Abstract
Recent findings illustrate a critical role for ankyrin-B function in normal cardiovascular physiology. Specifically, decreased expression of ankyrin-B in mice or human mutations in the ankyrin-B gene (ANK2) results in potentially fatal cardiac arrhythmias. Despite the clear role of ankyrin-B in heart, the mechanisms underlying transcriptional regulation of ANK2 are unknown. In fact, to date there is no description of ANK2 genomic organization. The aims of this study were to provide a comprehensive description of the ANK2 gene and to evaluate the relative expression of alternative splicing events associated with ANK2 transcription in heart. Using reverse-transcriptase PCR on mRNA isolated from human hearts, we identify seven new exons associated with the ANK2 gene including an alternative first exon located ~145 kb upstream of the previously-identified first exon. In addition, we identify over thirty alternative splicing events associated with ANK2 mRNA transcripts. Using real-time PCR and exon boundary-spanning primers to selectively amplify these splice variants, we demonstrate that these variants are expressed at varying levels in human heart. Finally, ankyrin-B immunoblot analysis demonstrates the expression of a heterogeneous population of ankyrin-B polypeptides in heart. ANK2 consists of 53 exons that span ~560 kb on human chromosome 4. Additionally, our data demonstrates that ANK2 is subject to complex transcriptional regulation that likely results in differential ankyrin-B polypeptide function.
Keywords: ankyrin-B, ANK2, cardiomyocytes, alternative splicing
1. Introduction
Ankyrins are adapter proteins that link integral membrane proteins to the actin/spectrin-based cytoskeleton in excitable and non-excitable cells [1]. In humans, three ankyrin genes ANK1, ANK2, and ANK3 encode polypeptides termed ankyrin-R, ankyrin-B, and ankyrin-G, respectively. Ankyrins –B and –G are expressed in most tissues, while ankyrin-R displays a restricted expression pattern [1].
A canonical ankyrin is comprised of three functional domains that interact with a variety of proteins including integral membrane proteins, cytoskeletal elements, and signaling molecules. The ankyrin ‘membrane-binding domain’ (MBD, comprised of 24 consecutive ANK repeats, see Figure 1A, yellow) interacts with a host of integral membrane proteins including ion channels, transporters, and cell adhesion molecules. Some of these membrane proteins include voltage-gated Na+ and K+ channels, Na/K ATPase, anion exchanger, Na/Ca exchanger, InsP3 receptor, as well as L1 family cell adhesion molecules [1, 2]. The 62 kD ankyrin ‘spectrin-binding domain’ (SBD, Figure 1A, red) displays binding activity for β-spectrin isoforms [3] and B56α, the regulatory subunit of protein phosphatase 2A [4]. Finally, the ‘death’ (DD) and ‘C-terminal’ domains (CTD) constitute the ‘regulatory domain’ (Figure 1A, green and blue) that mediates both ankyrin inter- and intra-molecular interactions [5–8].
Fig.1. Organization of ANK2 and ankyrin-B, and location of ANK2 exons on human chromosome 4.
(A), Ankyrin-B domains include a membrane-binding domain (MBD, yellow), spectrin-binding domain (SBD, red), death domain (DD, green), and C-terminal domain (CTD, blue). Diagram denotes ankyrin-B-binding sites for NCX, InsP3R, β-spectrin, and Hdj-1. B56αbind to ankyrin-B SBD residues 305–486 [4]. (B), Previous ANK2 exon organization. Exons in white have not been identified in cardiac ANK2 transcripts. Exon 38/40 (previous/current) encodes the 220 kD insert found in brain-specific 440 kD ankyrin-B [29]. Numbered lines represent ten overlapping regions PCR-amplified to identify alternative ANK2 transcripts. (C), Revised ANK2 organization. Newly identified exons encoding ankyrin-B are labeled in black. Preceding exon 26, black bar represents an alternative 3′-splice acceptor site. In exon 51, ‘b’ represents an alternative stop site. Exons are not drawn to scale. (D), Previous location of ANK2 exons. The 46 exons of ANK2 span ~330 kb on human chromosome 4 (114,190,334–114,522,076 on NC_000004.10, represents NCBI Build 36) with substantial intronic sequence (~124.6 kb) separating exons 1 and 2. (E), Current location of ANK2 exons. With the identification of non-coding exon (E0) and an alternative first exon (E1′), ANK2 now spans ~560 kb and includes 53 exons 113,958,714–114,522,245 on NC_000004.10). New exons are indicated in red. Note extensive intronic sequence that separates E1′ from E1 (~145.3 kb) and E0 from E1′ (~86.2 kb).
Ankyrins are critical for normal vertebrate physiology. Ankyrin-R dysfunction results in loss of erythrocyte membrane integrity, spherocytosis, and hemolytic anemia in humans and mice [9–12]. Disruption of ankyrin-G in the cerebellum causes abnormal targeting of voltage-gated Na+ and K+ channels in Purkinje and granule cell neurons, irregular action potentials, and ataxia [13–16]. Mice lacking ankyrin-B manifest significant central nervous system defects including degeneration of long axon tracts and die at post-natal day 1 [17]. Recently, we demonstrated that ankyrin-B plays a critical role in normal cardiovascular physiology. Specifically, ankyrin-B+/− mice display bradycardia, heart rate variability, polymorphic arrhythmia, syncope, and sudden cardiac death [18]. In humans, ANK2 (encodes ankyrin-B) loss-of-function variants are linked to dominantly-inherited arrhythmia termed “ankyrin-B syndrome” [18–20]. Phenotypes associated with this syndrome include bradycardia, atrial fibrillation, and ventricular arrhythmia. To date, nine human ankyrin-B loss-of-function variants in six different ANK2 exons have been identified that result in arrhythmia, or susceptibility to drug-induced arrhythmia [18–20].
Despite the critical role for ankyrin-B in normal cardiac physiology, virtually nothing is known regarding the regulation of ANK2 transcription and translation in heart. Furthermore, there has yet to be a detailed analysis of ANK2 genomic organization. This report describes the first analysis of ANK2 genomic organization and the complex alternative splicing events associated with the assembly of a diverse set of cardiac ANK2 mRNA transcripts. We report the unanticipated identification of seven novel human ANK2 exons including a previously unidentified alternative first exon nearly 145 kb upstream of the previous first exon. Using human left ventricular tissue, we characterize a host of cardiac ANK2 mRNAs that result in the addition or subtraction of key functional domains throughout the ankyrin-B polypeptide. Together, our new data demonstrates that ANK2 is subject to complex transcriptional regulation that likely result in differential ankyrin-B function.
2. Methods
2.1 Human tissue, RNA isolation, and reverse transcription
RNA was isolated from left ventricular muscle tissue from six healthy donor hearts that were not suitable for transplantation (subclinical atherosclerosis, age, no matching recipients) through the Iowa Donors Network and through the National Disease Research Interchange. The investigation conforms with the principles outlined in the Declaration of Helsinki [21]. Age and sex were the only identifying information acquired from the tissue providers, and the Iowa Human Subjects Committee deemed that informed consent from each patient was not required. None of the patients died from cardiac-related causes. RNA was isolated from the apex of flash frozen left ventricular samples (fat, coronary arteries excluded). RNA (with a 260/280 ratio of 1.9 to 2.1) was reverse-transcribed into cDNA using SuperScript III reverse transcriptase (20 μl). 1 μl of this reaction was used in subsequent PCR or real-time reactions. Human tissue for RNA experiments in Figures 2–5 was isolated at the University of Iowa (six hearts). Additional assays to search for novel ANK2 exons in humans were conducted in two non-diseased human hearts obtained from the University of Szeged (Szeged, Hungary) that were technically unusable for transplantation based on logistic considerations. Before cardiac explantation, organ donor patients did not receive medication except dobutamine, furosemide, and plasma expanders. The investigations conformed to the principles outlined in the Helsinki Declaration of the World Medical Association. All experimental protocols were approved by the Ethical Review Board of the Medical Center of the University of Szeged (no. 51–57/1997 OEJ). Total RNA from each cardiac sample was isolated and DNase treated with the RNeasy Fibrous Tissue Mini Kit (QIAGEN) following the manufacturer’s instructions. The quality of total RNA was assessed with PAGE (2100 Bioanalyzer; Agilent Technologies). Absence of genomic DNA contamination was verified by PCR.
Fig.2. PCR amplification of alternative splice variants.
PCR products for specific alternative splice variants are only amplified when exon boundary-spanning primers are used. (A), Transcripts containing the exon junctions of 6/7 and 6/8 are only amplified using the full-length boundary-spanning primers (column 4,5). In contrast, no products are detected using half the boundary-spanning primer (columns 1–3). Asterisk underlies a faint PCR product detected for boundary-spanning primer E6/7-3′. Findings are in agreement with the real-time PCR results presented in Fig.3D. (B), Transcripts containing the exon junctions of 23/24 and of 23/25 are only amplified using the full-length boundary-spanning primers (columns 4–5). In contrast, no PCR products are detected using half the boundary-spanning primer (columns 1–3).
Fig.5. Relative mRNA expression of alternative ANK2 transcripts encoding ankyrin-B CTD.
(A), Alternative splicing of ANK2 exons encoding ankyrin-B CTD. Exon size represents relative exon length. Exon 40 is not included in this diagram because it encodes the 220 kD insert found in brain-specific AnkB 440 kD. Note complex splicing events associated with exons 45–51. (B), Relative mRNA expression of ANK2 splice variants encoding ankyrin-B CTD. Bars represent real-time PCR analysis in triplicate of mRNA isolated from four human hearts. Ct values were weighted according to primer efficiency (Supplemental Table 1) and normalized to Ct values of E22/E23 that was set to 100%. Relative mRNA expression is presented for splice variants ±exon 46 and ±exon 48. Relative mRNA expression is also displayed for alternative transcripts that form splice junctions with exon 51 including E45/E51, E47/E51, E48/E51, E49/E51, and E50/E51. Error bars represent standard deviation (n = 3).
2.2 PCR amplification and isolation of alternative ANK2 cardiac transcripts
PCR products were amplified from reverse-transcribed ventricular mRNA and human heart cDNA library using Advantage 2 polymerase (Clontech). Eleven primer sets were designed to subdivide ankyrin-B cDNA into overlapping sections of 600 to 800 bp in length.
The primers sets are as follows: 1: (GACAGTGGAGAGAAGTTCAACGGC, GGTGTCATTCTCCAAGAGGATGGC), 2: (CCTTTATACATGGCTGCCCAAGAG, CTTCACACATTCCACGTGGTCTCC), 3: (CACTTCACTGTGCTGCACGAAGTG, CCATACTTGGCTGCTACATGCAGG), 4: (CAGTCCTATTGGAAGCAGGAGCAG, GACGTTGATGATGTGCGTGTGACC), 5: (GTGGCCTGTCACTATGGAAATGTG, CATTGTGTCTGCATCCTCGCATAG), 6: (CACTGAGAACTTAGACAACGTGGC, GCCTAGGATCTTCTTAACCAGCTC), 7: (CAGTGGTGTCTCGTATCAAACAGG, GATAGTCGTCCGCAAGGTTCCTGA), 8: (TCAGGAACCTTGCGGACGACTATC, GTCCAGCTGAAGCCAAGGTGATCA), 9: (TGATCACCTTGGCTTCAGCTGGAC, GGAGAGCTGGGTACTATCATAGCC), 10: (CCTGCCTGAAGAGTCATCTCTGG, GCCCTCTTCTGTGTGATGGCTTTACTC), 11: (ATGTTGCAAAAGTCTGACAGCAAT, GGTGTCATTCTCCAAGAGGATGGC). PCR products were separated and extracted from 2.5% agarose gel, then ligated into pCR2.1-TOPO vector (Invitrogen) for sequence analysis.
2.3 Quantitative real time (qt)-PCR analysis of alternative ANK2 transcripts
Based on the alternative ANK2 transcripts amplified from reverse-transcribed ventricular mRNA and human heart cDNA library, PCR primers that spanned exon boundaries were designed to selectively amplify particular ANK2 transcripts. For example, to amplify the ANK2 transcript that lacks exon 7, we used a 5′-primer internal to exon 6 and a 3′-primer (24 bp) that spanned the junction of exons 6 (12 bp) and 8 (12 bp). To test the specificity of exon-spanning primers, we repeated the PCR amplification using a 3′-primer to either half of the exon-spanning primer (e.g. first 12 bp corresponding to exon 6 or last 12 bp corresponding to exon 8). A detailed list of the 35 primer sets used for real-time PCR analysis is provided in Supplemental Table 1. We used a modified ΔCt method that accounts for primer efficiencies to calculate the mRNA expression of ANK2 splice variants relative to the expression of the exon 22/23 splice variant [22]. Specifically, to measure the fold difference, we calculated the primer efficiency (of the particular splice variant) to the power of the average of the Ct values for the particular splice variants minus the average of the Ct values of exon 22/23 transcripts. We expressed this number as a percentile in which expression of exon 22/23 was normalized to 100%. Experiments were replicated twice and each condition was performed in triplicate. Error bars represent standard deviation with a sample set of 3.
2.4 Immunoblot and co-immunoprecipitation assays
Immunoblots and co-immunoprecipitation assays were performed as described using ankyrin-B and NHERF1 (Sigma) Igs [23]. Briefly, hearts were extracted from post-natal day one ankyrin-B+/+ and ankyrin-B−/− mice and lysed in four volumes of 50 mM Tris pH 7.4, 10 mM NaCl, 0.32 M sucrose, 5 mM EDTA, 2.5 mM EGTA, 1 mM PMSF, and 1X protease inhibitor cocktail (Sigma). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Protein levels were measured using the Bicinchoninic Acid protein assay kit (Pierce) and equal amounts of protein lysate were separated by SDS-PAGE on 3–8% Tris-Acetate or 10% Bis-Tris gels (Invitrogen). Lysates were transferred to a nitrocellulose membrane (Whatman) which was incubated with affinity-purified ankyrin-B Ig (2 μg/ml) or Na+/H+ exchange regulatory factor 1 (NHERF1) (Sigma) overnight at 4°C. Ankyrin-B and NHERF1 immunoreactive polypeptides were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce).
2.5 In vitro binding assays
In vitro translated (IVT) products of alternative ankyrin-B SBDs were prepared using the TnT T7-Coupled Reticulate Lysate System (Promega) and binding experiments were performed as described [24]. Briefly, IVT products were incubated with GST-fusion proteins at 4°C in 500 μl binding buffer (50 mM Tris pH 7.4, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 0.1% Triton X-100) for 3 hours (for β1-spectrin). Binding reactions were washed five times in wash buffer (binding buffer supplemented with 1% Triton X-100 and 1 M NaCl). Binding reactions were pelleted, resuspended in SDS-sample buffer, separated by SDS-PAGE, and visualized with a phosphorimage machine (courtesy of Dr. Michael Welsh, University of Iowa).
3. Results
Identification of novel ANK2 exons
Ankyrin-B is critical for targeting and stabilization of ion channels and transporters at transverse-tubule (T-tubule) and sarcoplasmic reticulum (SR) membranes in ventricular cardiomyocytes [18, 23, 25, 26]. Furthermore, variants in the human ANK2 gene may lead to fatal cardiac arrhythmias [18–20]. Surprisingly, we know very little regarding the regulatory mechanisms of transcription and translation of ANK2 in heart. To identify cardiac-specific ANK2 transcripts, we performed reverse-transcriptase PCR on mRNA isolated from left ventricular tissue from human hearts. PCR products were also amplified from a human heart cDNA library. We used ten different over-lapping primer sets that spanned the entire ankyrin-B cDNA (Figure 1B). We unexpectedly identified seven novel ANK2 exons (numbered 0, 1′, 24, 28, 46, 49, and 50) (Figure 1C and Table 1). The presence of these seven exons was confirmed in human genomic sequence (Table 1) and homologous exons were identified in mouse Ank2. Table 1 displays the most current account of human ANK2 genomic organization including the new exon nomenclature, 5′- and 3′-splice sites, exon length, proceeding intron length, and the corresponding functional domain.
Table 1.
ANK2 Exon Organization
| Exon # | Previous exon # | 5′ splice | 3′ splice | Length (bp) | 3′ intron (bp) | Domain | Comments |
|---|---|---|---|---|---|---|---|
| 0 | TCCCA/gt | 86190 | non-coding | ||||
| 1′ | ag/GTAAG | AAAAG/gt | 60/21* | 269901 | MBD | alt. start | |
| 1 | 1 | AGAAG/gt | 84* | 124603 | MBD | start | |
| 2 | 2 | ag/TCTGA | ATCAG/gt | 102 | 21850 | MBD | ANK repeat 1 |
| 3 | 3 | ag/AATGG | CTAAG/gt | 99 | 2544 | MBD | ANK repeat 2 |
| 4 | 4 | ag/AAGGG | CTCAG/gt | 99 | 33051 | MBD | ANK repeat 3 |
| 5 | 5 | ag/AATGG | CAGAG/gt | 99 | 4727 | MBD | ANK repeat 4 |
| 6 | 6 | ag/GATGG | CCAAG/gt | 186 | 426 | MBD | ANK repeats 5 & 6 |
| 7 | 7 | ag/ATGAT | CTGAG/gt | 24 | 2862 | MBD | |
| 8 | 8 | ag/AGTGG | CCAGG/gt | 99 | 1527 | MBD | ANK repeat 7 |
| 9 | 9 | ag/AATGG | CTAGG/gt | 99 | 7554 | MBD | ANK repeat 8 |
| 10 | 10 | ag/GATGG | CTAAG/gt | 99 | 5872 | MBD | ANK repeat 9 |
| 11 | 11 | ag/AATGG | CCCTG/gt | 198 | 2117 | MBD | ANK repeats 10 & 11 |
| 12 | 12 | ag/AATGG | CAGAG/gt | 99 | 164 | MBD | ANK repeat 12 |
| 13 | 13 | ag/TCTGG | ACATT/gt | 99 | 6485 | MBD | ANK repeat 13 |
| 14 | 14 | ag/CGTGG | CCAGG/gt | 99 | 9456 | MBD | ANK repeat 14 |
| 15 | 15 | ag/GAGGA | CCAAG/gt | 198 | 3187 | MBD | ANK repeats 15 & 16 |
| 16 | 16 | ag/AAGGG | GGAAG/gt | 99 | 524 | MBD | ANK repeat 17 |
| 17 | 17 | ag/AACGG | CCAAG/gt | 99 | 4116 | MBD | ANK repeat 18 |
| 18 | 18 | ag/AATGG | CTAAG/gt | 198 | 4732 | MBD | ANK repeats 19 & 20 |
| 19 | 19 | ag/AGTGG | CAAAG/gt | 99 | 684 | MBD | ANK repeat 21 |
| 20 | 20 | ag/CTTGG | CCAAG/gt | 99 | 3929 | MBD | ANK repeat 22 |
| 21 | 21 | ag/AACGG | CTGCG/gt | 99 | 925 | MBD | ANK repeat 23 |
| 22 | 22 | ag/AATGG | CCACA/gt | 99 | 9228 | MBD | ANK repeat 24 |
| 23 | 23 | ag/ACTAT | AGAGG/gt | 73 | 1384 | MBD | |
| 24 | ag/CTCTT | TTCAG/gt | 54 | 6977 | MBD | ||
| 25 | 24 | ag/GTGAT | GACAG/gt | 145 | 6307 | SBD | |
| 26a | 25 | ag/CCTTC | ACCAG/gt | 103 | 707 | SBD | |
| 26b | ag/TTCCG | ACCAG/gt | 88 | 707 | SBD | alt. 3′ splice site | |
| 27 | 26 | ag/GTGTC | TCAGG/gt | 104 | 5138 | SBD | |
| 28 | ag/CCGCG | AGCAG/gt | 36 | 6451 | SBD | ||
| 29 | 27 | ag/TTTCC | CTTGG/gt | 225 | 1501 | SBD | Zu-5 motif |
| 30 | 28 | ag/TAAAC | CTTGG/gt | 99 | 983 | SBD | |
| 31 | 29 | ag/GCCTG | TGAAG/gt | 155 | 2637 | SBD | Zu-5 motif |
| 32 | 30 | ag/TACTG | TGCAG/gt | 212 | 519 | SBD | Zu-5 motif |
| 33 | 31 | ag/GCTCA | AACAG/gt | 205 | 2444 | SBD | |
| 34 | 32 | ag/GTGGA | GCCAG/gt | 97 | 2365 | SBD | |
| 35 | 33 | ag/GTTCT | TGGAG/gt | 229 | 1100 | SBD | |
| 36 | 34 | ag/GTGTT | TCAAG/gt | 126 | 2757 | SBD | |
| 37 | 35 | ag/GTACG | CAAAG/gt | 123 | 2253 | SBD | |
| 38 | 36 | ag/GAATC | AAGAG/gt | 33 | 1919 | SBD | |
| 39 | 37 | ag/ATCGA | AAAAA/gt | 22 | 2795 | SBD | |
| 40 | 38 | ag/ATGAT | TGAAG/gt | 6255 | 1523 | 220 kD insert | |
| 41 | 39 | ag/ATCCA | GACAG/gt | 75 | 2440 | DD | |
| 42 | 40 | ag/AATTA | TACAG/gt | 132 | 1569 | DD | |
| 43 | 41 | ag/ATACC | TGAAG/gt | 144 | 2383 | DD | |
| 44 | 42 | ag/GGTTC | TATTT/gt | 286 | 1662 | CTD | |
| 45 | 43 | ag/TGTGA | AAAAG/gt | 292 | 2727 | CTD | |
| 46 | ag/GAGCT | TTCAG/gt | 93 | 464 | CTD | ||
| 47 | 44 | ag/GGAGA | AGAAG/gt | 84 | 111 | CTD | α-helix |
| 48 | 45 | ag/GTTAC | CAGAG/gt | 165 | 1314 | CTD | α-helix |
| 49 | ag/GTCAC | AAGAG/gt | 183 | 3143 | CTD | ||
| 50 | ag/GCTTT | AAAAG/gt | 92 | 3275 | CTD | ||
| 51a | 46 | ag/GACAA | 15** | CTD | stop | ||
| 51b | ag/GACAA | 184** | CTD | alt. stop |
Fields represent: new and previous exon numbering, 5′- and 3′-splice sites, exon length (bp), proceeding intron length (bp), corresponding functional domain, and additional comments. For exon 1′ and 1, the length is measured from the beginning of the open reading frame and is denoted with an asterisk. Exon 26a/b represents alternative 3′-splice acceptor sites that allow for the addition or subtraction of 15 bp. The Zu-5 motif constitutes the minimal binding domain for β-spectrin [3]. Exon 40 encodes the 220 kD insert that is found in brain-specific 440 kD ankyrin-B [29]. Exon 51a/b represents alternative stop sites depending on the open reading frame. The terminal amino acid sequence for 51a is “DNNE”, while the terminal sequence for 51b is “QEAK”. Length of exons 51a/b corresponds to the distances to the in-frame stop codons and is marked with double asterisks.
A novel alternative first exon (E1′) was characterized in addition to a non-coding exon (E0) that is located ~86.2 kb upstream of E1′. These exons were initially identified in a BLAST search of translated expressed sequence tags (DA809405). The new alternative first exon lies ~269.9 kb upstream of exon 2 and ~145 kb upstream of the previously-reported first exon (Figure 1E and Table 1) [18]. The non-coding exon (E0) is only found in transcripts containing E1′ and is not associated with the previously-reported first exon. With the identification of these two new exons, the region encompassing the ANK2 gene locus on human chromosome 4 dramatically increased in size from ~330 kb to ~560 kb (Figure 1D–E).
Regarding the other newly-identified exons, exon 24 is 54 bp in length and is located at the junction of the membrane-binding and spectrin-binding functional domains (Figure 1C). Exon 28 is located in the spectrin-binding domain and, given its close proximity to the Zu-5 motif, may modulate β-spectrin binding [3]. Novel exons 46, 49, and 50 are located in the ankyrin-B C-terminal domain and may contribute to the regulatory function of this domain (Figure 1C). Interestingly, exon 50 is the only exon that shifts the open reading frame (+2) such that an alternative stop codon is utilized in exon 51. This shift extends the open reading frame for an additional 61 amino acids that terminates with the sequence “Gln-Glu-Ala-Lys”. In the absence of exon 50, the open reading frame terminates in exon 51 after four amino acid residues (Asp-Asn-Asn-Glu). Finally, we identified an alternative 3′-splice acceptor site in exon 26 that removes the first 15 bp, but has no effect on the open reading frame.
Characterization of novel ANK2 splice variants
While screening for cardiac-specific ANK2 transcripts, we identified a surprising number of novel alternative splice variants. Alternative ANK2 splice variants were identified in regions encoding the domains MBD, SBD, and CTD. To measure the relative mRNA expression of individual ANK2 transcripts, we performed real-time PCR analysis of mRNA isolated from human heart using exon boundary-spanning primers [27]. Exon boundary-spanning primers allow for the selective PCR-amplification of individual alternative transcripts. For example, ANK2 transcripts lacking exon 7 were selectively amplified using a 3′-primer that spanned the splice junction of exons 6 and 8 (Figure 2A, column 5). Likewise, transcripts containing exon 7 were selectively amplified using a boundary-spanning primer consisting of the junction of exons 6 and 7 (Figure 2A, column 4). To validate that specific transcripts were only amplified when using the full-length exon boundary-spanning primer, we repeated PCR using each individual half of the exon-spanning primer (Figure 2A, columns 1–3). These findings demonstrate that a full-length exon boundary-spanning primer is necessary for the amplification of a PCR product. Moreover, transcripts containing exon 7 are expressed markedly less than transcripts lacking this exon. These findings are corroborated by results from real-time PCR analysis (Figure 3D). Another representative example of selective PCR-amplification using full-length exon boundary-spanning primers is presented in Figure 2B that demonstrates the amplification of ANK2 transcripts ±exon 24. This technique was used to confirm the selective amplification of individual ANK2 transcripts for all novel alternative splice variants identified. For all transcripts, full-length exon boundary-spanning primers are necessary for the amplification of PCR products.
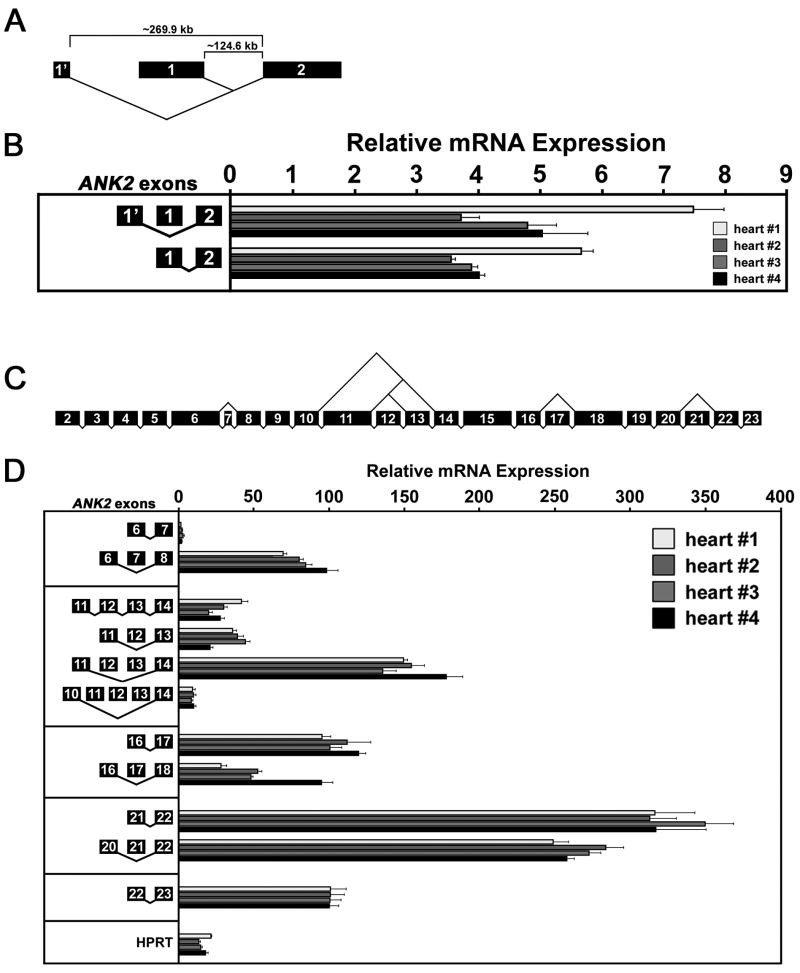
Fig.3. Relative mRNA expression of alternative ANK2 transcripts encoding ankyrin-B MBD.
(A), Alternative splicing of ANK2 first exons. Exon size represents relative exon length. Note lengthy intronic sequences separating the first exons from the second exon (~269.9 kb, ~124.6 kb). (B), Relative mRNA expression of splice variants with alternative first exons. Bars represent real-time PCR analysis in triplicate of mRNA isolated from four human hearts. (C), Alternative splicing of ANK2 exons encoding ankyrin-B MBD. Exon size represents relative exon length. Note complex splicing events associated with exons 10–14. (D), Relative mRNA expression of ANK2 splice variants encoding ankyrin-B MBD. Bars represent real-time PCR analysis in triplicate of mRNA isolated from four human hearts. Relative mRNA expression is presented for splice variants ±exon 7; with various combinations of exons 11, 12, and 13; ±exon 17; and ±exon 21. Relative expression of reference gene hypoxanthine-guanine phosphoribosyl transferase (HPRT) is also presented. For all data, cycle threshold (Ct) values were weighted according to primer efficiency (Supplemental Table 1) and normalized to Ct values of E22/E23 that was set to 100%. Error bars represent standard deviation (n = 3).
To ensure the accurate analysis of mRNA expression levels within a 1000-fold concentration gradient, we optimized the annealing temperatures for each primer set such that the efficiency fell within the range of 90 to 110% (see Supplemental Table 1 for annealing temperatures and primer set efficiencies). In calculating the relative mRNA expression levels, we used a modified ΔCt technique to incorporate primer efficiency (see Methods). Values were expressed as a percentage of the expression of the ANK2 transcript containing exons 22 and 23, which was normalized to 100%. Based on the previous observation that disrupting exon 23 effectively eliminates all ankyrin-B isoforms [17, 28], we propose that all ANK2 transcripts contain exon 22.
Relative mRNA expression levels of alternative ANK2 splice variants were evaluated in ventricular tissue from four different human hearts. Experiments were replicated twice with each condition performed in triplicate. For each alternative transcript, results were displayed for individual hearts to observe any variability in expression between hearts. Finally, we performed real-time PCR analysis of the reference gene hypoxanthine-guanine phosphoribosyl transferase (HPRT) to assess relative cDNA amounts between hearts.
Novel alternative first exon in ANK2 transcript
The novel alternative first exon E1′ is 60 nucleotides long, but the coding sequence begins with the terminal 21 nucleotides (Table 1). This exon is ~269.9 kb upstream of exon 2, while the previously-identified first exon (E1) is ~124.6 kb upstream of exon 2 (Figure 3A). Real-time PCR analysis using exon boundary-spanning primers demonstrated that ANK2 transcripts containing alternative first exons E1 or E1′ were expressed at approximately the same level (Figure 3B). It is noteworthy that expression of these transcripts was significantly less (3.5–7.5% of E22/23 expression that is normalized to 100%) than all other ANK2 transcripts. Considering the tremendous expanse of intronic sequence separating the first and second exons (~269.9 kb and ~124.6 kb for E1′/E2 and E1/E2, respectively), it is possible that these transcripts may in fact be significantly less abundant.
Novel ANK2 splice variants encoding ankyrin-B membrane-binding domain
For the MBD, we identified novel ANK2 transcripts that removed individual exons such as exons 7, 17, and 21 (Figure 3C). We also identified complex splice variants consisting of various combinations of exons 11, 12, and 13 corresponding to ANK repeats 10 and 11, 12, and 13, respectively (Figure 3C). Based on our previous studies, we predict that splicing events that remove exon 17 (encoding ANK repeat 18) or exon 21 (encoding ANK repeat 23) will disrupt the binding sites for Na/Ca exchanger (ANK repeats 16–18) [25] or InsP3R (ANK repeats 22–24) [26].
In terms of relative mRNA expression, the general trend for each transcript is consistent across the four human hearts. In all hearts, the expression of −E7 transcripts (lacking exon 7) is much more abundant (>50 fold) than expression of +E7 transcripts (Figure 3D). While exon 7 is not abundantly expressed in cardiac transcripts, it was found in transcripts isolated during the screen of human ventricular mRNA; therefore, it was not designated as non-cardiac like exons 30 and 40. Expression of −E12/13 transcripts is the most abundant followed by −E12 transcripts and +E12/13 transcripts that are expressed at similar levels (Figure 3D). Transcripts lacking exons 11, 12 and 13 are the least abundant. In general, +E17 transcripts are expressed more abundantly than −E17 transcripts (~2 fold), although there is greater variability in −E17 transcript expression between the four hearts (Figure 3D). Both +E21 and −E21 transcripts are expressed at similar levels in all four hearts (Figure 3D). Together, these findings challenge the notion that the heart solely expresses a single, multivalent ankyrin-B polypeptide (e.g. 220 kD ankyrin-B), which contains distinct binding sites for the Na/Ca exchanger, Na/K ATPase, and InsP3R [18, 23, 25, 26]. In fact, these findings suggest that alternative splicing of ANK2 may produce distinct polypeptides that interact exclusively with the Na/Ca exchanger or InsP3R.
Novel ANK2 splice variants encoding ankyrin-B spectrin-binding domain
When screening ANK2 for alternative splice variants, we identified multiple transcripts of the spectrin-binding domain that included or excluded exons 24, 28, or 38 (Figure 4A). In addition, we also characterized an alternative 3′-splice acceptor site within exon 26 that removes the first 15 bp. Exons 30 and 40 were never found in any ANK2 cardiac transcript. Furthermore, we were unable to detect exons 30 or 40 in ventricular mRNA by reverse transcriptase PCR using internal primers to exons 30 or 40; therefore, these exons are not likely expressed in cardiac ANK2 transcripts. Interestingly, the inclusion of exon 30 would disrupt the Zu-5 motif, which is encoded by exons 29, 31, and 32 and is important for β-spectrin binding activity [3]. Exon 40 encodes the large 220 kD insert that is found in the brain-specific 440 kD ankyrin-B isoform [29].
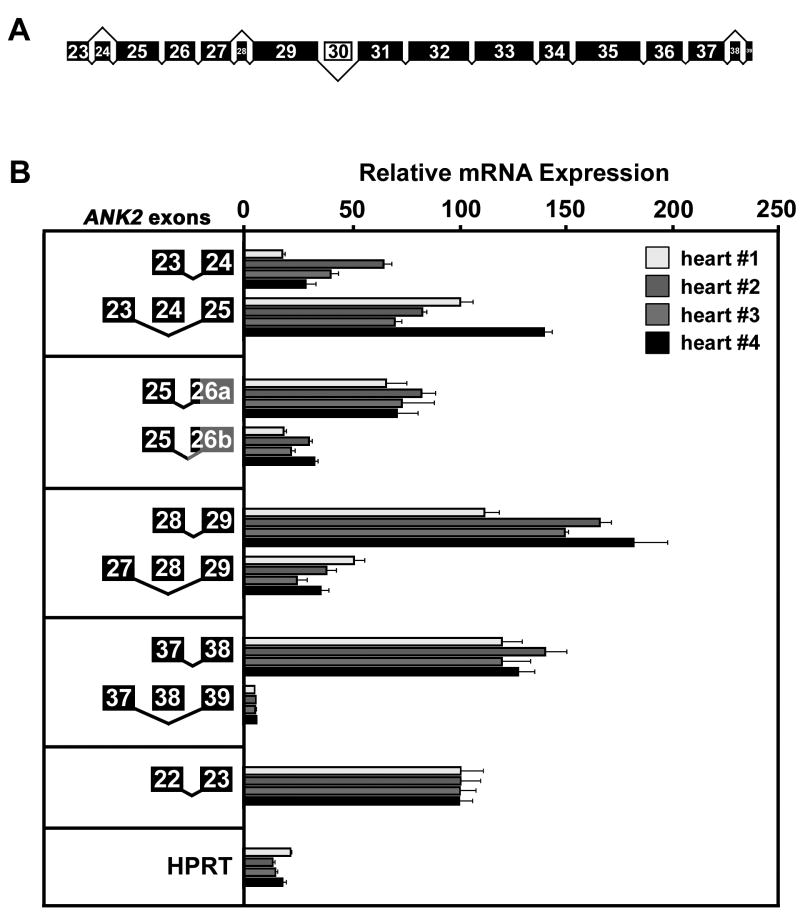
Fig.4. Relative mRNA expression of alternative ANK2 transcripts encoding ankyrin-B SBD.
(A), Alternative splicing of ANK2 exons encoding ankyrin-B SBD. Exon size represents relative exon length. Exon 30 (white) is considered non-cardiac. (B), Relative mRNA expression of ANK2 splice variants encoding ankyrin-B SBD. Bars represent real-time PCR analysis in triplicate of mRNA isolated from four human hearts. Ct values were weighted according to primer efficiency (Supplemental Table 1) and normalized to Ct values of E22/E23 that was set to 100%. Relative mRNA expression of transcripts ±exon 24, using alternative 3′-splice acceptor sites (a or b) in exon 26, ±exon 28, and ±exon 38. Relative expression of HPRT is also presented. Error bars represent standard deviation (n = 3).
We performed real-time PCR analysis to determine the relative mRNA expression levels of these alternative splice variants. While there was variability between hearts in the expression of transcripts ± exon 24, transcripts lacking this exon were more abundant than +E24 transcripts (Figure 4B). For exon 26, transcripts with the first 3′-splice acceptor site are more prevalent (~3 fold) than transcripts with the second 3′-splice acceptor site (Figure 4B). In addition, +E28 transcripts are more abundant (~2–5 fold) than −E28 transcripts (Figure 4B). Finally, transcripts containing exon 38 (+E38) are far more prevalent (~25 fold) than −E38 transcripts (Figure 4B).
Novel ANK2 splice variants encoding ankyrin-B C-terminal domain
ANK2 CTD is subject to complex alternative splicing events (Figure 5A). In fact, eleven distinct ANK2 transcripts were identified during the screen. We observed splice variants that simply include or exclude individual exons such as exon 46 or exon 48. +E46 transcripts are more common (~7.5–9 fold) than −E46 transcripts (Figure 5B), while + E48 transcripts are more abundant (~5–6 fold) than −E48 transcripts (Figure 5B). The majority of alternative transcripts are the result of many exons (45, 47, 48, 49, and 50) forming independent splice junctions with the final exon (exon 51; Figure 5A). All of these transcripts display similar expression levels with the exception of the transcript lacking exons 48, 49, and 50, which is the least abundant (Figure 5B). As mentioned above, inclusion of exon 50 alters the open reading frame (+2) such that an alternative stop codon is introduced in exon 51. It is noteworthy that the most extensive alternative splicing occurs within the C-terminal domain and that this domain regulates the intra- and inter-molecular interactions of ankyrin-B.
The heart expresses a heterogeneous population of ankyrin-B polypeptides
To determine whether the complex mRNA splicing events detailed in Figures 3–5 actually result in a diverse set of ankyrin-B polypeptides, we performed immunoblot analysis on protein lysates from ankyrin-B+/+ and ankyrin-B−/− neonatal hearts. Mouse hearts were lysed on ice and in the presence of protease inhibitors to prohibit protein degradation. Equal loading of protein lysate was validated by Coomassie Blue stain (Figure 6B) and immunoblot analysis of the reference protein Na+/H+ exchanger regulatory factor 1 (NHERF1) (Figure 6C). To maximize the resolution of high molecular weight products, protein lysates were separated on a 3–8% Tris-Acetate gel, while low molecular weight products were resolved on a 10% Bis-Tris gel (Figure 6A, upper and lower panels). These findings demonstrate that ankyrin-B+/+ hearts express a heterogeneous population of ankyrin-B polypeptides. Specifically, canonical 220 kD and 160 kD ankyrin-B isoforms are only detected in the ankyrin-B+/+ hearts (Figure 6A, upper panel). Furthermore, numerous ankyrin-B immunoreactive polypeptides ranging in size from 45 to 105 kD are solely detected in ankyrin-B+/+ hearts. Interestingly, we also observed a 100 kD band in protein lysates from the ankyrin-B null hearts. To evaluate the expression of alternative ankyrin-B polypeptides in human heart, we performed immunoblot analysis on protein lysate isolated from human left ventricular tissue. Consistent with our findings from the mouse heart, the human heart expresses multiple isoforms of ankyrin-B, including the predominant 220 kD isoform (Figure 6D). Small variations between the alternative ankyrin-B isoforms identified in mouse heart versus human heart likely represent minor differences in tissue source (whole mouse heart versus pure human left ventricular wall muscle tissue) and chemistry of the SDS-PAGE resolving gels (resolution of low molecular weight ankyrin-B polypeptides is improved on 10% Bis-Tris gels). These findings support the hypothesis that ANK2 is subject to complex mRNA splicing events that translate into a heterogeneous population of ankyrin-B polypeptides.
Fig. 6. Heterogeneous population of ankyrin-B polypeptides in heart.
(A) Ankyrin-B immunoblot of protein lysates from ankyrin-B+/+ and ankyrin-B−/− hearts. Upper panel, lysates were separated on a 3–8% Tris-Acetate gel, while the lower panel depicts lysates separated on a 10% Bis-Tris gel. Equal loading was demonstrated by Coomassie Blue (B) and NHERF1 immunoblot (C). Note multiple ankyrin-B polypeptide products in wild-type hearts. Asterisks denote 220 kD and 160 kD ankyrin-B isoforms. (D) Ankyrin-B immunoblot of protein lysate from left ventricular tissue of human heart. Arrows indicate multiple ankyrin-B isoforms. Asterisk denotes the predominant 220 kD ankyrin-B isoform.
4. Discussion
This is the first study to detail the large genomic organization and complex transcriptional regulation of human ANK2. Molecular analysis of human heart tissue revealed seven previously unidentified ANK2 exons. To date, ANK2 now includes 53 exons that span over 560 kb, with an alternative first exon nearly 270 kb upstream of exon 2. Our results show a complex splicing profile for ANK2 in human cardiac muscle. In fact, we observed thirty new splicing events in key functional domains of ankyrin-B. Based on known sites for ankyrin-B interacting partners, our results suggest that ankyrin-B polypeptides are dynamically regulated at the transcriptional level for specific functions (e.g. NCX targeting to T-tubule membrane vs. InsP3 receptor targeting to SR membrane). Together, our findings detail the complex transcriptional regulation of ANK2 and identify new exons to be screened for disease-variants in the human population.
Our study characterized multiple splicing events in the ANK2 gene that would alter ankyrin-B topology. The newly identified exon 1′ lies ~145 kb upstream of the previously identified first exon. Alternative first exons have also been identified for ANK1 [30] and ANK3 [31]. Obvious future goals are to characterize the full-length ANK2 transcripts associated with these alternative first exons and to address the possibility of differential gene regulation mediated by distinct promoter regions. In MBD, there are three predominant sites of alternative splicing that would alter ANK repeat organization. Interestingly, splicing at two of these sites disrupts known binding sites for Na/Ca exchanger and InsP3R [25, 26]. The third site of complex splicing consists of exons 10–13 encoding ANK repeats 9–13. To date, this region has not been associated with an ankyrin-B interacting partner. In the related polypeptide ankyrin-R, a binding site for the cell adhesion molecule neurofascin has been broadly localized to ANK repeats 7–18 [32]. Future experiments to characterize binding partners and binding sites will focus on ANK repeats 9–13.
We identified two novel exons (24 and 28) located in the coding region for the spectrin-binding domain. An alternative 3′-splice acceptor site was also characterized in exon 26 that removes the first 15 bp. Considering the different possible splicing events characterized in the spectrin-binding domain (Figure 4), we were intrigued by the prospect that alternative splicing could differentially regulate association of ankyrin-B with β-spectrin. For example, deletion of 70 amino acids preceding the minimal spectrin-binding domain in ankyrin-R reduced β-spectrin binding affinity by 20-fold [33]. In ankyrin-B, the minimal spectrin-binding domain is encoded by exons 29, 31, and 32 [3]. Using an in vitro binding assay, we were unable to detect differences in the interactions of β1-spectrin with four different alternative isoforms of ankyrin-B SBD (Figure 7). While these results suggest that alternative splicing of the SBD does not influence ankyrin-B/β1-spectrin interactions, another intriguing possibility is that alternative SBD isoforms may differentially associate with specific β-spectrin isoforms in heart. Furthermore, exon 30 was not identified in any cardiac ANK2 transcripts. Inclusion of this exon would disrupt the minimal spectrin-binding domain. Future experiments will address whether exon 30 modifies ankyrin/β-spectrin interactions in a tissue/cell-specific manner.
Fig. 7. β1-spectrin binding to alternative ankyrin-B SBD isoforms.
(A–D), Diagram represents the alternative ankyrin-B SBD splice variant evaluated for binding to β1–spectrin. Representative binding between 35S-labeled ankyrin-B SBD and GST-fusion β1–spectrin is presented in the phosphorimage. Conditions were repeated in triplicate and quantified results are presented in the graph. Error bars represent standard deviation (n = 3).
We report the association of three new exons (46, 49, and 50) with human cardiac ANK2 transcripts encoding the C-terminal domain, the site of nearly all human ANK2 gene variants associated with cardiac arrhythmias [18–20]. The C-terminal domain plays an important role in regulating the intra- and inter-molecular interactions of ankyrins. For example, the C-terminal and membrane-binding domains of ankyrin-B have been shown to interact by yeast 2-hybrid analysis [5]. This interaction is necessary for the proper subcellular targeting of InsP3 receptor in cardiomyocytes [5]. Furthermore, the localization of the InsP3 receptor at the SR membrane specifically requires the C-terminal domain of ankyrin-B and cannot be recapitulated with the C-terminal domain of ankyrin-G [8].
Studies addressing the association of ankyrin-R with the anion exchanger and β-spectrin suggest that intra-molecular interactions between the functional domains negatively regulate inter-molecular interactions. Specifically, an alternative ankyrin-R isoform lacking 161 residues in the C-terminal domain exhibits increased affinity for the anion exchanger and β-spectrin [6, 7]. Together, these findings suggest that the C-terminal domain regulates the association of ankyrin with integral membrane and cytoskeletal proteins. The addition of exons 46, 49, and 50 likely add further complexity to the regulatory functions of the ankyrin-B C-terminal domain. For example, exon 46 contains a site homologous to an obscurin binding domain found in small Ank1.5 [34, 35] leading to the intriguing possibility that ankyrin-B interacts with the large sarcomeric protein obscurin.
On a clinical note, the three new exons in the C-terminal domain will provide additional sequences to analyze for genetic variants associated with human cardiac arrhythmias. The nine loss-of-function variants previously associated with arrhythmia are illustrated in the context of our current understanding of ANK2 genomic organization (Figure 8). Furthermore, we propose a revised nomenclature for these variants. Specifically, we suggest that the variants be referred to using the nucleotide change and location starting from exon 1 (e.g. E1425G becomes A4463G). This new nomenclature will hopefully mitigate any potential confusion arising from the existence of multiple cardiac ankyrin-B polypeptides.
Fig. 8. Revised nomenclature for ankyrin-B loss-of-function variants.
Nine loss-of-function (LOF) variants have been previously characterized in ankyrin-B encompassing the CTD (blue), DD (green), and a neighboring portion of the SBD (red) [18–20]. The locations of these variants are indicated on the corresponding exons for previous and updated ANK2. An updated nomenclature for the LOF variants includes the nucleotide change and its location in full-length ANK2 that includes all exons starting with E1.
In the ankyrin family, there is precedence for tissue expressing multiple alternative splice variants of ankyrin polypeptides. For example, multiple isoforms of ankyrin-R have been characterized in erythrocytes, brain, kidney, and muscle [30, 36–38]. Multiple isoforms of ankyrin-G have also been identified in epithelial tissue, muscle, and neural tissue [31, 39–42]. For ankyrin-B, multiple isoforms have been identified in brain and thymus [28, 29, 43, 44]. Many of the alternative isoforms of ankyrin polypeptides display differential subcellular localization and most likely are associated with distinct functions. For example, two alternative isoforms of ankyrin-G express a unique serine-rich domain that facilitates the subcellular localization of these isoforms to the axon initial segments [45].
In metazoans, ankyrin genes are uniquely designed to facilitate the modular assembly of polypeptides reflecting a diversity of combinatorial possibilities. For example, one or two ANK repeats are encoded by individual exons. In ANK2, alternative splicing of these exons (as we observed for exons 17 and 21, Figure 3C) would disrupt the binding sites for Na/Ca exchanger or InsP3R. Instead of the heart expressing a single canonical 220 kD ankyrin-B with numerous functions, our data suggests that the heart expresses multiple ankyrin-B polypeptides specifically tailored for unique functions and distinct subcellular targeting. Moreover, these alternative splicing events could regulate the ability of ankyrin to interact with cytoskeletal elements (e.g. β-spectrin). Prior studies addressing ankyrin-B function/dysfunction in heart have generally relied on the ‘canonical’ 220 kD ankyrin-B polypeptide [3, 4, 8, 18–20, 23, 25, 26, 46]. These experiments were likely successful as this polypeptide is the primary ankyrin-B isoform in heart and as past assays have primarily focused on well-characterized 220 kD ankyrin-B protein partners includingInsP3 receptor, β-spectrin, and Na/Ca exchanger [3, 25, 26]. Based on our new findings, it will be important in future studies to characterize specific functions associated with distinct ankyrin-B polypeptides in the cardiomyocyte. In summary, the modular exon organization and diverse combinatorial assembly of ANK2 would allow for the multifaceted ankyrin-B gene products to function in highly specialized domains in cardiomyocytes such as costameres, SR/T-tubule junctions, A-bands, and intercalated discs [18, 31, 42, 47–50].
Supplementary Material
Acknowledgments
Funding: National Institutes of Health (HL084583 and HL083422 to PJM); Pew Scholars Trust to PJM. Fondation Leducq Trans-Atlantic network of Excellence grant (05 CVD 01, Preventing Sudden Death) and Association Française contre les Myopathies (JJS).
We thank the following people for their assistance: Jordan Miller (UI) for coordinating the procurement of human hearts, Madhu Singh (UI) for helpful advice on real-time PCR, and Dr. Michael Welsh (UI) for the use of the phosphorimage machine. We thank Andras Varro (University of Szeged) and Sophie Demolombe (l’institut du thorax, Nantes, France) for providing the human tissues.
Footnotes
Conflict of Interest: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunha SR, Mohler PJ. Cardiac ankyrins: Essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res. 2006 Jul 1;71(1):22–9. doi: 10.1016/j.cardiores.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 3.Mohler PJ, Yoon W, Bennett V. Ankyrin-B Targets {beta}2-Spectrin to an Intracellular Compartment in Neonatal Cardiomyocytes. J Biol Chem. 2004 Sep 17;279(38):40185–93. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56{alpha} targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H109–19. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 5.Abdi KM, Mohler PJ, Davis JQ, Bennett V. Isoform Specificity of Ankyrin-B: A site in the divergent C-terminal domain is required for intramolecular association. J Biol Chem. 2006 Mar 3;281(9):5741–9. doi: 10.1074/jbc.M506697200. [DOI] [PubMed] [Google Scholar]
- 6.Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem. 1992;267(26):18966–72. [PubMed] [Google Scholar]
- 7.Hall TG, Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem. 1987;262(22):10537–45. [PubMed] [Google Scholar]
- 8.Mohler PJ, Gramolini AO, Bennett V. The Ankyrin-B C-terminal Domain Determines Activity of Ankyrin-B/G Chimeras in Rescue of Abnormal Inositol 1,4,5-Trisphosphate and Ryanodine Receptor Distribution in Ankyrin-B (−/−) Neonatal Cardiomyocytes. J Biol Chem. 2002;277(12):10599–607. doi: 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- 9.Costa FF, Agre P, Watkins PC, Winkelmann JC, Tang TK, John KM, et al. Linkage of dominant hereditary spherocytosis to the gene for the erythrocyte membrane-skeleton protein ankyrin. New England Journal of Medicine. 1990;323(15):1046–50. doi: 10.1056/NEJM199010113231507. [DOI] [PubMed] [Google Scholar]
- 10.Eber SW, Gonzalez JM, Lux ML, Scarpa AL, Tse WT, Dornwell M, et al. Ankyrin-1 mutations are a major cause of dominant and recessive hereditary spherocytosis. Nat Genet. 1996;13(2):214–8. doi: 10.1038/ng0696-214. [DOI] [PubMed] [Google Scholar]
- 11.Lux SE, Tse WT, Menninger JC, John KM, Harris P, Shalev O, et al. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990;345(6277):736–9. doi: 10.1038/345736a0. [DOI] [PubMed] [Google Scholar]
- 12.White RA, Birkenmeier CS, Lux SE, Barker JE. Ankyrin and the hemolytic anemia mutation, nb, map to mouse chromosome 8: presence of the nb allele is associated with a truncated erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 May;87(8):3117–21. doi: 10.1073/pnas.87.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004 Oct 15;119(2):257–72. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155(5):739–46. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, et al. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007 Mar 15;120(Pt 6):953–63. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- 16.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143(5):1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scotland P, Zhou D, Benveniste H, Bennett V. Nervous system defects of AnkyrinB (-/-) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD AnkyrinB in premyelinated axons. J Cell Biol. 1998;143(5):1305–15. doi: 10.1083/jcb.143.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003 Feb 6;421(6923):634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 19.Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007 Jan 30;115(4):432–41. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 20.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):9137–42. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.anonymous. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovascular research. 1997;35(1):2–3. [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B Coordinates the Na/K ATPase, Na/Ca Exchanger, and InsP(3) Receptor in a Cardiac T-Tubule/SR Microdomain. PLoS Biol. 2005 Dec;3(12):e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kline CF, Cunha SR, Lowe JS, Hund TJ, Mohler PJ. Revisiting ankyrin-InsP(3) receptor interactions: Ankyrin-B associates with the cytoplasmic N-terminus of the InsP(3) receptor. Journal of cellular biochemistry. 2008 Feb 14; doi: 10.1002/jcb.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of na/ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem. 2007 Feb 16;282(7):4875–83. doi: 10.1074/jbc.M607096200. [DOI] [PubMed] [Google Scholar]
- 26.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem. 2004 Mar 26;279(13):12980–7. doi: 10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 27.Vandenbroucke II, Vandesompele J, Paepe AD, Messiaen L. Quantification of splice variants using real-time PCR. Nucleic Acids Research. 2001;29(13):E68–8. doi: 10.1093/nar/29.13.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol. 1999;147(5):995–1008. doi: 10.1083/jcb.147.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otto E, Kunimoto M, McLaughlin T, Bennett V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol. 1991;114(2):241–53. doi: 10.1083/jcb.114.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkenmeier CS, White RA, Peters LL, Hall EJ, Lux SE, Barker JE. Complex patterns of sequence variation and multiple 5′ and 3′ ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem. 1993;268(13):9533–40. [PubMed] [Google Scholar]
- 31.Hopitzan AA, Baines AJ, Ludosky MA, Recouvreur M, Kordeli E. Ankyrin-G in skeletal muscle: tissue-specific alternative splicing contributes to the complexity of the sarcolemmal cytoskeleton. Exp Cell Res. 2005 Sep 10;309(1):86–98. doi: 10.1016/j.yexcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Michaely P, Bennett V. Mechanism for binding site diversity on ankyrin. Comparison of binding sites on ankyrin for neurofascin and the Cl-/HCO3- anion exchanger. J Biol Chem. 1995;270(52):31298–302. doi: 10.1074/jbc.270.52.31298. [DOI] [PubMed] [Google Scholar]
- 33.Davis LH, Bennett V. Mapping the binding sites of human erythrocyte ankyrin for the anion exchanger and spectrin. J Biol Chem. 1990;265(18):10589–96. [PubMed] [Google Scholar]
- 34.Armani A, Galli S, Giacomello E, Bagnato P, Barone V, Rossi D, et al. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Experimental cell research. 2006;312(18):3546–58. doi: 10.1016/j.yexcr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Borzok MA, Catino DH, Nicholson JD, Kontrogianni-Konstantopoulos A, Bloch RJ. Mapping the binding site on small ankyrin 1 for obscurin. Journal of Biological Chemistry. 2007;282(44):32384–96. doi: 10.1074/jbc.M704089200. [DOI] [PubMed] [Google Scholar]
- 36.Birkenmeier CS, Sharp JJ, Gifford EJ, Deveau SA, Barker JE. An alternative first exon in the distal end of the erythroid ankyrin gene leads to production of a small isoform containing an NH2-terminal membrane anchor. Genomics. 1998 May 15;50(1):79–88. doi: 10.1006/geno.1998.5305. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher PG, Forget BG. An alternate promoter directs expression of a truncated, muscle-specific isoform of the human ankyrin 1 gene. J Biol Chem. 1998 Jan 16;273(3):1339–48. doi: 10.1074/jbc.273.3.1339. [DOI] [PubMed] [Google Scholar]
- 38.Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol. 1997;136(3):621–31. doi: 10.1083/jcb.136.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doctor RB, Chen J, Peters LL, Lux SE, Mandel LJ. Distribution of epithelial ankyrin (Ank3) spliceoforms in renal proximal and distal tubules. Am J Physiol. 1998 Jan;274(1 Pt 2):F129–38. doi: 10.1152/ajprenal.1998.274.1.F129. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Davis JQ, Carpenter S, Bennett V. Structural requirements for association of neurofascin with ankyrin. J Biol Chem. 1998;273(46):30785–94. doi: 10.1074/jbc.273.46.30785. [DOI] [PubMed] [Google Scholar]
- 41.Devarajan P, Stabach PR, Mann AS, Ardito T, Kashgarian M, Morrow JS. Identification of a small cytoplasmic ankyrin (AnkG119) in the kidney and muscle that binds beta I sigma spectrin and associates with the Golgi apparatus. J Cell Biol. 1996;133(4):819–30. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagelin C, Constantin B, Deprette C, Ludosky MA, Recouvreur M, Cartaud J, et al. Identification of Ank(G107), a muscle-specific ankyrin-G isoform. J Biol Chem. 2002 Apr 12;277(15):12978–87. doi: 10.1074/jbc.M111299200. [DOI] [PubMed] [Google Scholar]
- 43.Hortsch M, Paisley KL, Tian MZ, Qian M, Bouley M, Chandler R. The axonal localization of large Drosophila ankyrin2 protein isoforms is essential for neuronal functionality. Molecular & Cellular Neurosciences. 2002;20(1):43–55. doi: 10.1006/mcne.2002.1113. [DOI] [PubMed] [Google Scholar]
- 44.Kunimoto M. A neuron-specific isoform of brain ankyrin, 440-kD ankyrinB, is targeted to the axons of rat cerebellar neurons. J Cell Biol. 1995;131(6 Pt 2):1821–9. doi: 10.1083/jcb.131.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Bennett V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J Cell Biol. 1998;142(6):1571–81. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, et al. Isoform Specificity among Ankyrins: An amphipathic alpha-helix in the divergent regulatory domain of ankyrin-B interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem. 2004 Jun 11;279(24):25798–804. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- 47.Beck KA, Buchanan JA, Nelson WJ. Golgi membrane skeleton: identification, localization and oligomerization of a 195 kDa ankyrin isoform associated with the Golgi complex. J Cell Sci. 1997;110(Pt 10):1239–49. doi: 10.1242/jcs.110.10.1239. [DOI] [PubMed] [Google Scholar]
- 48.Hoock TC, Peters LL, Lux SE. Isoforms of ankyrin-3 that lack the NH2-terminal repeats associate with mouse macrophage lysosomes. J Cell Biol. 1997;136(5):1059–70. doi: 10.1083/jcb.136.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michaely P, Kamal A, Anderson RG, Bennett V. A requirement for ankyrin binding to clathrin during coated pit budding. J Biol Chem. 1999;274(50):35908–13. doi: 10.1074/jbc.274.50.35908. [DOI] [PubMed] [Google Scholar]
- 50.Mohler PJ, Rivolta I, Napolitano C, Lemaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17533–8. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.