Abstract
The ability to modulate bilateral finger tapping in time to different frequencies of an auditory beat was studied. Twenty children, 7 years of age, ten with and ten without Developmental Coordination Disorder (DCD), and ten adults tapped their left index and right middle fingers in an alternating pattern in time with an auditory signal for 15 s (4 trials each, randomly, at 0.8, 1.6, 2.4, 3.2 Hz per finger). Dominant and non-dominant finger data were collapsed since no differences emerged. All three groups were able to modulate their finger frequency across trials to closely approximate the signal frequency but children with DCD were unable to slow down to the lowest frequency. Children with DCD were more variable in tap accuracy (SD of relative phase) and between-finger coordination than typically developing children who were respectively more variable than the adults. Children with DCD were unable to consistently synchronize their finger with the beat. Adults were tightly synchronized and often ahead of the beat while children without DCD tended to be behind the beat. Overall, these results indicated that children with DCD can only broadly match their finger movements to an auditory signal with variability and poor synchronicity as key features of their auditory-fine motor control. Individual inspection of the data revealed that 5 children with DCD had difficulty matching the slowest frequencies and that these children also had higher variability and lower percentile MABC scores from the Movement Assessment Battery for Children (MABC) than other children with DCD. Three children with DCD were more variable only at higher frequencies and two performed like typically developing children.
Keywords: Developmental Coordination Disorder, Bilateral Tapping, children, Interlimb coordination
Introduction
According to Williams (2002), rhythmic timing and coordination of movements is a well-recognized deficit in children with Developmental Coordination Disorder (DCD). Yet relatively few empirical studies exist of this deficit. A meta-analysis by Wilson and McKenzie (1998) suggests that motor coordination difficulties have primarily been associated with poor visuo-spatial and kinesthetic processing although it did not assess the few studies of auditory temporal processing. Even the Movement Assessment Battery for Children (Henderson & Sugden, 1992), which is the most commonly used standardized test for assessing DCD, does not include a rhythmic activity as one of the items. In this study, we address timing and coordination in children with DCD by focusing on the perception-action coupling between an auditory beat set at different tempos and bilateral finger tapping.
Previous studies on finger movements in children with DCD have mainly used one of two experimental paradigms influenced by different theoretical approaches. From an information processing perspective, the continuation paradigm involves participants keeping time by tapping to a specific beat and subsequently trying to maintain the same frequency of tapping without the beat. In these studies, the primary finding has been that children with DCD are more variable in maintaining their timing than children without DCD particularly in unilateral tapping (Lundy-Ekman, Ivry, Keele, & Woollacott, 1991; Williams, Woollacott, & Ivry, 1992) and possibly in bilateral tapping (Geuze & Kalverboer, 1994). In addition, children with DCD are less successful in discriminating sounds (Williams et al., 1992) and those with cerebellar soft signs have increased difficulty in timing leading many to suggest that atypical cerebellar development contributes to the difficulties seen in these children (Lundy-Ekman et al., 1991). Using the Wing and Kristofferson (1973) approach to decomposing the beats into central and peripheral components, Williams et al. (1992) suggested that the primary deficits in children with DCD lie in motor programming or central timekeeping.
The second approach has been to use a “dynamic pattern” paradigm appropriate to bilateral coordination where participants are asked to maintain alternating (antiphase) or simultaneous (inphase) bilateral finger movements to a constant auditory beat before being perturbed, or they are asked to match their movements to the increased frequency of an auditory or visual cue (Volman & Geuze, 1998a, 1998b). Again, the primary finding is that children with DCD are more variable, both spatially and temporally, than control children in their ability to maintain a stable coordination pattern at constant speed, and they demonstrate, also, an increased relaxation time after perturbation and a tendency to transition earlier from the less stable antiphase to an inphase pattern. From these experiments, Volman and Geuze (1998a, 1998b) argued that the deficit may not be entirely central in nature and is better characterized as a dynamic control deficit with the underlying structural nature of this deficit unknown although the cerebellum is still suggested as a strong candidate. Taken together, the evidence from these two experimental paradigms suggests that children with DCD are more variable in their ability to maintain timing of a single finger and/or coordination between fingers.
An additional paradigm has been to explicitly investigate visual influences on rhythmic timing (Lord & Hulme, 1988). Volman and Geuze (1998a) looked at the perception-action coupling between a visual cue and unilateral finger movements using the dynamic pattern approach. They found the predictable increase in variability between the DCD group and children without DCD in matching the finger flexion/extension to the cue. Interestingly, however, there was no difference in the absolute error between finger movements and the visual cue. This suggests that the timing problem for children with DCD, at least for visual-motor timing, is not one of being able to synchronize with a sensory cue. In the present study, we pursue a similar strategy of testing perception-action coupling, but measure auditory-motor coupling. Thus the primary purpose of the current study is to characterize the ability of children with DCD to match and synchronize with a range of auditory signals as well as to investigate the stability of bilateral antiphase finger tapping to an auditory beat.
One aspect of assessing the synchronicity between an environmentally-specified driving signal and an individual trying to move in time to the signal is that one can assess whether the movements are primarily ahead of or behind the signal. The former indicates some anticipation on the part of the mover, that is, a feedforward control mechanism, while the latter suggests no anticipation, and indicates use of feedback control mechanisms. Contemporary theorists argue that the anticipatory perception-action relationships can be represented as maps or “internal models” that the CNS uses to accurately control movements (Horak, 1996; Imamizu et al., 2000; Shadmehr & Mussa-Ivaldi, 1994; Wolpert, Ghahramani, & Jordan, 1995). Typically developing children, we speculate, naturally acquire these relationships or internal models by acting upon and perceiving the world around them, discovering the relevant properties of their environments and the tasks they seek to perform. Children with DCD, we hypothesize, may have systematic problems in their sensorimotor system that result in poor mappings between perception and action. Therefore, we expect to see poor synchronization as well as the typical high variability of finger movements as seen in previous studies.
Whether poor synchronicity and high variability will also reflect a poor adaptation or matching to the change in frequency is an empirical question. Matching the beat and synchronicity are separate, but related, abilities. For example, one may be able to detect and adjust to a change of frequency without being closely synchronized with the stimulus. On the other hand, one might also time a movement to coincide with a beat but not “hit” every one. These coupling differences have been termed frequency-locking (matching the beat but not necessarily the phase) and phasing or synchronization (timing the stimulus and response together) in the adult literature (Kay, Kelso, Saltzman, & Schöner, 1987; Turvey, Rosenblum, Schmidt, & Kugler, 1986).
For basic frequency-locking or matching of the beat, we hypothesize that children with DCD may be able to match the slower frequencies overall but will have increasing difficulty with matching higher steady state frequencies as found in Geuze and Kalverboer (1987) in a unilateral tapping task between two targets. One reason for this difficulty would be that children with DCD are slow processors of information (Geuze & Kalverboer, 1994; van Dellen & Geuze, 1988). For phasing or synchronization to the beat, we suspect that children with DCD will be less synchronized to the beat and also less able to synchronize their two fingers to alternate tapping. As found in earlier studies we predict an increased variability of phasing between finger/beat or finger/finger and across increasing frequencies we expect increasing variability of tapping inline with the theoretical predictions and experimental evidence of the Haken, Kelso and Bunz model of bilateral finger tapping (Haken, Kelso, & Bunz, 1985; Kelso, 1984).
Finally, an additional purpose of this study is to investigate individual differences within the group of children with DCD because the heterogeneity of this population makes group comparisons alone of limited value (Larkin & Hoare, 1992). In this respect, we may expect to identify a sub-group with particular difficulties in auditory-motor coupling (Volman & Geuze, 1998a, 1998b).
2. Method
Participants
Ten children with a DCD diagnosis (mean age = 7.04+/- 0.42 years, range of age = 6.2~7.6 years), ten typically developing children gender- and age-matched within 4 months to the DCD group (mean age = 7.08 +/- 0.60 years, range of age = 6.0~8.0 years), and ten adults (age between 21-35 years old) participated in this study. Each group consisted of 7 males and 3 females, one male in each group was left-handed.
Potential DCD participants were evaluated for the following inclusion criteria (1) A diagnosis given by a pediatrician through a developmental and medical history and a neurodevelopmental exam based on the Neurological Exam for Subtle Signs (NESS; Denckla, 1985). There was no cut-off score for the NESS; the pediatrician used her judgement of the performance and history to make her assessment; (2) A Movement Assessment Battery for Children (MABC) score of ≤ 15% given by trained testers (Henderson & Sugden, 1992). This is normative, standardized assessment instrument designed to identify children with motor impairment with the ≤ 15% cut-off proposed to indicate children with potential DCD (Geuze et al., 2001) (3) Normal cognitive functioning (i.e., no mental retardation) on the Woodcock-Johnson Psycho-Educational Assessment Battery (W-J) given by an education specialist (Woodcock & Johnson, 1989, 1990). Exclusion criteria included (1) motor difficulties due to a general medical condition such as cerebral palsy, hemiplegia or muscular dystrophy and (2) meeting the criteria for a Pervasive Developmental Disorder. These exclusion criteria were assessed by the pediatrician through parental questioning in conjunction with the neurodevelopmental examination. Of the 15 participants initially evaluated, five were excluded for failure to meet inclusion or exclusion criteria.
Typically developing children were included with the criteria of normal cognitive ability, no diagnosis of a learning disability based on the Woodcock-Johnson or parent report and >20th percentile score on MABC. In addition, they were age (+/- 3 months) and gender matched to the DCD participants. Table 1 includes a description of the child participants. Potential adult participants were excluded only if they had received extensive musical training (professional or more than 10 years training). All adults and the guardians of participants read and signed a consent form approved by the Institutional Review Board at the University of Maryland, Baltimore. Children gave verbal assent to participate in the study.
Table 1.
Description of Children Participants
| Participant | DCD Children | Control Children | ||||||
|---|---|---|---|---|---|---|---|---|
| Age | Sex | MABC | % | Age | Sex | MABC | % | |
| 1 | 6.7 | F | 25.5 | <1 | 6.3 | F | 8 | 22 |
| 2 | 6.2 | M | 20 | <1 | 6.0 | M | 6 | 36 |
| 3 | 7.1 | F | 16 | 2 | 7.2 | F | 5.5 | 40 |
| 4 | 7.0 | M | 28 | <1 | 7.3 | M | 0 | 96 |
| 5 | 7.6 | M | 23 | <1 | 7.6 | M | 4 | 54 |
| 6 | 7.6 | M | 13 | 6 | 8.0 | M | 4.5 | 49 |
| 7 | 7.1 | M | 11 | 11 | 6.9 | M | 0 | 96 |
| 8 | 6.9 | M | 20.5 | <1 | 6.8 | M | 0 | 96 |
| 9 | 7.1 | F | 16.5 | 2 | 7.1 | F | 5 | 45 |
| 10 | 7.3 | M | 10.5 | 13 | 7.6 | M | 3 | 65 |
|
| ||||||||
| Mean | 7.0 | 18.4 | 7.1 | 3.6 | ||||
| SD | 0.4 | 5.7 | 0.6 | 2.8 | ||||
Experimental Setup and Apparatus
The participants were seated at a table in a quiet, enclosed area with their forearms comfortably resting on the table with slight flexion of both elbows. The participants’ forearms and proximal ends of the metacarpal bones were strapped to the table to restrict extraneous movement. Finger tapping was measured with the MotionMonitor™ system (MM), a magnetic tracking system that provides 3D positional data. This motion system includes “mini-bird” magnetic based kinematic sensors, a 12-bit and16-channel A/D data collection module, and sensor-detected transmitter. “Mini-bird” sensors were taped to the left index and right middle fingernails. A Q-tip was taped across the interphalangeal joint to restrain movement to the metacarpophalangeal joint. The MM transmitter was placed within 0.5 m and equidistant to each hand. All the motion data were sampled at 100 Hz. Auditory signals were produced via a Hewlett Packard waveform generator (set to 0.8, 1.6, 2.4 or 3.2 Hz) attached to an amplifier and speaker. The frequency of each signal was also fed into the MM system via the A/D board. Each session was video recorded using a Panasonic VHS recorder for later verification purposes.
Procedure
Once participants were introduced to the experimental set up, they were given a practice trial to tap their left index and right middle fingers in an alternating pattern without an auditory signal to ensure that the task could be accomplished. A non-homologous pair of fingers (left index and right middle fingers) was chosen to increase the attention demands of the task. To ensure sound production when tapping, a small piece of plastic was taped under the participants’ fingertip. Following the practice tapping trial, an auditory signal was then introduced. The participant was instructed to listen to the beat for 5 s, and then they were verbally cued to begin tapping in alternation, one finger in time with each beat. Testing began after confirming the participant’s ability to tap with a beat. The session consisted of four blocks of four 15-s trials, for a total of sixteen trials. Each block consisted of one 0.8, 1.6, 2.4, and 3.2 Hz trial, the order of which was randomized. Short rest intervals were given in between blocks. All the child participants were rewarded and motivated with prizes (market value of one to three dollars) and a small financial compensation of twenty dollars.
Data Reduction and Measures
All tapping data were filtered by recursive low-pass filter (4th order Butterworth; f3db = 10 Hz). Custom-designed Matlab programs derived the following variables for both dominant and non-dominant hands for each trial. After an initial test verifying no effect of dominance on all variables, dominant and non-dominant finger data were collapsed for each variable for further statistical analysis.
Mean intertap interval (ITI)/Normalized ITI (NITI)
ITI was defined as the average time interval (sec) between each tap onset within a trial. We defined a finger-tap onset based on one-dimensional (vertical) position data recorded with the MM system. The tap onset is an extreme point of position data, which is determined by derived velocity within the algorithm. To determine the frequency effect and potential interaction effect of group by frequency on participant’s performance associated with ITI, we compared the normalized ITI (NITI) across frequencies. NITI was defined as ITI divided by the interstimulus interval (2.5, 1.25, 0.83, 0.625 s for 0.8, 1.6, 2.4, 3.2 Hz respectively). A value of 1 on NITI indicates that the tapping frequency a participant performs is equal to the stimulus frequency. In addition, individual data were investigated comparing control children and children with DCD.
Mean phase between signal and finger tap (Phase)
To calculate how the participants matched their taps to the signal, the mean phase relationship between the auditory signal and finger tap for a trial was measured. Phase was calculated in the unit of percent cycle by the following formula:
| (1) |
where Rn indicates the reference signal and Tn indicates the finger tap for the nth cycle in the time series. Then the phase was normalized with a value of 100% corresponding to precise synchronicity between the tap and the signal. A phase value smaller than 100% indicates that the tap was ahead of the signal and vise versa. After the initial analysis, further analysis of this variable was deemed necessary in order to investigate the masking effects of averaging responses within and between individuals (see results).
Phase Variability within a trial (VPhase)
To examine the variability of the phasing relationship within a trial, the standard deviation of the phasing relationship between each tap corresponding to the signal within a trial was calculated. In addition, individual comparisons were investigated between control children and children with DCD
Mean phase between fingers (BTW)
Formula 1 was used to measure the relative phase between the fingers where Rn indicates the finger tap of non-dominant hand and Tn indicates the finger tap of dominant hand for the nth cycle in the time series. The phase then was normalized with a value of 50% corresponding to precise anti-phase between two fingers.
BTW phase variability within a trial (VBTW)
The standard deviation of relative phase between fingers was calculated within a trial to examine variability of between finger phase relationship.
Statistic Analysis
Linear mixed model techniques (Proc Mixed, SAS, version 8.2) were applied to the variables associated with the effects of group and frequency. This method was chosen because it provides tools to control correlated measures as well as variance heterogeneity. The mixed model also accounts for both random (e.g., within- and between-subject) and fixed sources (e.g., experimental groups) of variances. In our model, group, frequency condition, and their interaction were included as a class-level fixed effect. The random portions of our model included participant, participant by frequency and participant by group. To account for the correlated measures of the independent variable in our study (frequency condition), the mixed model technique provided the goodness-of-fit statistic to assess how well the random portion of the model fit the residuals and provided the proper variance-covariance structure. A goodness-of-fit statistic (Akaike’s Information Criterion Correction, AICC; smaller is better) was recorded from each covariance structure to find the better-fit variance-covariance structure. Heterogeneous variance models were also considered for each factor. Residual variances were pooled when the AICC indicated that it was appropriate to do so. Post hoc analyses (adjusted by Bonferroni procedures) were applied when significant main or interaction effects were found. All effects were tested at a significance level of p = .05.
To further understand the individual differences within the DCD group, we compared the individual results of children with DCD with the mean performance of the control group in variables NITI and VPhase. We used the criteria of two standard deviations as a cut-off point to determine which children with DCD deviated from the average performance of control children.
3. Results
We collapsed across hand for the single finger measures because an initial analysis of hand as a variable within a group, across conditions and between groups found no differences for any comparison except for the variable NITI in which there was an overall difference (i.e., collapsed across groups) in the 3.2 Hz condition only.
Normalized intertap interval (NITI)
To determine whether participants could grossly match the frequency across a trial for each frequency condition, NITI was examined. Fig. 1 illustrates the NITI of three groups across frequency conditions. We found a significant frequency main effect, F(3, 81) = 4.37, p < .01, and a significant group by frequency interaction effect, F(6, 81) = 3.19, p < .01. Group main effect was not significant (F(2, 27) = 1.64, p = .21). Post hoc analysis revealed that DCD children were less able to match the frequency at 0.8 Hz than both adult (p < .001) and control groups (p < .01). Within a group, DCD children at 0.8 Hz were less able to match that frequency compared to other frequencies (p < .01) but adult and control groups did not differ across frequencies.
Fig. 1.
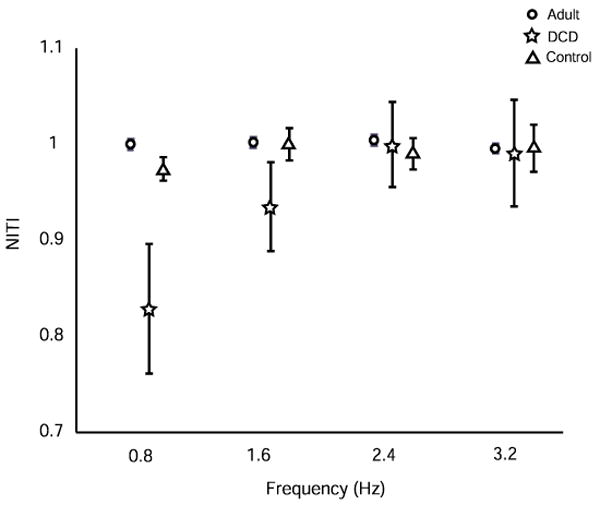
Normalized mean intertap interval (NITI) at each frequency for adult, DCD and control groups.
Fig. 2 shows NITI of individual participants in both children’s groups for each frequency condition. A confidence interval of +/- 2SD around the mean of the control children allows us to detect exactly which children had the most difficulty in matching a frequency. Inspection of children with DCD outside this confidence interval reveals that 4 children (#1,4,5,8) had a problem with slowing the frequency to 0.8 Hz (3 markedly so) and one (#2) was also close to the line. Two children (#1, 2) basically had problems with all frequencies being either slower or faster while three of the four who were initially too fast at 0.8 Hz were more accurate with the faster frequencies. The remaining four children were relatively accurate across all frequencies except for one who had problems in being too slow to catch up with the faster frequencies (#3).
Fig. 2.
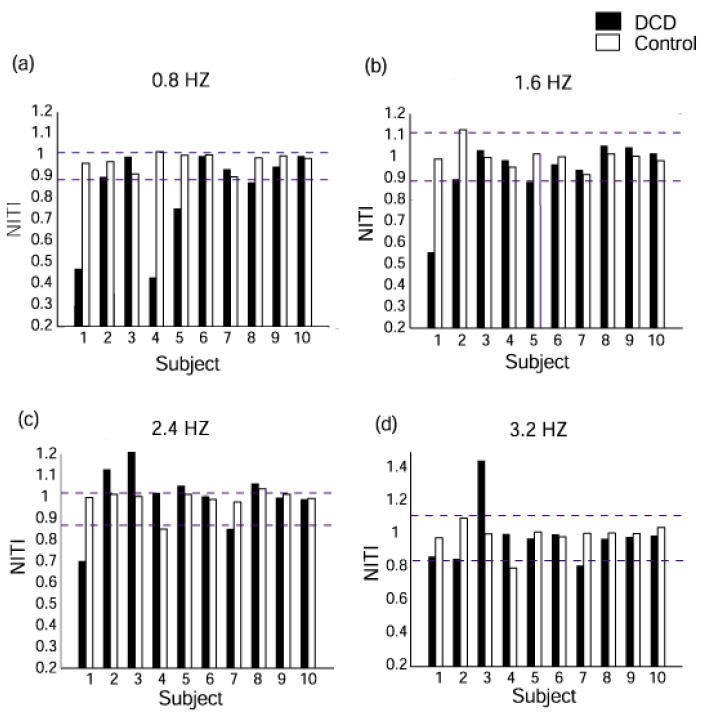
Individual comparison between children with DCD and their matched controls on NITI at different frequency conditions: (a) 0.8 Hz (b) 1.6 Hz (c) 2.4 Hz (d) 3.2 Hz. Dashed lines represent +/- 2 SD around the mean of the TD children.
Mean Phase Relationship between Signal and Finger (Phase)
To determine how closely the participants matched their tap (initial contact) to the auditory signal, we measured the normalized phase relationship between the signal and tap with a tap contact precisely synchronized with the driving signal yielding a value of 100%. Fig. 3 illustrates the mean phase for each group across conditions. There were no significant main or interaction effects (group: F(2, 12.9) = 3.17, p = .07, frequency: F(3, 31.6) = 1.35, p = .26 and group by frequency: F(6, 38.9) = 1.9, p = .10), although the group main effect was close to significant and indicated a trend for control children, but not those with DCD, to be behind the signal compared to the adult group. These data are misleading, however, because averaging within and across trials (as well as participants) will distort the actual synchronization relationship.
Fig. 3.
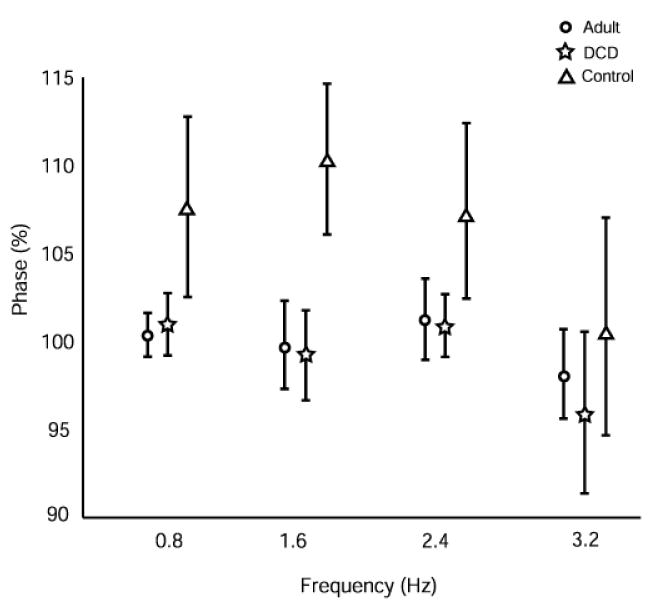
Mean phase relationship between signal and finger (phase) at each frequency for adult, DCD and control groups.
To eliminate the distortion we constructed histogram plots to observe the phase distributions across each tap summed across individuals within a group at all frequencies. Figs. 4 and 5 show examples of such histograms at frequencies 0.8 Hz and 3.2 Hz (the middle frequencies do not provide additional information). At 0.8 Hz (Fig. 4), the adults are tightly synchronized with the beat with most taps slightly ahead and some slightly behind the beat. The typically developing children have less tightly synchronized tapping with some taps ahead but more taps behind the beat. The children with DCD are spread out across the entire phase distribution with a modal value just ahead of the beat. At 3.2 Hz (Fig. 5) the adults still show a relatively tight clustering of taps around the beat although the modal value is now behind the beat and there are some less synchronized taps appearing. The control children are now spread across the phase distribution with a modal value that is also behind the beat. The children with DCD have no one modal value and appear equally distributed across the entire phase distribution. Data were checked for phase wandering but no systematic instances of this were found across a trail in any of the participants.
Fig. 4.
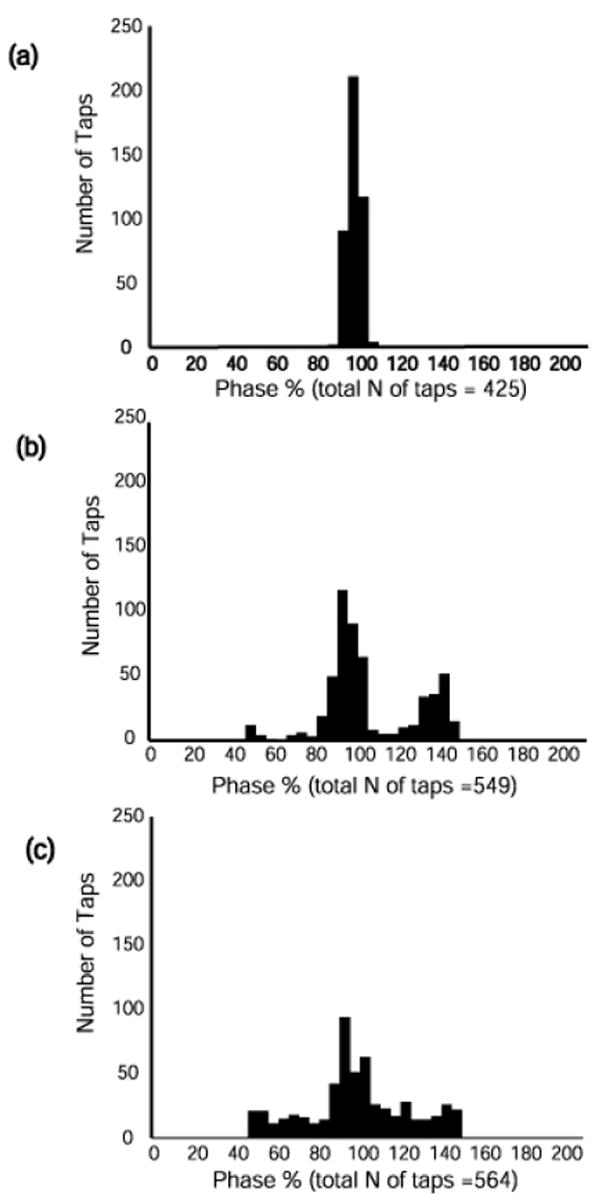
Histogram plots of individual tap phase distribution at 0.8 Hz summed across trials for all participants in each group: (a) adult, (b) control children (c) DCD children
Fig. 5.
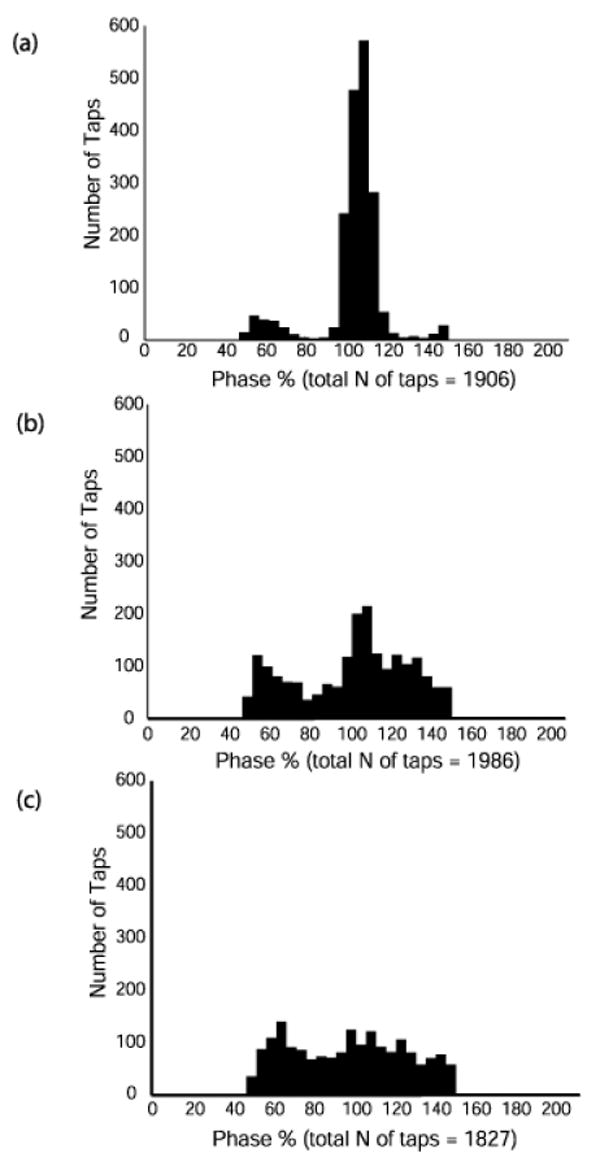
Histogram plots of individual tap phase distribution at 3.2 Hz summed across trials for all participants in each group: (a) adult, (b) control children (c) DCD children
Variability of Phasing between Signal and Finger (V-Phase)
To determine the variability of the phasing relationship within a trial, the standard deviation of the mean phase for each participant was calculated. Fig. 6 illustrates the results of v-phase. Significant main effects were found for both group, F(2, 19.4) = 52.49, p < .0001, and frequency, F(3, 48.5) = 14.41, p < .0001. The group by frequency interaction was close to significance (F(6, 51.7) = 2.16, p = .06). Post hoc analysis of the main effects revealed that the adults were significantly less variable than the control and the DCD groups (p < .0001) and that the typical developing children group was significantly less variable than the DCD group (p < .0001). Across frequencies, all the conditions were significantly different from each other (p < .05) except 1.6 Hz and 2.4 Hz.
Fig. 6.
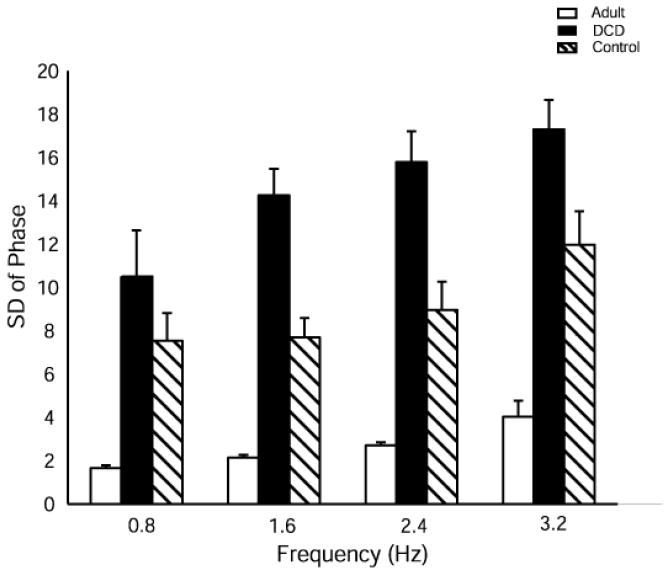
Mean variability of phasing between finger and signal (VPhase) within a trial at each frequency for adult, DCD and control groups.
Fig. 7 shows V-Phase of individual participants in both children’s groups for each frequency condition. A confidence interval of + 2SD around the mean of the control children allows us to detect exactly which children were most variable in their attempts to synchronize to the beat. Inspection of the children with DCD reveals that 7 children were outside the upper limit of the confidence interval for at least one frequency condition and all but two were consistently more variable than their matched control. These two (#6 & 10) also had relatively accurate frequency matching (see the results of NITI).
Fig. 7.
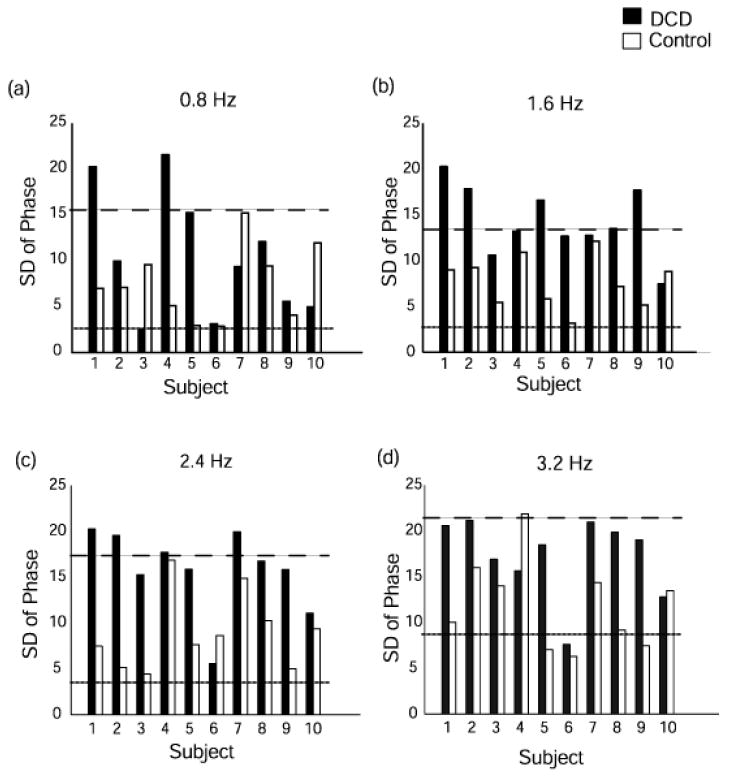
Individual comparison between DCD and control children on VPhase at different frequency conditions. (a) 0.8 Hz (b) 1.6 Hz (c) 2.4 Hz (d) 3.2 Hz. Dashed lines represent +/- 2 SD around the mean of the TD children.
Mean Phase between fingers (BTW)
There were no significant main and interaction effects (group: F(2, 27) = 1.9, p = .17; frequency: F(3, 81) = 0.76, p = .52; group by frequency: F(6, 81) = 0.48, p = .82). Overall means for each group were 50.1 ± 1.3 (adult), 49.3 ± 2.8 (typically developing) and 46.6 ± 12.2 (DCD).
BTW phase variability within a trial (VBTW)
We found significant main effects for both group, F(2, 27) = 22.29, p < .0001, and frequency, F(3, 81) = 19.42, p < .0001, but no significant group by frequency interaction (F(6, 81) = 1.47, p = 0.2). Fig. 8 shows the results of BTW phase variability. Post hoc analysis revealed that the adults were significantly less variable than the control and the DCD groups (p = .002 and < .0001 respectively) and that the typically developing children’s group was significantly less variable than the DCD group (p = .02). Across frequencies, all the conditions were significantly different from each other (p < .05) except 1.6 Hz and 2.4 Hz and 2.4 Hz and 3.2 Hz..
Fig. 8.
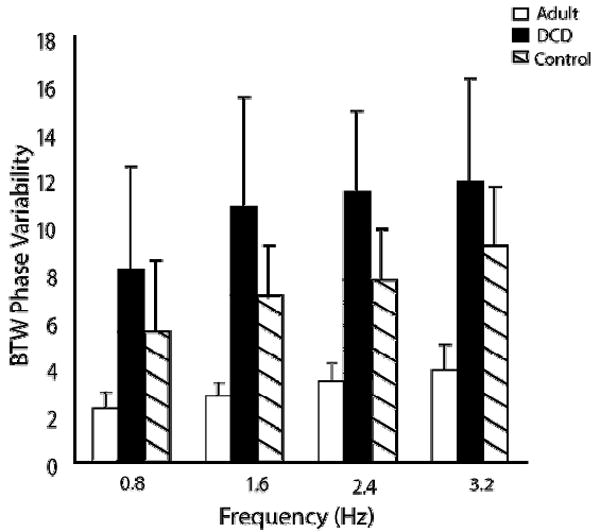
Mean variability of phasing between fingers (VBTW) at each frequency for adult, DCD, and control groups.
Summary of Group and Individual Performance Analyses
In combination with an inspection of individual phase data (not shown) the following conclusions can be made (1) adults are tightly frequency-matched and synchronized across the trials and frequency conditions with some lessening of this coupling at higher frequencies; all individuals show many taps that are consistently ahead of the beat particularly at lower frequencies; (2) typically developing children are more variable than adults but consistently match the beat (although #7 has some trouble with lower frequencies and is more variable also in these conditions); some individuals synchronize close to the beat but most are behind the beat for lower frequencies and become less synchronized at higher frequencies; and, (3) most children with DCD have problems with matching the beat, particularly at the lower frequencies and are more variable than control children; they are also less able to synchronize to the beat at any frequency. However, at least 2 individuals perform equivalent to the average control child and are considerably better than control #7.
Discussion
Children with DCD are known to have problems with moving to a beat but this relationship has been little investigated beyond the frequent observation that these children are more variable than age-matched controls. The primary aim in this study was to assess the auditory-motor coupling of children with and without DCD by asking the children to match a series of auditory beats at different frequencies with bilateral finger tapping. These data were compared to an adult group and a group of age- and gender-matched controls to better characterize the developmental status of the children with DCD.
In general, children with DCD can broadly match the different frequencies although some have a problem with slowing down to match the slowest 0.8 Hz frequency. Both groups of children are inaccurate in synchronizing their finger tap with the beat, but children with DCD appear to have no consistent relationship to the beat while control children, in general, appear to tap behind the beat. As found in previous studies, children with DCD are more variable than their matched controls who, in turn, are more variable than adults.
Children with DCD have poor auditory-motor coupling in all aspects
Contemporary conceptualizations of motor development contend that it is the acquisition of sensorimotor (or perception-action) relationships between the dynamics of the external world and the musculoskeletal system that is critical to our understanding of the development of stable, adaptive action patterns (Bertenthal, 1998; Bloch, 1990; Thelen, 1990). The present experiment uses the well-studied task of paced bilateral finger tapping as a sensorimotor task. Our results replicate earlier studies of tapping (Lundy-Elkman et al. 1991 and Geuze & Kalverboer, 1994) and frontal plane finger flexion/extension movements (Volman & Geuze, 1998a, 1998b) in demonstrating that children with DCD produce a more variable performance. This study adds to these findings, by investigating frequency matching and synchronization (coupling) to the auditory cue.
Contrary to our expectations, children with DCD had a problem with matching the slowest frequency and not the faster ones (except for one individual who had more trouble with the latter). This is not consistent with the results of Geuze & Kalverboer (1987) who found that many of their clumsy children could not keep up with faster frequencies when tapping unilaterally between two targets. One plausible contributing factor to the differences between studies is that the criteria for inclusion were different in that the Geuze and Kalverboer (1987) study consisted of a population with less disordered coordination. Another reason for these differing results might be the nature of bilateral finger tapping vs. unilateral tapping between two targets. The former has more complexity with two hands, and in this experiment the complexity was increased with the tapping of two non-homologous fingers. Slowing this task may have required actively inhibiting the contralateral homologous finger which became more difficult at a very slow frequency. On the other hand, the Geuze and Kalverboer study involved moving the arm between two targets and, since increasing speed accelerates the need to control the initial forces produced, this control of the dynamics may contribute to the difficulties of children with DCD. In fact, it could be that we did not reach a high enough frequency to obtain a detrimental effect in our children with DCD. If we had increased the frequency, the children with DCD may have shown an earlier inability to match the higher frequencies, compared to matched controls as in the Volman and Geuze (1998b) study.
Although, in general, children with DCD appear to be slow processors of information, there is surprisingly little data suggesting that they accomplish goal-directed tasks at a slower speed. In fact, in a parallel study to the present one, the children with and without DCD chose the same preferred speed (around 1.1 Hz on average) for bilateral tapping (Roche, Clark, Wilms-Floet, & Whitall, 2007). Nevertheless, our data suggest that slowing down to 0.8 HZ is a problem for these children, at least when their tapping is cued by an auditory beat. Excessively fast responses to cued tapping are also found in many children diagnosed with Attention Deficit Hyperactive Disorder (ADHD). In two studies, Rubia et al. (1999) and Ben-Pazi et al. (2003) found that many children of a similar age as in this study (mostly boys) tapped faster than the auditory stimulus. However, unlike the present study this phenomenon tended to occur above the 2Hz frequency. Below 2Hz frequency, children with ADHD could match the frequency. Ben-Pazi et al. (2003) speculate that abnormal sub-cortical oscillatory mechanisms may contribute to their findings as seen in parallel “hastening” with Parkinson’s patients. Given that with DCD the accelerated tapping occurs at a slower frequency it is unlikely that the same dysfunctional mechanisms would underlie the DCD and ADHD populations. Nevertheless, from a behavioral perspective we can speculate that abnormal oscillatory mechanisms, of some kind, are also involved with DCD because of how poorly the children with DCD are coupled to the signal.
Analysis of individual taps in Figs. 4 and 5 shows that children with DCD rarely are able to couple their finger movements close to the beat. Unlike typically developing children they do not seem to congregate in specific parts of the phase distribution but use the whole timing range. Thus, the increased variability appears to be related to a lack of precise timing to the environmental stimulus. This is particularly true of the faster frequencies at which, conversely, they are better at frequency matching on average. This apparent discrepancy is solved by realizing that with slower frequency cues some of these children with DCD are equally as poor at synchronizing as they are at the fast frequency but others are able to synchronize better at the higher beats resulting in a modal distribution around the beat.
Although our results of increased variability are in common with previous findings, the synchronization errors do not correspond to the results of Volman & Geuze (1998a) who found no group differences in absolute error for either inphase or antiphase coordination between unilateral finger flexion movements and a visual cue at 1.25Hz. There are several plausible reasons for this discrepancy including different constraints between the two experiments and different methodological analyses.
For example, visuo-motor coupling is likely more difficult than auditory-motor coupling. Visual cues require visual attentional focus and therefore constrain the finger movement to be monitored only from kinesthetic information (although the cue and finger both appeared to be in the line of sight in the Volman study). In an auditory cueing paradigm, vision is available as an additional sensory cue to aid in producing and monitoring the finger tapping. In addition, it is far more likely that participants have experience with auditory rather than visual cues in timing movements, again promoting auditory-motor coupling as a likely stronger than visual-motor coupling. Experimental results in adults confirm the superiority of audio-motor coupling. Semjen & Ivry (2001) found that the well-known advantage of tight coupling to in-phase and antiphase patterns is much more pronounced for auditory cues during experiments that determine whether adults can match different externally cued phasing relationships. Thus if visual-motor coupling is harder for adults, it may be that much harder for children. Therefore, the lack of differences between groups in the Volman & Geuze (1998a) experiment might reflect the fact that both sets of children have not developed this kind of visuo-motor coupling relationship yet either because it is intrinsically harder or for lack of practice, or both.
Other constraint differences between the two experiments are the use of unilateral vs. bilateral non-homologous finger movements, and the use of free movements vs. those that collide with a surface (table). Unilateral tapping becomes stable more quickly than bilateral tapping (Wolff et al.,1998) and non-homologous fingers are more difficult still. These two factors suggest that our study constraints are more difficult and would exacerbate differences between the two groups whereas in the Volman and Geuze (2001) study both groups of children might perform more similarly with unilateral tapping. On the other hand, tapping has extra cutaneous information from the table surface that also provides a stabilizing force (Whitall et al., 1999). Thus one could make the reverse argument for these differences since tapping is a more stable activity and both groups of children might reach a plateau. Further research where constraints are changed systematically, is needed to sort out these potential influences on the performance of children with and without DCD. Finally, an alternative reason behind the lack of differences between groups in the Volman & Geuze (1998a) study compared to the present study is the choice of absolute error to reflect synchronization. This error demonstrates the overall accuracy of timing but masks the true tendency of where the timing error lies. In this study we chose to present constant error for this reason. In addition, using either absolute or constant errors, group and individual differences can be washed out by averaging within and between trials.
Taken together, our results suggest that the increased timing variability between fingers and auditory signals of children with DCD is a reflection, in part, of their lack of ability to couple the auditory stimulus with the motor response. Furthermore, even though they can adapt in a general way to a change of frequency stimulus, and therefore appear to perceive the change of stimulus, at least half of these children find it hard to slow down enough to match the frequency (on average) especially when it is below 2 Hz. These specific children are particularly poor and/or, delayed, in learning how to couple their fingers to a beat. When or whether they will ever progress along a developmental profile towards “adult-like” ability is an empirical question that requires future longitudinal study.
Developmental Profile of Paced Bilateral Finger Tapping and Implications
Although our age range of children is small, the inclusion of an adult group that can be compared to the control group allows us to make some inferences about the developmental profile of paced bilateral finger tapping. Given the lack of differences between adults and controls, the ability to match a frequency (frequency-locking) would appear to be acquired first. This ability requires a detection of the change in stimulus frequency and an ability to make a matched response to the change in the stimulus frequency. A study of children’s ability to produce a range of frequencies without cueing, albeit using a drumstick, showed that children from 4 to 10 years could not produce the same range of frequencies as an adult and had trouble with both lower and higher frequencies (Drake, Jones, & Baruch, 2000). In particular, the younger children were unable to slow down initially so it is perhaps not surprising that many of our group with DCD had problems with slowing down.
Second, since control children are less well-synchronized than adults and tend to be behind the beat even at .8Hz, it clearly takes time to turn the basic matching process into an ability to anticipate the response in a feedforward fashion (or build an internal model or representation of antiphase movement as some would argue). Finally, a natural consequence of this ability to fine-tune the synchronization into an accurate modal response will be to reduce the variability of timing. As seen in our data set, not one of the control children was near the kind of stable response demonstrated by the adults. Whether these children would demonstrate a more adult-like anticipatory response for unilateral tapping, since this becomes stable earlier than bilateral tapping, is an open question although absolute error is related to increasing stability in unilateral tapping (Wolff et al., 1998).
To our knowledge, this is only the second study in typically developing children that examined and compared the three abilities of matching frequency, synchronizing signals (and the first from a constant error perspective) and producing a tight coupling (low variability) for bilateral antiphase tapping around an auditory signal. For variability, our results are consistent with the previous study that included paced bilateral antiphase finger tapping in showing typically developing 7-year-olds to be more variable than adults (Wolff, Kotwica, & Obregon, 1998). Similarly they reported adult-like frequency matching for 1.5 and 2Hz. However, Wolff et al. (1998) did not report constant error so a direct comparison cannot be made. Indeed, as Wolff et al. (1998) also observe, it is important to recognize that distinctions such as the number of fingers/hands (unilateral, bilateral), the type of phasing (antiphase; inphase) and the mode and existence of cues (paced, continuation, self-paced) may each have an effect on performance measures even if frequency is kept constant. For example, in adults, pacing will reduce the between-finger phasing variability of bilateral inphase but not antiphase tapping compared to a self-paced condition (Forrester & Whitall, 2000). From the present results, one can speculate that typically-developing children cannot initially use auditory cueing to stabilize their finger movements (either anti or inphase) but need to practice frequency-matching first.
If our speculation is correct, it implies that initial learning of auditory-motor skills should be facilitated by providing a variety of different frequencies in order to force practicing of frequency change. Subsequently, an attempt to actually synchronize with the beat would be suggested and this would lead to naturally and gradually to tighter coupling between the signal and movement. This suggestion is counter to learning theories (principles) that promote stabilization before adaptation but fits better with theories that promote, for example, variability of practice, contextual interference and mobility before stability (Schmidt, 2005). This proposed progression of auditory-motor skills also has implications for specific intervention strategies for children with DCD if their poor performance seems to be a case of developmental delay rather than an atypical developmental profile. That is, initially these children should be encouraged to change movements in time to changes of beat rather than worry about synchronizing to the beat.
Individual Profiles for Children with DCD indicate potentially different neural or experiential bases?
As previously reported, children with DCD are far from a homogenous group of individuals that have a predictable developmental performance profile based on a specific neurological profile (Kaplan, Wilson, Dewey, & Crawford, 1998). Our study results illustrate three different performance profiles: five children (#, 1,2,4,5,8; avg. MABC = < 1) were poor at slowing their frequency to match the slowest speed, with poor synchronization and high variability across most frequencies; three children (# 3, 7 and 9; avg MABC = 5) were generally able to match the lower speeds fairly well but became less stable and/or less able to match the higher frequencies (although it should be noted that 9 was near but not outside the range for the higher frequencies); and finally two children (6 & 10; avg MABC = >9) performed like controls with good matching and lower (but not adult-like) variability. These latter two children also had good synchronicity (average of 91 and 98%) and reasonable scores on the fine-motor section of the MABC (9 & 7). These results have several implications.
For example, first it is apparent that between 50 to 80% of our small sample (depending on criteria used) does have problems with the fine-motor task of bilateral finger tapping compared to typically developing children. This percentage is higher than the 37.5% reported by Geuze & Volman (1998b) and may reflect the difficulty of using non-homologous muscles or of needing to adapt to a variety of different frequencies with little time to stabilize performance. It also reflects the fact that Geuze & Volman (1998b) used more strict criteria of two parameters/conditions being deviant to place their children in the sub-group with specific problems in tapping performance. In this study, we were able to unequivocally categorize two children with DCD who performed normally in our tapping task suggesting that we have at least two sub-types of DCD in our sample, those with a profile of inferior tapping and those with a profile like typical developing children. Secondly, since the 8 children with poor performance included three children with somewhat different, less deviant, profiles from the rest and higher MABC scores, this could mean either that those three are somewhere between initial acquisition and age-appropriate performance (implicating an experiential basis for the profile differences) or that the differences themselves are from an atypical profile (suggesting different atypical brain development). In order to test these hypotheses, we will need a developmental landscape of tapping performance under these conditions across childhood and neuroanatomical studies that compare specific performance deficits with brain function.
Conclusions
Limitations of this study include the small sample size which allowed us to detect interaction effect sizes of .8 with 80% confidence at .05 alpha level. It is plausible, for example, that we would have detected a group by frequency interaction for variability of phasing between finger and signal with more participants. We also cannot be certain that children were primarily attending to the instructions to time their fingers to the beat versus attending to producing the antiphase beat. If the children were attending only to their fingers and not the beat as instructed, this could explain the lack of synchronization. Finally, we made the choice to calculate the dependent measures across a constant time period rather than across a finite number of taps. Thus, any frequency effects could be attributable to the different numbers of taps that contribute to the measure. In general, and apart from the Group by Frequency interaction, the frequency effects were constant across groups and followed the well established pattern of increasing phasing variability as the frequency increased (Haken et al., 1985; Kelso, 1984).
This study demonstrated that 7-year-old children with DCD have poor auditory-motor coupling between external cues and bilateral antiphase finger tapping with non-homologous fingers. Although these children seem able to detect the change in frequency of the external auditory cues about half are unable to slow to .8 Hz., and most are highly variable and lack the ability to synchronize. Only two children performed like TD children so the majority had difficulty with this task. Individual differences suggest perhaps 3 different performance profiles supporting the concept of heterogeneity and different etiologies of atypical brain development (Kaplan et al., 1998). The results also lead us to speculate that initial interventions aimed at improving rhythmic repetitive performance, particularly in finger movements, might benefit from initially varying the frequency at which external cues are presented rather than concentrating on synchronization at one frequency.
Acknowledgments
We thank the children and parents who contributed their time to this study. We also thank Renee Wachtel, MD for her initial assistance with the project, Kevin McQuade, Ph.D., PT for technical assistance and the following for help with pilot work and data collection: Steve Kim, Karen van der Poel, Jennifer Boast and Jenna Mace. This work was supported by a grant from the National Institutes of Health (RO3 HD38337, J.Whitall and RO1 HD42527, J. Clark).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Pazi H, Gross-Tsur V, Bergman H, Shalev RS. Abnormal rhythmic motor response in children with attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2003;45:743–745. doi: 10.1017/s0012162203001385. [DOI] [PubMed] [Google Scholar]
- Bertenthal BIC. Perception and action. In: Kuhn DS, editor. Handbook of child psychology, Vol.2. Cognition, perception, and language. New York: Wiley; 1998. [Google Scholar]
- Bloch HB. Sensory-motor organizations and development in infancy and early childhood. Dordrecht, The Netherlands: Kluwer; 1990. [Google Scholar]
- Forrester L, Whitall J. Bimanual finger tapping: Effects of frequency and auditory information on timing consistency and coordination. Journal of Motor Behavior. 2000;32:176–191. doi: 10.1080/00222890009601369. [DOI] [PubMed] [Google Scholar]
- Geuze RH, Kalverboer AF. Inconsistency and adaptation in timing of clumsy children. Journal of Human Movement Studies. 1987;13:421–432. [Google Scholar]
- Geuze RH, Kalverboer AF. Tapping a rhythm: A problem of timing for children who are clumsy and dyslexic? Adapted Physical Activity Quarterly. 1994;11:203–213. [Google Scholar]
- Haken H, Kelso JA, Bunz H. A theoretical model of phase transitions in human hand movements. Biological Cybernetics. 1985;51:347–356. doi: 10.1007/BF00336922. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden D. Movement Assessment Battery for Children. London: The Psychological Corporation; 1992. [Google Scholar]
- Horak FM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of physiology, Sec 12, Exercise: regulation and integration of multiple system. New York: Oxford UP; 1996. pp. 255–292. [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Wilson BN, Dewey D, Crawford SG. DCD may not be a discrete disorder. Human Movement Science. 1998;17:471–490. [Google Scholar]
- Kay BA, Kelso JAS, Saltzman EL, Schöner G. Space-time behavior of single and bimanual rhythmical movements: Data and limit cycle model. Journal of Experimental Psychology: Human Perception and Performance. 1987;13:178–192. doi: 10.1037//0096-1523.13.2.178. [DOI] [PubMed] [Google Scholar]
- Kelso JAS. Phase transitions and critical behavior in human bimanual coordination. American Journal of Physiology. 1984;246(6 Pt 2):R1000–1004. doi: 10.1152/ajpregu.1984.246.6.R1000. [DOI] [PubMed] [Google Scholar]
- Lord R, Hulme C. Visual perception and drawing ability in clumsy and normal children 785. British Journal of Developmental Psychology. 1988;6:1–9. [Google Scholar]
- Lundy-Ekman L, Ivry R, Keele S, Woollacott M. Timing and force control deficits in clumsy children 1253. Journal of Cognitive Neuroscience. 1991;3:367–376. doi: 10.1162/jocn.1991.3.4.367. [DOI] [PubMed] [Google Scholar]
- Roche R, Clark JE, Wilms-Floet A, Whitall J. Bilateral self-selcted frequency finger tapping in children with and without developmental coordination disorder and adults. 2007 Manuscript submitted for publication. [Google Scholar]
- Rubia K, Taylor A, Taylor E, Sergeant JA. Synchronization, anticipation, and consistency in motor timing of children with dimensionally defined attention deficit hyperactivity behaviour. Perceptual and Motor Skills. 1999;89(3 Pt 2):1237–1258. doi: 10.2466/pms.1999.89.3f.1237. [DOI] [PubMed] [Google Scholar]
- Schmidt RA. Motor control and learning: A behavioral Emphasis. 4. Human Kinetics; 2005. [Google Scholar]
- Semjen A, Ivry RB. The coupled oscillator model of between-hand coordination in alternate-hand tapping: A reappraisal. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:251–265. doi: 10.1037//0096-1523.27.2.251. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. Journal of Neuroscience. 1994;14(5 Pt 2):3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Coupling perception and action in the development of skill: A dynamic approach. In: Bloch H, B BI, editors. Sensory-motor organization and development in infancy and early childhood. Dordrecht, The Netherlands: Kluwer; 1990. pp. 39–56. [Google Scholar]
- Turvey MT, Rosenblum LD, Schmidt RC, Kugler PN. Fluctuations and phase symmetry in coordinated rhythmic movements. J Exp Psychol Hum Percept Perform. 1986;12(4):564–583. doi: 10.1037//0096-1523.12.4.564. [DOI] [PubMed] [Google Scholar]
- van Dellen T, Geuze RH. Motor response processing in clumsy children. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1988;29:489–500. doi: 10.1111/j.1469-7610.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Volman MJM, Geuze RH. Relative phase stability of bimanual and viuomanual rhythmic coordination patterns in children with a Developmental Coordination Disorder. Human Movement Science. 1998a;17:541–572. [Google Scholar]
- Volman MJM, Geuze RH. Stability of rhythmic finger movements in children with a developmental coordination disorder. Motor Control. 1998b;2:34–60. doi: 10.1123/mcj.2.1.34. [DOI] [PubMed] [Google Scholar]
- Williams H. Motor control in children with developmental coordination disorder. In: Cermak S, L D, editors. Developmental coordination disorder. Albany, NY: Delmar, Thomson Learning; 2002. pp. 117–138. [Google Scholar]
- Williams HG, Woollacott MH, Ivry R. Timing and motor control in clumsy children. Journal of Motor Behavior. 1992;24:165–172. doi: 10.1080/00222895.1992.9941612. [DOI] [PubMed] [Google Scholar]
- Wilson PH, McKenzie BE. Information processing deficits associated with developmental coordination disorder: A meta-analysis of research findings. Journal of Child Psychology Psychiatry. 1998;39(6):829–840. [PubMed] [Google Scholar]
- Wing AM, Kristofferson AB. Responses delays and the timing of discerte motor responses. Perception and Psychophysics. 1973;14:5–12. [Google Scholar]
- Wolff PH, Kotwica K, Obregon M. The development of interlimb coordination during bimanual finger tapping. International Journal of Neuroscience. 1998;93:7–27. doi: 10.3109/00207459808986408. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]


