Abstract
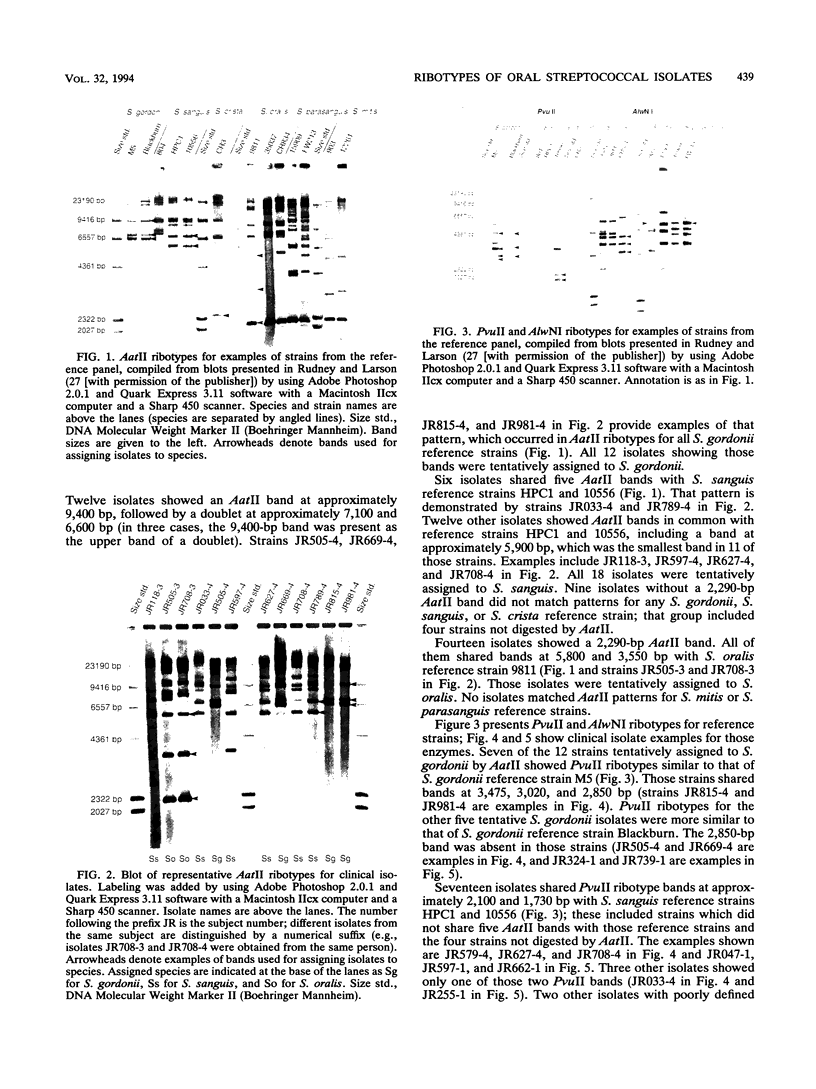
This study evaluated restriction fragment length polymorphisms of rRNA genes (ribotyping) for genotypic identification of 53 oral isolates classified as "Streptococcus sanguis" by colony morphology. Isolates were from 8-h buccal plaque on lower first permanent molars of 20 subjects. DNA was digested with AatII and hybridized with digoxygenin-labeled cDNA of Escherichia coli 16S and 23S rRNA. Strains were ribotyped again with AlwNI or PvuII on the basis of the presence or absence of a 2,290-bp AatII band. Band patterns were compared with reference ribotypes for Streptococcus gordonii, Streptococcus sanguis, Streptococcus crista, Streptococcus oralis, Streptococcus mitis, and Streptococcus parasanguis strains. Forty-eight isolates could be assigned to a species (22 S. sanguis, 14 S. oralis, 12 S. gordonii). Multiple species were seen in 14 subjects; multiple strains of the same species occurred in 11 subjects. Our findings suggest that ribotyping can be used for genotypic identification of S. sanguis, S. oralis, and S. gordonii isolates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baloga A. O., Harlander S. K. Comparison of methods for discrimination between strains of Listeria monocytogenes from epidemiological surveys. Appl Environ Microbiol. 1991 Aug;57(8):2324–2331. doi: 10.1128/aem.57.8.2324-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D., Hardie J. M., Whiley R. A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991 Dec;35(6):367–372. doi: 10.1099/00222615-35-6-367. [DOI] [PubMed] [Google Scholar]
- Beighton D., Whiley R. A. Sialidase activity of the "Streptococcus milleri group" and other viridans group streptococci. J Clin Microbiol. 1990 Jun;28(6):1431–1433. doi: 10.1128/jcm.28.6.1431-1433.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield P. W., Cutter G. R., Dasanayake A. P. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993 Jan;72(1):37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- Caufield P. W., Ratanapridakul K., Allen D. N., Cutter G. R. Plasmid-containing strains of Streptococcus mutans cluster within family and racial cohorts: implications for natural transmission. Infect Immun. 1988 Dec;56(12):3216–3220. doi: 10.1128/iai.56.12.3216-3220.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield P. W., Walker T. M. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989 Feb;27(2):274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Classification and identification of the viridans streptococci. Clin Microbiol Rev. 1989 Jul;2(3):315–328. doi: 10.1128/cmr.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Grimont F., el Solh N. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Apr;135(4):989–999. doi: 10.1099/00221287-135-4-989. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Pease A. A., Whiley R. A. Amylase-binding as a discriminator among oral streptococci. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):193–197. doi: 10.1016/0378-1097(90)90281-t. [DOI] [PubMed] [Google Scholar]
- Facklam R. R., Rhoden D. L., Smith P. B. Evaluation of the Rapid Strep system for the identification of clinical isolates of Streptococcus species. J Clin Microbiol. 1984 Nov;20(5):894–898. doi: 10.1128/jcm.20.5.894-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. V., Pedrazzoli V., Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991 Jun;6(3):129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Grimont F., Lefèvre M., Ageron E., Grimont P. A. rRNA gene restriction patterns of Legionella species: a molecular identification system. Res Microbiol. 1989 Nov-Dec;140(9):615–626. doi: 10.1016/0923-2508(89)90193-9. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Duke B., Guiney M., Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992 Apr;30(4):915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P., Coykendall A., Beighton D., Hardie J. M., Whiley R. A. Streptococcus crista sp. nov., a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int J Syst Bacteriol. 1991 Oct;41(4):543–547. doi: 10.1099/00207713-41-4-543. [DOI] [PubMed] [Google Scholar]
- Hinnebusch C. J., Nikolai D. M., Bruckner D. A. Comparison of API Rapid Strep, Baxter MicroScan Rapid Pos ID Panel, BBL Minitek Differential Identification System, IDS RapID STR System, and Vitek GPI to conventional biochemical tests for identification of viridans streptococci. Am J Clin Pathol. 1991 Oct;96(4):459–463. doi: 10.1093/ajcp/96.4.459. [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Nyvad B. Ability to bind salivary alpha-amylase discriminates certain viridans group streptococcal species. J Clin Microbiol. 1990 Nov;28(11):2576–2577. doi: 10.1128/jcm.28.11.2576-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Reinholdt J., Nyvad B., Frandsen E. V., Mikkelsen L. IgA1 proteases of oral streptococci: ecological aspects. Immunol Invest. 1989 Jan-May;18(1-4):161–170. doi: 10.3109/08820138909112235. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Alpert C. A., Chassy B. M., Michalek S. M. Repeated DNA sequence involved in mutations affecting transport of sucrose into Streptococcus mutans V403 via the phosphoenolpyruvate phosphotransferase system. Infect Immun. 1991 Apr;59(4):1535–1543. doi: 10.1128/iai.59.4.1535-1543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moureau P., Derclaye I., Gregoire D., Janssen M., Cornelis G. R. Campylobacter species identification based on polymorphism of DNA encoding rRNA. J Clin Microbiol. 1989 Jul;27(7):1514–1517. doi: 10.1128/jcm.27.7.1514-1517.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24(4):267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987 Oct;95(5):369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Pérolat P., Grimont F., Regnault B., Grimont P. A., Fournié E., Thevenet H., Baranton G. rRNA gene restriction patterns of Leptospira: a molecular typing system. Res Microbiol. 1990 Feb;141(2):159–171. doi: 10.1016/0923-2508(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Rudney J. D., Krig M. A., Neuvar E. K., Soberay A. H., Iverson L. Antimicrobial proteins in human unstimulated whole saliva in relation to each other, and to measures of health status, dental plaque accumulation and composition. Arch Oral Biol. 1991;36(7):497–506. doi: 10.1016/0003-9969(91)90142-h. [DOI] [PubMed] [Google Scholar]
- Rudney J. D., Larson C. J. Species identification of oral viridans streptococci by restriction fragment polymorphism analysis of rRNA genes. J Clin Microbiol. 1993 Sep;31(9):2467–2473. doi: 10.1128/jcm.31.9.2467-2473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudney J. D., Neuvar E. K., Soberay A. H. Restriction endonuclease-fragment polymorphisms of oral viridans streptococci, compared by conventional and field-inversion gel electrophoresis. J Dent Res. 1992 May;71(5):1182–1188. doi: 10.1177/00220345920710051001. [DOI] [PubMed] [Google Scholar]
- Ruoff K. L., Kunz L. J. Use of the Rapid STREP system for identification of viridans streptococcal species. J Clin Microbiol. 1983 Nov;18(5):1138–1140. doi: 10.1128/jcm.18.5.1138-1140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Callihan D. R. Analysis of rRNA restriction fragment length polymorphisms from Bacteroides spp. and Bacteroides fragilis isolates associated with diarrhea in humans and animals. J Clin Microbiol. 1992 Apr;30(4):806–812. doi: 10.1128/jcm.30.4.806-812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tardif G., Sulavik M. C., Jones G. W., Clewell D. B. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect Immun. 1989 Dec;57(12):3945–3948. doi: 10.1128/iai.57.12.3945-3948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley R. A., Fraser H. Y., Douglas C. W., Hardie J. M., Williams A. M., Collins M. D. Streptococcus parasanguis sp. nov., an atypical viridans Streptococcus from human clinical specimens. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):115–121. doi: 10.1111/j.1574-6968.1990.tb04133.x. [DOI] [PubMed] [Google Scholar]