Abstract
Background and Aims
Given the limitations of conventional therapies and restrictions imposed on newer pharmacological agents, there is an urgent need to develop efficacious and efficient treatments that teach patients behavioral self management skills for relieving irritable bowel syndrome (IBS) symptoms and associated problems.
Method
75 Rome II diagnosed IBS patients (86% female) without comorbid GI disease were recruited from local physicians and the community and randomized to either 2 versions of cognitive behavior therapy (10 session, therapist administered CBT vs. 4 session, patient administered CBT) or a wait list comparison (WLC) that controlled for threats to internal validity Final assessment occurred two weeks after the 10 week treatment phase ends. Outcome measures included adequate relief from pain and bowel symptoms; global improvement of IBS symptoms (CGI-Improvement Scale); IBS symptom severity (IBS SSS); quality of life (IBSQOL); psychological distress (Brief Symptom Inventory); patient satisfaction (Client Satisfaction Scale).
Results
At week 12, both CBT versions were significantly (p < .0001) superior to WLC in the percentage of participants reporting adequate relief (e.g., MC-CBT = 72%, S-CBT = 60.9%, WLC = 7.4%) and improvement of symptoms. CBT treated patients reported significantly improved quality of life and IBS symptom severity but not psychological distress than WL patients (p < .0001)
Conclusions
Data from this pilot study lend preliminary empirical support to a brief patient administered CBT regimen capable of providing short term relief from IBS symptoms largely unresponsive to conventional therapies.
Irritable bowel syndrome (IBS) is a common, oftentimes disabling functional GI disorder whose full range of symptoms (abdominal pain/discomfort with altered bowel habits such as diarrhea and/or constipation) are generally unresponsive to conventional therapies. The few efficacious medications developed specifically for IBS have either been withdrawn or severely restricted in response to concerns about safety, creating an urgent need to develop behavioral self management treatments that train patients to adopt effective strategies for relieving symptoms unresponsive to available dietary or pharmacological agents. The broader disease management literature 1 indicates that effectively managing the day to day burden of chronic medical conditions involves the adoption of specific behavioral self care skills (self monitoring, goal setting and problem solving, education, managing negative emotions and other aversive somatic states such as pain). These skills form the basis of a psychological treatment approach called cognitive behavior therapy (CBT). CBT’s overarching goal is to teach patients skills for taking a more proactive role in controlling symptoms, coping with their emotional unpleasantness, and improving quality of life.
A number of clinical trials support the efficacy of CBT when administered over multiple (weekly) sessions by trained therapists in tertiary care settings 2, 3 4.Treatment gains associated with CBT include improvements in key GI symptoms (pain, bowel dysfunction)4, quality of life 5, 6, and psychological distress4, 6. In its standard form, however, CBT has practical limitations (high cost, shortage of adequately trained therapists, long waiting lists, time requirements) that hamper its clinical utility. As the “second generation” of IBS treatments under development, it is increasingly clear that efficacy demonstration is a necessary but not sufficient condition of treatment viability for IBS treatments. An unmet need exists for a brief form of CBT that is less costly, time intensive and more transportable, yet retains the clinical efficacy of the “gold standard” CBT delivered in routine office settings.
One strategy for economizing CBT is to decrease therapist contact time through the use of primarily self-administered or “home based” treatments. In a minimal-contact (MC) treatment (e.g.7), self-management skills are introduced in periodic clinic sessions but most of what is learned occurs at home using self-study materials. As a result, MC-CBT requires only 4 clinic sessions rather than the 10–20 weekly sessions featured in the literature. Potential advantages of MC-CBT include compatibility with the number of counseling sessions (N = 6) most patients attend 8, greater patient involvement, a reduction in patient costs (direct and opportunity), expanded availability of services, lower stigma, easier scheduling and penetration into underserved areas, and more rapid integration into routine clinical settings subject to yearly HMO limits on outpatient counseling visits. In a health care culture that emphasizes stepped care, an MC-CBT treatment represents a logical first step intervention for individuals who require more than advice, reassurance or simple lifestyle changes but a less complex, and costly option than more intensive behavioral treatments administered in specialty care settings.
The primary goal of this pilot study was to test the acute treatment effects of self administered CBT with reference to a waitlist control condition. On an exploratory level, we compared the self-administered CBT to a standard-of-care, therapist administered CBT condition to examine comparability of effects. We predicted a priori that the brief CBT would show evidence of overall relief of GI symptoms, improvement of quality of life, and reduction of severity of IBS symptoms as compared to a wait list control and comparable effects to patients assigned to therapist based CBT.
Patients and Methods
Study Design
This study is a single site, three arm randomized clinical trial pitting a 4 session, self administered version of CBT against a “passive” wait list control group that controlled for several threats to internal validity (passage of time, maturation, the effects of repeated assessment) and a 10 session, therapist administered version of CBT. The study was approved by the IRB at the University at Buffalo, SUNY and all participants signed informed consent.
Patients
Females and males between the ages of 18 and 70 years were recruited primarily through referral from local specialists (e.g., gastroenterology) and primary care physicians, media coverage and advertisements in local media. Recruitment began in February 2005 and ended in April 2006. A total of 327 subjects contacted the Behavioral Medicine Clinic at the University at Buffalo and underwent a telephone screening interview to assess basic eligibility criteria. Those who qualified were scheduled for formal medical and psychological evaluations to determine their standing on inclusion/exclusion criteria. Inclusion criteria included Rome II IBS diagnosis9 established during a medical examination conducted by a gastroenterologist; IBS symptoms of at least moderate severity; willingness to maintain a stable dosage of IBS medications during the pre treatment baseline period; ability to provide written consent; and a minimum 6th grade reading level10. Exclusion criteria were: presence of a comorbid organic GI disease (e.g., IBD, lactose intolerance) or mental retardation; concomitant or lifetime participation in psychotherapy featuring cognitive-behavioral techniques; current or past diagnosis of schizophrenia or other psychotic disorders; current diagnosis of unipolar depression with suicidal ideation; current diagnosis of psychoactive substance abuse. Moderate to severe IBS was operationally defined 4, 11, 12 as a score of 2 or greater on physician administered global ratings scale of IBS severity (1 = mild, 2 = moderate, 3 = severe, 4 = very severe). The average duration of IBS symptoms for the study sample was 16.5 years. Patients’ predominant bowel habit was determined after medical examination using Rome II guidelines 9 and clinical impression: 53% were diarrhea-predominant; 25% constipation-predominant, and 21% were alternating or mixed. Additional demographic data are presented in Table 1. Of 138 individuals whose eligibility was formally assessed in clinic, 75 completed pretreatment assessments that ended with the completion of 4 weeks of daily symptom monitoring. At this point, the study research coordinator who had no therapeutic relations with any of the participants used a computer generated treatment allocation scheme to randomly assign patients to one of the three conditions. Because it is not possible to disguise the “contents” of psychosocial treatments, we adopted the practice 6, 13 of assessing patients’ perceived credibility of the treatments to which they were assigned. Participants were allocated to therapists, with the goal of balancing the number of patients treated in each condition by each therapist.
Table 1.
Sample baseline characteristics for total sample and by treatment group.
| Treatment Group | ||||
|---|---|---|---|---|
| Total Sample (N = 75) | MC-CBT (n = 25) | S-CBT (n = 23) | Wait List (n = 27) | |
| Age (mean, sd) | 46.6 (16.7) | 41.9 (13.6) | 48.13 (18.2) | 49.7 (17.6) |
| Gender (%) | ||||
| Male | 13.3% | 24.0% | 8.7% | 7.4% |
| Female | 86.7% | 76.0% | 91.3% | 92.6% |
| Marital Status (%) | ||||
| Single | 24.0% | 24.0% | 30.4% | 18.5% |
| Married | 45.3% | 48.0% | 39.1% | 48.1% |
| Divorced | 22.7% | 24.0% | 17.4% | 25.9% |
| Widowed/separated | 2.7% | 0.0% | 4.3% | 3.7% |
| Living w/ partner | 5.3% | 4.0% | 8.7% | 3.7% |
| Ethnicity (%) | ||||
| Asian | 1.3% | 4.0% | 0.0% | 0.0% |
| African American | 2.7% | 4.0% | 0.0% | 3.7% |
| White | 94.7% | 92.0% | 100% | 92.6% |
| Hispanic | 1.3% | 0.0% | 0.0% | 3.7% |
| Education | ||||
| Some High School | 2.7% | 0.0% | 8.7% | 0.0% |
| High School Grad/GED | 8.0% | 8.0% | 8.7% | 7.4% |
| Some College | 17.3% | 16.0% | 13.0% | 22.2% |
| Vo-Tech/Associate’s | 17.3% | 28.0% | 8.7% | 14.8% |
| College Grad | 18.7% | 16.0% | 30.4% | 11.1% |
| Some Post-Grad | 8.0% | 0.0% | 4.3% | 18.5% |
| Master’s Degree | 21.3% | 16.0% | 21.7% | 25.9% |
| Doctoral Degree | 6.7% | 16.0% | 4.3% | 0.0% |
| Years with IBS (mean, sd) | 16.5 (15.3) | 12.00 (12.1) | 16.2 (12.2) | 20.9 (19.2) |
| Bowel habit (%) | ||||
| Diarrhea | 53.3% | 48.0% | 60.9% | 51.9% |
| Constipation | 25.3% | 32.0% | 21.7% | 22.2% |
| Mixed | 21.3% | 20.0% | 17.4% | 25.9% |
| IBS-SSS | 294.0 (79.4) | 312.4 (81.2) | 282.9 (77.3) | 286.6 (79.5) |
Note. IBS-SSS Categories: Mild = 75–175; Moderate = 175–300; Severe = > 300.
Treatment Conditions
All treatments were standardized and conducted on an individual, outpatient basis at a tertiary care setting at an academic medical setting.
Standard CBT (S-CBT) is a skills–based training program 14 delivered to patients in 10 weekly, one hour sessions. Treatment involved six overlapping phases: (1) education regarding stress and its relationship to IBS (2) self monitoring of stressful situations associated with IBS episodes (3) muscle relaxation exercises to increase physiological self regulation and to cultivate a sense of self control over symptoms (4) learning to identify, reevaluate, and change negatively skewed thoughts (e.g.,catastrophizing15) associated with IBS, (5) changing underlying “core” beliefs (e.g., perfectionism) that fuel threatening cognitions; (6) formal training in problem solving to strengthen the ability to cope with realistic stressors associated with IBS. Weekly home exercises were assigned to facilitate skills acquisition.
Minimal Contact CBT (MC-CBT) covers the same range of procedures featured in S-CBT but relies extensively on self-study materials16. Whereas S-CBT involved ten, 1 hour clinic visits, MC-CBT meets for only four, 60-minute clinic visits over the same period. At the first MC-CBT session, treatment is explained, self-study materials 16 are provided and muscle relaxation and self-monitoring are introduced. The second treatment session introduces cognitive coping techniques (e.g. decatastrophizing through prediction testing). At the third session, patients learn problem solving training and advanced cognitive coping skills (e.g., modifying core beliefs such as perfectionism). The fourth session introduces relapse prevention skills. Two 10 minute phone contacts were scheduled at week 3 and 7 to troubleshoot any problems.
Wait List Control Subjects in this condition were placed on a 10-week delayed treatment wait list, during which time they engaged in daily self-monitoring of GI symptoms
Therapists and Treatment Integrity
Three doctoral level clinical psychologists (2 male, 1 female) with an average of 10 years of experience in the psychological treatment of painful medical disorders provided treatment under the supervision of an experienced Ph.D level psychologist (JML). All therapists received extensive training which included review of both concepts and techniques of CBT, topic-by-topic review of the manuals, listening to audio taped examples of treatment, role playing and practicing exercises, discussion of case examples and rehearsing strategies for difficult or challenging cases; and practice cases. To ensure fidelity of treatment delivery, therapists followed a manualized treatment protocol that provided detailed session by session guidance. A checklist of procedures specific to each session was used to assure compliance. Review of checklist revealed no violation of treatment procedures. No adverse events were reported.
Statistical Power
The primary focus was to compare a brief CBT to a wait list control to demonstrate its effectiveness. Prior research comparing IBS specific CBT to wait-list control6 suggests a standardized effect size mean difference comparable to a “large” effect should be observed (namely, a d of 0.80 as defined by Cohen17). The study therefore targeted a sample size of 25 per group in each of the three conditions, which yields approximate power of 0.80 to detect a large standardized effect size difference between means when contrasting two groups. To establish comparability between the brief and standard CBT, one needs considerably larger sample sizes that support formal equivalence testing paradigms. This facet of the research is therefore exploratory. We expect to observe effect sizes in these two conditions that are comparable and, if this is the case, this justifies a future more expensive clinical trial that can support such equivalence tests
Measures
Primary outcome variables
Clinical efficacy was assessed with two adequate relief mesaures18, 19. The first asks “In the past 7 days, have you had adequate relief from irritable bowel syndrome pain or discomfort?”(Yes/No)18. Because IBS is a multisymptom problem that involves other (non pain) symptoms, we created a second item assessing adequacy of relief from bowel symptoms for which they initially sought treatment. Per Rome III guidelines 20, we also assessed clinical efficacy using the Clinical Global Impressions - Improvement Scale (CGI-I)21: “Compared to how you felt prior to entering the study, how would you rate the IBS symptoms for which you sought treatment during the past week?” (1 = substantially improved, 4= no change, 7 = substantially worse). Consistent with past research22 we defined treatment responders a priori by a score of 1 (substantially) or 2 (moderately improved).
Secondary outcome variables
Additional clinical endpoints included overall psychological distress (Global Severity Index of the Brief Symptom Inventory23), IBS symptom severity (IBS Symptom Severity Scale24), overall patient satisfaction (Client Satisfaction Form25), and health related quality of life (IBS Quality of Life26). Outcome measures were obtained at intake and post treatment with the exception of global relief measures and patient satisfaction measures that were administered only at post treatment. Patients returned to the clinic 14–16 days after their last scheduled CBT session to provide post treatment efficacy data.
Results
Preliminary Data Analyses
Treatment Dropouts and Missing Data
The number of individuals assigned to MC-CBT, S-CBT and WLC conditions was 25, 23 and 27, respectively. Sixteen individuals (21%) dropped out of the study during the course of treatment. Five were in the MC-CBT condition, 6 were in the S-CBT condition and 5 were in the WLC condition. Dropouts identified 27 “getting to the session” as the most common reason for dropping out of treatment. Forty three percent (7/16) of dropouts terminated treatment before session one. To identify dropout bias, a dummy variable (1= dropped out, 0 = did not drop out) was correlated with a variety of demographic variables (e.g., gender, ethnicity, education) and pre-treatment assessments. Drop-out was related to the baseline clinical severity measure (r = 0.40, p < 0.05) in the direction that patients with higher severity scores were more likely to drop out of treatment. There were no other statistically significant predictors of treatment dropout.
Analyses used both an intent-to-treat (ITT) framework and a dosage-response (DR) framework. The DR analyses were based only on individuals who completed the treatments, whereas ITT analyses were based on all individuals who were randomized to condition. For the ITT analyses, of the 16 individuals who dropped out of treatment, follow-up assessments were obtained for 5 of them (31%). All 5 of these individuals responded negatively to achieving adequate relief for abdominal pain as well as adequate relief for bowel symptoms and all five indicated “no change” (a score of zero) on the global improvement (CGI) measure. These values were therefore imputed for individuals who dropped out of treatment.
Follow-up data were missing for 12 of the 75 clients (16%). Seven of the twelve individuals were in the S-CBT condition and 5 were in the MC-CBT condition. Eleven of the twelve individuals were treatment drop-outs. For the single individual who completed treatment but for whom no follow-up assessment was obtained (who was in the S-CBT condition), for the adequate relief measures, we randomly selected and imputed a score from a binomial distribution with a mean and variance equal to the observed mean and variance for treatment completers in the S-CBT condition. For the global improvement measure, we imputed a randomly selected score from a normal distribution with a mean and variance equal to the mean and variance for treatment completers in the S-CBT condition. A multiple imputation strategy was used to estimate parameters, standard errors and confidence intervals 28, but because multiple imputations were pursued for only a single individual, the effect of imputation based variability was negligible.
Primary Analyses
Comparison of Treatment Groups: Dosage Response Analysis
The top half of Table 2 presents the percentage of individuals reporting adequate relief of abdominal pain, the percentage of individuals reporting adequate relief of bowel symptoms, and mean patient global improvement for the three treatment conditions for people who completed treatment and who completed the WLC. In terms of adequate relief of abdominal pain, the percentage difference between S-CBT and the WLC was 73.3 (p < 0.05), with a 95% Bayes adjusted confidence interval (CI) of 51.6 to 95.0. The percentage difference between MC-CBT and the WLC was 80.9 (p < 0.05), with a 95% Bayes adjusted CI of 63.1 to 98.7. The percentage difference between S-CBT and MC-CBT was −7.6 (ns) with a 95% Bayes adjusted CI of −30.0 to 14.8. Thus there were sizeable differences between the two treatment conditions and the WLC, but minimal differences between the two treatment conditions. There were no between group differences in treatment credibility or expectancy of improvement at baseline.
Table 2.
Comparison of Groups on Primary Outcomes
| Outcome Variable | WLC | S-CBT | MC-CBT |
|---|---|---|---|
| Adequate Relief – Abdominal Pain: DR | 9.1 | 82.4a | 90.0a |
| Adequate Relief – Bowel Symptoms: DR | 0.0 | 82.4a | 75.0a |
| Global Symptom Improvement : DR | 0.14 | 2.12a | 2.10a |
| Adequate Relief – Abdominal Pain: ITT | 7.4 | 60.9a | 72.0a |
| Adequate Relief – Bowel Symptoms: ITT | 0.0 | 60.9a | 60.0a |
| Global Symptom Improvement: ITT | 0.11 | 1.56a | 1.68a |
Note: DR = Dosage response analysis; ITT = Intent-To-Treat Analysis; WLC = wait list control, S-CBT = standard cognitive-behavior therapy; MC-CBT = minimal contact cognitive-behavior therapy. Groups within a row that do not share a common superscript are statistically significantly different, p < 0.05.
For adequate relief of bowel symptoms, no one in the WLC reported adequate relief whereas 81.3% and 75% reported such relief in the S-CBT and MC-CBT conditions, respectively. The zero frequency for the WLC precludes formal significance tests contrasting the S-CBT and MC-CBT conditions with the WLC group, but the lower limit of the 95% CI for the S-CBT and MC-CBT conditions were both well above zero (53.1 and 59.0, respectively), suggesting sizeable improvement over the WLC condition. The percentage difference between S-CBT and MC-CBT was 7.4 (ns) with a 95% Bayes adjusted CI of −18.8 to 33.6.
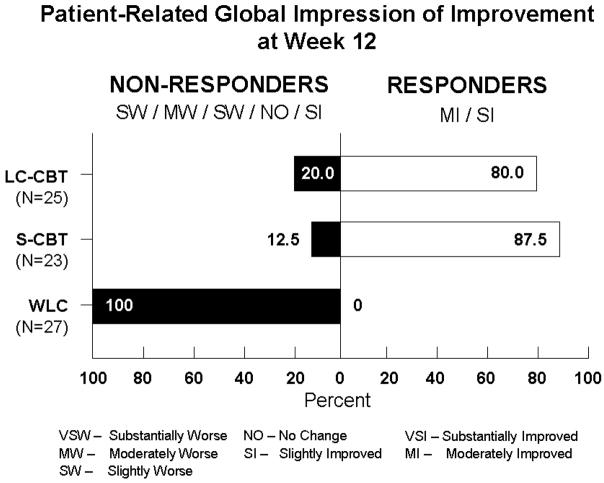
Finally, the mean difference between S-CBT and the WLC on patient impression of improvement was 1.99 (p < 0.05), with a 95% CI of 1.50 to 2.48. The mean difference between MC-CBT and the WLC was 1.96 (p < 0.05), with 95% CI of 1.50 and 2.42. The mean difference between S-CBT and MC-CBT was 0.025 (ns) with a 95% CI of −0.52 to 0.47. This pattern of statistical significance maintained when an analysis of covariance (ANCOVA) strategy was used comparing posttest means adjusted for pretest scores. When we analyzed the proportion of responders by classifying patients reporting “moderately” or “substantially” improvement (scored 2 and 1, respectively) as responders and those reporting “slight” improvement or above (3>) as nonresponders, 87.5% of the individuals in the S-CBT condition reported very much to much improvement, 80% of the individuals in the MC-CBT condition did so and 0% of the WLC individuals did so. The former two percentages did not differ significantly from one another (p > 0.55). These data are presented in Figure 1. CBT treated participants were uniformly satisfied with the care they received as evidenced by their close to maximum possible scores (MC-CBT M = 30.1, SD= 1.8; S-CBT M = 30.3, SD = 2.3) on the Client Satisfaction Scale (range = 8 to 32)
Figure 1.
Comparison of Treatment Groups: Intent-To-Treat Analysis
The bottom half of Table 2 presents relevant data summaries for all individuals randomly assigned to conditions. In terms of adequate relief of abdominal pain, the percentage difference between S-CBT and the WLC was 53.5 (p < 0.05), with a 95% Bayes adjusted CI of 31.2 to 75.8. The percentage difference between MC-CBT and the WLC was 64.6 (p < 0.05), with a 95% Bayes adjusted CI of 44.4 to 84.8. The percentage difference between S-CBT and MC-CBT was −11.1 (ns) with a 95% Bayes adjusted CI of −37.7 to 15.5. As with the dosage response analyses, there were sizeable differences between the two treatment conditions and the WLC, but minimal differences between the two treatment conditions.
For adequate relief of bowel symptoms, no one in the WLC reported adequate relief whereas 60.9% and 60% reported such relief in the S-CBT and MC-CBT conditions, respectively. The presence of a zero frequency for the WLC again precludes a formal significance test contrasting the S-CBT and MC-CBT conditions with the WLC group. The lower limit of the 95% confidence interval for the S-CBT and MC-CBT conditions were both well above zero (41.0 and 40.8, respectively), suggesting a sizeable improvement relative to the WLC condition. The percentage difference between S-CBT and MC-CBT was −0.9 (ns) with a 95% Bayes adjusted confidence interval of −28.5 to 26.8.
Finally, the mean difference between S-CBT and the WLC on global symptom improvement was 1.45 (p < 0.05), with a 95% CI of 0.90 to 2.00. The mean difference between MC-CBT and the WLC was 1.57 (p < 0.05), with 95% CI of 1.03 and 2.10. The mean difference between S-CBT and MC-CBT was 0.11 (ns) with a 95% CI of −0.44 to 0.67. This pattern of statistical significance maintained when the aforementioned ANCOVA strategy was used to compare posttest means adjusted for pretest scores. When translated into the traditional dichotomy of reporting “moderate” or “substantial” improvement (scored 1) versus assigning a rating category of “moderate” improvement or above, the results were essentially the same as the dosage response analyses.
Sensitivity Analysis and Equivalence Tests
The above contrasts were pursued from a variety of alternative analytic perspectives to ensure that results were robust across forms of analysis in the spirit of sensitivity analysis. For example, small sample logistic regression methods based on exact probabilities tested group contrasts in conjunction with covariates that included gender, education level and age. Similarly, different strategies for dealing with missing data were evaluated, such as an expectation-maximization (EM) approach. In all cases, the fundamental conclusions remained unchanged.
An interesting result was the near equivalence of effects for the S-CBT and MC-CBT conditions. It is, of course, not possible to make statements of equivalence using traditional hypothesis testing, because one can never accept the null hypothesis. A better approach is to use formal equivalence testing methods, which involves specifying a threshold value (TV) for defining equivalence and then examining confidence intervals relative to that threshold. For example, for adequate relief for abdominal pain, it might be argued that if the percent of patients claiming adequate relief in the respective group populations is within 10% of each other, then the therapies should be judged to be “functionally equivalent.” The value of 10% is the threshold value. If the lower limit of the sample confidence interval for the percent difference is larger than −10% and the upper limit of the confidence interval is less than +10%, then the therapies are declared functionally equivalent. If either the upper or lower limit of the confidence interval is within the interval defined by –TV to +TV, then one can not make a definitive statement about functional equivalence. This was the case for the present data. Essentially, the small sample sizes in the study yielded confidence intervals that were too wide to make definitive statements about functional equivalence between the S-CBT and MC-CBT conditions.
Supplementary Analyses
In addition to the primary outcome variables, we compared treatment conditions on several variables that were of secondary interest. Relevant dosage-based means for these variables are in Table 3. The S-CBT and MC-CBT group means differed statistically significantly (p < 0.05) from the WLC at the follow-up for the quality of life outcome and the IBS symptom severity ratings, but not for the index of distress. For the former two outcomes, the change was in the direction of improvement. Behaviorally treated patients reduced the severity of IBS symptoms and their quality of life impairment. None of the contrasts between the S-CBT and MC-CBT groups were statistically significant and the means for these two conditions tended to be similar.
Table 3.
Supplementary Analyses on Secondary Outcomes: Dosage Response Means
| QOL | BSI-GSI | IBS-SSS | Satisfaction | |
|---|---|---|---|---|
| WLC: Baseline | 62.0 | 57.7 | 286.6 | |
| WLC: FU | 64.2b | 56.9a | 266.3 | |
| WLC: Change | 2.2 | −0.8 | −20.3 | |
| S-CBT: Baseline | 52.0 | 60.3 | 282.9 | |
| S-CBT: FU | 74.0a,b | 55.6a | 158.1a | 30.3a |
| S-CBT: Change | 18.0* | −4.7* | −124.8* | |
| MC-CBT: Baseline | 58.7 | 58.7 | 312.4 | |
| MC-CBT: FU | 78.3a | 52.5a | 162.7a | 30.1a |
| MC-CBT: Change | 19.6* | −6.2* | −149.7* |
Note: WLC = wait list control, S-CBT = standard cognitive-behavior therapy; MC-CBT = minimal contact cognitive-behavior therapy; Baseline = baseline assessment; FU = follow-up assessment; Change = follow-up minus baseline; QOL = quality of life measure; Satisfaction = client satisfaction rating.
= mean change is statistically significantly different from 0, p < 0.05; Empty cells indicate measure not administered at the designated time point. For follow-up assessments, groups within a column that do not share a common superscript are statistically significantly different, p < 0.05. None of the group differences at baseline were statistically significant.
Discussion
The goal of this study was to test experimentally the feasibility, acceptability, and clinical efficacy of a brief, patient administered behavioral self management treatment for patients with severe IBS. At the conclusion of a 10 week treatment phase, a majority of patients assigned to brief CBT described their IBS symptoms as significantly improved and reported adequate relief from IBS symptoms. The proportion of MC-CBT patients who reported adequate relief from IBS symptoms was comparable to that of patients assigned to therapist administered, clinic based CBT (60% – 72%) and exceeded that of patients assigned to a wait list control condition (0%–7%). A similar pattern of data was observed for treatment response on secondary endpoints. CBT treated patients showed statistically significant improvements in their quality of life (IBS QOL) and severity of IBS symptoms that well exceeded gains of WL patients at post treatment. Our failure to show prospective changes in mental well being may appear somewhat counterintuitive but is consistent with the results of a recent large scale CBT trial 29 and 20 years of treatment outcome research 30 showing that CBT’s most robust effect is improvement in GI symptoms not distress variables.
These data compare favorably to two recently published trials of brief behaviorally oriented programs for IBS. Robinson et al 31 examined the efficacy of a self help guidebook and found that while patients perceived themselves as globally improved (CGI-I) at the 12 month follow up, their actual level of IBS symptom severity remained statistically unchanged from baseline. Similarly, Heitkemper et al 32 found no change in IBS symptoms in patients treated with a single session behavioral treatment program, although select dimensions of QOL improved. These studies echo the disappointing track record of most single session behavioral treatments developed for a range of mental (e.g., anxiety) and physical (e.g., headache) problems. While very low intensity behavioral treatments have obvious logistical appeal, their efficiency typically involves reducing therapist contact below a level than is required for behavior change to occur33–38. For this reason, we were careful to design an MC-CBT treatment that retained a therapeutic level of therapist contact that provided patients corrective feedback around problems they encountered between sessions, as well as other program features (e.g., goal setting, follow up, menu of strategies34) common across empirically validated, self administered behavioral interventions for diverse health problems (e.g., headache, alcohol, depression, anxiety, smoking, binge eating, depression). This may explain why CBT treated patients reported improvement on global improvement of and adequate relief from IBS symptoms as well as reduction in their actual symptom severity immediately following CBT. Additionally, the MC-CBT protocol benefited from a year long therapy development process that included (a) initial writing of a working manual specifying techniques to be used and excluded, (b) use of the manual by well trained, well supervised therapists with 10 cases, (c) revision of the manual, based on feedback from outside experts, therapists conducting the sessions, and patients, (d) use of the updated manual with new cases, and (e) final revision of the manual based on further feedback from outside experts, therapists, and participants. We also believe that the methodological rigor, systematic approach to protocol development, reliance on psychometrically sound outcome measures, and attention to quality control throughout the trial may explain the discrepancy between our largely positive findings and the more equivocal findings of two recent behavioral trials39, 40.
While traditional CBT is not typically associated with adverse side effects, it is not without its costs. If one assumes that (1) CBT is delivered weekly for 12 weeks (e.g., 39) and (2) per session costs are $100, treatment costs amount to $1200 per patient. By comparison, the average wholesale price of a 12-week regimen 39 of the tricyclic antidepressant desipramine is $207.78 41. Beyond cost, logistical problems complicate CBT. CBT requires experienced clinicians who number only several hundred worldwide. Psychotherapy requires a significant investment of time on the part of patients who must often travel long distances -- often in inclement weather and or during the work day – for traditional face to face treatment over a three month period. Patients undergoing intensive CBT are often required to pay high co-payment charges, deductibles and/or out of pocket expenses depending on insurance restrictions. Patients who are candidates for psychotherapy are also subject to stigma, especially IBS patients 42 whose medically benign symptoms are often viewed as psychological and “not real”. It is likely that many people choose not to pursue behavioral treatments because they neither desire to be labeled a “head case” nor wish to endure the stigma this entails. By reducing travel and therapist time, we believe we have developed a brief CBT that may incur fewer costs without any apparent loss of efficacy. Formal cost effectiveness studies are required to monetize the true economic value and impact of CBT on health care utilization, work productivity, and other “real world” endpoints.
While we found a statistically non-significant difference between the 4 session and 10 session CBT interventions, these data should be interpreted cautiously. Our data are based on a small, exploratory study with only 25 patients assigned to the two CBT conditions at a single investigative site. The statistical power to observe a statistically significant difference between the groups was low. We are therefore are not on sufficiently strong scientific grounds to conclude that both versions of CBT are equivalent. Our study was not designed to permit an adequate equivalence test analysis. Rather, it was a tightly controlled feasibility study that sought to use rigorous experimental design and conservative statistical methods to provide perspectives on whether the 4 session CBT intervention produces sufficient treatment effects to warrant their investigation in an appropriately powered, randomized controlled trial. There are characteristics of the sample that may limit generalizability of our findings. Our sample represented severely affected IBS patients with a relatively homogeneous demographic profile and results may not generalize to a more diverse sample or one treated by other therapists at other sites. Because we used a wait list control comparison, we can conclude that treatment effects are not due to measurement artifact or temporal processes such as spontaneous remission, life events, or passage of time. While the WLC has limitations, it is an ideal control for ruling out whether treatment effects are due to treatment or simply the fluctuating nature of IBS. Whether treatment gains attributed to CBT are due to its specific techniques and strategies or nonspecific factors common to all therapies (e.g. provision of a convincing rationale for the treatment, support and encouragement, the quality of the therapeutic relationship, positive expectancy of improvement) is an important research question beyond the scope of this study. Despite this, we believe that even in its preliminary state the information from this trial lays the foundation of future trials for determining the effectiveness of self directed behavioral treatments that minimally supervised paraprofessionals can administer.
This work was based on the stage model of behavioral therapies research 43. Developed by the National Institute on Drug Abuse 44, the model is an effort to create an organizational framework for facilitating the transition of clinically useful, novel, scientifically rigorous treatment from pilot work, through RCT, to tests in “real life” clinical settings. The model specifies three progressive stages that roughly parallel those for the development of pharmacologic therapies. Stage I involves initial development and pilot or feasibility testing of new and untested treatments. Stage II consists principally of randomized controlled clinical trials to evaluate efficacy of treatments that have shown promise or efficacy in initial studies. Stage III, which corresponds to phase IV research for drug trials, aims to address issues of transportability of treatments whose efficacy has been demonstrated in at least two stage II trials. This study is concerned with the Stage 1 whose primary measures of success is whether a treatment is feasible to administer, whether it is acceptable and tolerable to patients, and whether it evidences desirable treatment effects based on a recommended ample size of 15–30 patients per cell to warrant a large scale clinical trial (Stage 2)45.
The present study suggests that a brief, patient administered version of CBT is a well tolerated, efficient, and clinically useful treatment associated with global relief from more severe IBS, symptom reduction, and improvement in quality of life. This is an important finding that comes at a critical time when few empirically validated therapies are available to more severely affected IBS patients. Further research is needed to establish the therapeutic potential and limitations of this novel approach to IBS management.
Acknowledgments
Grant support: This research was supported by NIH/NIDDK Grant 67878
Abbreviations
- IBS
irritable bowel syndrome
- CBT, cognitive behavioral therapy
MC, minimal contact
- WL
wait list
- ITT
intent to treat
- DR
dosage-response
Footnotes
Competing Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative Management of Chronic Illness. Annals of Internal Medicine. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–58. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health. Treatment Choice in Psychological Therapies and Counseling: Evidence Based Clinical Guidelines. UK Department of Health; 2001. [Google Scholar]
- 4.Lackner JM, Morley S, Dowzer C, Mesmer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:1100–13. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 5.Lackner JM, Gudleski GD, Zack MM, Katz LA, Powell C, Krasner S, Holmes E, Dorscheimer K. Measuring health-related quality of life in patients with irritable bowel syndrome: can less be more? Psychosom Med. 2006;68:312–20. doi: 10.1097/01.psy.0000204897.25745.7c. [DOI] [PubMed] [Google Scholar]
- 6.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Blanchard EB. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. 2007;133:433–44. doi: 10.1053/j.gastro.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holroyd KA, O’Donnell FJ, Stensland M, Lipchik GL, Cordingley GE, Carlson BW. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial. [see comment] Jama. 2001;285:2208–15. doi: 10.1001/jama.285.17.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro DA, Barkham M, Stiles WB, Hardy GE, Rees A, Reynolds S, Startup M. Time is of the essence: A selective review of the fall and rise of brief therapy research. Psychol Psychother. 2003;76:211–35. doi: 10.1348/147608303322362460. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA, Corazziari E, Talley NJ, Thompson GW, Whitehead WE, Rome II. Diagnosis, pathophysiology and treatment: A multinational consensus. Degnon Associates; 2000. The functional gastrointestinal disorders. [Google Scholar]
- 10.Jastak S, Wilkinson G. Wide Range Achievement Test - Revised. Wilminton, DE: Jastak Associates; 1984. [Google Scholar]
- 11.Drossman DA. Do psychological factors define symptom severity and patient status in irritable bowel syndrome? American Journal of Medicine. 1999;107:41S–50S. doi: 10.1016/s0002-9343(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 12.Lackner JM, Gurtman MB. Pain catastrophizing and interpersonal problems: A circumplex analysis of the communal coping model. Pain. 2004;110:597–604. doi: 10.1016/j.pain.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.van Tulder MW, Ostelo R, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioral treatment for chronic low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine. 2000;25:2688–99. doi: 10.1097/00007632-200010150-00024. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard EB. Irritable bowel syndrome: Psychosocial assessment and treatment. APA; 2000. [Google Scholar]
- 15.Lackner JM, Quigley BM, Blanchard EB. Depression and abdominal pain in IBS patients: the mediating role of catastrophizing. Psychosomatic Medicine. 2004;66:435–441. doi: 10.1097/01.psy.0000126195.82317.46. [DOI] [PubMed] [Google Scholar]
- 16.Lackner JM. Controlling IBS the drug-free way: A 10-step plan for symptom relief. Stewart, Tabori, & Chang; 2007. [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 18.Mangel AW, Hahn BA, Heath AT, Northcutt AR, Kong S, Dukes GE, McSorley D. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76–81. doi: 10.1177/030006059802600203. [DOI] [PubMed] [Google Scholar]
- 19.Bijkerk CJ, de Wit NJ, Muris JW, Jones RH, Knottnerus JA, Hoes AW. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122–7. doi: 10.1111/j.1572-0241.2003.07158.x. [DOI] [PubMed] [Google Scholar]
- 20.Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of Treatment Trials for Functional Gastrointestinal Disorders. Gastroenterology. 2006;130:1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 21.Klein KB. Assessment of treatment outcome in the functional gastrointestinal disorders. In: Corazziari E, editor. Approach to the patient with chronic gastrointestinal disorders. Milano, Italy: Messaggi; 1999. [Google Scholar]
- 22.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. Jama. 2000;283:2529–36. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 23.Derogatis LR. Brief Symptom Inventory. National Computer Systems; 1993. [Google Scholar]
- 24.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 25.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 26.Patrick DL, Drossman DA, Frederick IO. A Quality of Life Measure for Persons with Irritable Bowel Syndrome (IBS-QOL): User’s Manual and Scoring Diskette for United States Version. Seattle, Washington: University of Washington; 1997. [Google Scholar]
- 27.Lackner JM. IBS Treatment Non Completer Questionnaire. Buffalo: University at Buffalo, SUNY; 2004. [Google Scholar]
- 28.King G, Honaker J, Joseph A, Scheve K. Analyzing incomplete political science data: An alternative algorithm for multiple imputation. American Political Science Review. 2001;95:49–69. [Google Scholar]
- 29.Lackner JM, Jaccard J, Krasner SS, Katz LA, Blanchard EB. How does cognitive behaioral therapy for IBS work: A structural equation modeling analysis of results from a randomized controlled trial. Gastroenterology. 2006;130:A-504. [Google Scholar]
- 30.Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:1100–13. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 31.Robinson A, Lee V, Kennedy A, Middleton L, Rogers A, Thompson DG, Reeves D. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of IBS. Gut. 2005 doi: 10.1136/gut.2004.062901. gut.2004.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heitkemper MM, Jarrett ME, Levy RL, Cain KC, Burr RL, Feld AD, Barney P, Weisman P. Self-management for women with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2004;2:585–596. doi: 10.1016/s1542-3565(04)00242-3. [DOI] [PubMed] [Google Scholar]
- 33.Holroyd KA. Assessment and psychological management of recurrent headache disorders. J Consult Clin Psychol. 2002;70:656–77. doi: 10.1037//0022-006x.70.3.656. [DOI] [PubMed] [Google Scholar]
- 34.Miller WR, Sanchez VC. Motivating young adults for treatment and lifestyle change. In: Howard G, editor. Issues in Alcohol Use and Misuse in Young Adults. South Bend, IN: University of Notre Dame Press; 1993. [Google Scholar]
- 35.Edwards G, Orford J, Egert S, Guthrie S, Hawker A, Hensman C, Mitcheson M, Oppenheimer E, Taylor C. Alcoholism: a controlled trial of “treatment” and “advice”. J Stud Alcohol. 1977;38:1004–31. doi: 10.15288/jsa.1977.38.1004. [DOI] [PubMed] [Google Scholar]
- 36.Mains JA, Scogin FR. The effectiveness of self-administered treatments: a practice-friendly review of the research. J Clin Psychol. 2003;59:237–46. doi: 10.1002/jclp.10145. [DOI] [PubMed] [Google Scholar]
- 37.Sanders KA. Psychology. Albany: University at Albany, SUNY; 2006. Self-managed treatment for IBS: Comparison to a wait list control; p. 70. [Google Scholar]
- 38.Saito YA, Prather CM, Van Dyke CT, Fett S, Zinsmeister AR, Locke GR., 3rd Effects of multidisciplinary education on outcomes in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004;2:576–84. doi: 10.1016/s1542-3565(04)00241-1. [DOI] [PubMed] [Google Scholar]
- 39.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, Le T, Meyer K, Bradshaw B, Mikula K, Morris CB, Blackman CJ, Hu Y, Jia H, Li JZ, Koch GG, Bangdiwala SI. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 40.Blanchard EB, Lackner JM, Sanders K, Krasner S, Keefer L, Payne A, Gudleski GD, Katz L, Rowell D, Sykes M, Kuhn E, Gusmano R, Carosella AM, Firth R, Dulgar-Tulloch L. A controlled evaluation of group cognitive therapy in the treatment of irritable bowel syndrome. Behaviour Research and Therapy. 2007;45:633–648. doi: 10.1016/j.brat.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 41.FirstData Bank. National Drug Data File. Author, 2003.
- 42.Havlicek T, Bratten J, Keefer L, Jones MP. Subjective experiences of Irritable Bowel Syndrome (IBS) patients: an exploratory study of the social stigma of IBS. Gastroenterology. 2007:132. [Google Scholar]
- 43.Carroll KM, Nuro KF. One Size Cannot Fit All: A Stage Model for Psychotherapy Manual Development. Clinical Psychology: Science and Practice. 2002;9:396–406. [Google Scholar]
- 44.Onken LS, Blaine JD, Battjes R. Behavioral therapy research: a conceptualization of a process. In: Henggeler SW, Santos AB, editors. Innovative Approaches for Difficult-to-Treat Populations. Washington, D. C.: American Psychiatric Press; 1996. pp. 477–485. [Google Scholar]
- 45.Rounsaville BJ, Carroll KM, Onken LS. Clinical Psychology: Science and Practice. 2001. A Stage Model of Behavioral Therapies Research: Getting Started and Moving on From Stage I; p. 8. [Google Scholar]