Abstract
Adiponectin is one of several, important metabolically active cytokines secreted from adipocytes. Low circulating levels of this adipokine have been associated epidemiologically with obesity, insulin resistance, type II diabetes, and cardiovascular disease. To determine if adiponectin can modulate lipid metabolism in macrophages, we expressed the adiponectin gene in human THP-1 macrophage foam cells using a lentiviral vector expression system and demonstrated that macrophages transduced with the adiponectin gene had decreased lipid accumulation compared with control macrophages transduced with the LacZ gene. Macrophages transduced with the adiponectin gene also exhibited decreased oxidized low-density lipoprotein (oxLDL) uptake and increased HDL-mediated cholesterol efflux.
Additional studies suggest two potential mechanisms for the reduced lipid accumulation in these adiponectin-transduced macrophage foam cells. The first mechanism involves the PPARγ and LXR signaling pathways which up-regulate the expression of ABCA1 and promote lipid efflux from these cells. The second mechanism involves decreased lipid uptake and increased lipid hydrolysis which may result from decreased SR-AI and increased SR-BI and HSL gene activities in the transformed macrophage foam cells. We demonstrated also that the expression of two proatherogenic cytokines, MCP-1 and TNFα, were decreased in the adiponectin transduced macrophage foam cells.
These results suggest that adiponectin may modulate multiple pathways of lipid metabolism in macrophages. Our studies provide new insights into potential mechanisms of adiponectin-mediated alterations in lipid metabolism and macrophage foam cell formation which may impact the development of atherosclerosis.
Keywords: adiponectin, lipid accumulation, macrophage foam cells, atherosclerosis
1. Introduction
Atherosclerosis is a chronic inflammatory disease as well as a disorder of lipid metabolism [1]. The complex physiopathologic process is initiated by the formation of cholesterol-rich lesions in the arterial wall. The accumulation of cholesterol-rich lipoproteins in the artery wall results in the recruitment of circulating monocytes, their adhesion to the endothelium, and their differentiation into tissue macrophages. Macrophages play a crucial role in this process because they accumulate large amounts of lipid to form the foam cells that initiate the formation of the lesion and participate actively in the development of the atherosclerotic lesion. Since the transformation of macrophages to foam cells is a critical component of atherosclerotic lesion formation [2], the prevention or reversal of cholesterol accumulation and/or the production of inflammatory mediators in macrophage foam cells could result in protection from multiple pathological effects of atherosclerosis and abnormal lipid metabolism. A well-characterized cell model system to study this critical transformation of macrophages to foam cells is the human THP-1 monocytic cell line [3]. These THP-1 monocytic cells can be induced to differentiate into macrophages following treatment with phorbol myristate acetate (PMA) and the resulting macrophages can then be induced to form foam cells following treatment with modified LDL [4].
Adiponectin (also known as apM1, AdipoQ, Gbp28 and Acrp30), an adipocytokine exclusively expressed and secreted by adipocytes and circulating in plasma in a high concentration, has been shown to inhibit macrophage foam cell formation by down-regulating scavenger receptor A (SR-A) expression and acyl-coenzyme A: cholesterol-acyltransferase 1 (ACAT1) expression [5, 6]. It has also been reported that adiponectin may inhibit both the inflammatory process and atherogenesis by suppressing the migration of monocytes/macrophages and their transformation into macrophage foam cells in the vascular wall [5, 7]. These results suggest that adiponectin may play an inhibitory role in foam cell formation from human monocyte-derived macrophages. Thus, the low adiponectin levels associated with elevated plasma levels of atherogenic triglyceride-rich lipoproteins and their lipolytic remnants may contribute to foam cell formation.
Experiments conducted in vitro using cell cultures have also shown that adiponectin suppresses the expression of endothelial adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin [8], and the proliferation of vascular smooth muscle cells [9]. In addition, there is increasing evidence in vivo to demonstrate that incidence of cardiovascular death is higher in patients with low plasma adiponectin levels compared with those with high plasma levels [10] and that hypertrophic signals in the heart can be mediated by adiponectin [11]. Thus, dysfunction of adiponectin is linked to atherosclerosis and the vascular complications of diabetes [12, 13]. Some insights have also been gained through work with transgenic and knockout mouse models. Recently, it has been reported that overexpression of the adiponectin gene protected apoE-deficient mice from atherosclerosis by reducing lesion formation in the aortic sinus by 30% [14]. All these investigations point to the anti-inflammatory and anti-atherogenic role of adiponectin during atherosclerosis.
In this paper, we provide evidence that adiponectin inhibits macrophage foam cell formation by increasing high density lipoprotein (HDL)-mediated cholesterol efflux from the macrophage foam cells through the PPARγ/LXR/ABCA1 signaling pathways. We also demonstrate that adiponectin gene expression in macrophage foam cells significantly decreases cholesterol and triglyceride accumulation in these cells. Adiponectin gene expression effects these changes in macrophage lipid metabolism both by reducing lipid uptake and also by enhancing HDL-mediated cholesterol efflux from these cells. These data suggest that adiponectin may play a critical atheroprotective role by altering macrophage lipid metabolism and by promoting cholesterol efflux via critical lipid metabolic signaling pathways and genes for prevention of macrophage foam cell formation.
2. Methods
2.1. Experimental materials
Human monocytic leukemia THP-1 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Tissue culture media were purchased from Life Technologies (Gaithersburg, MD). Phorbol myristate acetate (PMA) was purchased from the Sigma Chemical Company (St. Louis, MO). Horseradish peroxidase (HRP)-conjugated antibodies to the V5 epitope were purchased from Invitrogen (Carlsbad, CA). PPARγ, SR-AI and SR-BI specific antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); LXRα and ABCA1 specific antibodies from Abcam (Cambridge, MA); MCP-1 and TNFα specific antibodies from R&D Systems (Minneapolis, MN). Unless otherwise specified, all other reagents were purchased from the Sigma Chemical Company.
2.2. Cell culture
Human monocytic leukemia THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Tissue Culture Biologicals, Tulare, CA), penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37 °C in 5% CO2. THP-1 monocytes were treated with 100 nM PMA for 24 hours to facilitate monocytes differentiating into macrophages. After PMA treatment, the adherent macrophages were washed three times with phosphate-buffered saline (PBS) and incubated with cell culture medium for 24 hours at 37°C until addition of oxLDL for another 24 hours to transform these macrophages into foam cells. All reagents were added to the culture medium in a minimal volume (<0.1 %) of dimethyl sulfoxide (DMSO) or ethanol, and, in each case the carrier was shown to not affect the measured parameters. For each experiment, a minimum of three independent experiments with the triplicate samples (each sample with 3 × 106 cells/ 60 × 15 mm dish) was performed.
2.3. LDL isolation and modification
Human peripheral blood from healthy volunteers was collected in 10 mM ethylenediaminetetraacetic acid (EDTA). Native LDL (1.019<d<1.063 g/ml) and HDL (1.063<d<1.21 g/ml) were isolated from the plasma by sequential ultracentrifugation at 60,000 rpm for 24 hours at 10°C in a 60Ti rotor (Beckman Coulter, Inc., Palo Alto, CA). Native LDL was washed and concentrated by ultracentrifugation at 40,000 rpm for 24 hours and HDL for 40 hours at 10°C in an SW41 rotor (Beckman). The washed and concentrated LDL and HDL were dialyzed against saline (0.15 M NaCl and 300 μM EDTA, pH 7.4) at 4°C for 36 hours. LDL modified by oxidation (oxLDL) was prepared by incubation of LDL (2 mg of LDL protein/ml) with 40 mM CuCl2 in PBS for 24 hours at 37°C followed by the addition of 300 μM EDTA, and dialysis against saline at 4°C for 36 hours. The degree of LDL oxidation was determined by measurements of conjugated dienes [15] and fluorescent compounds [16]. The formation of conjugated dienes and fluorescent compounds, as surrogates for oxidation of lipid and protein moieties, in oxLDL was 5.8- and 15-fold of those in native LDL, respectively. Protein concentrations were determined using a modification of the Lowry protein assay with bovine serum albumin used as a standard [17].
2.4. Recombinant lentiviruses and lentiviral transduced cell lines
Fusion cDNAs containing the full length human adiponectin coding sequence and a V5 epitope tag were cloned into a ViraPower-CMV vector (Invitrogen). The recombinant adiponectin and control LacZ lentiviral plasmids were transfected into HEK293 cells to generate the lentiviruses. Western blot and X-gal staining were performed to confirm that the HEK293 cell transfections were successful and infectious virus particles were produced after 72 hours. To establish stable THP-1 cell lines which express adiponectin or LacZ genes, recombinant adiponectin or LacZ lentiviral stocks were used to infect THP-1 cells with Polybrene (Specialty Media, Phillipsburg, New Jersey) at a final concentration of 6 μg/ml. Forty-eight hours post-transduction, these cells were placed under blasticidin selection (10 μg/ml) for 20 days. Western blot analyses were performed using antibody against the V5 epitope tag to test for stable adiponectin or LacZ gene expression after antibiotic selection of these cell lines. From selected each four cell lines for adiponectin or LacZ gene expression, we used each two clones that have the similar gene expression levels to perform the experiments.
2.5. Oil Red O staining and cholesterol and triglyceride measurements of human THP-1 macrophage foam cells
Lipid contents in macrophage foam cells were stained with Oil Red O essentially as described previously [18]. The macrophage foam cells were fixed in 10% formalin for 90 minutes. After washing thoroughly with distilled water, these cells were incubated with a working solution of Oil Red O for 3 hours [18]. The staining of lipid droplets in foam cells was quantified using phase contrast microscope and Image-Pro Plus software from Media Cybernetics (Carlsbad, CA).
The contents of cholesterol and triglyceride in macrophage cells were measured quantitatively by enzymatic colorimetric assays with the kits from Wako (Richmond, VA) according to the manufacturer's protocols. Briefly, 20 μl of each sample (cell lysate), standard (cholesterol 200 mg/dL) and blank (distilled water) were added into the pre-labeled tubes and added with 2 ml of color reagent (containing cholesterol oxidase, cholesterol ester hydrolase, peroxidase, 4-aminoantipyrine, ascorbate oxidase and DAOS); then these reactions were mixed well and incubated at 37°C for 5 minutes. Finally, the measurements of the absorbance of the samples and standard against the blank were performed at 600 nm. The total cholesterol concentrations corresponding to the absorbance of samples were calculated from the standard calibration curve. The contents of triglyceride in macrophage cells were measured quantitatively with a similar assay process but with a different sample volume (3 μl), triglyceride standard and Enzyme color A (260 μl) and Enzyme color B (130 μl) solutions provided by the manufacturer (Wako). The concentrations of cellular proteins from these cells were measured with a protein assay kit from Bio-Rad (Hercules, CA).
2.6. OxLDL uptake and HDL-mediated cholesterol efflux analysis
OxLDL containing radiolabeled cholesterol (3H-cholesterol, MP Biomedicals, Solon, OH, specific radioactivity, 47 Ci/mmol) was prepared by slowly adding 3H-cholesterol (about 0.01 μCi) in ethanol (2-4 μl) to 5% albumin PBS buffer while stirring. The albumin-3H-cholesterol mixtures were then incubated with oxLDL at 37°C for about 4 hours to permit the diffusion of 3H-cholesterol from the albumin into the oxLDL. The 3H-cholesterol-oxLDL mixtures were then separated from albumin by ultracentrifugation as described above for preparation of LDL. The 3H-cholesterol-oxLDL preparation was then passed through a Sephadex G-25 desalting column to remove potassium bromide. Finally, the desalted 3H-cholesterol-oxLDL preparation was passed through a 0.2 micron filter for sterilization before use in cell culture experiments.
3H-oxLDL uptake and HDL-mediated cholesterol efflux were measured both in cell culture media and macrophage cells which had been preincubated with 3H-oxLDL (100 μg/ml) in the culture medium for 24 hours at 37°C. HDL-mediated cholesterol efflux analyses were performed by removing the media containing 3H-oxLDL and replacing it with fresh media containing non-radiolabeled HDL (10 μg/ml) for an additional 4 hours. After the incubation, cells were washed twice with culture medium containing 1% BSA, then washed three times with extended rinses of cells with PBS buffer containing albumin, and harvested with cell lysis buffer (1 × PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing freshly added protease inhibitor cocktail (Sigma). Cell total protein was determined [17] and cell-associated radioactivity was measured in media and cell extracts by scintillation counting.
2.7. Western blot analysis
Lentiviral transduced and control macrophage foam cells were harvested from the culture plates with cell lysis buffer containing freshly added protease inhibitor cocktail (Sigma). Cell lysate proteins (25μg protein) were separated by SDS-polyacrylamide gel electrophoresis and then electrophoretically transferred onto nitrocellulose membranes and incubated overnight at 4°C with blocking solution (5% nonfat milk in TBS). The blocked membranes were separately incubated with the specific antibodies (1:5000 dilutions with 1% nonfat milk in TBS) for 1 hour at room temperature, and washed three times with TBS buffer containing 0.1% Tween 20 for 15 minutes at room temperature with shaking. The secondary antibody conjugated to horseradish peroxidase (HRP) (Santa Cruz Biotechnology) against to the primary antibody was added, incubated, and washed as described steps above for the first antibody. Immunodetection analyses were accomplished using the Enhance Chemiluminescence Kit (New England Nuclear Life Science Products, Boston, MA).
2.8. Isolation of adiponectin multimers
Human serum was obtained from the American Red Cross in Birmingham, Alabama. Serum density was increased to d = 1.21g/ml using solid potassium bromide and all the lipoprotein fractions in serum were removed by ultracentrifugation of the serum (40 hours, 4°C, 50,000rpm, 50.2Ti) to form lipoprotein deficient serum (LPDS). The LPDS was exhaustively dialyzed against Column Running Buffer (0.1M Tris, 150mM NaCl, 0.001M NaN3, pH 8.0) and sample containing approximately 1 mg total adiponectin was applied to a chromatography column (AcA22, 2.6X200 cm) from Pall Corp (East Hills, NY) equilibrated in the same buffer. Protein eluting from the column was monitored continuously by quantitating the absorbance at 280nm (OD280). Adiponectin protein was quantitated in each fraction eluted from the column using ELISA (R&D Systems, Inc., Minneapolis, MN). Adiponectin concentration in each fraction is reported as micrograms per milliter. The distribution of adiponectin multimers in each fraction was investigated using polyacrylamide gel electrophoresis as described previously [19]. Column fractions containing predominantly the high molecular form of adiponectin (HMW) were pooled separate from those containing predominantly the low molecular weight form (LMW).
2.9. Statistics
Experimental results are reported as the Mean ± SE. Statistical analyses were conducted using the unpaired Students' T–test assuming unequal variance unless otherwise indicated. Significance was defined as the p< 0.05.
3. Results
3.1. Generation of recombinant lentiviruses and expression of adiponectin in human THP-1 macrophage foam cells
To investigate the function and regulation of the adiponectin gene in macrophage foam cells, we first demonstrated that the expression of the adiponectin or LacZ genes in macrophage foam cells was stable after the antibiotic selection, PMA- and oxLDL–treatment of the THP-1 macrophages by Western blot analyses to investigate expression of adiponectin protein (Figure 1). We detected adiponectin protein both in cell lysates and in culture media indicating that we stably expressed adiponectin in macrophage foam cells and that the expressed adiponectin is secreted into cell culture medium.
Figure 1. Detection of adiponectin in lentivirus transduced human THP-1 macrophage foam cells and culture media.
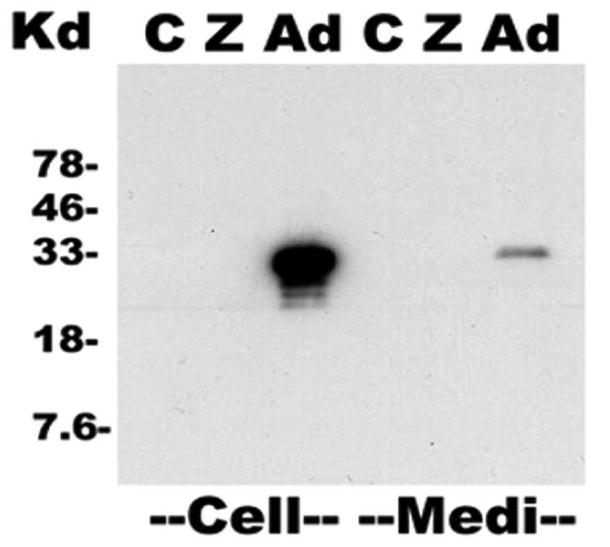
The expression of adiponectin protein was examined in macrophage foam cells and in the culture media by Western blot analyses with a human adiponectin antibody. Fusion cDNAs containing the full length human adiponectin coding sequence and a V5 epitope tag were cloned into a ViraPower-CMV vector and lentiviral plasmid was transfected into HEK293 cells to generate the lentiviruses. Western blot and X-gal staining confirmed that the HEK293 cell transfections were successful and infectious virus particles were produced after 72 hours. To establish stable THP-1 cell lines transfected with adiponectin or LacZ genes, THP-1 cells were transfected with the lentivirus and 48 hours post-transduction, these cells were placed under blasticidin selection for 20 days. Figure shows representative Western blot analyses performed using antibody against the V5 epitope tag to test for stable adiponectin or LacZ gene expression in cells or cell culture media.
C - Control macrophage foam cells; Z - LacZ transduced macrophage foam cells; Ad - adiponectin transduced macrophage foam cells. “Cell” denotes samples of macrophage foam cell lysates, each lane loaded with 20 μg total protein and “Medi” denotes aliquots of macrophage foam cell culture media, each lane loaded with 20 μl of non-concentrated medium, approximately 4 μg total protein.
3.2. Adiponectin decreases cholesterol and triglyceride accumulation in human THP-1 macrophage foam cells
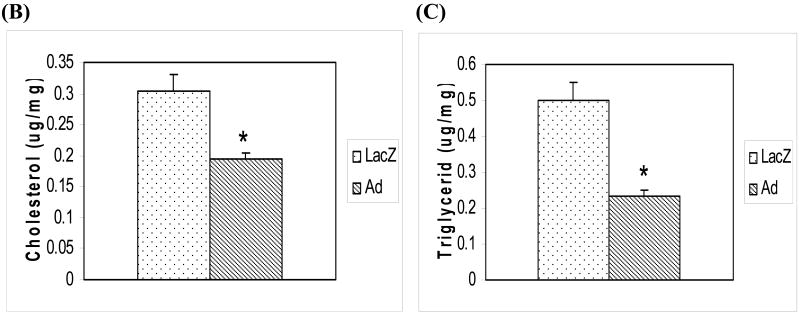
To investigate the function of adiponectin in macrophage foam cells, we cultured adiponectin or LacZ transduced THP-1 cells that had been treated initially with PMA for 24 hours to differentiate THP-1 cells into macrophages and then for an additional 24 hours with ox-LDL to transform the macrophages into foam cells. We then stained these lipid laden macrophage foam cells with Oil Red O and analyzed the staining intensity to quantify the lipid droplets inside these cells (Figure 2A) or harvested the cells to determine cellular cholesterol and triglyceride contents (Figure 2B and C). These analyses demonstrated that macrophage foam cells expressing the adiponectin gene had less Oil Red O staining (31%, p<0.05) than the control macrophage foam cells (stained lipid accumulation) when analyzed using quantitative image analysis software. We also determined the intracellular accumulation of cholesterol and triglyceride in the adiponectin or LacZ transduced macrophage foam cells to confirm the apparent decrease in lipid accumulation suggested by the quantitative histological analyses. Cholesterol accumulation was reduced 30-40% (p<0.05) in macrophage foam cells transduced with the adiponectin gene compared to control cells transduced with the LacZ gene (Figure 2B). The expression of adiponectin in macrophage foam cells also led to a significant 40-50 % (p<0.05) decrease in triglyceride accumulation when compared with levels in control cells (Figure 2C).
Figure 2.
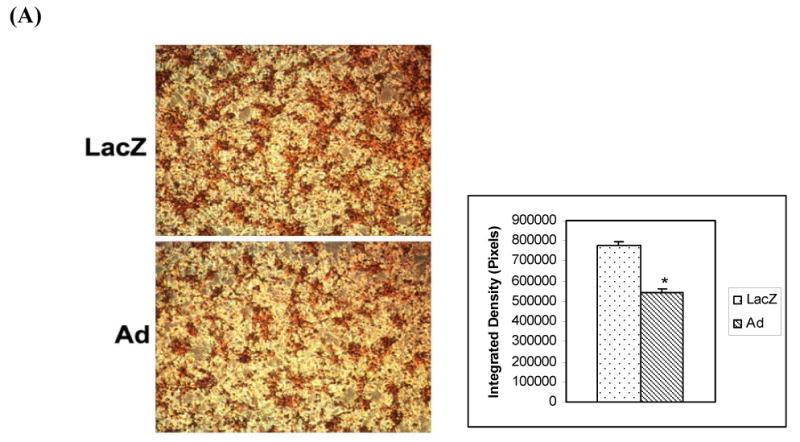
(A) Effects of adiponectin on lipid droplet accumulation in human THP-1 macrophage foam cells. Oil Red O staining was performed to examine the content of lipid in lentiviral transduced macrophage foam cells. LacZ denotes lentiviral LacZ transduced macrophage foam cells for 24 hours of oxLDL treatment; Ad denotes adiponectin transduced macrophage foam cells after incubation with oxLDL for 24 hours. Adiponectin expressing macrophage foam cells showed less Oil Red O staining (31%, p<0.05) than the control macrophage foam cells (stained lipid accumulation) when analyzed by Image-Pro Plus software. (B) Decreased cholesterol accumulation in human THP-1 macrophage foam cells due to the expression of adiponectin. Cellular accumulation of cholesterol was assessed in transduced human THP-1 macrophage foam cells. Cholesterol mass was quantitated using an enzymatic colorimetric method. LacZ - control macrophage foam cells transduced with LacZ lentivirus; Ad – macrophage foam cells transduced with adiponectin lentivirus. (C) Decreased triglyceride accumulation in human THP-1 macrophage foam cells due to the expression of adiponectin. Cellular accumulation of triglycerides was measured in transduced human THP-1 macrophage foam cells. LacZ - control THP-1 macrophage foam cells transduced with LacZ lentivirus; Ad - THP-1 macrophage foam cells transduced with adiponectin. All of the data represent the mean ± SE from three separate experiments with triplicate samples. *, p< 0.05.
These results demonstrate that expression of adiponectin in human THP-1 macrophage foam cells can significantly decrease cholesterol and triglyceride accumulation in these cells. In addition, when conditioned medium from the adiponectin transduced cell cultures was added to the control LacZ cell cultures, a similar decrease in cholesterol and triglyceride mass accumulation was also observed (data not shown).
3.3. Reduced oxLDL uptake and increased HDL-mediated cholesterol efflux in the adiponectin transduced human THP-1 macrophage foam cells
Adiponectin may decrease cholesterol and triglyceride accumulation in human THP-1 macrophage foam cells by reducing lipid uptake or by facilitating cholesterol efflux. To investigate these potential mechanisms whereby adiponectin reduced cholesterol and triglyceride accumulation in THP-1 macrophage foam cells, we examined the uptake of 3H-cholesterol labeled oxLDL in both LacZ and adiponectin transduced foam cells.
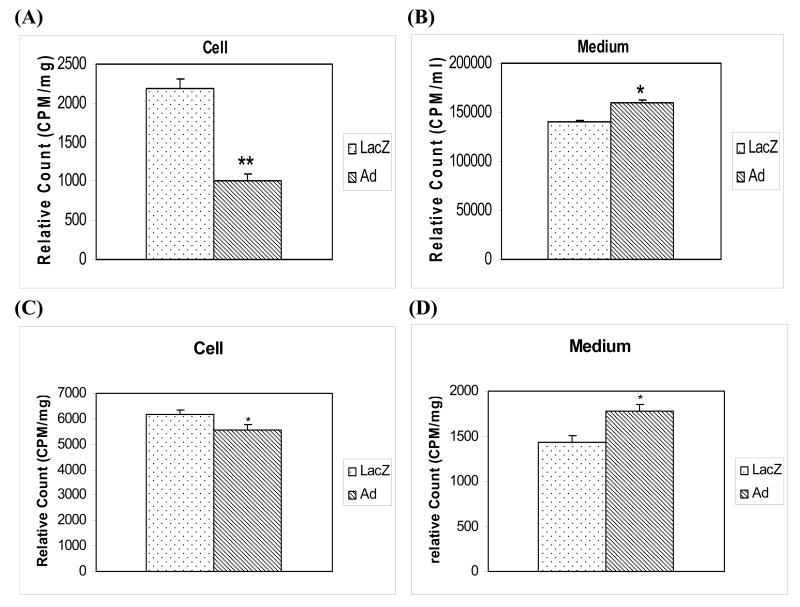
The accumulation of 3H-cholesterol in human THP-1 macrophage foam cells transduced with adiponectin gene was significantly reduced (p<0.01) compared to that in control macrophage foam cells transduced with control LacZ gene (Figure 3A). These results were accompanied by a significantly (p<0.05) higher level of 3H-cholesterol remaining in the media from adiponectin transduced macrophage foam cells when compared to control cells (Figure 3B). These data suggest that adiponectin in macrophage foam cells facilitates reduced oxLDL uptake by these cells.
Figures 3. Accumulation of oxLDL 3H-cholesterol in media and cell pellets in transduced human THP-1 macrophage foam cells.
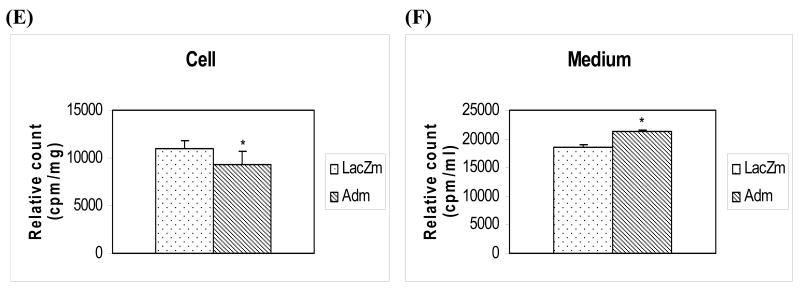
(A) Levels of 3H-radioactivity measured in cell pellets from human THP-1 macrophage foam cells transduced with LacZ or adiponectin genes and preincubated with 3H-oxLDL. The adiponectin or LacZ transduced macrophages were treated with 3H-labeled oxLDL for 24 hours. These cells were then washed tree times with PBS, and the levels of 3H-radioactivity in the cell pellets were determined. (B) Levels of 3H-radioactivity measured in culture media from human THP-1 macrophage cells transduced with LacZ or adiponectin genes and which were preincubated with 3H-oxLDL (100 μg/ml) in the culture medium for 24 hours at 37°C. Media were removed for measurement of the levels of 3H-radioactivity. (C) and (D) HDL-mediated cholesterol efflux from human THP-1 macrophage foam cells transduced with the adiponectin gene. Adiponectin or LacZ gene transduced THP-1 macrophage cells were incubated with 3H-labeled oxLDL for 24 hours. Media were removed for measurement and normalization of the levels of 3H-radioactivity. These macrophage foam cells were then washed three times with PBS, and HDL-mediated cholesterol efflux was determined by adding fresh media with 10 μg/ml of HDL for 4 hours. PANEL C – Decrease of 3H-cholesterol in the cell pellets after the HDL-mediated cholesterol efflux. PANEL D – Increase of 3H-cholesterol in media after the HDL-mediated cholesterol efflux from THP-1 macrophage foam cells transduced with the adiponectin (Ad) gene when compared to the control LacZ gene (LacZ). (E) and (F) HDL-mediated cholesterol efflux in human THP-1 macrophage foam cells. THP-1 macrophage cells were incubated with 3H-labeled oxLDL for 24 hours transforming into macrophage foam cells. These macrophage foam cells were then washed three times with PBS, and HDL-mediated cholesterol efflux was determined by adding either LacZ conditioned media (LacZm) or adiponectin conditioned media (Adm) with 10 μg/ml of HDL for 4 hours. PANEL E – Decrease of 3H-cholesterol in the cell pellets after the HDL-mediated cholesterol efflux. PANEL F – Increase of 3H-cholesterol in media after the HDL-mediated cholesterol efflux from THP-1 macrophage foam cells. All of the data represent the mean ± SE from three separate experiments with triplicate samples. *, p< 0.05; **, p< 0.01.
We also determined if adiponectin altered the HDL-mediated cholesterol efflux in foam cells transfected with the adiponectin gene. Transduced human THP-1 macrophage foam cells were preincubated with 3H-labeled oxLDL for 24 hours, the cultures were washed, and fresh media containing 10 μg/ml of HDL (which acts as acceptor for lipid efflux) was added for an additional 4 hours. We quantitated the levels of 3H-radioactivity in these macrophage foam cell pellets (Figure 3C) and in the culture media (Figure 3D) normalized the results (Figure 3A and B) with radioactivity levels before HDL added to the cell culture media. The results of these experiments were similar to the data described in the Figure 3A and B, an increase of 3H-cholesterol from oxLDL has been observed in the cell culture media of adiponectin transduced macrophage foam cells, and a decrease of 3H-cholesterol of oxLDL detected in the cell pellets of adiponectin transduced macrophage foam cells when compared to the controls. When we used adiponectin conditioned medium to control LacZ macrophage foam cell cultures for HDL-mediated cholesterol efflux experiments (10 μg/ml of HDL for 4 hours), we observed a similar result as described above (Figure 3E and 3F). These results demonstrate that adiponectin expressed in human THP-1 macrophage foam cells can modulate the lipid accumulation in these cells through decreased uptake of oxLDL and increased HDL-mediated cholesterol efflux from the cells.
3.4. Expression of proteins affected by adiponectin in human THP-1 macrophage foam cells
Since uptake of oxLDL into macrophage foam cells is reportedly regulated by PPARγ ligands, we further examined if the expression of the PPARγ gene was altered in these lentiviral transduced human TPH-1 macrophage foam cells. Our data showed that the protein levels of PPARγ were upregulated in adiponectin transduced macrophage foam cells when compared to the control LacZ transduced macrophage foam cells (Figure 4, UP-Panel). Previous reports suggest that transcription of ABCA1 gene, a member of the ATP binding cassette (ABC) family of energy-dependent transporter proteins, is regulated by nuclear oxysterol receptor, LXR [20, 21] and that the PPARγ and LXR ligands can also cooperate to induce expression of ABCA1, and therefore, promote cholesterol efflux from human THP-1 cell lines and macrophages derived from mouse embryonic stem cells [22]. Based on these investigations, we performed Western blot analyses to examine whether expression of LXR and ABCA1 proteins would be also affected in these transduced macrophage foam cells. As shown in Figure 4, the expression of both LXR and ABCA1 proteins was up-regulated in adiponectin transduced macrophage foam cells when compared to those of control LacZ transduced macrophage foam cells.
Figure 4. Regulation of protein expression by adiponectin in human THP-1 macrophage foam cells.
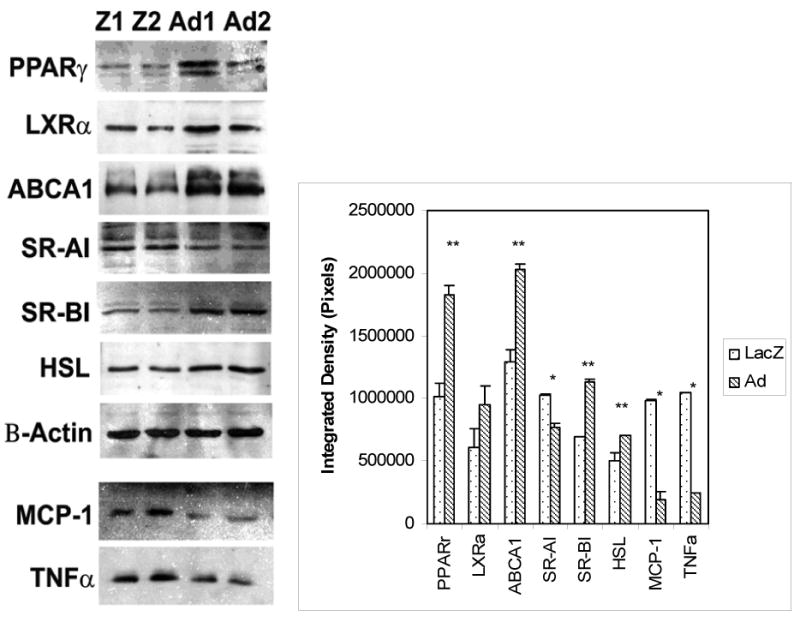
Western blot analyses were performed to examine the levels of proteins: including PPARγ, LXRα, SR-A1, and SR-BI receptors; ABCA1 and HSL; two proinflammatory cytokines secreted in the media which were concentrated 10-folḍ, MCP-1 and TNFα; in adiponectin transduced macrophage foam cells (from Ad1 and Ad2 cell lines) and in the LacZ transduced control macrophage foam cells (from Z1 and Z2 cell lines). The data of Western blots from three experiments were scanned and analyzed with NIH ImageJ software. Results represent the mean ± SE from three separate experiments. *, p< 0.05; **, p< 0.01.
As shown in Figure 4, we also detected a down-regulation of scavenger receptor type AI (SR-AI) in adiponectin-transduced macrophage foam cells when compared with levels in control LacZ transduced macrophage foam cells. This observation is consistent with the role of this scavenger receptor in oxidized lipid uptake in macrophage cells. Conversely, the scavenger receptor BI (SR-BI), which promotes HDL-mediated cholesterol efflux, and the hormone sensitive lipase (HSL), which helps to hydrolyze triglycerides in macrophage foam cells, both were significantly (p<0.01) upregulated in adiponectin-transduced macrophage foam cells. Proatherogenic cytokines are well known to exert effects on the transformation of macrophage into foam cells; therefore, we next examined the impact of adiponectin on MCP-1 and TNFα release from macrophages. The expression of adiponectin in these transformed macrophages led to reduced production of MCP-1 and TNFα proteins in cell culture media when compared to those from the controls (Figure 4, DOWN-Panels). Thus, the expression and secretion of adiponectin in macrophage foam cells resulted in the regulation of multiple important regulatory components in lipid metabolic signaling pathways, lipid metabolism related genes, and proatherogenic cytokines for facilitating oxLDL uptake decrease and lipid efflux increase. In concert, these changes in macrophage metabolism all serve to reduce macrophage foam cell formation.
3.5. Isolation adiponectin multimers and their effects on macrophage foam cell lipid metabolism
Since direct expression of adiponectin or increased adiponectin concentration in conditioned medium both can influence lipid accumulation in THP-1 macrophages, we determined the effects of the different multimers of adiponectin, e.g. low molecular weight (LMW) and high molecular weight (HMW) of adiponectin, on lipid metabolism in macrophage foam cells. The low molecular weight form of adiponectin was separated from higher weight forms using size exclusion chromatography. A representative column elution profile showed in Figure 5A. The concentration of adiponectin in each fraction was determined using ELISA (data not shown). Total adiponectin applied to the column was well resolved into two distinct peaks with the majority of contaminating plasma proteins (OD 280) eluting after the second adiponectin peak. The distribution of adiponectin multimers in serum was similar to that in lipoprotein deficient serum (LPDS) indicating that the purification and concentration of adiponectin by ultracentrifugation did not alter adiponectin multimer distribution.
Figure 5. Effect of LMW and HWM adiponectin on cholesterol metabolism in human THP-1 macrophage foam cells.
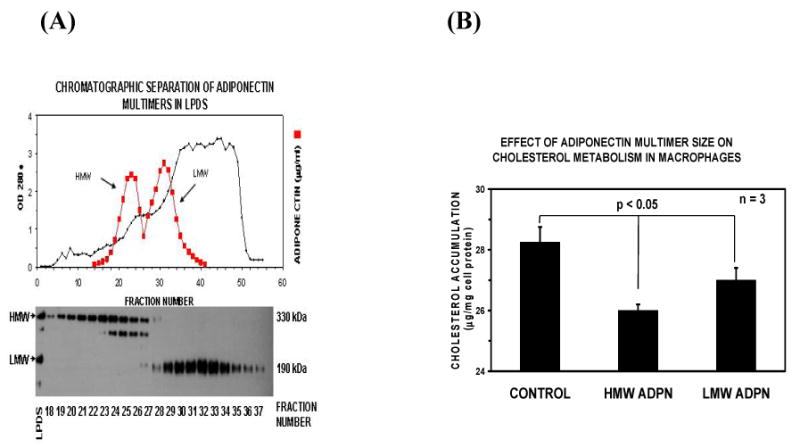
PANEL A – Preparation of LMW and HMW adiponectin. Lipoprotein deficient serum was exhaustively dialyzed against column running buffer and sample containing approximately 1 mg total adiponectin was applied to a chromatography column (AcA22, 2.6X200 cm) equilibrated in the same buffer. Protein eluting from the column was continuously monitored by quantitating absorbance at 280nm (●, OD280, left Y-axis). The concentration of adiponectin protein in each fraction was determined using ELISA and is reported as micrograms per milliter (■, right Y-axis). The distribution of adiponectin isoforms in each column fraction was assayed using Western blot [19]. Fractions containing predominantly LMW adiponectin (fractions 18-23) were pooled separately from those containing HMW adiponectin (fractions 29-37) and used for subsequent experiments. PANEL B. Effects of adiponectin isoforms on lipid accumulation in macrophage foam cells. Fractions containing predominantly LMW adiponectin or HMW adiponectin were added to the macrophage foam cell culture media without adiponectin (CONTROL) or with LMW adiponectin (15μg/ml) or HMW adiponectin (15μg/ml) during oxLDL treatment for 24 hours. Lipids were measured utilizing an enzymatic colorimetric method for the analysis. Cellular protein was measured using a protein analysis kit from Bio-Rad. Results represent the mean ± SE from three separate experiments.
We then determined the effects of LMW compared to HMW adiponectin on macrophage foam cell cholesterol metabolism by determining cholesterol accumulation in macrophage foam cells incubated 24 hours with oxLDL (100μg/ml) without adiponectin added to the medium (CONTROL) or with LMW adiponectin (15μg/ml) or HMW adiponectin (15μg/ml). As shown in Figure 5B, cholesterol accumulation in THP-1 foam cells incubated with HMW adiponectin was significantly (p<0.05) reduced compared to cells incubated with LMW adiponectin or to control incubations without adiponectin added to the media. Thus, adiponectin multimers differentially inhibit macrophage foam cell formation with HMW having the most potent activity. The HMW adiponectin will be probably more atheroprotective in the development of atherosclerosis by inhibiting cholesterol accumulation in the macrophage foam cells.
4. Discussions
Adiponectin is exclusively expressed and secreted from adipocytes and adiponectin expression has not been previously detected in macrophage cells. To investigate the mechanisms of adiponectin-mediated alterations of lipid metabolism in macrophage foam cells, we expressed the gene coding for human adiponectin in macrophage foam cells by using a lentiviral vector. Furthermore, we demonstrated that, endogenous adiponectin produced by macrophages exerts similar alterations in lipid metabolism in human THP-1 macrophage foam cells as exogenous (adipocyte-derived) adiponectin.
Our studies have shown that expression of adiponectin in macrophage foam cells can significantly decrease triglyceride and cholesterol accumulation in these cells by reducing oxLDL uptake into the cells while enhancing HDL-mediated cholesterol efflux. These results suggest that adiponectin plays an important role in lipid metabolism and cholesterol efflux by modulating lipid metabolic signaling pathways for preventing macrophage foam cell formation.
The conversion of macrophages embedded in the intima of arterial walls to cholesterol-laden foam cells is a critical step in the progression of atherosclerosis and this process involves probably not only the internalization of oxLDL particles by scavenger receptor-A, but also PPARγ signaling pathway as recently implicated in this process [23]. PPARγ is expressed at high levels in macrophages, including the foam cells of athermanous lesions [23, 24], and treatment of LDL receptor–deficient mice, a standard model of atherosclerosis, with PPARγ ligands led to the prevention of atherosclerotic lesion formation in these animals [25]. On the other hand, cholesterol efflux from macrophages is reportedly regulated by ABCA1, a member of the ATP binding cassette (ABC) family of energy-dependent transporter proteins, and transcription of the ABCA1 gene is regulated by the nuclear oxysterol receptor, LXR [20, 21]; and PPARγ and LXR ligands probably cooperate to induce expression of ABCA1 and thus promote cholesterol efflux from human THP-1 cell lines and macrophages derived from mouse embryonic stem cells [22]. In our studies, both the PPARγ and LXRα protein levels were up-regulated in the adiponectin-transduced macrophage foam cells. The probable mechanism for this joint molecular cooperation appears to be the LXRα gene, a direct target of the PPARγ/RXR heterodimer [22]. Activation of PPARγ results in increased LXRα expression which in turn leads to increased ABCA1 expression and cholesterol efflux as we have observed in our results. Thus, joint PPARγ/LXR/ABCA1 signaling pathways appear to reduce uptake of oxLDL from macrophage foam cells coupled with increased efflux of free cholesterol which can bind to HDL. During this process, decreased SR-AI and increased SR-BI and HSL may also contribute to reduction of lipid accumulation in adiponectin transduced macrophage foam cells. Since macrophage cells usually do not have high level of SR-BI expression, a higher SR-BI expression is observed in adiponectin transduced cells (compared to lacZ cells) as these cells accumulate less cholesterol; a higher SR-BI expression could probably explain a higher cholesterol efflux to HDL based on the fact that the expression of this receptor, that is involved in cholesterol efflux to mature HDL, is highly correlated to the cholesterol cellular content. Here, adiponectin transduced macrophage cells finely regulate SR-BI expression on the basis of their intracellular cholesterol content.
A broader role for PPARγ activation in the regulation of monocyte/macrophage biology and in inflammation has been also suggested by studies showing that treatment of monocytes or macrophages with PPARγ agonists, including 15d-PGJ2 and glitazones, result in reduced expression of proinflammatory cytokines such as TNFα and interleukin 6 (IL-6) and thus, inhibit macrophage activation [26, 27]. We also demonstrate decreased TNFα and MCP-1 (which are two proatherogenic cytokines) levels in adiponectin-transduced macrophage foam cells which may further reduce the impact of macrophage foam cell formation.
The molecular basis of the cellular action of adiponectin became more evident with the recent identification of two trans-membrane proteins, adiponectin receptor 1 (AdipoR1) and 2 (AdipoR2), which serve as receptors for adiponectin [28]. Recently, these two adiponectin receptors have been also found to be expressed in human macrophages [29]. In addition, the expression of these receptors in macrophages is regulated by agonists of the PPARs and LXR. It is generally considered that the binding of adiponectin to its receptors, and subsequent signaling events initiated by this binding, are probably responsible for the physiological effects. However, using our unique macrophage foam cell model system, we have demonstrated that the bulk of the macrophage-expressed adiponectin remains inside the cells and, thus, exerts its effects on macrophage metabolism without binding to the adiponectin receptors., However, we can not exclude possibility that the small fraction of adiponectin secreted from the adiponectin-transduced macrophage foam cells (Figure 1) binds to macrophage cell receptors and is responsible for the observed alterations in macrophage metabolism. It is important to determine effect of large amounts of intracellular adiponectin on the expression of adiponectin receptors and, furthermore, to determine if there exists a feedback inhibition of the receptor expression as we have previously reported in 3T3-L1 adipocytes [30]. The study of cells with reduced adiponectin receptor expression but which express adiponectin would clarify whether adiponectin has any cellular effect that is not related to its receptor binding in our cell models. The finding that high molecular weight (HMW) adiponectin is more effective than low molecular weight (LMW) one in reducing lipid accumulation is interesting. However, additional studies are required to determine potential mechanisms whereby differently sized adiponectin molecules may differentially affect macrophage metabolism.
Acknowledgments
This work was supported by grants from the National Institutes of Health (PO1-HL55782, DK38764) and the Merit Review Program of the Medical Research Service of the Department of Veterans Affairs to WTG and RLK; by grants from National Institutes of Health (P20 RR16434) and American Diabetes Association (1-07-RA-49) to YF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Auwerx J. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa K, Hori M, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Miyazaki A, Nakayama H, Horiuchi S. Adiponectin down-regulates acyl-coenzyme A: cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochemical and Biophysical Research Communications. 2004;317:831–836. doi: 10.1016/j.bbrc.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 9.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 11.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nature Medicine. 2004;10:13841389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and apoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 15.Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6:67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- 16.Cominacini L, Garbin U, Davoli A, Micciolo R, Bosello O, Gaviraghi G, Scuro LA, Pastorino AM. A sample test for predisposition of LDL oxidation based on the fluorescence development during copper-catalyzed oxidative modification. J Lipid Res. 1991;32:349–358. [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 20.Repa JJ, Turley SD, Lobaccaro JMA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 21.Venkateswaran A, Laffitte BA, Joseph SB, Mak A, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 23.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, Witztum JL, Auwerx J, Palinski W, Glass CK. Expression of the peroxisome proliferator-activated receptor γ (PPARγ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator–activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor–deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 27.Jiang CY, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Tekekawa S, Waki H, Tsuno N, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Aritaira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–768. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 29.Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARα, PPARγ, and LXR. Biochemical & Biophysical Research Communications. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 30.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]