SUMMARY
Plasminogen activator inhibitor-1 (PAI-1) paradoxically enhances tumor progression and angiogenesis, however the mechanism supporting this role is not known. Here we provide evidence that PAI-1 is essential to protect endothelial cells (EC) from FasL-mediated apoptosis. In the absence of host-derived PAI-1, human neuroblastoma cells implanted in PAI-1 deficient mice form smaller and poorly vascularized tumors containing an increased number of apoptotic EC cells. We observed that knockdown of PAI-1 in EC enhances cell-associated plasmin activity, and increases spontaneous apoptosis in vitro. We further demonstrate that plasmin cleaves FasL at Arg144 - Lys145, releasing from the surface of EC a soluble pro-apoptotic FasL fragment. The data provide a mechanism explaining the pro-angiogenic activity of PAI-1.
SIGNIFICANCE
PAI-1, the central regulator of plasmin generation is a predictor of poor clinical outcome in cancer patients. We previously reported that PAI-1 has a pro-angiogenic function but the mechanism has remained poorly understood. Here we describe a mechanism for the pro-angiogenic function of PAI-1 by providing evidence that PAI-1 protects EC from Fas/FasL-mediated apoptosis. We demonstrate that in the absence of PAI-1 in EC, there is an increase in plasmin generation and the release by plasmin of a soluble FasL fragment. This plasmin-generated soluble FasL activates Fas and is a potent inducer of apoptosis in EC. Our results suggest that PAI-1 could be a target for anti-angiogenic and anti-vascular therapies.
Keywords: plasminogen activator inhibitor-1, plasmin, FasL, apoptosis, angiogenesis, neuroblastoma
INTRODUCTION
Angiogenesis, the process leading to the formation of new blood vessels, plays a central role in solid tumor growth and metastatic dissemination (Carmeliet, 2003). This process requires a coordinated and temporal regulation of adhesive, proteolytic and migratory events. During angiogenesis, EC stimulated by hypoxia and angiogenic factors leave a state of quiescence to proliferate and migrate through the extracellular matrix (ECM). During this process EC become more sensitive to apoptosis (Folkman, 2003). The Fas/FasL pathway is a key mediator of EC apoptosis and controls angiogenesis. The expression of Fas and FasL is upregulated in activated EC and upon EC detachment, hypoxia or exposure to vascular endothelial growth factor (VEGF) (Bouchet et al., 2002; Cardier et al., 1999; Chavakis and Dimmeler, 2002). Natural inhibitors of angiogenesis like thrombospondin 1 (TSP1) and pigment epithelium-derived factor derive their specific anti-angiogenic function from upregulating FasL and enhancing apoptosis in EC (Volpert et al., 2002). The ability of EC to become resistant to Fas-mediated apoptosis is therefore critical for angiogenesis to succeed. Among known mechanisms of resistance is EC attachment to the ECM which modulates Fas-mediated apoptosis by downregulating Fas and upregulating c-Flip, an endogenous antagonist of caspase-8 (Aoudjit and Vuori, 2001; Aoudjit and Vuori, 2000). However beyond this mechanism, little is known of other potential ways for EC to escape.
During angiogenesis, EC express and activate enzymes that degrade the ECM as they invade surrounding tissues. Stimulated EC make urokinase-type plasminogen activator (uPA), which converts plasminogen into its active form, plasmin (Pepper, 2001). uPA itself is secreted as an inactive precursor form (pro-uPA) that binds with high affinity to a specific cell surface glycosylphosphatidylinositol-anchored receptor designated uPAR (Blasi and Verde, 1990). When activated, uPAR-bound uPA enhances the generation of plasmin at the cell surface, promoting ECM degradation, the activation of matrix metalloproteinases (MMP) and the release and activation of ECM- and cell surface-anchored growth factors (Dano et al., 2005). PAI-1 is the physiological inhibitor of uPA, and therefore controls the activation of plasminogen into plasmin. In addition to interacting with uPA, PAI-1 also binds to vitronectin. The binding of PAI-1 to vitronectin results in its stabilization but also interferes with the binding of integrins of the αv family to the RGD domain of vitronectin and therefore with cell migration (Deng et al., 1996). The two inhibitory functions of PAI-1 on proteolysis and on cell attachment initially led to the prediction that PAI-1 would have an anti-angiogenic function. It was therefore surprising to discover that high, rather than low, levels of PAI-1 were predictive of poor outcome in patients suffering from several types of cancers like breast, colon and neuroblastoma (Foekens et al., 1995; Ganesh et al., 1994; Sugiura et al., 1999). Consistent with a positive effect of PAI-1 on cancer progression, we and others published data that provided some explanation for the paradoxical effect of PAI-1 in cancer. We demonstrated that in the absence of host-derived PAI-1 there is a defect of tumor vascularization in mice implanted subcutaneously with transformed keratinocytes (Bajou et al., 1998; Bajou et al., 2001) and that this is related to the anti-proteolytic activity of PAI-1. The effect of PAI-1 on angiogenesis is dose dependent, with a stimulatory role reported at low physiological concentrations and an inhibitory role at pharmacological concentrations (Bajou et al., 2004). The exact mechanism by which inhibition of proteolysis by PAI-1 promotes angiogenesis has however not been discovered. Here we provide evidence for such a mechanism by demonstrating that PAI-1 expression by EC is critical to protect them from FasL-mediated apoptosis.
RESULTS
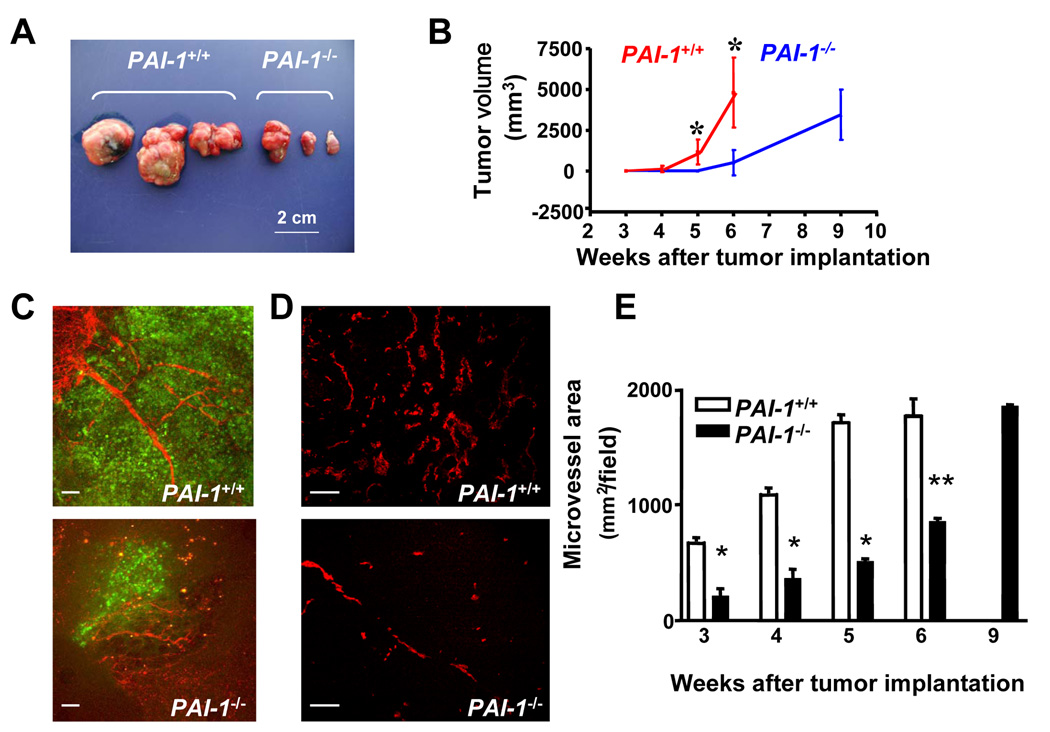
Absence of Host-derived PAI-1 Reduces Human Neuroblastoma Tumor Growth and Angiogenesis
To investigate the importance of PAI-1 in tumor progression, we took advantage of the fact that neuroblastoma cells do not produce PAI-1 (Sugiura et al., 1999) and orthotopically implanted fragments from human SK-N-BE(2) neuroblastoma tumors grown subcutaneously in the adrenal gland of PAI-1+/+ Rag−/− mice and PAI-1−/− Rag−/−mice. The growth of these tumors was monitored by sacrificing mice 3, 4, 5, 6 and 9 weeks after implantation, measuring the tumor volume and examining the tumors for vascularization, malignant cell proliferation and apoptosis. We observed a significant difference in the tumor volume between the 2 groups of mice with the presence of much smaller tumors in PAI-1−/− mice when compared with PAI-1+/+ mice at week 5 and 6 after implantation (Figure 1A and B). By week 6, all tumor-bearing PAI-1+/+ mice had to be sacrificed due to the presence of large tumors (average volume of 5 cm3). However by week 9 post implantation, PAI-1−/− mice had developed tumors of similar size to PAI-1+/+ mice at week 6. Because PAI-1 has been shown to play a regulatory role in angiogenesis, the vasculature of these xenotransplanted tumors was specifically examined. This analysis revealed a delay in the formation of the vasculature between week 3 and 6 in tumors derived from PAI-1−/− mice when compared with tumors derived from PAI-1+/+ mice as indicated by angiographies (Figure 1C) and measurements of the microvessel area in tumor sections immunostained for the platelet/endothelial cell adhesion molecule-1 (PECAM-1)/CD31 antigen (Figure 1D and E). At week 9, the mean microvessel area of tumors from PAI-1−/− mice was similar to values observed in tumors from PAI-1+/+ mice at week 6. The inhibition of angiogenesis in tumors derived from PAI-1−/− mice was associated with an inhibition of tumor cell proliferation as indicated by a lower percentage of BrdU positive tumor cells between week 3 and 5 (supplemental data Figure 1A and B). In contrast, the presence or absence of PAI-1 in host cells did not affect tumor cell apoptosis (supplemental data Figure 1C and D). However we observed a higher percentage of apoptotic EC in tumors obtained from PAI-1−/− mice at week 3 and 4 after tumor implantation when compared to tumors implanted in PAI-1+/+ mice (supplemental data Figure 1E and F). This increase in apoptotic EC was not observed in tumors obtained at week 5 and 6 after implantation. The data thus indicate that in the absence of host-derived PAI-1 there is a delay in tumor growth associated with an inhibition of angiogenesis and an increase in EC apoptosis. They point to a possible protective effect of PAI-1 on EC apoptosis.
Figure 1. Absence of Host-derived PAI-1 Reduces Human Neuroblastoma Tumor Growth and Angiogenesis.
(A) Picture of representative neuroblastoma tumors collected 6 weeks after orthotopic implantation in Rag−/− PAI-1+/+ or Rag−/− PAI-1−/− mice.
(B) Tumor volume in PAI-1+/+ (n= 6 to 8) and PAI-1−/− (n = 6 to 8) mice over time. Means ± standard deviation (SD) .
(C) Representative images of fluorescent angiographies obtained 3 weeks after tumor implantation. Tumor cells are EGFP positive (green) and blood vessels (red) are recognized by the presence of the biotinylated tomato lectin-Texas red avidin D complex.
(D) Representative microphotographs of tumor sections stained for PECAM-1/CD31, 4 weeks after tumor implantation.
(E) Microvessel area values were determined on tumor sections stained for PECAM-1/CD31 at indicated time points as previously described. The data represent the means (± SD) of 6 sections examined each in 6 to 8 tumors per time point. Scale bars are 100 µm in C and D (*p<0.05, ** p<0.01 in B and E).
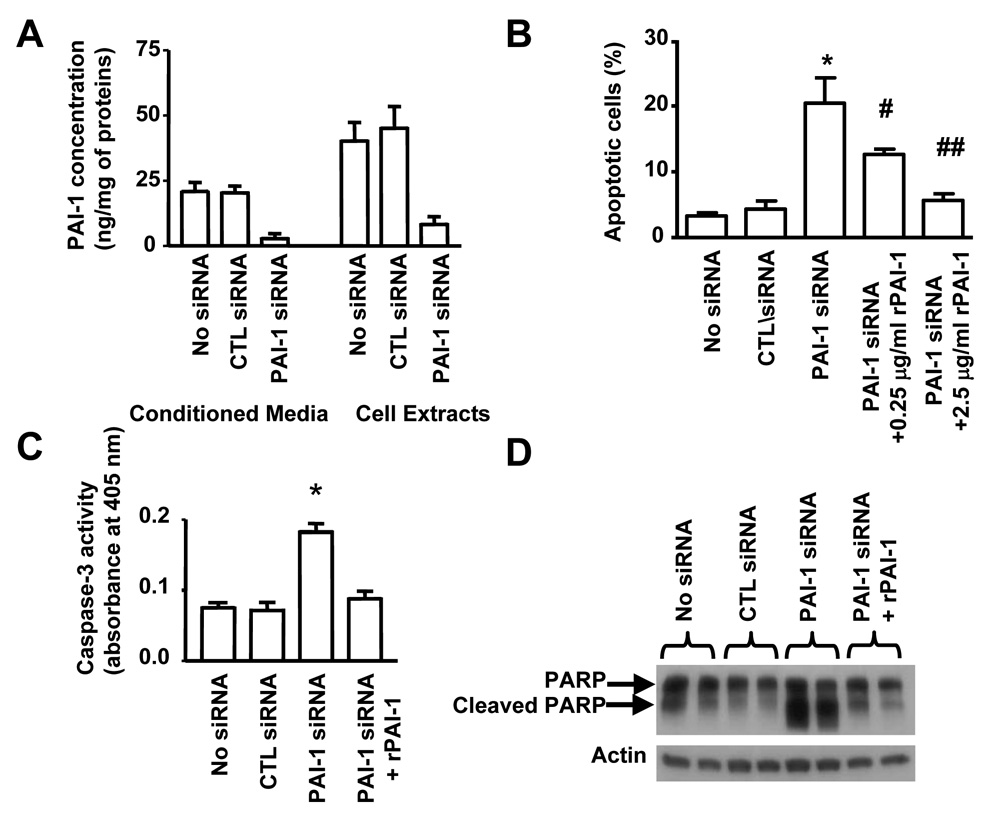
PAI-1 Protects EC from Apoptosis
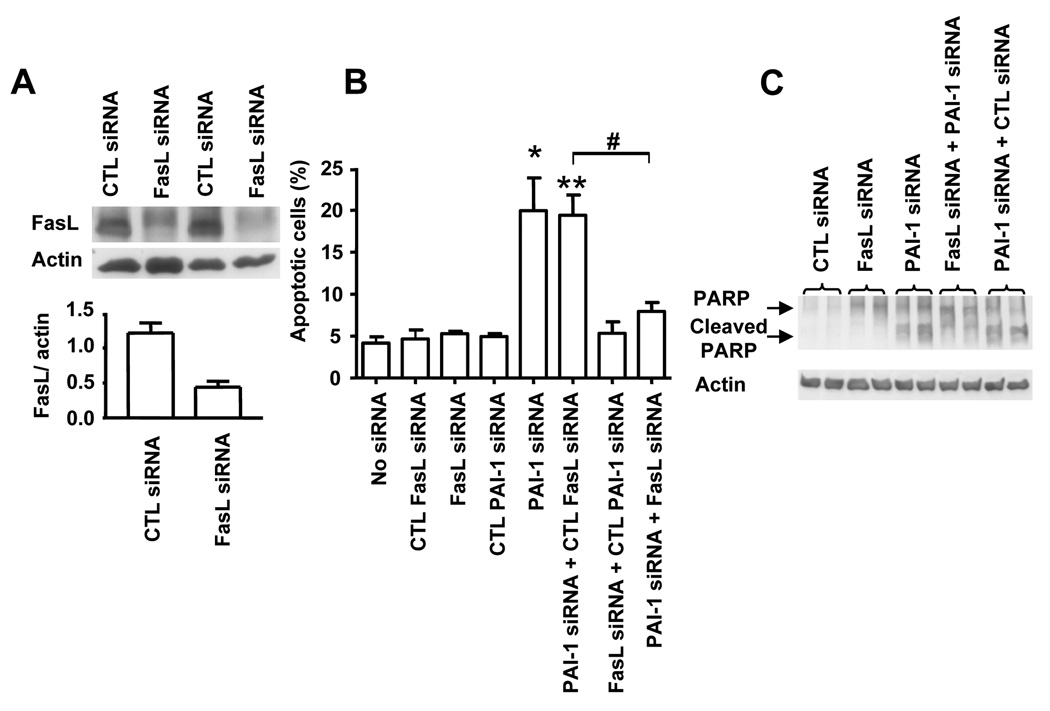
To test this possibility, we examined the effect of downregulation of PAI-1 expression on apoptosis in human brain microvascular EC (HBMEC) using small interfering (si)RNA. Transfection of HBMEC with a PAI-1 siRNA inhibited the expression of PAI-1 (protein) in cell extracts and conditioned media by 80% 72 hr after transfection, when compared with untransfected cells or cells transfected with a scrambled (control) siRNA (Figure 2A). Inhibition of PAI-1 expression in HBMEC transfected with PAI-1 siRNA was associated with a 5 fold increase in spontaneous apoptosis (Figure 2B). Depending on the conditions, an 8 to 9 fold increase in apoptosis was observed in other experiments. A 9 fold increase in apoptosis was also documented when another PAI-1 siRNA sequence was tested (data not shown).
Figure 2. PAI-1 Protects EC from Apoptosis.
(A) The levels of PAI-1 in the conditioned medium (CM) and in cell extracts (cell associated, CA) of EC transfected with different siRNAs were measured by ELISA. The data represent the means (± SD) of triplicate samples.
(B) The percentage of apoptotic EC was determined 72 hr after transfection with siRNA by TUNEL assay. rPAI-1 (0.25 and 2.5 µg/ml) was added 24 hr after transfection. The data represent the means (± SD) of triplicate samples.
(C) Caspase-3 activity in experimental conditions described in (B) was determined by colorimetric assay as described in Experimental Procedures. The data represent the means (± SD) of triplicate samples.
(D) PARP cleavage was examined by Western blot analysis in the same experimental conditions as in (B). rPAI-1 was added at 2.5 µg/ml 24h after siRNA transfection. (* p<0.01 compared to CTL siRNA, # p<0.05, ## p<0.01 compared to PAI-1 siRNA, B and C).
Consistent with this effect being the result of an inhibition of PAI-1 production and secretion in the culture medium, the addition of 0.25 µg/ml of stable recombinant (r) PAI-1 to the culture medium decreased the percentage of apoptotic HBMEC by 40% and to baseline levels at a concentration of 2.5 µg/ml. The induction of HBMEC apoptosis upon PAI-1 downregulation was associated with a 2 fold increase in caspase-3 activity when compared with HBMEC transfected with scrambled siRNA (Figure 2C) and with the detection of poly-ADP-ribose-polymerase (PARP) cleavage products detected by Western blot analysis (Figure 2D). These two effects were suppressed by the addition of exogenous rPAI-1. Altogether the data suggest that PAI-1 has a protective effect on spontaneous apoptosis in EC that is associated with an inhibition of caspase-3 activation.
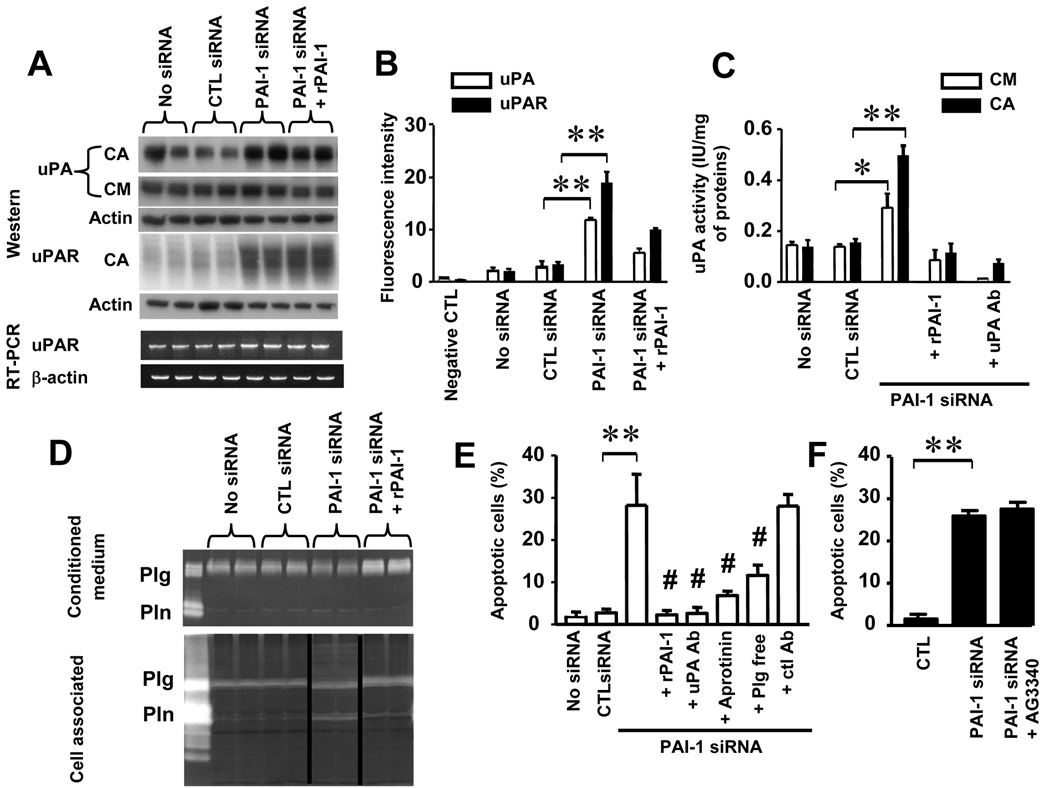
uPA-mediated Plasminogen Activation Induces Apoptosis in HBMEC
To determine whether this protective effect on apoptosis involved the anti-proteolytic activity of PAI-1, we first examined whether downregulation of PAI-1 affected the expression of uPA and its receptor uPAR by Western blot analysis. The data (Figure 3A) revealed higher levels of cell-associated uPA and uPAR in HBMEC transfected with PAI-1 siRNA. The addition of stable rPAI-1 to HBMEC transfected with PAI-1 siRNA decreased the amount of cell-associated uPA although not uPAR. Changes in uPAR levels were not associated with changes in uPAR transcription since we did not observe changes in uPAR mRNA upon PAI-1 downregulation by RT-PCR (Figure 3A, lower panel). When binding to uPA, PAI-1 promotes the internalization of a uPA/uPAR/PAI-1 complex which results in an initial decrease in the expression of uPAR at the cell surface (Nykjaer et al., 1997). Consistently, an analysis by flow cytometry revealed an increase in cell surface expression of uPA and uPAR in HBMEC upon downregulation of PAI-1 that reflects a decrease in internalization of the uPA/uPAR complex in the absence of PAI-1 (Figure 3B). Downregulation of PAI-1 was also associated with higher levels of uPA-associated proteolytic activity in cell extracts and conditioned media that were not observed upon the addition of rPAI-1 or a function-blocking antibody against the proteolytic activity of uPA (Figure 3C). As anticipated and documented by casein zymography, higher levels of cell-associated uPA activity in HBMEC transfected with PAI-1 siRNA resulted in an increase in plasminogen activation, which was suppressed in the presence of stable rPAI-1 (Figure 3D) or an anti-uPA antibody (data not shown). Thus, the data indicate that PAI-1 controls the proteolytic activity of uPA at the surface of EC not only through inhibition of uPA activity but also through inhibition of uPA and uPAR expression. They raise the question whether the protective effect of PAI-1 on HBMEC apoptosis can be a direct consequence of its inhibitory effect on uPA and indirectly on plasmin generation, or in other words, whether there is a causal link between EC apoptosis and cell-associated plasmin activity. To test this possibility, we examined the effect of blocking uPA and plasmin activity on apoptosis in HBMEC transfected with PAI-1 siRNA. This experiment indicated a suppression of spontaneous apoptosis in PAI-1-deficient HBMEC in the presence of an antibody against the proteolytic function of uPA but not a control normal IgG. There was also a significant decrease in apoptosis in the presence of aprotinin or when plasminogen was depleted from the serum in the culture medium. The data thus confirm that the increase in uPA-generated plasmin upon PAI-1 downregulation is responsible for inducing apoptosis in HBMEC (Figure 3E). Because plasmin has been shown to activate other proteases, in particular matrix metalloproteinase (MMP)-3/ stromelysin-1 (Nagase et al., 1990) and indirectly MMP-9/gelatinase B (Ramos-DeSimone et al., 1999), it was conceivable that the effect of plasmin on apoptosis may have been an indirect effect of MMP activation by plasmin. To eliminate this possibility, we performed experiments in the presence of an inhibitor of MMPs (AG3340). The data indicate that treatment with AG3340 did not prevent EC from undergoing apoptosis upon PAI-1 downregulation (Figure 3F), indicating therefore that the effect of plasmin on apoptosis is MMP-independent.
Figure 3. uPA-mediated Plasminogen Activation Induces EC Apoptosis.
(A) Top: Western blot analysis of cell-associated (CA) and secreted (CM) uPA and CA uPAR in EC transfected with siRNA as indicated on the top. rPAI-1 was added at 2.5 µg/ml. Bottom: RT-PCR analysis of uPAR and β-actin expression in the cell extracts obtained in the same conditions.
(B) The presence of uPA and uPAR at the cell surface of cells treated as in (A) was examined by flow cytometry. The data represent the means (± SD) of triplicate samples and are representative of 3 experiments showing similar results.
(C) The proteolytic activity of uPA in cell extracts (CA) and CM was determined by colorimetric assay as indicated in Experimental Procedures. The data are the means (± SD) of triplicate samples.
(D) The presence of plasminogen (Plg) and plasmin (Pln) in the same experimental conditions as in (A) was examined by casein zymography in CM and cell extracts (CA).
(E,F) The percentage of apoptotic EC transfected with PAI-1 siRNA and control siRNA and treated as indicated was determined by flow cytometry. The data represent the means (± SD) of triplicate dishes and are representative of 2 separate experiments showing similar results. rPAI-1 was added at 2.5 µg /ml, aprotinin at 200 µg/ml and anti uPA antibody or mouse IgG control at 7 µg/ml. AG3340 was added at 10 µg/ml (* p<0.05, ** p<0.01 for PAI-1 siRNA versus control siRNA in B,C,E and F, # p<0.01 for values versus PAI-1 siRNA in E).
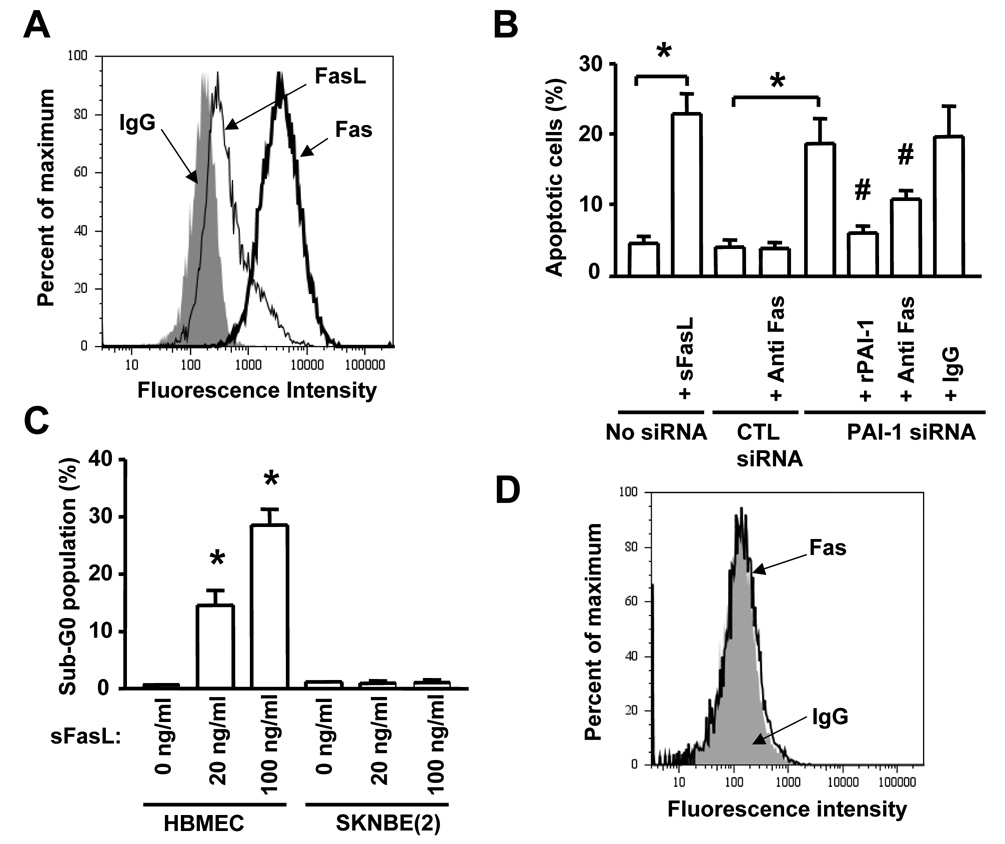
Increase in FasL-mediated EC Apoptosis in the Absence of PAI-1
We then determined whether the protective effect of PAI-1 on apoptosis involved interference with the intrinsic or the extrinsic apoptotic pathway. In a first experiment we examined the effect of PAI-1 downregulation on the expression of phospho-Bcl-2, Bcl-XL, Bak, Bax, and the activation of Akt and ERK 1/2. This analysis revealed no differences in the expression and activation of these proteins upon downregulation of PAI-1 by siRNA (supplemental data Figure 2). In contrast, we observed an increase in cleaved active caspase-8 and in caspase-8 activity in the absence of activation of caspase-9. This effect was suppressed by the addition of stable human rPAI-1. Altogether these data suggest that in the absence of PAI-1 there is an activation of the extrinsic apoptotic pathway in EC. Considering the central role played by the Fas/FasL pathway in EC apoptosis, we asked the question whether plasmin could affect the activity of Fas/FasL. We first documented that HBMEC expressed Fas and FasL under unstimulated conditions (Figure 4A). The Fas receptor was functional because the cells underwent apoptosis when exposed to a recombinant soluble FasL (sFasL) (Figure 4B and C). A more direct evidence supporting the involvement of FasL in HBMEC apoptosis upon downregulation of PAI-1 was then obtained by demonstrating that blocking FasL-Fas interaction with an anti-Fas antibody (ZB4) inhibited apoptosis in HBMEC transfected with PAI-1 siRNA (Figure 4B) whereas a nonspecific mouse IgG had no effect. In contrast, treatment of SK-N-BE(2) neuroblastoma cells with sFasL did not induce apoptosis (Figure 4C), because these cells did not constitutively express Fas as determined by FACS analysis (Figure 4D). In a second experiment, we tested whether PAI-1 downregulation would induce apoptosis in HBMEC in the absence of FasL. To accomplish this, we generated a FasL siRNA and first demonstrated that it inhibited the expression of FasL in HBMEC by 65% (Figure 5A). We then examined the effect of simultaneously downregulating PAI-1 and FasL in HBMEC by dual siRNA transfection. This experiment (Figure 5B) indicated a significant reduction in the percentage of apoptotic HBMEC when FasL was simultaneously downregulated with PAI-1 but not when a control FasL siRNA scrambled sequence was used. Downregulation of FasL alone had no effect, which is consistent with Fas/FasL having no effect on basal apoptosis but having an effect of apoptosis in the absence of PAI-1. Consistently, the cleavage of PARP observed upon transfection of EC with PAI-1 siRNA was significantly reduced upon simultaneous transfection with a FasL siRNA but not a control (scrambled) siRNA (Figure 5C). These data thus provide evidence that FasL is a downstream target of PAI-1 and a necessary intermediate in the induction of apoptosis in EC upon PAI-1 downregulation. On the basis of these observations, we postulated that the increase in plasmin activity in the absence of PAI-1 might solubilize sFasL and enhance Fas-dependent apoptosis.
Figure 4. Increase in FasL-mediated Apoptosis in the Absence of PAI-1.
(A) The expression of Fas and FasL in HBMEC was determined by flow cytometry.
(B) The percentage of apoptotic HBMEC transfected with siRNAs in the absence or presence of an anti-Fas blocking antibody or rPAI-1 (2.5 µg/ml) was determined by flow cytometry after staining with propidium iodide. The data are the means (± SD) of triplicate samples.
(C) Apoptosis in HBMEC and SK-N-BE(2) cells was determined by flow cytometry as in (B) after the cells were treated for 24 hr with rsFasL at indicated concentrations. The data are the means (± SD) of triplicate samples.
(D) The expression of Fas in SK-N-BE(2) tumor cells was determined by flow cytometry (* p<0.001, # p<0.01 in B and C).
Figure 5. FasL is Required for EC Apoptosis upon PAI-1 Downregulation.
(A) HBMEC were transfected with a FasL siRNA or a scrambled siRNA sequence (control) and examined after 72 hr for the presence of FasL in the cell lysate by Western blot. A quantitative analysis of the data obtained by the scanning of 3 separate blots is shown in the lower panel. The data are the mean ratios (± SD) of FasL/actin.
(B) HBMEC were transfected with PAI-1 siRNA, FasL siRNA or a combination of both siRNAs and their respective scrambled controls and examined after 72 hr for apoptosis by flow cytometry. The data represent the mean numbers (± SD) of apoptotic cells from triplicate samples (* p<0.025, ** p<0.01, # p<0.005).
(C) Cell lysates of HBMEC treated as indicated in the figure were examined for PARP cleavage by Western blot analysis.
Plasmin Cleaves FasL at Arg144 - Lys145
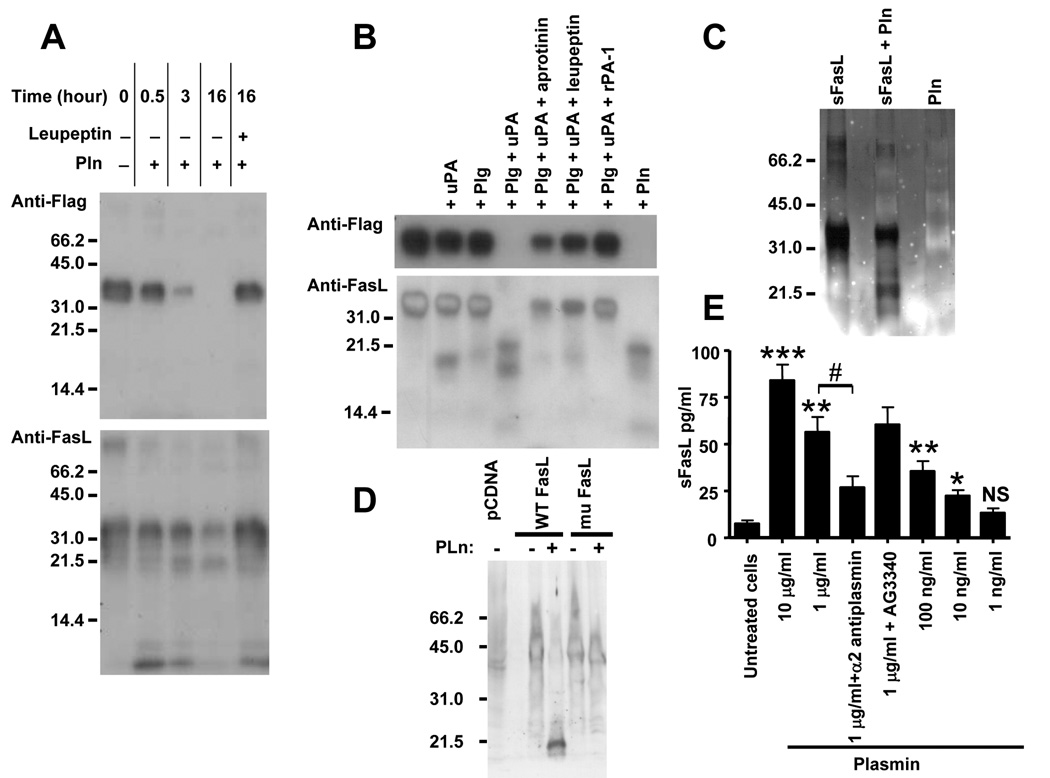
This possibility was first tested by examining the proteolytic activity of plasmin on a soluble recombinant glycosylated FLAG-FasL protein extending from residue 103 to 281. The data revealed a time-dependent cleavage of the 36 kDa FLAG-FasL in the presence of plasmin and the generation of a cleavage product with a relative MW (Mr) of approximately 21.5 (Figure 6A). This cleavage was inhibited by leupeptin. Neither uPA nor plasminogen cleaved FasL but cleavage occurred in the presence of both plasminogen and uPA and was blocked in the presence of aprotinin, leupeptin or rPAI-1 (Figure 6B). To identify the plasmin-cleavage site of FasL, the N-terminal amino acid sequence of the ~21.5 kDa FasL plasmin-generated fragment was determined by Edman degradation (Figure 6C and supplemental data Figure 3A). This analysis revealed the following N-terminus sequence: Lys-Val-Ala-His-Leu-Thr indicating that there is a plasmin cleavage site in FasL at Arg144 - Lys145 in the trimerization domain, located in the extracellular segment of the protein. This analysis predicted the release of a soluble FasL fragment of 137 amino acid with a molecular mass of 15.7 kDa (non-glycosylated) and higher pending on the degree of glycosylation. We next examined whether plasmin could also cleave native membrane-associated FasL. For this experiment, human HT1080 cells (that do not express FasL) were transfected with a pcDNA plasmid containing either the full length sequence of wild-type (WT) FasL or the same sequence in which the plasmin cleavage site Arg144 - Lys145 was mutated to Val144 - Ala145 (mu FasL). To demonstrate that this mutation did not affect the apoptotic activity of FasL, we co-cultured WT FasL and mu FasL expressing HT1080 cells with Jurkat T-cells, and examined the effect on apoptosis in Jurkat T-cells (Cappellesso et al., 2000). This experiment (supplemental data Figure 4) revealed similar increases in apoptosis of Jurkat T-cells in the presence of either WT FasL or mu FasL expressing HT1080 cells. Thus the mutation at Arg144 - Lys145 does not affect FasL apoptotic activity. To demonstrate that the mutation affected the cleavage of FasL, we submitted membrane extracts from HT1080 cells expressing WT or mu FasL to plasmin digestion and examined the extracts for the presence of FasL by Western blot (Figure 6D). The data indicated a loss of the 40 kDa membrane-associated FasL protein and the presence of a ~21.5 kDa fragment in HT1080 cells expressing WT FasL but not in cells overexpressing mu FasL. The data thus confirm that plasmin cleaves cell-associated FasL at the specific Arg144 - Lys145 site. To further demonstrate that plasmin promoted the cleavage and release of FasL, we performed a series of experiments in cultured HBMEC measuring the amount of FasL released in the culture medium upon exposure to plasmin. The data (Figure 6E) indicated an increase in sFasL in the medium in the presence of plasmin at a concentration of 10 to 0.01 µg/ml that was inhibited by the addition of α2 anti-plasmin but not by the addition of an MMP inhibitor. They are consistent with plasmin having a direct effect on releasing sFasL from the surface of HBMEC that does not involve MMP activation.
Figure 6. Cleavage of Recombinant and Membrane-associated FasL by Plasmin.
(A,B) Western blot analysis of FLAG-FasL (10 µg/ml) treated in the conditions indicated on top using an anti-FLAG antibody (upper blots) or an anti-FasL antibody (lower blots). The concentrations used were as follows: plasmin (Pln), 1 µg/ml; plasminogen (Plg), 1 µg/ml; leupeptin, 500 µg/ml; aprotinin, 1 mg/ml; uPA, 600 IU/ml; and rPAI-1, 50 µg/ml.
(C) Silver staining of a polyacrylamide gel of recombinant sFasL incubated in the presence or absence of Pln as indicated in A and B.
(D) Western blot analysis with an anti-FasL antibody of membrane proteins extracted from HT1080 cells expressing WT FasL or mu FasL and incubated for 1 hr in the presence or absence of plasmin (1 µg/500 µg of lysate) at 37°C before SDS-PAGE.
(E) Levels of sFasL in the serum-free culture medium of HBMEC incubated in the presence of plasmin and inhibitors as indicated. α2-antiplasmin was added at a concentration of 100 µg/ml and AG3340 at a concentration of 10 µg/ml. The data represent the mean concentrations (±SD) of sFasL in the culture medium of triplicate samples and are representative of 2 experiments showing similar results (* p<0.025, ** p<0.01, *** p<0.005 versus untreated cells, # p<0.005 versus plasmin treated cells).
The 21.5 kDa Plasmin-generated FasL Fragment is Released upon PAI-1 Downregulation and is Pro-Apoptotic
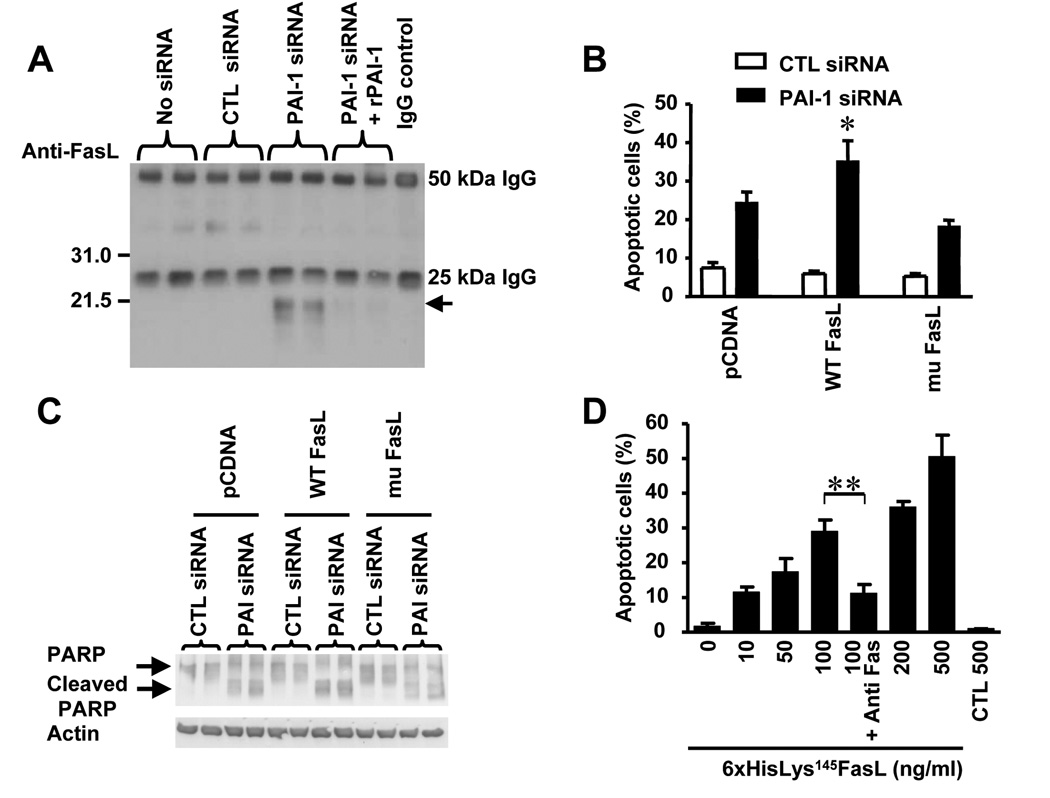
Using immunoprecipitation, we then documented the presence of the ~21.5 kDa plasmin-generated FasL peptide in the conditioned medium of HBMEC upon downregulation of PAI-1. Consistent with our previous data, this fragment was detected in the supernate of HBMEC transfected with a PAI-1 siRNA but not with the control siRNA or upon addition of rPAI-1 (Figure 7A). Further evidence supporting a key role played by the cleavage of FasL by plasmin in increasing apoptosis in PAI-1 deficient EC was obtained by comparing the effect of PAI-1 downregulation in HBMEC expressing either WT FasL or mu FasL. This experiment (Figure 7B and C) indicated a significantly higher level of apoptosis associated with an increased presence of cleaved PARP in cells that overexpressed WT FasL and in which PAI-1 was downregulated. Such increase, above the level of apoptosis typically observed in EC expressing endogenous FasL and transfected with PAI-1 siRNA, was not observed in EC that overexpressed mu FasL. In these conditions, the level of apoptosis was similar to the level observed in cells expressing endogenous FasL. Altogether the data provide direct evidence that plasmin cleaves membrane-associated FasL at Arg144 - Lys145 to release a ~21.5 kDa soluble fragment in the supernate of HBMEC upon PAI-1 knockdown, and that apoptosis upon PAI-1 downregulation is dependent on the release of sFasL
Figure 7. The 21.5 kDa Plasmin-generated FasL is Pro-apoptotic.
(A) The presence of the 21.5 kDa sFasL plasmin-generated fragment was detected by immunoprecipitation and Western blot analysis with an anti-FasL antibody in the CM of HBMEC treated as indicated on top.
(B) HBMEC were transfected with a pcDNA plasmid containing either a WT FasL or a mu FasL. Stable transfected cells were selected and re-transfected with a PAI-1 siRNA or a control siRNA and tested for apoptosis by FACS analysis after 72 hr. The data represent the means (± SD) of triplicate samples (* p< 0.05).
(C) Cells treated as described in (B) were examined for the presence of cleaved PARP by Western blot analysis.
(D) The recombinant 6xHisLys145FasL protein that corresponds to the 21.5 kDa plasmin-generated FasL obtained as shown in supplemental data Figure 2C was added at indicated concentrations to HBMEC for 48 hr and the percentage of apoptotic cells was measured by flow cytometry. An anti-Fas antibody (ZB4) was added at 500 ng/ml. The data represent the means (±SD) from triplicate samples (** p<0.005).
To obtain confirmation that the soluble FasL fragment generated by plasmin is pro-apoptotic in HBMEC, we generated a recombinant FasL fragment starting at Lys145 and containing a 6xHis tag on its N-terminus using the pRSET bacterial expression vector. This plasmid was transfected into competent BL21 E. coli and the 6xHis tagged protein (6xHisLys145FasL) was extracted from the bacterial lysate and purified in a single step on a nickel-chelating column by elution with 250 mM imidazole (supplemental data Figure 3B and C). A preparation containing 70 µg/ml of the protein was tested for its effect on EC apoptosis. The data indicated that 6xHisLys145sFasL protein was a potent inducer of apoptosis in HBMEC at concentrations ranging from 10 to 500 ng/ml and that its pro-apoptotic activity was inhibited in the presence of an anti-Fas blocking antibody (Figure 7D). This apoptotic effect was confirmed by the detection of PARP cleavage products by Western blot analysis (supplemental data Figure 3D). Altogether the data are consistent with PAI-1 having a protective effect on EC apoptosis by inhibiting the release by plasmin of a sFasL fragment that is an activator of caspase-8 and 3 in EC. Because it has been suggested that FasL needs to form a trimer to be active and plasmin cleaves FasL in the trimerization domain, we examined whether 6xHisLys145FasL formed a trimer. FLAG-sFasL and 6xHisLys145FasL were incubated with the cross-linking agent disuccinimidyl suberate (DSS) and examined for trimer formation by Western blot (supplemental data Figure 5A). The data show that FLAG-sFasL forms a trimer in the presence of DSS, but that 6xHisLys145FasL does not, indicating that cleavage in the trimerization domain by plasmin prevents trimer formation. However, we show that 6xHisLys145FasL is pro-apoptotic (Figure 7D). To confirm the pro-apoptotic activity, we tested whether 6xHisLys145FasL activated Fas by examining the presence of Fas/FADD complexes in lysates of HBMEC exposed to FLAG-sFasL or to 6xHisLys145FasL. This experiment (supplemental data Figure 5B) indicated the presence of FADD associated with Fas in HBMEC treated with FLAG-sFasL and 6xHisLys145FasL, and thus confirms that 6xHisLys145FasL is active in the absence of trimer formation.
The Control of PAI-1 on Angiogenesis in vitro is Fas-dependent
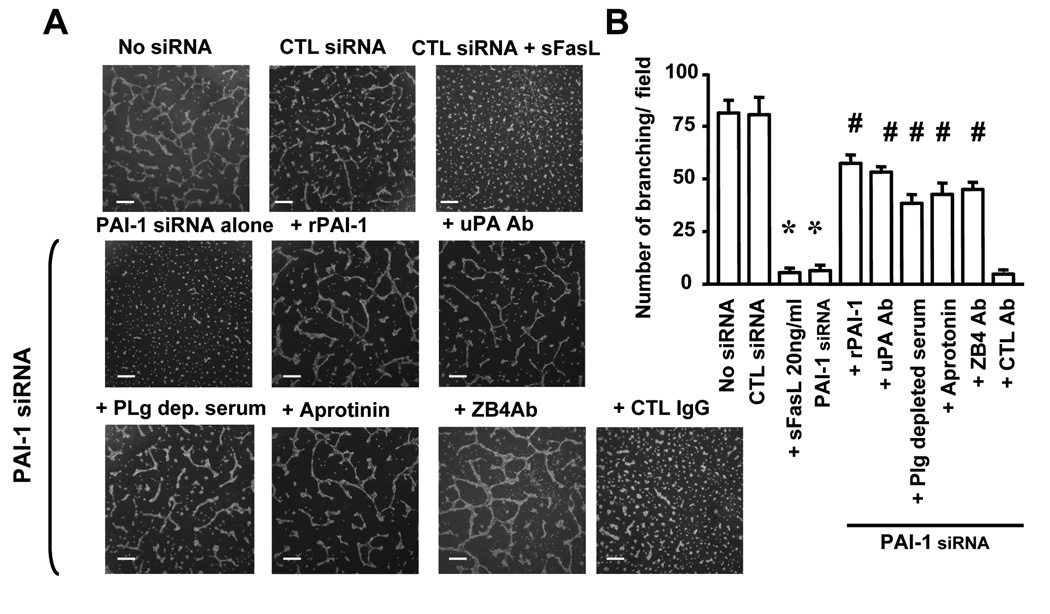
It was important to determine whether the control of PAI-1 on in vitro angiogenesis was FasL-dependent. We therefore plated HBMEC on Matrigel and examined the effect of PAI-1 downregulation on the formation of a network of cords of EC that mimic a vascular network. The data (Figure 8A and B) revealed a significant inhibition in the number of branches in the network formed by HBMEC in the presence of sFasL or when cells were transfected with PAI-1 siRNA. The reduction in branching in HBMEC transfected with PAI-1 siRNA was markedly prevented when rPAI-1 was added or in the presence of a blocking antibody against uPA, aprotinin or when plasminogen was depleted from the serum. Consistent with the effect of PAI-1 on angiogenesis being dependent on Fas/FasL, the addition of a blocking antibody against Fas also prevented the reduction in branching. The data are consistent with our data on apoptosis, and indicate that PAI-1 affects angiogenesis in vitro by a mechanism that is Fas-dependent.
Figure 8. FasL Inhibits EC Branching in Matrigel.
(A) Representative photomicrographs of HBMEC plated on Matrigel and treated as indicated.
(B) Quantitative analysis of the data. The data represent the mean (± SD) numbers of branches per microscopic field from a total of 6 fields each examined in 3 different dishes (* p<0.001, # p<0.001). Bar = 400 µm
Fas, FasL, uPA and uPAR are expressed in neuroblastoma tumors orthotopically implanted in mice
To obtain evidence that Fas/FasL and the plasminogen system contribute to EC apoptosis in tumors, we examined by immunofluorescence the expression of FasL, Fas, uPA and uPAR in neuroblastoma tumors orthotopically implanted in mice. The data (supplemental data Figure 6) revealed a strong expression of uPAR in cells aligned along the basement membrane (type IV collagen) of blood vessels consistent with uPAR being expressed in vascular EC. uPA also co-expressed with PECAM-1/CD31 positive EC in the tumor vasculature. There was no obvious difference in uPA and uPAR expression between tumors grown in WT or PAI-1−/− mice. We also demonstrated the co-expression of PECAM-1/CD31 and Fas and FasL in vascular structures in these tumors. The data thus indicate that Fas/FasL and uPA/uPAR are expressed in EC in vivo. Although they do not demonstrate that they are responsible for the increased apoptosis observed in the absence of PAI-1, the selective expression of these proteins in vascular EC in tumors is consistent with their role in controlling EC apoptosis in vivo.
In summary, our data identify a cleavage site for plasmin in FasL that results in the release of a pro-apoptotic sFasL peptide and provide a mechanism that explains the previously reported pro-angiogenic activity of PAI-1 (supplemental data Figure 7).
DISCUSSION
Our data provide a mechanism that explains the protective effect of PAI-1 on apoptosis through its control of the pericellular activity of plasmin and FasL solubilization. Evidence for such a FasL-dependent mechanism is provided by the following data: (1) induction of apoptosis in PAI-1 deficient EC is blocked in the presence of an anti-Fas antibody, (2) PAI-1 deficient EC do not undergo apoptosis in the absence of FasL, (3) there is no additional increase in apoptosis in PAI-1 deficient EC overexpressing a FasL in which the plasmin cleavage sequence was mutated, and (4) FasL is released as a 21.5 kDa soluble protein in the culture medium of EC upon PAI-1 downregulation or plasmin treatment. We also report a cleavage site in FasL that is sensitive to plasmin. Membrane-associated FasL (mFasL) is typically solubilized by MMPs and its cleavage by plasmin has not been reported so far. The effect of sFasL on apoptosis is complex, differs among cell types and is in part governed by cell polarity. In non-polarized cells like hematopoietic and mesenchymal cells and lymphocytes the solubilization of FasL results in Fas inactivation (Tanaka et al., 1995). In contrast, in polarized epithelial cells, sFasL is active and induces apoptosis (Powell et al., 1999). The cleavage of mFasL by MMP-7 in prostate epithelial cells induces Fas-mediated apoptosis (Vargo-Gogola et al., 2002), whereas it protects tumor cells from drug cytotoxicity (Mitsiades et al., 2001). In EC under hypoxia, FasL is solubilized by MMPs and forms a 70 kDa trimeric complex that inhibits Fas and prevents hypoxia-induced apoptosis (Mogi et al., 2001). Our data are different, as we report here the cleavage of FasL not by an MMP but by plasmin, and demonstrate that this cleavage enhances rather than prevents apoptosis in EC. Because plasmin is a known activator of several MMPs, it was important to demonstrate that the effect of plasmin on the solubilization of sFasL and apoptosis in vivo was also a direct effect as shown in a test tube assay. Our data demonstrating an absence of effect of AG3340 on the release of sFasL by plasmin in cultured HBMEC and on HBMEC apoptosis upon PAI-1 downregulation are consistent with plasmin having a direct effect independent of MMP activation. The cleavage of FasL by plasmin was demonstrated not only for a recombinant protein but also for FasL expressed by HT1080 cells. The absence of cleavage in cells overexpressing mu FasL also confirms the specificity of the cleavage. It is interesting to note that in the case of MMP-7, two cleavage sites have been reported. A primary cleavage site at a Leu residue in the sequence ELAELR between the transmembrane domain and the self-assembly (trimerization) domain generates a 25 kDa fragment, and a secondary ELR site in the self-assembly domain generates an approximately 21 kDa fragment. In the case of plasmin, the cleavage site was identified close to the secondary MMP-7 cleavage site at RK in the KELRKV sequence in the self-assembly domain and generated a 21.5 kDa fragment. Consistent with the plasmin cleavage site being within the trimerization domain, we demonstrate an absence of trimer formation in the recombinant 6xHisLys145FasL protein corresponding to the plasmin-generated sFasL. However, we demonstrate that this fragment is pro-apoptotic in EC and activates Fas. These data are also consistent with the observation of Vargo-Gogola et al. who demonstrated that MMP-7, which also cleaves FasL in its trimerization domain, increases apoptosis in prostate epithelial cells and indicate that trimerization of sFasL is not necessary for its activity. This may explain why our results differ from the data of Mogi et al. Although these authors did not identify the MMP cleavage site in FasL, they reported the presence of soluble FasL trimers that prevented Fasmediated apoptosis. It is thus conceivable that the absence of trimerization domain in the case of cleavage by plasmin (and MMP-7) may be responsible for the pro-apoptotic activity of sFasL.
The demonstration that plasmin cleaves Fas-L in vitro does not necessarily mean that plasmin also cleaves Fas-L in vivo, as plasmin is inhibited in vivo by a series of physiological inhibitors other that PAI-1, like α2 anti-plasmin. Although α2 anti-plasmin primarily acts on the fibrinolytic system inside the vasculature, it is present in the extravascular space that is invaded by sprouting EC during angiogenesis and could inhibit the release of sFas-L by plasmin as suggested by our in vitro data (Figure 6E). However PAI-1 may play a more predominant role because it is made by EC and controls pericellular plasmin activity. The question of the role of PA-1 as the only regulator of angiogenesis remains open. Nevertheless our data showing an increase in apoptosis in EC in PAI-1 null mice, indicate that PAI-1 has a contributory function. The central role played by Fas/FasL in controlling EC apoptosis and angiogenesis is well recognized. Although quiescent EC express low levels of Fas, they become increasingly sensitized to Fas-mediated apoptosis under hypoxic conditions or when stimulated by VEGF or TNF-α (Sata and Walsh, 1998), and attachment of EC to ECM proteins is a known mechanism that protects them from Fas-mediated apoptosis. Hypoxia and VEGF have been shown to upregulate PAI-1 expression in EC (Dimova and Kietzmann, 2006), which raises the possibility that upregulation of PAI-1 under hypoxia or by VEGF could be critical for the survival of stimulated EC. This hypothesis is currently explored in our laboratory.
The protective role of PAI-1 on EC apoptosis could also be critical for conditions other than tumor angiogenesis. For example, it has been demonstrated that PAI-1 inhibits apoptosis in vascular smooth muscle cells during the formation of atherosclerotic plaques. When mice that are deficient in apolipoprotein E (apolipo E−/−) and show an increased formation of atherosclerotic plaque are crossed with PAI-1 deficient mice, there is a decrease in plaque formation that corresponds to an increase in apoptosis in vascular smooth muscle cells (Luttun et al., 2002). Our data suggest that in the absence of plasminogen/plasmin, there would be a stimulation of angiogenesis and wound healing. This is however not necessarily the case as in plasminogen deficient mice, there is an impairment in wound healing (Kortlever and Bernards, 2006) and an acceleration in PAI-1 deficient mice (Chan et al., 2001). However a critical feature in these models is the presence of thrombus and fibrin, as suggested by the observation that in mice deficient in plasminogen and fibrinogen there is a stimulation rather than inhibition of wound healing (Bugge et al., 1996). Our studies have not addressed the effect of PAI-1 deficiency on fibrin and have focused on pericellular plasmin activity and its local control over the release of cell surface-associated proteins like FasL. Although the formation of a provisional fibrin matrix is critical in wound healing, its role in tumor angiogenesis is less well understood. It is conceivable that the selective presence of PAI-1 stabilized by vitronectin in the pericellular space is a mechanism allowing plasmin to act distantly to degrade the provisional fibrin matrix without solubilizing FasL at the surface of EC. These aspects clearly deserve further investigation. Our data demonstrating a significant delay in angiogenesis in absence of host-derived PAI-1 provide an important insight into other vascular diseases than tumor angiogenesis. Whether PAI-1 could be a valuable target in controlling angiogenesis is an interesting but presently unanswered question that deserves further investigation.
EXPERIMENTAL PROCEDURES
Orthotopic Tumor Model
PAI-1 deficient mice (PAI-1−/−) and their corresponding wild type mice (PAI-1+/+) with a mixed genetic background of 87% C57BL/6 and 13% 129 strain (Carmeliet et al., 1993) were mated with Rag-1 deficient mice (Rag−/−: B6; 129s-Rag-1tm/Mom/J) purchased from Charles Rivers Laboratories (L’Arbresle, France), to generate PAI-1−/− and PAI-1+/+ in Rag-1−/− immunodeficient mice. Mice were anesthetized with Avertine and a 1 mm3 SK-N-BE(2) cl10 subcutaneous tumor fragment was sown on the left adrenal gland as described previously (Chantrain et al., 2004). Mice were sacrificed between 3 and 9 weeks after tumor implantation. Ex vivo angiographies and analysis of tumors were performed as previously described (Chantrain et al., 2004), and reported in supplemental procedures. All animal experiments were performed in accordance with the procedures established by the Institutional Animal Care and Usage Committee of Childrens Hospital Los Angeles under an approved protocol #41-05.
RNA Interference
A first siRNA duplex with sequences corresponding to nucleotides 457–477 of human PAI-1 cDNA (GenBank accession number: X12701) 5’-AAGGACGAGATCAGCACCACA-3’ was used for most of the experiments. A second nucleotide sequence corresponding to nucleotides 286–306 of human PAI-1 cDNA (5'-AACGTGGTTTTCTCACCCTAT-3') was used to confirm the effect of PAI-1 knock-down on apoptosis in HBMEC. The siRNA sequence for FasL was 5'-AACTGGGCTGTACTTTGTATA-3' (Ji et al., 2005). All siRNA duplex oligonucleotides were synthesized by Qiagen Sciences, Inc. (Valencia, CA). HBMEC were transfected over 5 hr with 40 nM of a PAI-1 siRNA, a FasL siRNA or a corresponding scrambled siRNA using either Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or TransMessenger Transfection Kit (Qiagen Sciences, Inc.). FITC-labeled siRNA was used to assess transfection efficiency.
Measurement of PAI-1 and sFasL Levels
PAI-1 and sFasL levels were determined using commercially available ELISA kits for human PAI-1 (American Diagnostica Inc., Stamford, CT) and human sFasL (R&D Systems Inc., Minneapolis, MN). The amount of protein in each sample was determined using a BCA protein assay kit (Pierce, Rockford, IL) and PAI-1 values were corrected for the amount of protein present in each sample.
Apoptosis
To evaluate apoptosis in HBMEC in culture, we used a TUNEL Apo-Direct Kit (BD Biosciences Pharmingen, San Diego, CA) or flow cytometry analysis of cells labeled with propidium iodide (5 µg/ml). After non-adherent cells were washed with PBS, adherent cells were harvested using trypsin-EDTA, resuspended in medium containing serum, collected by centrifugation and resuspended with non-adherent cells in 1 ml of 1% paraformaldehyde in PBS. After several washes with PBS, the cells were resuspended in 70% ethanol and stored at −20°C prior to being stained and analyzed by flow cytometry.
Flow Cytometry
Analysis of HBMEC by flow cytometry was done using the following primary antibodies: a mouse monoclonal antibody against human uPA (American Diagnostica, Inc.), a mouse monoclonal antibody against human uPAR (American Diagnostica, Inc.), a mouse monoclonal antibody against Fas (Alexis, San Diego, CA), a mouse monoclonal antibody against FasL (Alexis). We used a FITC-conjugated horse anti-mouse IgG (Vector Laboratories) as a secondary antibody. Cells were analyzed on a Beckman-Coulter Epics ESP cell sorter (Miami, FL) equipped with a 488 nm argon laser. Data were acquired and analyzed with EXPO32 software (Applied Cytometry Systems, Dinnington, Sheffield, UK).
Caspase-3, 8 and 9 Activities
Caspase-3 and caspase-8 activities were measured using the ApoTarget Caspase-3/CPP32 and Caspase-8/FLICE Colorimetric Protease Assay (Biosource, Camarillo, CA). The activity of each caspase was determined in 200 µg of cell lysate proteins suspended in 50 µl of extraction buffer. Absorbance at 405 nm using a spectrophotometer was determined after 24 hr of incubation with the substrate for each caspase. The presence of cleaved caspase-8 and 9 was examined by Western blot analysis using antibodies from Cell Signaling Technology (Danvers, MA).
uPA Proteolytic Activity
Ten µl of conditioned media or cell lysates were incubated with bovine plasminogen (Sigma, 0.1 mg/ml) and a chromogenic substrate for plasmin (Spectrozyme Pl; 0.5 mM; American Diagnostica, Inc.) in PBS. The reactions (100 µl) were carried out in the presence or the absence of amiloride (2 mM) in 96 well microtitration plates at room temperature for 20 min and the release of free p-nitroaniline from the chromogenic substrate was measured spectrophotometrically. The activity was determined in reference to a standard curve generated by the activity of a two chain uPA standard, corrected for the amount of protein in each sample and expressed in International Units (IU)/mg of proteins.
Zymographic Analysis of Plasmin Generation
α-casein 1 mg/ml (INC Biomedicals, Costa Mesa, CA) was incorporated in 0.1% SDS, 8% polyacrylamide gels. Aliquots of conditioned media and cell lysates containing equal amounts of proteins were mixed with loading buffer (10% SDS, 4% sucrose, 0.25 M Tris-HCl pH 6.8, 0.1% bromophenol blue). After electrophoresis, SDS was eluted from the gel by 2 washes (30 min each) of 100 mM glycine, 2.5% Triton X-100 buffer (pH 8.0). The gels were incubated overnight at 37°C in 100 mM glycine buffer (pH 8.0), stained in 0.1% Coomassie Brilliant Blue and destained in 20% methanol-10% acetic acid.
Mutagenesis of FasL
The two amino acid substitutions Arg144 → Val144 and Lys145 → Ala145 were introduced in the plasmin cleavage site of FasL using the Quick Change Mutagenesis kit (Promega, Madison, WI) according to the manufacturer's instructions and a human FasL cDNA subcloned into BamHI pcDNA 3.1-Neo (Invitrogen) expression vector (Vargo-Gogola et al., 2002) kindly provided by Dr. L. Matrisian, (Vanderbilt University, Nashville, TN). For the substitution we used the following pair of primers: forward 5'-CCTGAAAAAAAGGAGCTGGTGGCAGTGGCCCATTTAACAGGC-3'; reverse 5'-GCCTGTTAAATGGGCCACTGCCACCAGCTCCTTTTTTTCAGG-3'.
Matrigel Angiogenic Assay
Matrigel (Becton Dickinson) was added (300 µl) to each well of a 24-well plate and allowed to polymerize for 1 hr at 37°C. 4 × 104 HBMEC in culture medium were then added into the coated wells. Cells were incubated for 24 hr at 37°C, viewed under an Olympus CKX 41 microscope, and photographed using PictureFrame 2.1 software (Optronics, Goleta, CA). The numbers of branches were counted in five different fields in triplicate wells.
Statistical Evaluation
Results are represented as mean ± standard deviation. For in vivo data, statistical analysis was performed using a nonparametric Mann-Whitney U test. In vitro experiments were performed in triplicate and repeated at least two times, and the analysis was done using a two-tailed student's test. P values ≤ 0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a NIH grant CA81403 to Y.A.D. We thank F. Codrea and J. Barnhart of The Saban Research Institute FACS Core Facility for processing the FACS samples, and J. Rosenberg for assistance in preparing the manuscript. K.B. was the recipient of a Career Development Fellowship Award from The Saban Research Institute of CHLA and of a Postdoctoral Travel Fellowship Award from the Rose and Jean Hoguet Foundation, Kankerologische Stichting St Michiel Foundation, Mr et Mm F. Braconier-Lamarche, Centre Anticancereux of Liege. K.B. is currently a recipient of a Return Grant from the Federal Office for Scientific Research (Belgium). We thank Prof. P. Declerck and A. Gils (Leuven, Belgium) for providing us with stable rPAI-1, and Dr. D. Shalinsky (Agouron Pharmaceuticals, Inc., San Diego, CA) for the gift of AG3340.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aoudjit F, Vuori K. Engagement of the alpha2beta1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood. 2000;95:2044–2051. [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, Carmeliet P, Foidart JM, Noel A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23:6986–6990. doi: 10.1038/sj.onc.1207859. [DOI] [PubMed] [Google Scholar]
- Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noel A. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J. Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- Blasi F, Verde P. Urokinase-dependent cell surface proteolysis and cancer. X. Semin. Cancer Biol. 1990;1:117–126. [PubMed] [Google Scholar]
- Bouchet D, Tesson L, Menoret S, Charreau B, Mathieu P, Yagita H, Duisit G, Anegon I. Differential sensitivity of endothelial cells of various species to apoptosis induced by gene transfer of Fas ligand: role of FLIP levels. Mol. Med. 2002;8:612–623. [PMC free article] [PubMed] [Google Scholar]
- Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- Cappellesso S, Thibault G, Hoarau C, Watier H, Bardos P, Lebranchu Y. Induction of Jurkat T-cell apoptosis by Fas ligand-transfected endothelial cells. Transplant. Proc. 2000;32:2737–2738. doi: 10.1016/s0041-1345(00)01858-3. [DOI] [PubMed] [Google Scholar]
- Cardier JE, Schulte T, Kammer H, Kwak J, Cardier M. Fas (CD95, APO-1) antigen expression and function in murine liver endothelial cells: implications for the regulation of apoptosis in liver endothelial cells. FASEB J. 1999;13:1950–1960. doi: 10.1096/fasebj.13.14.1950. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Stassen JM, Schoonjans L, Ream B, Van den Oord JJ, De Mol M, Mulligan RC, Collen D. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J. Clin. Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Duszczyszyn DA, Castellino FJ, Ploplis VA. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am. J. Pathol. 2001;159:1681–1688. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2002;22:887–893. doi: 10.1161/01.atv.0000017728.55907.a9. [DOI] [PubMed] [Google Scholar]
- Dano K, Behrendt N, Hoyer-Hansen G, Johnsen M, Lund LR, Ploug M, Romer J. Plasminogen activation and cancer. Thromb. Haemost. 2005;93:676–681. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J. Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova EY, Kietzmann T. Cell type-dependent regulation of the hypoxia-responsive plasminogen activator inhibitor-1 gene by upstream stimulatory factor-2. J Biol. Chem. 2006;281:2999–3005. doi: 10.1074/jbc.M512078200. [DOI] [PubMed] [Google Scholar]
- Foekens JA, Look MP, Peters HA, van Putten WL, Portengen H, Klijn JG. Urokinase-type plasminogen activator and its inhibitor PAI-1: predictors of poor response to tamoxifen therapy in recurrent breast cancer. J. Natl. Cancer Inst. 1995;87:751–756. doi: 10.1093/jnci/87.10.751. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis and apoptosis. Semin. Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- Ganesh S, Sier CF, Griffioen G, Vloedgraven HJ, de Boer A, Welvaart K, van de Velde CJ, van Krieken JH, Verheijen JH, Lamers CB. Prognostic relevance of plasminogen activators and their inhibitors in colorectal cancer. Cancer Res. 1994;54:4065–4071. [PubMed] [Google Scholar]
- Ji J, Wernli M, Mielgo A, Buechner SA, Erb P. Fas-ligand gene silencing in basal cell carcinoma tissue with small interfering RNA. Gene Ther. 2005;12:678–684. doi: 10.1038/sj.gt.3302453. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Bernards R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle. 2006;5:2697–2703. doi: 10.4161/cc.5.23.3510. [DOI] [PubMed] [Google Scholar]
- Luttun A, Lupu F, Storkebaum E, Hoylaerts MF, Moons L, Crawley J, Bono F, Poole AR, Tipping P, Herbert JM, Collen D, Carmeliet P. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:499–505. doi: 10.1161/hq0302.104529. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- Mogi M, Fukuo K, Yang J, Suhara T, Ogihara T. Hypoxia stimulates release of the soluble form of fas ligand that inhibits endothelial cell apoptosis. Lab Invest. 2001;81:177–184. doi: 10.1038/labinvest.3780225. [DOI] [PubMed] [Google Scholar]
- Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 1997;16:2610–2620. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 1999;9:1441–1447. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J. Biol. Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- Sata M, Walsh K. TNFalpha regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat. Med. 1998;4:415–420. doi: 10.1038/nm0498-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Ma LQ, Sun B, Shimada H, Laug WE, Seeger RC, DeClerck YA. The plasminogen-plasminogen activator (PA) system in neuroblastoma: Role of PA inhibitor-1 in metastasis. Cancer Res. 1999;59:1327–1336. [PubMed] [Google Scholar]
- Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch. Biochem. Biophys. 2002;408:155–161. doi: 10.1016/s0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat. Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.