosis to unmask MF-SLIN LTP, the results do not reveal whether endogenously released glutamate can trigger the metaplastic switch. Despite optimal localization to the active zone of presynaptic terminals (Shigemoto et al., 1997; Shigemoto et al., 1996), the extremely low affinity of mGluR7 for glutamate (0.1–1 mM, Conn and Pin, 1997) indicates that significant receptor internalization may only proceed under conditions of prolonged intense presynaptic stimulation. To determine whether MF-SLIN LTP can be revealed in such a use-dependent fashion we monitored the effects of multiple closely spaced HFS episodes on MF-SLIN transmission. Consistent with our prior findings (Pelkey et al., 2005), two rounds of HFS simply produced saturating LTD (Fig. 3C). In contrast, subsequent HFS yielded significant potentiation, reversing synaptic efficacy from depressed levels to near baseline control values obtained prior to any HFS (Fig 3C). Both the initial depression and subsequent potentiation were accompanied by PPR and CV changes consistent with alterations in presynaptic function: during LTD PPR and CV increased to 159±22% and 353±80% of pre-tetanus baseline control levels respectively and following potentiation PPR and CV returned to 99±4% and 125±22% of baseline control respectively (p < 0.05 for both PPR and CV LTD vs. LTP). In some recordings potentiation was evident with only three episodes of HFS (eg. Fig 3C, upper panel), and all recordings exhibited significant potentiation by the fourth bout of HFS (Fig. 3C, lower panel). Importantly, slices treated with DDOA to prevent AC activity exhibited only saturating depression with four episodes of HFS (Fig. 3D). Thus, like L-AP4 treatment, multiple bursts of presynaptic activity can trigger an AC-dependent reversal in the polarity of presynaptic MF-SLIN plasticity, suggesting that excess glutamate release with intense presynaptic activation triggers mGluR7 internalization converting MF-SLIN release sites into a cAMP responsive state.
How does surface mGluR7 control cAMP sensitivity of presynaptic release at MF-SLIN synapses? Previously, we hypothesized that surface mGluR7 sequesters a putative presynaptic substrate necessary for LTP generation and that receptor endocytosis could trigger release of this substrate making MF-SLIN release sites LTP competent (Pelkey et al., 2005). In addition to AC and PKA activities prototypic presynaptic LTP, typified by MF-PYR synapses, requires the presynaptic scaffold protein RIM1α. Initially RIM1α was thought to be the critical PKA substrate for presynaptic LTP (Castillo et al., 2002; Lonart et al., 2003), however recent evidence indicates that presynaptic LTP proceeds normally in mice expressing a mutant form of RIM1α lacking the presumed critical PKA phosphorylation site (Kaeser et al., 2008). Nonetheless, the absolute requirement for RIM1α in presynaptic LTP at diverse synapses throughout the CNS remains undisputed (Castillo et al., 2002; Fourcaudot et al., 2008; Lonart et al., 2003; Kaeser et al., 2008). Similarly, we found that RIM1α is necessary for MF-SLIN LTP (Fig. 4A–B). In slices from RIM1α knockout mice (RIM1α−/−) L-AP4 treatment produced typical MF-SLIN depression comparable to that observed in recordings from wild-type (RIM1α+/+) littermate controls: 5 minutes after L-AP4 washout EPSCs were 54±8% and 56±11% of control for RIM1α−/− and RIM1α+/+ recordings respectively (p > 0.5; RIM1α−/−: n = 6 recordings from 4 mice born to 4 different litters, Fig. 4B; RIM1α+/+: n = 4 recordings from 3 mice born to 3 different litters, data incorporated into Fig. 1B). However, subsequent HFS failed to produce any lasting potentiation in RIM1α−/− slices (EPSCs remained at 43±6% of baseline control responses 10–15 minutes post-HFS) revealing a significant LTP deficit compared to RIM1α+/+ controls (EPSCs returned to 90±6% of baseline control, p < 0.01 RIM1α−/− vs. RIM1α+/+). Given this requisite role for RIM1α in presynaptic LTP and the strong localization ofboth mGluR7 and RIM1α to the presynaptic active zone (Kaeser and Sudhof, 2005; Shigemoto et al., 1996) we considered RIM1α to be a logical candidate LTP mediator sequestered by mGluR7. To determine if mGluR7b, the dominant splice variant in hippocampal MFs (Shigemoto et al., 1997), and RIM1α associate within a molecular complex we attempted to co-immunoprecipitate the two proteins. Using a polyclonal antibody (Supplemental Fig. 3) we immunoprecipitated mGluR7b from tissue homogenates of acute brain slices identical to those utilized for electrophysiological recordings. In homogenates from control slices that had been incubated with regular extracellular solution anti-mGluR7b reliably co-precipitated RIM1α (Fig. 4C). Remarkably, however, brief treatment (10 minutes) of slices with L-AP4 supplemented extracellular solution significantly reduced the efficiency of the mGluR7b-RIM1α co-precipitation without altering precipitation of mGluR7b itself or overall levels of RIM1α (Fig. 4C–D). These findings suggest that mGluR7b and the critical LTP substrate RIM1α exist within the same molecular complex in a fashion that can be disrupted by receptor activation/internalization providing a potential molecular mechanism to explain how mGluR7 internalization primes MF-SLIN terminals to become LTP competent.
Figure 4. RIM1αis required for MF-SLIN LTP and interacts with mGluR7b in a fashion regulated by L-AP4 treatment.
A,B. Representative example (A) and normalized group data time course plots (B, n = 6) for MF-SLIN recordings in slices from RIM1α−/− mice. L-AP4 induced depression proceeds normally but subsequent HFS fails to induce LTP/dedepression. C. Immunoblots from a representative experiment showing immunprecipitation of RIM1alpha from brain slice lysates incubated with the anti-mGluR7b antibody characterized in Supplemental Figure 3. Lysates from control and L-AP4 treated slices as indicated were incubated with anti-mGluR7b antibody (middle blots) or non-specific IgG (right blots) under non-denaturing conditions and immunoprecipitated proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes and probed with anti-RIM1α (upper blots), or anti-GluR7b (lower blots) antibodies. Precipitation of RIM1α by anti-GluR7b antibody was less efficient in L-AP4 treated slices. Blots on the left show immunoblot analyses of total slice lysates (input) with anti-RIM1α or anti-mGluR7b antibodies showing that L-AP4 treatment does not alter the overall levels of RIM1α or mGluR7b. Blots are all from a single representative experiment with control and L-AP4 treated slices obtained from the same brain run in parallel. D. Summary histogram for quantitative data obtained in six experiments similar to that illustrated in A. The amount of mGluR7b or RIM1α detected in L-AP4 treated tissue is expressed as a percentage of that detected in control slices from the same brains run in parallel. * denotes p<0.05.
In summary, our findings reveal a state-dependent cAMP sensitivity of release at MF-SLIN synapses. Naïve, surface mGluR7 expressing MF-SLIN terminals exhibit activity-induced LTD and are completely insensitive to elevations of cAMP despite exquisite cAMP sensitivity of neighboring MF-PYR terminals. In contrast, MF-SLIN terminals that have internalized mGluR7 are competent to undergo AC/cAMP/PKA/-mediated LTP revealing a breakdown in the compartmentalization of cAMP sensitivity between PYR and SLIN targeting MF release sites. While this control of cAMP sensitivity by surface expressed mGluR7 could arise in part through Gi coupling of mGluR7, our data also suggest that surface mGluR7 may sequester RIM1α, a requisite molecule for cAMP-dependent forms of presynaptic plasticity. The polarity reversal of MF-SLIN plasticity has dramatic consequences for hippocampal information propagation as the MF-SLIN pathway provides a highly efficient feedforward inhibitory circuit controlling dentate-mediated recruitment of the autoassociative CA3 PYR network (Acsady et al., 1998; Henze et al., 2002; Mori et al., 2004). Initial depression of feedforward inhibition may be critical for excitation-spike generation in PYR targets, however, subsequent reversal of MF-SLIN LTD is likely crucial for restoring balance to CA3 excitation/inhibition dynamics to prevent runaway excitation as a consequence of continued disinhibition (see also Froemke et al., 2007).
Experimental Procedures
Detailed experimental procedures are outlined in the supplemental data available online.
Electrophysiology
Hippocampal slices (300–350μm thick) were prepared from 2–3 week old C57BL/6, RIM1α−/−, and RIM1+/+ mice as previously described (Pelkey et al., 2005, 2006). After recovery slices were transferred to a recording chamber and perfused with extracellular solution (in mM): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, 10 glucose, and 0.005 bicuculline methobromide saturated with 95%O2/5%CO2, pH 7.4. DL-AP5 (50–100μM) was added to the perfusate ensuring that only NMDAR-independent presynaptic MF-SLIN LTD was assayed (Lei and McBain, 2002). SLINs and PYRs were visually identified and whole-cell recordings were performed (multiclamp 700A amplifier, Axon Instruments, Foster City, CA) in voltage-clamp mode at a holding potential of –60mV using electrodes (3–5MΩ) pulled from borosilicate glass (WPI, Sarosota, FL) filled with (in mM): 100 Cs-gluconate, 5 CsCl, 0.6 EGTA, 5 MgCl2, 8 NaCl, 2 Na2ATP, 0.3 GTPNa, 40 HEPES, 0.1 spermine, and 1 QX-314, pH 7.2–7.3, 290mOsm. MF-SLIN/PYR responses were evoked at 0.33Hz by low intensity stimulation (150μs/10–30μA) in the dentate gyrus, or stratum lucidum, via a constant current isolation unit (A360, WPI) connected to a patch electrode filled with oxygenated extracellular solution. HFS consisted of 100Hz stimulation for 1s given 3 times at an interval of 10s. Data acquisition and analysis were performed using a PC equipped with pClamp 9.0 software (Axon Instruments). Group data are presented as means±s.e.m. and statistical significance was assessed using both parametric (paired and unpaired t-tests) and non-parametric (Mann-Whitney or Wilcoxon signed-rank tests) statistical analyses as appropriate.
Presynaptic Ca2+ imaging
MF terminals in acute slices were loaded with a membrane-permeable form of Ca2+ indicator Oregon Green-488-BAPTA-1-AM (OGB1-AM) to monitor presynaptic CaTs as previously described (Pelkey et al., 2006). Calcium imaging was performed using a two-photon laser scanning microscope based on a mode-locked Ti:Sapphire laser operating at 800nm, 76-MHz pulse repeat, <200fsec pulse width and pumped by a solid state source (Mira 900 and 5W Verdi argon ion laser, Coherent, Santa Clara, CA). Using a long-range water-immersion objective (40×, NA 0.8) fluorescence was detected through a short-pass filter (cut-off 680nm) in non-descanned detection mode and images were acquired using the LSM 510 software (Carl Zeiss, Kirkland, QC). MF stimulation was provided throughout experiments (0.33 Hz) and CaTs (average of 3 responses) were monitored at 1–5 min intervals by scanning a line along the tip of filipodial extensions emanating from large mossy fiber boutons. For CaT analysis fluorescence background was subtracted from fluorescence intensity averaged over the line. Changes in fluorescence were calculated relative to baseline and expressed as %ΔF/F=[(F−Frest)/Frest]×100. CaT group data are presented as mean± s.e.m. unless indicated otherwise.
Immunoprecipitation from acute brain slices
Following a 1 hour recovery period acute mouse brain slices were moved to an incubation chamber containing the same extracellular solution used to perfuse slices for electrophysiological experiments and allowed to equilibrate for 10–15 minutes. Then slices were separated into 2 groups and processed in parallel as control and L-AP4 treated. Control slices remained in the extracellular solution containing chamber while L-AP4 treated slices were moved to another incubation chamber containing extracellular solution supplemented with L-AP4 (400 μM). Following 10 additional minutes of incubation in control or L-AP4 supplemented extracellular solution slices were homogenized and then lysed. Supernatants following centrifugation (14,000×g at 4°C for 15 minutes) were collected and protein quantity was determined by BCA assay. For immunoprecipitation slice lysates were pre-cleared and incubated with affinity-purified rabbit anti-mGluR7b antibody (or normal rabbit IgG in controls) at 4°C for 12–16 hours. Antigen-antibody complexes were immobilized and precipitated by centrifugation. After multiple washes immune complexes were eluted and immunoprecipitates were subjected to electrophoresis on a 3–8% NuPAGE Tris-Acetated gel (Invitrogen) under denaturing and reduced conditions. Resolved proteins were transferred to PVDF membrane, and probed with rabbit anti-myc antibody (Santa Cruz, CA), mouse anti-Rim1α antibody (BD Biosciences, CA), and affinity-purified rabbit anti-mGluR7b antibody as indicated. IRDye680 conjugated goat anti-mouse IgG, or IRDye680 conjugated goat anti-rabbit IgG were used as secondary antibodies and western blot results were detected using an Odyssey Infrared Imaging System (LI-COR Biosciences, NE).
Supplementary Material
Acknowledgments
The authors thank Brian Jeffries, for expert technical assistance, and Richard Robitaille for expertise and guidance with two photon confocal imaging. Myc-mGluR7a/b constructs were a generous gift from Drs. Laurent Fagni and Frederica Bertaso. Affinity purified mGluR7b antibody was kindly provided by Drs. Katherine Roche and YoungHo Suh. We are very grateful to Drs. Tom Sudhof and Pascal Kaeser for providing RIM1α mice. This work was supported by an NICHD intramural award to C.J.M., CIHR and FRSQ awards to J.-C.L. and an HFSP award to both C.J.M. and J.-C.L. J.-C.L. is the recipient of the Canada Research Chair in Cellular and Molecular Neurophysiology. K.A.P. is an NIH Fellow. L.T. was supported by the Savoy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. Presynaptic long-term potentiation in corticothalamic synapses. J Neurosci. 1999;19:9090–9097. doi: 10.1523/JNEUROSCI.19-20-09090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL. Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science. 2008;319:101–104. doi: 10.1126/science.1143808. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Humeau Y, Casassus G, Shaban H, Poulain B, Luthi A. cAMP/PKA signaling and RIM1α mediate presynaptic LTP in the lateral amygdala. PNAS. 2008;105:15130–15135. doi: 10.1073/pnas.0806938105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Sudhof TC. RIM function in short- and long-term synaptic plasticity. Biochem Soc Trans. 2005;33:1345–1349. doi: 10.1042/BST0331345. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Kwon H–B, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Sudhof TC. RIM1α phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. PNAS. 2008;105:14680–14685. doi: 10.1073/pnas.0806679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Umeda K, Ozawa S, Manabe T. Presynaptic Ca2+ entry is unchanged during hippocampal mossy fiber long-term potentiation. J Neurosci. 2002;22:10524–10528. doi: 10.1523/JNEUROSCI.22-24-10524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33:921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Two Loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci. 2004;24:2112–2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- Mayford M, Wang J, Kandel ER, O’Dell TJ. CAMKII regulates the frequency repsonse function of hippocampal synapses for the production of both LTD and LTP. Cell. 1995;81:891–904. doi: 10.1016/0092-8674(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron. 2002;33:765–777. doi: 10.1016/s0896-6273(02)00606-2. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. Discrete synaptic states define a major mechanism of synapse plasticity. Trends Neurosci. 2004;27:744–750. doi: 10.1016/j.tins.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Mori M, Abegg MH, Gahwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Differential regulation at functionally divergent release sites along a common axon. Curr Opin Neurobiol. 2007;17:366–373. doi: 10.1016/j.conb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Target-cell dependent plasticity within the mossy fiber-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites of a common axon. J Physiol. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron. 2006;52:497–510. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory Role of NR2A for Metaplasticity in Visual Cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. The maintenance of LTP at hippocampal mossy fiber synapses is independent of sustained presynaptic calcium. Neuron. 1991;7:451–459. doi: 10.1016/0896-6273(91)90297-d. [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.