Abstract
Purpose
Energy balance seems to be important in the pathogenesis of colon cancer. Fatty acid synthase (FASN) is physiologically regulated by energy balance and is often upregulated in colorectal cancer. Nonetheless, the influence of FASN expression on patient outcome is uncertain.
Patients and Methods
Using the database of 647 patients with colon cancer in two independent cohort studies, FASN overexpression was detected in 84 tumors (13%) by immunohistochemistry. Cox proportional hazards models calculated hazard ratios (HRs) of colon cancer–specific and overall mortalities, adjusted for patient characteristics and related tumoral features, including KRAS, BRAF, p53, microsatellite instability and the CpG island methylation phenotype.
Results
There were 279 deaths, including 160 colon cancer–specific deaths. FASN overexpression was associated with a significant reduction in colon cancer–specific mortality by both univariate and multivariate analyses (adjusted HR, 0.41; 95% CI, 0.19 to 0.89) and an insignificant trend toward improved overall mortality (adjusted HR, 0.75; 95% CI, 0.50 to 1.13). Notably, the effect of FASN expression on mortality might be different according to body mass index (BMI; Pinteraction = .019); the adjusted HR of overall mortality for FASN overexpression was 0.63 (95% CI, 0.39 to 1.02) among patients with BMI less than 27.5 kg/m2 and 2.91 (95% CI, 1.19 to 7.12) among those with BMI ≥ 27.5 kg/m2. Moreover, the adverse effect of moderate overweight/obesity on overall survival was limited to FASN-positive tumors (adjusted HR, 4.10; 95% CI, 1.14 to 14.8; BMI ≥ 27.5 kg/m2 v < 27.5 kg/m2).
Conclusion
Among nonobese patients with colon cancer, tumoral FASN overexpression is associated with improved survival, whereas among moderately overweight or obese patients (BMI ≥ 27.5 kg/m2), FASN overexpression may predict a worse outcome.
INTRODUCTION
Energy balance, or the ability to maintain body weight by balancing energy intake with energy expenditure, seems to be important in the pathogenesis of human cancers, including colon cancer.1,2 Animal studies find that restricting energy intake reduces tumor development.3 In humans, prospective observational studies demonstrate that obesity increases the risk of colon cancer, whereas regular physical activity is associated with a reduced risk.4 Studies of patients with colon cancer indicate that regular physical activity and lean body mass significantly reduce the risk of cancer recurrence and mortality.5-9
Fatty acid synthase (FASN) plays an important role in de novo lipogenesis and is physiologically regulated by energy balance.1,10 High-carbohydrate/low-fat diets upregulate FASN, whereas exercise and energy restriction downregulate FASN.11 A missense mutation of the FASN gene is protective against obesity,12 suggesting the important role of FASN in regulating energy retention. FASN overexpression is commonly observed in human cancers,10,13-15 including colon cancer.16-19 Downregulation of FASN results in increased apoptosis in cancer cells.20 Preclinical studies suggest that FASN inhibitors, such as C75 and orlistat, may possess both chemopreventive and antitumor activity.21-23 Although FASN expression has been associated with patient outcome in breast, ovarian, and prostate cancers,13-15 the influence of FASN expression on survival among patients with colon cancer remains uncertain.16,18
We therefore examined the impact of tumoral FASN expression on clinical outcome among patients with colon cancer in two cohort studies (the Nurses’ Health Study and the Health Professionals Follow-Up Study). Because detailed data on patient characteristics and other major tumoral molecular events were recorded in this population, we were able to analyze the independent effects of tumoral FASN expression as well as the joint effect of body mass index (BMI) and FASN expression on patient outcome.
PATIENTS AND METHODS
Study Population
We used the databases of two large prospective cohort studies, the Nurses’ Health Study (N = 121,700 women observed since 1976)24,25 and the Health Professional Follow-Up Study (N = 51,500 men observed since 1986).25 Every 2 years, participants have been sent follow-up questionnaires to update information on potential risk factors and to identify newly diagnosed cancer and other diseases. We calculated body mass index (BMI, kg/m2) using self-reported height from the baseline questionnaire and weight from the biennial questionnaire that immediately preceded the diagnosis of colon cancer. In validation studies in both cohorts, self-reported anthropometric measures were well correlated with measurements by trained technicians (r > 0.96).26 Informed consent was obtained from all patients in this study. This study was approved by the Human Subjects Committees at Brigham and Women's Hospital and the Harvard School of Public Health.
Measurement of Colon Cancer and Mortality
On each biennial follow-up questionnaire, participants were asked whether they had a diagnosis of colon cancer during the previous 2 years. When a participant (or next of kin for decedents) reported colon cancer, we sought permission to obtain medical records. Study physicians, although blinded to exposure data, reviewed all records related to colon cancer and recorded American Joint Committee on Cancer tumor stage and tumor location. For nonresponders, we searched the National Death Index to discover deaths and ascertain any diagnosis of colon cancer that contributed to death or was a secondary diagnosis. Approximately 96% of all incident colon cancer cases were identified through these methods. We collected paraffin-embedded tissue blocks from hospitals where patients with colon cancer underwent resections of primary tumors.25 Tissue sections from all colon cancer cases were reviewed by a pathologist (S.O.). Tumor grade was categorized as high (≤ 50% glandular area) or low (> 50% glandular area). On the basis of availability of tissue samples, we included a total of 647 colon cancer cases diagnosed up to 2002.
Patients were observed until death or June 2006, whichever came first. Ascertainment of deaths included reporting by the family or postal authorities. In addition, the names of persistent nonresponders were searched in the National Death Index.27 The cause of death was assigned by physicians blinded to other clinical and lifestyle information. In rare patients who died as a result of colon cancer not previously reported, we obtained medical records with permission from next of kin. More than 98% of deaths in the cohorts were identified by these methods.
Immunohistochemistry for FASN and p53
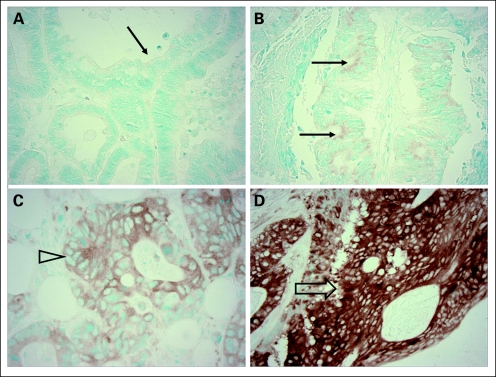
Tissue microarrays were constructed and immunohistochemistry for FASN and p53 was performed as previously described.17,19,28 FASN expression was categorized as negative (no or weak expression) or positive (strong expression; Fig 1). Appropriate positive and negative controls were included in each run of immunohistochemistry. All immunohistochemically stained slides were interpreted by a pathologist (S.O.) blinded to other data. Random samples of 246 and 118 tumors were examined for FASN and p53, respectively, by a second observer (K.N.) unaware of other data, and the concordances between the two observers were 0.93 for FASN (κ = 0.57, P < .0001) and 0.87 for p53 (κ = 0.75, P < .0001).
Fig 1.
Fatty acid synthase (FASN) expression in colon cancer. (A) No FASN expression in colon cancer (arrow). (B) Little FASN expression in colon cancer (negative for FASN overexpression; arrows). (C) Weak FASN expression in colon cancer (negative for FASN overexpression; open arrowhead). (D) Strong FASN expression in colon cancer (positive for FASN overexpression; open arrow).
DNA Extraction, KRAS/BRAF Sequencing, and Microsatellite Instability Analysis
DNA from paraffin-embedded tissue was extracted, and sequencing of KRAS codons 12 to 13 and BRAF codon 600 were performed as previously described.29,30 Microsatellite insability (MSI) status was determined as previously described,17 using a 10-marker panel (D2S123, D5S346, D17S250, BAT25, BAT26,31 BAT40, D18S55, D18S56, D18S67, and D18S487). MSI high was defined as the presence of instability in ≥ 30% of the markers.
Real-Time Polymerase Chain Reaction for Quantitative DNA Methylation Analysis
Sodium bisulfite treatment on DNA and subsequent quantitative polymerase chain reaction were performed as previously described32 to quantify DNA methylation in eight CpG island methylation phenotype (CIMP)–specific markers (CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1).33-35 CIMP high was defined as six or more of eight methylated markers using the eight-marker CIMP panel; CIMP low/0 was defined as five or fewer than eight methylated markers, according to the previously established criteria.34
Statistical Analysis
We used Cox proportional hazards models to calculate hazard ratios (HRs) of death according to tumoral FASN status, adjusted for age, sex, BMI, year of diagnosis, tumor location, stage, grade, and statuses of MSI, CIMP, KRAS, BRAF, and p53. For analyses of colon cancer–specific mortality, death as a result of colon cancer was the end point, and deaths as a result of other causes were censored. Among the covariates in multivariate Cox models, age and year of diagnosis were used as continuous variables, and the other covariates were used as categoric variables. In secondary analyses, we examined the joint effects of FASN expression and BMI on mortality. To maximize statistical power for these subgroup analyses as well as to effectively demonstrate influence of FASN overexpression in moderately overweight to obese patients, we categorized BMI dichotomously as less than 27.5 kg/m2 and ≥ 27.5 kg/m2, as this represented the midpoint between the upper limit of normal BMI and obesity, as defined by WHO.36 We also assessed the effect of FASN on survival in a range of categories of BMI (< 25 kg/m2, 25 to 27.5 kg/m2, 27.5 to 30 kg/m2, and ≥ 30 kg/m2). For the purposes of this analysis, a single multivariate Cox regression model computed HRs (in FASN-positive tumors compared with FASN-negative tumors) in different strata of the BMI categories under the assumption that coefficients for all covariates stay constant across the BMI categories. This method maximized power in analysis of multiple strata while adequately adjusting for covariates. When there was missing information on BMI (3.7% missing), tumor location (1.2% missing), stage (7.1% missing), grade (0.5% missing), MSI (2.2% missing), p53 (0.6% missing), KRAS (1.7% missing), or BRAF (4.1% missing), we assigned a separate (“missing”) indicator variable and included those cases in multivariate Cox models. We confirmed that excluding cases with a missing variable did not significantly alter results (data not shown). An interaction was assessed by including the cross-product of two variables of interest in a Cox model, and the likelihood ratio test was performed. To assess an interaction of FASN and stage, we dichotomized tumor stage (I to II v III to IV) and also dealt with stage as a linear ordinal variable (from I to IV) to confirm no significant effect modification. To assess an interaction of FASN and BMI, we used BMI categories (< 25 kg/m2, 25 to 27.5 kg/m2, 27.5 to 30 kg/m2, and ≥ 30 kg/m2) as a linear ordinal variable. The Kaplan-Meier method and the log-rank test were used to assess a difference in survival time distributions. The χ2 test was used to examine an association between categoric variables. The t test assuming unequal variances was used to compare mean age and BMI. All analyses used SAS version 9.1 (SAS Institute, Cary, NC), and all P values were two-sided.
RESULTS
FASN Expression in Colon Cancer and Patient Survival
Among 647 eligible patients with available colon cancer specimens, there were 279 deaths, including 160 colon cancer–specific deaths. Among all tumors, 84 (13%) demonstrated FASN overexpression, whereas 563 (87%) were negative for FASN overexpression. Table 1 lists clinical and molecular features of colon cancer according to FASN expression status. When compared with patients with FASN-negative cancers, those with FASN-positive tumors were less likely to present with stage IV disease (P = .01) and more likely to exhibit MSI high (P = .01).
Table 1.
Clinical and Molecular Features of Colon Cancer According to FASN Overexpression
| Clinical or Molecular Feature | All Cases
|
FASN Negative
|
FASN Positive
|
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total No. | 647 | 563 | 84 | ||||
| Sex | .68 | ||||||
| Male, HPFS | 287 | 44 | 248 | 44 | 39 | 46 | |
| Female, NHS | 360 | 56 | 315 | 56 | 45 | 54 | |
| Age, years | .37 | ||||||
| Mean | 66.6 | 66.7 | 65.7 | ||||
| SD | 8.3 | 8.0 | 10.0 | ||||
| Prediagnosis BMI, kg/m2 | .002 | ||||||
| Mean | 26.5 | 26.6 | 25.2 | ||||
| SD | 4.7 | 4.8 | 3.7 | ||||
| BMI, kg/m2 | .04 | ||||||
| < 25 | 262 | 42 | 219 | 40 | 43 | 52 | |
| 25-27.5 | 155 | 25 | 132 | 24 | 23 | 28 | |
| 27.5-30 | 98 | 16 | 89 | 16 | 9 | 11 | |
| ≥ 30 | 108 | 17 | 101 | 19 | 7 | 8.5 | |
| Year of diagnosis | .77 | ||||||
| Before 1990 | 100 | 15 | 86 | 15 | 14 | 17 | |
| 1990 to 1999 | 471 | 73 | 409 | 73 | 62 | 74 | |
| 2000 to 2002 | 76 | 12 | 68 | 12 | 8 | 9.5 | |
| Tumor location* | .17 | ||||||
| Proximal | 371 | 58 | 317 | 57 | 54 | 65 | |
| Distal | 268 | 42 | 239 | 43 | 29 | 35 | |
| Tumor stage | .01 | ||||||
| I | 135 | 21 | 118 | 21 | 17 | 20 | |
| II | 222 | 34 | 181 | 32 | 41 | 49 | |
| III | 162 | 25 | 143 | 25 | 19 | 23 | |
| IV | 82 | 13 | 79 | 14 | 3 | 3.6 | |
| Unknown | 46 | 7.1 | 42 | 7.5 | 4 | 4.8 | |
| Tumor grade | .78 | ||||||
| Low | 573 | 89 | 499 | 89 | 74 | 88 | |
| High | 71 | 11 | 61 | 11 | 10 | 12 | |
| MSI | 0.01 | ||||||
| Low/MSS | 514 | 81 | 455 | 83 | 59 | 71 | |
| High | 119 | 19 | 95 | 17 | 24 | 29 | |
| CIMP | .39 | ||||||
| Low/0 | 523 | 81 | 458 | 81 | 65 | 77 | |
| High | 124 | 19 | 105 | 19 | 19 | 23 | |
| KRAS mutation | .70 | ||||||
| Negative | 403 | 63 | 352 | 64 | 51 | 61 | |
| Positive | 233 | 37 | 201 | 36 | 32 | 39 | |
| BRAF mutation | .73 | ||||||
| Negative | 520 | 84 | 451 | 84 | 69 | 85 | |
| Positive | 100 | 16 | 88 | 16 | 12 | 15 | |
| p53 expression | .83 | ||||||
| Negative | 393 | 61 | 342 | 61 | 51 | 62 | |
| Positive | 250 | 39 | 219 | 39 | 31 | 38 | |
Abbreviations: FASN, fatty acid synthase; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; SD, standard deviation; BMI, body mass index; MSI, microsatellite instability; MSS, microsatellite stable; CIMP, CpG island methylator phenotype.
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to sigmoid colon.
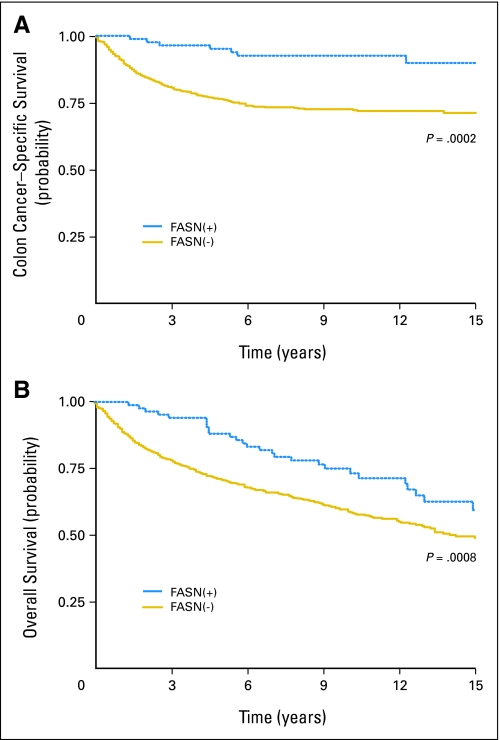
We assessed the influence of FASN expression on patient survival (Fig 2). Five-year colon cancer–specific survival was 76% among patients with FASN-negative tumors and 95% among patients with FASN-positive tumors (log-rank P = .0002). Similarly, 5-year overall survival was 71% among patients with FASN-negative tumors and 88% among patients with FASN-positive tumors (log-rank P = .008).
Fig 2.
Kaplan-Meier curves for (A) colon cancer–specific survival and (B) overall survival according to tumoral fatty acid synthase (FASN) expression.
In the Cox regression model, compared with patients with FASN-negative tumors, those with FASN-positive tumors experienced a significant reduction in unadjusted colon cancer–specific mortality (HR, 0.27; 95% CI, 0.12 to 0.57) as well as overall mortality (HR, 0.59; 95% CI, 0.40 to 0.88; Table 2). After adjusting for potential confounders, FASN positivity remained as a significant predictor of improved colon cancer–specific mortality (HR, 0.41; 95% CI, 0.19 to 0.89), with a trend toward an improved overall mortality (HR, 0.75; 95% CI, 0.50 to 1.13). The attenuation in the effect of FASN overexpression in the multivariate analysis was principally the result of adjusting for tumor stage; when we simply adjusted for tumor stage, the FASN overexpression was associated with HR of 0.37 (95% CI, 0.17 to 0.80) for colon cancer–specific mortality and HR of 0.71 (95% CI, 0.48 to 1.07) for overall mortality.
Table 2.
FASN Expression and Patient Survival in Colon Cancer
| FASN Overexpression | Total No. | Colon Cancer–Specific Mortality
|
Overall Mortality
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths/Person-Years | Univariate
|
Multivariate
|
Deaths/Person-Years | Univariate
|
Multivariate
|
||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Negative | 563 | 153/4,817 | 1 | Referent | 1 | Referent | 252/4817 | 1 | Referent | 1 | Referent |
| Positive | 84 | 7/915 | 0.27 | 0.12 to 0.57 | 0.41 | 0.19 to 0.89 | 27/915 | 0.59 | 0.40 to 0.88 | 0.75 | 0.50 to 1.13 |
The multivariate Cox model includes age, year of diagnosis, sex, body mass index, tumor location, stage, tumor grade, and statuses of KRAS, BRAF, p53, microsatellite instability, and the CpG island methylator phenotype.
Abbreviations: FASN, fatty acid synthase; HR, hazard ratio.
Interactive Effect of FASN and BMI on Patient Survival
We examined whether the effect of FASN expression on patient survival was modified by BMI (as reported within 2 years before the diagnosis of colon cancer). The effect of FASN expression on patient survival seemed to differ according to BMI (P for interaction = 0.019 in analysis of overall mortality). Multivariate HRs for overall mortality in FASN-positive tumors (compared with FASN-negative tumors) across a range of BMI categories were as follows: 0.55 (95% CI, 0.30 to 1.03) in BMI less than 25 kg/m2, 0.68 (95% CI, 0.32 to 1.46) in BMI 25 to 27.5 kg/m2, 1.70 (95% CI, 0.51 to 5.68) in BMI 27.5 to 30 kg/m2, and 2.41 (95% CI, 0.82 to 7.08) in BMI ≥ 30 kg/m2. Thus the adverse effect of FASN overexpression seemed to be limited to patients with BMI ≥ 27.5 kg/m2, whereas there was no apparent adverse effect of FASN overexpression among patients with BMI less than 27.5 kg/m2. Next we dichotomized patients according to BMI (< 27.5 kg/m2 v ≥ 27.5 kg/m2). In patients with BMI less than 27.5 kg/m2 (normal weight and minimally overweight), FASN positivity was associated with significantly improved cancer-specific mortality (adjusted HR, 0.37; 95% CI, 0.15 to 0.92) and a trend toward improved overall mortality (adjusted HR, 0.63; 95% CI, 0.39 to 1.02; Table 3). In contrast, among patients with BMI ≥ 27.5 kg/m2 (moderately overweight and obese), FASN positivity was associated with higher overall mortality (adjusted HR, 2.91; 95% CI, 1.19 to 7.12). Similar results of an interactive effect of FASN and BMI on survival were observed among patients with prostate cancer in the Health Professionals Follow-up Study and Physicians Health Study cohorts (Nguyen et al, unpublished data).
Table 3.
Joint Effects of FASN Expression and BMI on Patient Survival in Colon Cancer
| Variable | Total No. | Colon Cancer–Specific Mortality
|
Overall Mortality
|
||||
|---|---|---|---|---|---|---|---|
| Death/Person-Years | Multivariate
|
Deaths/Person-Years | Multivariate
|
||||
| HR | 95% CI | HR | 95% CI | ||||
| Effect of FASN by strata of BMI | |||||||
| BMI < 27.5 kg/m2 | |||||||
| FASN negative | 351 | 98/2,995 | 1 | Referent | 163/2,995 | 1 | Referent |
| FASN positive | 66 | 5/749 | 0.37 | 0.15 to 0.92 | 20/749 | 0.63 | 0.39 to 1.02 |
| BMI ≥ 27.5 kg/m2 | |||||||
| FASN negative | 190 | 47/1,658 | 1 | Referent | 78/1,658 | 1 | Referent |
| FASN positive | 16 | 2/147 | 1.31 | 0.29 to 5.90 | 7/147 | 2.91 | 1.19 to 7.12 |
| Effect of BMI by strata of FASN | |||||||
| FASN negative | |||||||
| BMI < 27.5 kg/m2 | 351 | 98/2,995 | 1 | Referent | 163/2,995 | 1 | Referent |
| BMI ≥ 27.5 kg/m2 | 190 | 47/1,658 | 0.91 | 0.63 to 1.30 | 78/1,658 | 0.86 | 0.65 to 1.14 |
| FASN positive | |||||||
| BMI < 27.5 kg/m2 | 66 | 5/749 | 1* | Referent | 20/749 | 1 | Referent |
| BMI ≥ 27.5 kg/m2 | 16 | 2/147 | 2.94* | 0.48 to 18.0 | 7/147 | 4.10 | 1.14 to 14.8 |
NOTE. The multivariate Cox model includes age, year of diagnosis, sex, tumor location, stage, tumor grade, and statuses of KRAS, BRAF, p53, microsatellite instability, and the CpG island methylator phenotype.
Abbreviations: FASN, fatty acid synthase; BMI, body mass index; HR, hazard ratio.
Adjusted for stage, as a result of the only seven total deaths in this subset analysis.
We examined whether the effect of BMI on survival was modified by FASN status (Table 3). Among patients with FASN-negative tumors, moderate overweight or obesity (BMI ≥ 27.5 kg/m2) was not significantly associated with increased mortality. In contrast, among patients with FASN-positive tumors, those with BMI ≥ 27.5 kg/m2 experienced a significantly higher overall mortality (HR, 4.10; 95% CI, 1.14 to 14.8) when compared with those with BMI less than 27.5 kg/m2.
FASN Expression, Other Patient and Tumoral Variables, and Prognosis
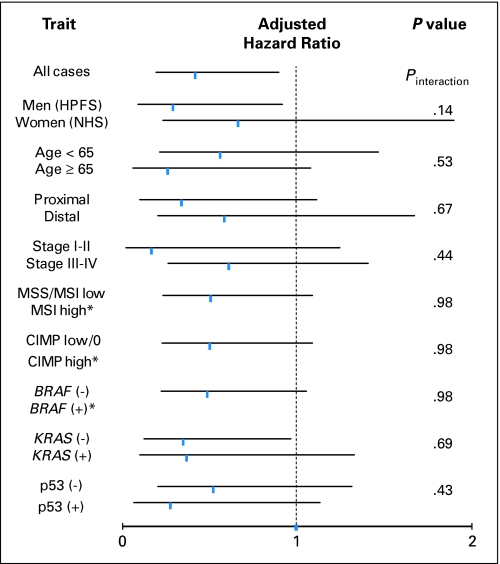
Finally, we examined whether the effect of FASN expression on mortality was modified by other patient or disease characteristics. Notably, the effect of FASN expression did not significantly differ between the male cohort (Health Professionals Follow-Up Study) and the female cohort (Nurses’ Health Study; Pinteraction = .14). The inverse relation between FASN positivity and mortality persisted across strata of other clinical and tumoral variables, and all point estimates of HRs for colon cancer–specific mortality were less than 1 (Fig 3). There was no evidence for significant effect modification by any of these variables (all Pinteraction > .13).
Fig 3.
Stratified analysis of fatty acid synthase (FASN) and colon cancer–specific mortality. Adjusted hazard ratios (HRs) with 95% CI for FASN-positive tumors (compared with FASN-negative tumors) in various strata are shown. Adjusted HRs are consistently less than 1, indicating lower mortalities associated with FASN-positive tumors. *95% CI is not shown because of no colon cancer–specific deaths among patients with FASN-positive tumors that were microsatellite instability (MSI) high, CpG island methylation phenotype (CIMP) high, or BRAF positive. HPFS, Health Professionals Follow-Up Study; MSS, microsatellite stable; NHS, Nurses’ Health Study.
DISCUSSION
In this large cohort of patients with colon cancer, we found that tumoral FASN overexpression was a significant predictor of reduced cancer-specific mortality, independent of various clinical and molecular variables including tumor stage and statuses of MSI, KRAS, BRAF, and p53. Moreover, the improved outcome associated with FASN overexpression was similar across strata of most patient and disease characteristics and was consistent across the two independent prospective cohort studies in this analysis.
Notably, the effect of FASN overexpression on clinical outcome seemed to be modified by patient BMI. Among normal weight and minimally overweight patients (BMI < 27.5 kg/m2), FASN overexpression was associated with a reduction in mortality, whereas among moderately overweight and obese patients (BMI ≥ 27.5 kg/m2), FASN overexpression conferred a significant increased in mortality. Similar findings on FASN expression, BMI, and survival were observed among patients with prostate cancer in the Health Professionals Follow-Up Study and Physicians Health Study cohorts (Nguyen et al, unpublished data). These data suggest that influence of FASN overexpression differs substantially according to the host milieu in which the overexpression occurs. Moreover, consistent with other studies among patients with colon cancer,6-9 higher BMI was associated with an increased mortality, although the deleterious effect of obesity was limited to patients with FASN overexpression.
FASN has been implicated in cancer pathogenesis.1,10 FASN inhibitors, such as C75 and orlistat, have been shown to exhibit antitumor activity.21,22,37 Downregulation of FASN through its enhanced proteasomal degradation results in increased apoptosis, indicating an addiction of tumor cells to FASN.20 Recent data suggest that increased FASN activity in tumor cells is important for function of endoplasmic reticulum to maintain membrane biogenesis.38 These data collectively support that FASN acts as an oncoprotein. In fact, FASN overexpression is commonly observed in human cancers,10,13-15 including colon cancer.16,18,19 Importantly, FASN overexpression confers chemoresistance in breast cancer cells, and inhibition of FASN may enhance the effect of chemotherapy.39,40 FASN overexpression has been reported to confer a significantly worse outcome in patients with breast, ovarian, lung, and prostate cancers,1,13-15 although none of these studies assessed patient BMI. Among patients with colorectal cancer, no significant relation between FASN expression and survival was demonstrated in two smaller studies.16,18
Potential prognostic factors and markers have been investigated in colon cancer.41-46 Dietary factors and altered energy balance represent important risk factors for cancer incidence, recurrence, and death.7-9 Among patients with colon cancer, obesity has been associated with poor clinical outcome.7-9 However, mechanisms of how obesity or altered energy balance influence cancer prognosis are poorly understood. Accumulating evidence suggests that FASN may play a role in the link between dietary and energy factors and pathogenesis of neoplasia; FASN is regulated by energy balance and FASN alterations seem to be important in carcinogenesis.1,10,11 Our current results are particularly intriguing in that the negative influence of obesity on survival may be limited to patients with FASN-overexpressing tumors. Our data support the importance of FASN in determining biologic behavior of colon cancer as well as a significant interaction between energy balance and tumor biology on clinical outcome. One may speculate that colon cancer cells with FASN upregulation may depend on excess energy for growth, leading to more aggressive tumor behavior among obese patients.
Although FASN has been considered to act as an oncoprotein,20-22,37 its overexpression seems to mark a subtype of colon cancer that is associated with indolent behavior among relatively normal-weight patients. It should be noted that upregulation of a particular oncoprotein or downregulation of a particular tumor suppressor does not necessarily imply a poor clinical outcome.47 For example, although MSI has been shown to mutate and inactivate a number of tumor suppressors, leading to cancer development, MSI-high colon cancer has been consistently associated with better outcome.48,49 It is plausible that, among normal-weight patients, colon cancer without FASN upregulation develops through an alternative nonenergy dependent pathway, which is associated with poor clinical outcome.
Our study has several advantages, including a large number of colon cancers derived from the two prospective cohorts as well as extensive data on patient characteristics, disease characteristics, and other important tumoral molecular events. Thus we have been able to demonstrate an effect of FASN on patient survival, independent of clinical and other tumoral characteristics.
In our cohorts, data on cancer treatment were limited. Nonetheless, it is unlikely that chemotherapy use differed according to tumoral FASN status, especially because such data were not available to patients or treating physicians. In addition, beyond cause of mortality, data on cancer recurrences were not available in these cohorts. However, given that the median survival for metastatic colon cancer was 10 to 12 months during much of the time period of this study,5 colon cancer–specific survival should be a reasonable surrogate for cancer-specific outcomes.
To date, there is no standardized classification scheme for FASN expression in colon cancer. Nevertheless, previous studies have demonstrated that the results of mRNA expression microarray and immunoblot analyses correlate well with immunohistochemical grading of FASN.13,50 In validation studies of the central, blinded review of tumor specimens, we observed substantial interobserver agreement (93%). Moreover, any random misclassification of FASN overexpression would be expected to bias our results toward the null hypothesis.
In conclusion, this large prospective study of patients with colon cancer suggests that FASN upregulation is a significant independent predictor of improved survival among nonobese patients with colon cancer, whereas among moderately overweight or obese patients (BMI ≥ 27.5 kg/m2), FASN overexpression may predict a worse outcome. Concurrently, the influence of obesity on patient survival may be modified by tumoral FASN expression. Our finding may have significant clinical implications and may offer a potential mechanism by which excess energy balance may influence tumorigenesis and cancer progression.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shuji Ogino, Massimo Loda, Charles S. Fuchs
Financial support: Shuji Ogino, Charles S. Fuchs
Administrative support: Gregory J. Kirkner, Edward Giovannucci, Charles S. Fuchs
Provision of study materials or patients: Shuji Ogino, Katsuhiko Nosho, Jeffrey A. Meyerhardt, Gregory J. Kirkner, Andrew T. Chan, Takako Kawasaki, Edward Giovannucci, Charles S. Fuchs
Collection and assembly of data: Shuji Ogino, Katsuhiko Nosho, Jeffrey A. Meyerhardt, Gregory J. Kirkner, Andrew T. Chan, Takako Kawasaki, Edward Giovannucci, Massimo Loda, Charles S. Fuchs
Data analysis and interpretation: Shuji Ogino, Katsuhiko Nosho, Jeffrey A. Meyerhardt, Gregory J. Kirkner, Andrew T. Chan, Takako Kawasaki, Edward Giovannucci, Massimo Loda, Charles S. Fuchs
Manuscript writing: Shuji Ogino, Katsuhiko Nosho, Jeffrey A. Meyerhardt, Edward Giovannucci, Charles S. Fuchs
Final approval of manuscript: Shuji Ogino, Katsuhiko Nosho, Jeffrey A. Meyerhardt, Gregory J. Kirkner, Andrew T. Chan, Takako Kawasaki, Edward Giovannucci, Massimo Loda, Charles S. Fuchs
Acknowledgments
We thank the Nurses’ Health Study and Health Professionals Follow-up Study cohort participants who have generously agreed to provide us with biologic specimens and information through responses to questionnaires; hospitals and pathology departments throughout the United States for generously providing archival tumor specimens; and Walter Willett, Susan Hankinson, and many other staff members who implemented and have maintained the cohort studies.
published online ahead of print at www.jco.org on October 27, 2008
Supported by National Institutes of Health Grants No. P01 CA87969, P01 CA55075, P50 CA127003, and K07 CA122826 (S.O.), and in part by the Bennett Family Fund for Targeted Therapies Research, and by the Entertainment Industry Foundation (EIF) through the EIF National Colorectal Cancer Research Alliance. K.N. was supported by a fellowship grant from the Japanese Society for Promotion of Science.
None of the funding agencies had any role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
S.O. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Menendez JA, Lupu R: Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763-777, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Chen YQ, Edwards IJ, Kridel SJ, et al: Dietary fat-gene interactions in cancer. Cancer Metastasis Rev 26:535-551, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, Nunez NP, Patel AC, et al: The utility of genetically altered mouse models for nutrition and cancer chemoprevention research. Mutat Res 576:80-92, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gunter MJ, Leitzmann MF: Obesity and colorectal cancer: Epidemiology, mechanisms and candidate genes. J Nutr Biochem 17:145-156, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Giovannucci EL, Holmes MD, et al: Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24:3527-3534, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Haydon AM, Macinnis RJ, English DR, et al: Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55:62-67, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dignam JJ, Polite BN, Yothers G, et al: Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 98:1647-1654, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Tamakoshi K, Wakai K, Kojima M, et al: A prospective study of body size and colon cancer mortality in Japan: The JACC Study. Int J Obes Relat Metab Disord 28:551-558, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Catalano PJ, Haller DG, et al: Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 98:484-495, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Kuhajda FP: Fatty acid synthase and cancer: New application of an old pathway. Cancer Res 66:5977-5980, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Motoshima H, Goldstein BJ, Igata M, et al: AMPK and Cell Proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574:63-71, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs P, Harper I, Hanson RL, et al: A novel missense substitution (Val1483Ile) in the fatty acid synthase gene (FAS) is associated with percentage of body fat and substrate oxidation rates in nondiabetic Pima Indians. Diabetes 53:1915-1919, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Rossi S, Graner E, Febbo P, et al: Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res 1:707-715, 2003 [PubMed] [Google Scholar]
- 14.Aló PL, Visca P, Marci A, et al: Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer 77:474-482, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Gansler TS, Hardman W 3rd, Hunt DA, et al: Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28:686-692, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Rashid A, Pizer ES, Moga M, et al: Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol 150:201-208, 1997 [PMC free article] [PubMed] [Google Scholar]
- 17.Ogino S, Brahmandam M, Cantor M, et al: Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol 19:59-68, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Visca P, Alo PL, Del Nonno F, et al: Immunohistochemical expression of fatty acid synthase, apoptotic-regulating genes, proliferating factors, and ras protein product in colorectal adenomas, carcinomas, and adjacent nonneoplastic mucosa. Clin Cancer Res 5:4111-4118, 1999 [PubMed] [Google Scholar]
- 19.Ogino S, Kawasaki T, Ogawa A, et al: Fatty acid synthase overexpression in colorectal cancer is associated with microsatellite instability, independent of CpG island methylator phenotype. Hum Pathol 38:842-849, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Graner E, Tang D, Rossi S, et al: The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 5:253-261, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Alli PM, Pinn ML, Jaffee EM, et al: Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene 24:39-46, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kridel SJ, Axelrod F, Rozenkrantz N, et al: Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res 64:2070-2075, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kridel SJ, Lowther WT, Pemble CW: Fatty acid synthase inhibitors: New directions for oncology. Expert Opin Investig Drugs 16:1817-1829, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Hankinson SE: The Nurses’ Health Study: Lifestyle and health among women. Nat Rev Cancer 5:388-396, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Chan AT, Ogino S, Fuchs CS: Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356:2131-2142, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Stampfer MJ, Colditz GA, et al: Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1:466-473, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Sathiakumar N, Delzell E, Abdalla O: Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med 40:808-813, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Ogino S, Brahmandam M, Kawasaki T, et al: Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia 8:458-464, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Kawasaki T, Brahmandam M, et al: Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn 7:413-421, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogino S, Kawasaki T, Kirkner GJ, et al: CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: Possible associations with male sex and KRAS mutations. J Mol Diagn 8:582-588, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boland CR, Thibodeau SN, Hamilton SR, et al: A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248-5257, 1998 [PubMed] [Google Scholar]
- 32.Ogino S, Kawasaki T, Brahmandam M, et al: Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn 8:209-217, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino S, Cantor M, Kawasaki T, et al: CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut 55:1000-1006, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, Kawasaki T, Kirkner GJ, et al: Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn 9:305-314, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisenberger DJ, Siegmund KD, Campan M, et al: CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 38:787-793, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Physical status: The use and interpretation of anthropometry: Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 854:1-452, 1995 [PubMed] [Google Scholar]
- 37.Li JN, Gorospe M, Chrest FJ, et al: Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res 61:1493-1499, 2001 [PubMed] [Google Scholar]
- 38.Little JL, Wheeler FB, Fels DR, et al: Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res 67:1262-1269, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Menendez JA, Lupu R, Colomer R: Inhibition of tumor-associated fatty acid synthase hyperactivity induces synergistic chemosensitization of HER-2/neu-overexpressing human breast cancer cells to docetaxel (taxotere). Breast Cancer Res Treat 84:183-195, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Furuta E, Pai SK, Zhan R, et al: Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68:1003-1011, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Press OA, Haiman CA, et al: Association of methylenetetrahydrofolate reductase gene polymorphisms and sex-specific survival in patients with metastatic colon cancer. J Clin Oncol 25:3726-3731, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Kim GP, Colangelo LH, Wieand HS, et al: Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol 25:767-772, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Khambata-Ford S, Garrett CR, Meropol NJ, et al: Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230-3237, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Sartore-Bianchi A, Moroni M, Veronese S, et al: Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol 25:3238-3245, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Gripp S, Moeller S, Bolke E, et al: Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 25:3313-3320, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Scartozzi M, Bearzi I, Pierantoni C, et al: Nuclear factor-kB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. J Clin Oncol 25:3930-3935, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ogino S, Goel A: Molecular classifi0cation and correlates in colorectal cancer. J Mol Diagn 10:13-27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popat S, Hubner R, Houlston RS: Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23:609-618, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Boland CR: Clinical uses of microsatellite instability testing in colorectal cancer: An ongoing challenge. J Clin Oncol 25:754-756, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Pflug BR, Pecher SM, Brink AW, et al: Increased fatty acid synthase expression and activity during progression of prostate cancer in the TRAMP model. Prostate 57:245-254, 2003 [DOI] [PubMed] [Google Scholar]