Abstract
Many ion channels are attractive therapeutic targets for the treatment of neurological or cardiovascular diseases; there is a continuous need for selective channel-antagonists and/or agonists. Recently, several technologies have been developed that make exploration of the enormous diversity of venom-derived peptidic toxins more feasible. Integration of exogenomics with synthetic methods such as diselenide or fluorous bridges, backbone spacers and N-to-C cyclization provides an emerging technology that promises to accelerate discovery and development of natural products-based compounds. These drug discovery efforts are complemented by novel approaches to modulate the activities of ion channels and receptors. Taken together, these technologies will advance our knowledge and understanding of ion channels and will accelerate their expansion as targets for first-in-class therapeutics.
Introduction
Ion channels and receptors are key membrane proteins that control excitability of cells. Precise regulation of their activities is crucial for normal function of the nervous system, muscles, and secretory organs; loss of the balance between excitability and inhibition leads to pathological states. Numerous ion channels and receptors are therapeutic targets for the treatment of neurological disorders (e.g. pain, epilepsy), cardiovascular and metabolic diseases, and over 13% of currently FDA-approved drugs act by modulation of voltage- and ligand-gated ion channels. In order to validate new therapeutic targets, highly selective and potent antagonists or agonists are a prerequisite. Intensive efforts by medicinal chemists have provided only a handful of small molecules that modulate activity of ion channels, but they often lack high selectivity and/or potency.
In search for new, highly-selective ligands targeting ion channels and receptors, peptide-based natural products, namely neurotoxins, continue to dominate a discovery pipeline [1]. Neurotoxins from venomous spiders, scorpions or mollusks comprise a group of millions of unique, disulfide-rich peptides. These peptides provide an evolutionary advantage for the venomous animals, since they are used to capture a prey and for self-defense. For example, Conus snails have spent the last 50 million years to master conotoxins that can effectively shut down the fish nervous system, allowing an “easy catch”. Although only a small fraction of naturally occurring toxins has been studied and characterized to-date, it is clear that venom peptides provide invaluable pharmacological tools to study structure and function of ion channels, as well as make very promising drug candidates, some already approved by the FDA [1].
How large is the pool of toxins that target ion channels? With over 500 Conus snails species, each producing 100–200 different conotoxins, the molecular diversity of compounds exceeds 50,000 from Conus alone. Moreover, novel peptide-based toxins were recently discovered from venomous mollusks from the turrid group (Turridae) that comprise approximately 10,000 species, suggesting a new source of diverse neurotoxins [2]. Venoms of sea anemone also contain a large variety of peptidic neurotoxins targeting ion channels [3,4]. 1,500 species of scorpions are “expected” to produce over 100,000 venom components, with greater than 99% yet to be characterized. However, the largest biodiversity of neurotoxins, measured in millions of peptides, is a result of 38,000 species of spiders evolving for the last 400 million years [5]. As illustrated in Figure 1, combined pools of toxins from Conus and Turridae snails, scorpions and spiders result in a large, yet biased, combinatorial library of neuroactive natural products. This review will focus on the latest technological developments that allow accelerated exploration of this mega-diverse source of novel ligands that target ion channels and receptors.
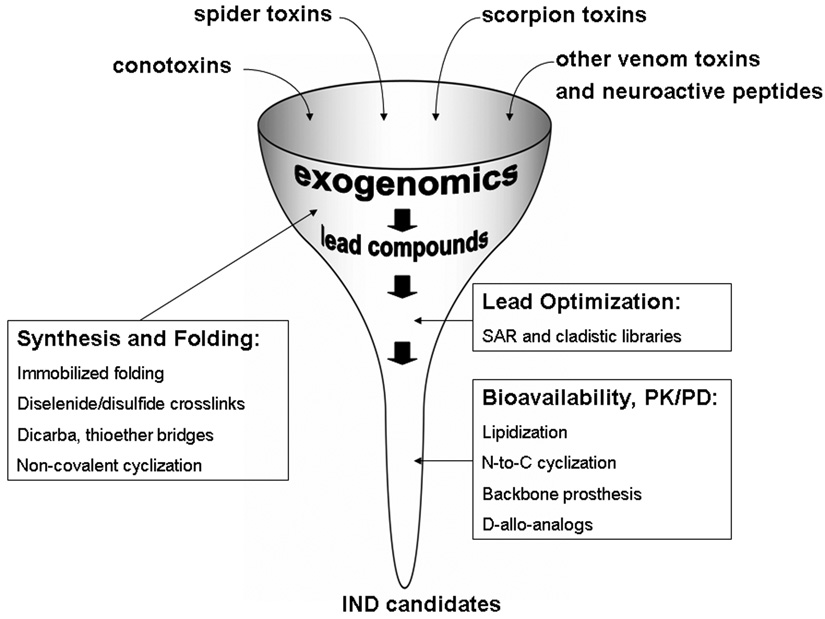
Figure 1.
Integrating the discovery pipeline for toxin-based compounds targeting ion channels and receptors. Conotoxins, spider and scorpion toxins provide access to hundreds of thousands of distinct peptide-based compounds targeting ion channels. Current efforts in molecular cloning and venomics are focused on structural characterization of individual components of the venoms. Phylogeny-based exogenomics strategy facilitates mining megadiverse groups of the toxins. Advances in the chemical synthesis and the oxidative folding provide faster access to hundreds of potential lead compounds. New approaches also accelerate lead optimization and improvement of pharmaceutical and pharmacological properties of future investigational new drug (IND) candidates.
Discovery via venomics and exogenomics
Two complementary strategies have been recently implemented to accelerate mining the molecular diversity of venom-derived toxins: venomics and exogenomics [6,7••]. Venomics employs advanced mass spectrometry techniques to obtain structural information about toxins [8]. MALDI-TOF MS or electrospray ionization MS, often coupled to liquid chromatography, allow to profile whole venoms (venom fingerprinting) or to de novo sequence individual venom components. Whereas venomics focuses on analyzing venom toxins by mass spectrometry, exogenomics described below, is based on studying exogenous genes obtained from cDNA and/or genomic libraries. Since both, venomics and exogenomics provide complementary information about structure of toxins, they will likely merge into an integrated discovery platform [9,10]. Indeed, the recent report on the discovery novel O-superfamily conotoxins was based on merging molecular cloning and mass spectrometry analysis [11]. As more sequences are currently being derived from the cloning efforts, the mass spectrometry based screening becomes a very useful tool to discover the native peptides from venoms.
Exogenomics is a drug-discovery strategy that employs phylogenetic analysis to rationalize and simplify screening of megadiverse, yet related, gene-based natural products. Evolutionarily rapidly diverging toxin families comprise similar peptides, yet with differing selectivity profiles; thus narrowing the number of possible peptides to be screened for a given target. This strategy, recently articulated by Olivera [7••], has been successfully used to identify novel conopeptides that target specific subtypes of sodium channels [12], as well nicotinic acetylcholine receptors [13]. In the case of sodium channel blocking conotoxins, the initial finding and characterization of µ-conotoxin SmIIIA [14,15], produced by Conus stercusmuscarum, led to a subsequent discovery of more selective toxins, KIIIA and SIIIA (Figure 2) derived from C. kinoshitai and C. striatus, respectively [12,16]. Exogenomics strategy played an important role in a discovery on new analgesic compound from Conus venoms, RgIA (Figure 2), that identified a novel analgesic mechanism: blocking nicotinic acetylcholine receptors (nAChRs) [13,17,18]. RgIA, which targets α9α10 nAChRs with low nanomolar potency, is from C. regius, a species closely related to C. imperialis (a source of similar conotoxins α-ImI and α-ImII, which target α7 nAChRs) [13]. Thus, the exogenomics-based discovery efforts have already resulted in several subtype-selective ligands for the ion channels and receptors: this approach is likely to accelerate an expansion of repertoire of peptides belonging to the individual gene families.
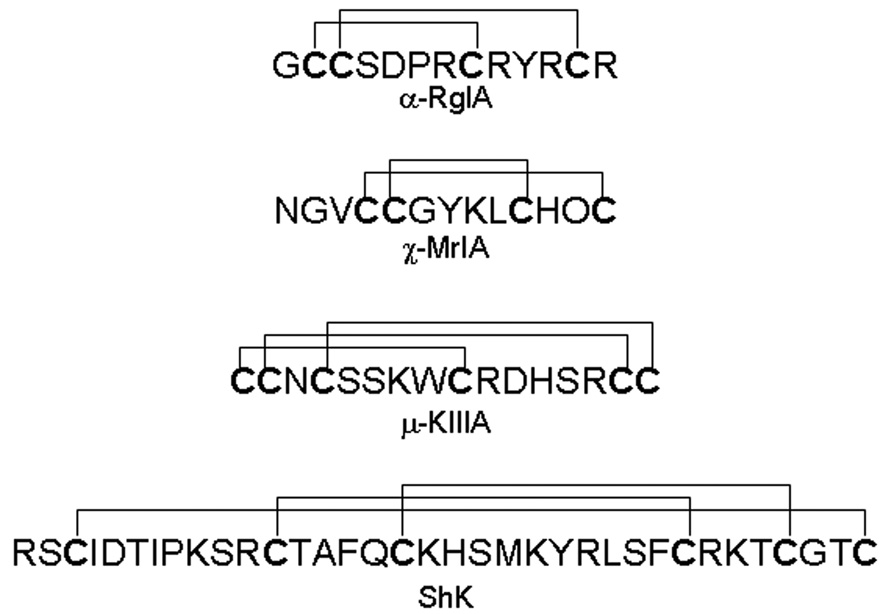
Figure 2.
Structures of selected toxins discussed in this review. Note the diversity of primary amino acid sequences and the disulfide scaffolds. α-Conotoxins target nicotinic acetylcholine receptors, whereas χ-conotoxin MrIA (currently in the human clinical trials) is an inhibitor of norepinephrine transporter. µ-Conotoxin KIIIA almost irreversibly blocks neuronal sodium channel Nav1.2 and exhibits potent analgesic activity in the animal model of inflammatory pain. ShK toxin derived from sea anemone blocks potassium channel subtypes Kv1.1 and Kv1.3 and has a therapeutic potential for the treatment of multiple sclerosis.
The phylogenetic (cladistic) analysis of conotoxins can also be used for designing biased combinatorial libraries, since sequence alignments of closely related toxins are effective in identification of conserved (“scaffold”) and hypervariable parts (“hot spots”) of the toxins [19]. The hypervariable “hot-spots” are arguably a result of a co-evolution of Conus toxins and the intended preys’ receptors/ion channels; thus replacements of amino acid residues within “hot-spots” might optimize interactions with a target ion channel. For example, synthesis and deconvolution of α-conotoxin-based libraries resulted in a novel ligand, α-exoconotide 1, that exhibited significantly longer off-rate toward α7 nAChRs [20]. Such “cladistic” libraries, although still in the prototype, rather than in the mainstream development stage, are more likely to facilitate the lead optimization efforts, when the target subtype selectivity or/and pharmaceutical properties need to be improved.
To facilitate exogenomics-based drug discoveries, comprehensive databases of toxins structure-function data must be developed. Recently established ConoServer compiled over 2,200 peptidic sequences of conotoxins [21]. Tox-Prot is a toxin-based annotation program that summarizes entries of toxins in the Swiss-Prot database [22], but as of 2008, “only” ~2,400 sequences were deposited. A slightly larger number of toxin sequences (over 3,000) currently exist in the Animal Toxin Database [23]. Analyzing the fast growing number of toxin sequences, their phylogenetic relationships and bioactivities will become critical in the next few years to advance exogenomics applications.
The challenge of oxidative folding of disulfide-rich toxins
Venom-derived toxins from spiders, scorpions, and cone and turrid snails can be highly crosslinked with disulfide bridges. The advantages of the disulfide-rich scaffolds exploited by these venomous animals are apparent: (1) increased stability of the bioactive conformation in the extracellular environment, and (2) availability of a repertoire of privilege scaffolds for evolving hypervariable sequences within the intercysteine loops. However, a significant disadvantage of the disulfide-rich peptides (for peptide chemists, not predatory animals) is their oxidative folding, the formation of the native disulfide bridges. The oxidative folding problem for small, disulfide-rich peptides, such as conotoxins was recently assessed [24], but it also applies to scorpion and spider toxins [25]. In particular, folding of peptides containing more than two disulfide bridges poses a challenge, since the regioselective folding of peptides with the orthogonal protection on pairs of Cys residues no longer is effective. Thus, the main bottleneck for exploring a molecular diversity of disulfide-rich toxins is an efficient, high-throughput (HT) synthetic method that allows production of hundreds of sequences that can be screened against target ion channels (Figure 1).
Three oxidation strategies may be compatible with a high-throughput synthesis of the Cys-rich peptides: oxidative folding chromatography [25], immobilized folding [26,27] and disulfide/diselenide crosslinks [28•]. The first two strategies are based on a pseudo-dilution principle, so that the formation of intramolecular disulfide bond is favored over the intermolecular bond formation. Perhaps, the most promising strategy for improved oxidative folding of Cys-rich peptides is the introduction of diselenide bridges; this strategy was previously applied to other proteins [29], but recently was used in α-conotoxin ImI [28•]. In a very elegant work, three analogs of α-conotoxin ImI were designed in which each of two pairs of Cys (or both pairs) were replaced by Sec (selenocysteine) residues. Resulting α-selenoconotoxins were not only very similar to the wild-type α-ImI, as judged by NMR, but they also exhibited comparable potency in blocking α7 nAChRs. Furthermore, since a diselenide bond is more stable than a disulfide bond, the Sec-containing analogs appeared more stable under different redox conditions. Compatibility of selenocysteines with the Fmoc-based chemistry makes this approach very attractive for HT synthesis protocols [30].
An alterative approach to simplify the oxidative folding of the disulfide-rich toxins is replacement of disulfide bridges by other covalent crosslinks: conotoxins containing dicarba or thioether bridges have been reported [31,32]. Since a preexisting crosslink can topologically affect the formation of subsequent disulfide bridges, this strategy might be optimized for HT synthesis of Cys-rich peptides. Recent publications on replacing disulfide bridges by noncovalent interactions of allyl glycine residues [33] and on stabilizing effects of fluorous amino acids in polypeptides [34,35] raised a possibility that disulfide bridges might be effectively replaced by “fluorous bridges” (interactions between fluorocarbons can significantly stabilize the native peptide conformation). Thus, replacing a pair of Cys residues that form the native disulfide bridge with, for example a pair of hexafluoroleucines, may provide a sufficient stabilizing effect that would: (1) facilitate the formation of the remaining disulfide bridges, and (2) stabilize the bioactive conformation via disulfide/fluorous bridges. Although still in a conceptual stage, such “non-covalent cyclization”, when in combination with diselenide/disulfide bridges may revolutionize future synthetic strategies for disulfide-rich peptides (Figure 3).
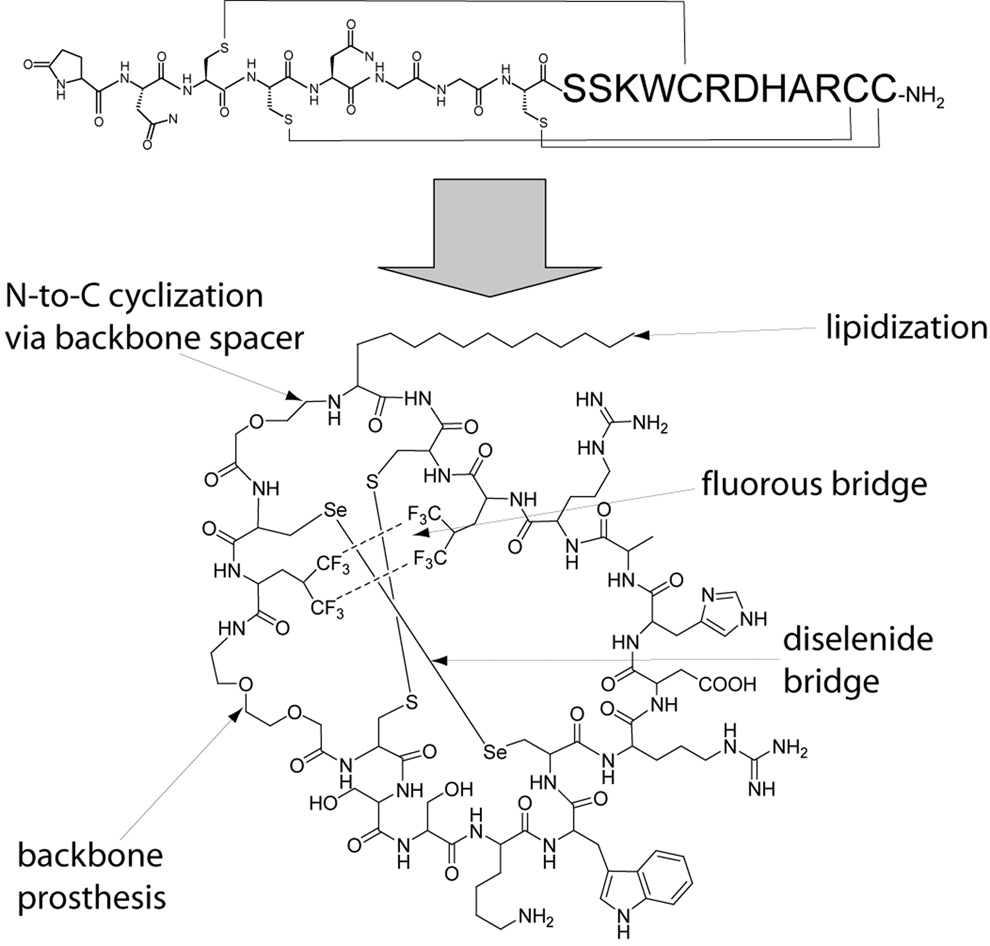
Figure 3.
Prototype conotoxin-based drug exhibiting oral bioavailability and simplified oxidative folding properties. Upper structure represents unmodified µ-conotoxin SIIIA, which is a blocker of neuronal sodium channels and a potent analgesic compound following systemic administration [42]. Lower structure represents a hypothetical polytide analog of µ-SIIIA [42] in which the N-terminal backbone spacer is coupled to both a lipoamino acid and the C-terminus. This lipidized and N-to-C cyclized analog should exhibit improved oral bioavailability. To simplify the oxidative folding of such an non-natural analog, two of the native disulfide bridges are replaced by a diselenide bridge and a “non-covalent crosslink” via fluorous hydrophobic-bonding interaction.
Improving bioavailability and pharmacology of lead compounds
Four different types of chemical modifications have been recently applied to neurotoxins: lipidization, N-to-C cyclization, backbone prosthesis and D-diastereomerization. Two lipoamino acid (LAA) analogs of α-conotoxin MII were synthesized in which 2-aminododecanoic acid was coupled to either the N-terminus or replaced Asn5 [36]. The peptide with LAA conjugated to the N-terminus maintained the overall conformation and the bioactivity similar to that of the native peptide. Interestingly, despite increased penetration of the LAA-MII across the Caco-2 cell monolayer, the lipidization improved neither oral nor CNS bioavailability, but increased the accumulation of the analog in the liver [37]. Since LAA-containing peptides are generally known to exhibit better bioavailability and stability, this modification is likely to be explored in other toxins.
N-to-C backbone cyclization, naturally occurring in disulfide-rich cyclotides, is a proven strategy to increase the stability and bioavailability of peptide drug candidates [38], and was recently applied to two conotoxins, α-MII and χ-MrIA (Figure 2) [39,40]. Both peptides possess two disulfide bridges, making the backbone cyclization as the third crosslink. Highly stable analogs of cyclized α-conotoxin MII were designed, synthesized and characterized using NMR and biological assay. The cyclized α-MII analog appeared as active as the unmodified MII in blocking nAChRs-mediated currents, but exhibited better stability against proteinases and plasma [39]. Another example of the bioactive cyclized conotoxin, χ-MrIA, confirmed that this strategy is applicable to a broader group of neuroactive toxins [40]. An excellent review summarizing chemical modifications applied to conopeptides was recently published [41]; it is apparent that both the stability and the activity of these peptides can be significantly improved.
To further improve the properties of toxins as potential therapeutics, the non-essential parts of three-disulfide bridged µ-conotoxin SIIIA were replaced with the isostere, nonpeptidic backbone spacers [42•]. This strategy, called “backbone prosthesis” resulted in two analogs of µ-SIIIA, namely PEG-SIIIA (containing two amino-3-oxapentanoic acid, PEG-spacer) and AHX-SIIIA (containing 6-aminohexanoic acid). Noteworthy, “non-essential” parts of µ-SIIIA were identified using the cladistic/exogenomic strategy, rather than more traditional structure-activity relationship studies [43]. Analogs of µ-SIIIA containing the backbone spacers appeared better blockers of neuronal sodium channels and very active analgesics in mice model of the inflammatory pain. Surprisingly, PEG-SIIIA was not only 20-fold more potent analgesic, as compared to the unmodified toxin, but also exhibited faster onset and significantly longer duration of analgesia. This work coined a concept of polytides (polymer-peptides hybrids); disulfide-rich scaffolds containing toxin’s pharmacophore amino acid residues and isostere polymeric spacers that replaced nonessential residues. As described later, such polytides can be further modified by N-to-C backbone cyclization and lipidization making them orally-bioavailable drug candidates (Figure 3).
The Norton group designed, synthesized and characterized the D-diastereomer of ShK toxin (Figure 2) that targets Kv1.3 and 1.1 subtypes of potassium channels [44•]. The native ShK toxin has a picomolar potency in blocking Kv1.3 that plays a critical role in activating the adaptive immune response via T lymphocytes. This immunosuppressive peptide is a promising lead for multiple sclerosis. Since ShK has a short in vivo half-life, the D-allo-ShK analog was designed to improve resistance to a proteolytic degradation. Interestingly, the all D-analog could form the three native disulfide bridges with high yields, exhibited nanomolar potency in blocking Kv1.3 and was resistant to proteolysis. Although the in vivo half-life was not significantly improved (suggesting renal clearance), a lack of immunogenicity provides an additional advantage of toxins containing all Damino acids.
Advances in alternative ligands and methods to modulate the activity of ion channels
An enormous diversity of venom-based peptidic toxins will continue to provide novel ligands for ion channels, but other marine natural products, such as hybrid polyketide/nonribosomal peptides may also emerge as a rich supply of neuromodulators. Although, the original discovery of mixed polyketide-peptide jamaicamides (sodium channel blockers derived from marine cyanobacteria) [45] has not (yet) been expanded into a larger source of ion channel modulators, the latest report on rapamycin analogs that blocked L-type voltage-gated calcium channels suggests that polyketide-peptide macrolides might be promising scaffolds for engineering ion channel blockers. Further exploration and optimization of macrolide-based peptides via combinatorial libraries, as recently reported by the Schmidt group [46,47], may lead to novel compounds with improved selectivity and potency for relevant voltage-gated ion channels.
Although beyond the scope of this review, current drug discovery efforts are complemented by novel biological and chemical means of modulating ion channels and receptors. In a very elegant work, Yang and coworkers [48] described genetically encoded Ras-like GTPases that could control calcium currents via small molecule-induced targeting to cell membranes. Such chemical genetic approach has the advantage over pharmacological intervention in transgenic animals in which specific parts of the nervous system could express the genetically engineered proteins. The spatial control of the activity of ion channels or receptors can also be accomplished by introduction of photoswitches [49]. For example, conjugation of a glutamate photoswitch containing azobenzene linker to ionotropic glutamate receptors allowed not only “on/off” control of receptor activity, but also the fraction of active channels was controllable by changing the wavelength of the light [50,51].
Conclusions and outlook
In summary, the unprecedented molecular diversity of the disulfide-rich neurotoxins will continue to provide novel compounds that exhibit superb selectivity and potency against therapeutically-relevant ion channels and receptors. Advances in genetic, chemical synthesis and HT screening methods will accelerate mining this megadiverse, yet biased library of the natural products. In particular, merging of advances in solid-phase synthesis and oxidative folding of cysteine-rich peptides will play a critical role in upcoming years. As structure-function relationship data for thousands of peptide-based toxins are being acquired, we can start defining the drug-design principles employed by “natural medicinal and peptide chemists” such as venomous gastropods, spiders and scorpions; this lesson will likely aid in better design of peptide-based therapeutics. For example, to improve pharmacological and pharmaceutical properties of conotoxins, Conus snails have been aggressively blending various posttranslational modifications [52]. However, this strategy has yet to be fully appreciated, much less exploited, by medicinal and peptide chemists, since all previous attempts to improve toxins as potential drugs included applications of a only single type of chemical modification at a time. To illustrate the challenge in developing toxin-based therapeutics in the near-future, Figure 3 shows a model drug that embodies current advances in designing next-generation, orally-bioavailable analgesic conotoxins whose manufacture is facilitated by simplified oxidative folding properties. Current technological advances being explored in both academic laboratories and in biotech companies will continue to provide new toxin-based channel-antagonists and/or agonists as future first-in-class therapeutics.
Acknowledgements
Financial support from NIH Program Project Grant GM 48677 and R21 NS055845 is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
GB is a scientific co-founder of NeuroAdjuvants, Inc.
References
- 1.Beeton CE, Gutman GA, Chandy KG. Targets and Therapeutic Properties of Venom Peptides. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Academic Press; 2005. pp. 403–414. [Google Scholar]
- 2.Watkins M, Hillyard DR, Olivera BM. Genes expressed in a turrid venom duct: divergence and similarity to conotoxins. J Mol Evol. 2006;62:247–256. doi: 10.1007/s00239-005-0010-x. [DOI] [PubMed] [Google Scholar]
- 3.Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon. 2007;49:271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Bosmans F, Tytgat J. Sea anemone venom as a source of insecticidal peptides acting on voltage-gated Na+ channels. Toxicon. 2007;49:550–560. doi: 10.1016/j.toxicon.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sollod BL, Wilson D, Zhaxybayeva O, Gogarten JP, Drinkwater R, King GF. Were arachnids the first to use combinatorial peptide libraries? Peptides. 2005;26:131–139. doi: 10.1016/j.peptides.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Escoubas P, Quinton L, Nicholson GM. Venomics: unravelling the complexity of animal venoms with mass spectrometry. J Mass Spectrom. 2008;43:279–295. doi: 10.1002/jms.1389. [DOI] [PubMed] [Google Scholar]
- 7. Olivera BM. Conus peptides: biodiversity-based discovery and exogenomics. J Biol Chem. 2006;281:31173–31177. doi: 10.1074/jbc.R600020200. This is the first publication that describes the phylogenetic-based strategy to accelerate discovery of new gene-products from megadiverse families. Definition, principles and applications of the exogenomics are provided, along with an example of a discovery of subtype selective ligands for nicotinic acetycholine receptors.
- 8.Favreau P, Menin L, Michalet S, Perret F, Cheneval O, Stocklin M, Bulet P, Stocklin R. Mass spectrometry strategies for venom mapping and peptide sequencing from crude venoms: case applications with single arthropod specimen. Toxicon. 2006;47:676–687. doi: 10.1016/j.toxicon.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Escoubas P, Sollod B, King GF. Venom landscapes: mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon. 2006;47:650–663. doi: 10.1016/j.toxicon.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Jakubowski JA, Keays DA, Kelley WP, Sandall DW, Bingham JP, Livett BG, Gayler KR, Sweedler JV. Determining sequences and post-translational modifications of novel conotoxins in Conus victoriae using cDNA sequencing and mass spectrometry. J Mass Spectrom. 2004;39:548–557. doi: 10.1002/jms.624. [DOI] [PubMed] [Google Scholar]
- 11.Gowd KH, Dewan KK, Iengar P, Krishnan KS, Balaram P. Probing peptide libraries from Conus achatinus using mass spectrometry and cDNA sequencing: identification of δ and ω-conotoxins. J Mass Spectrom. 2008;10:141–155. doi: 10.1002/jms.1377. [DOI] [PubMed] [Google Scholar]
- 12.Bulaj G, West PJ, Garrett JE, Watkins M, Zhang MM, Norton RS, Smith BJ, Yoshikami D, Olivera BM. Novel conotoxins from Conus striatus and Conus kinoshitai selectively block TTX-resistant sodium channels. Biochemistry. 2005;44:7259–7265. doi: 10.1021/bi0473408. [DOI] [PubMed] [Google Scholar]
- 13.Ellison M, Haberlandt C, Gomez-Casati ME, Watkins M, Elgoyhen AB, McIntosh JM, Olivera BM. α-RgIA: a novel conotoxin that specifically and potently blocks the alpha9alpha10 nAChR. Biochemistry. 2006;45:1511–1517. doi: 10.1021/bi0520129. [DOI] [PubMed] [Google Scholar]
- 14.West PJ, Bulaj G, Garrett JE, Olivera BM, Yoshikami D. µ-Conotoxin SmIIIA, a potent inhibitor of tetrodotoxin-resistant sodium channels in amphibian sympathetic and sensory neurons. Biochemistry. 2002;41:15388–15393. doi: 10.1021/bi0265628. [DOI] [PubMed] [Google Scholar]
- 15.Keizer DW, West PJ, Lee EF, Yoshikami D, Olivera BM, Bulaj G, Norton RS. Structural basis for tetrodotoxin-resistant sodium channel binding by µ-conotoxin SmIIIA. J Biol Chem. 2003;278:46805–46813. doi: 10.1074/jbc.M309222200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang MM, Green BR, Catlin P, Fiedler B, Azam L, Chadwick A, Terlau H, McArthur JR, French RJ, Gulyas J, et al. Structure/function characterization of µ-conotoxin KIIIA, an analgesic, nearly irreversible blocker of mammalian neuronal sodium channels. J Biol Chem. 2007;282:30699–30706. doi: 10.1074/jbc.M704616200. [DOI] [PubMed] [Google Scholar]
- 17.Ellison M, Feng ZP, Park AJ, Zhang X, Olivera BM, McIntosh JM, Norton RS. Alpha-RgIA, a novel conotoxin that blocks the α9α10 nAChR: structure and identification of key receptor-binding residues. J Mol Biol. 2008;377:1216–1227. doi: 10.1016/j.jmb.2008.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM. Molecular mechanism for analgesia involving specific antagonism of α9α10 nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A. 2006;103:17880–17884. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulaj G, Olivera BM. Method of Making a Library of Phylogenetically Related Sequences. US Patent. 2004
- 20.Ellison M, Nielsen J, Green BR, Buczek P, McIntosh JM, Olivera BM, Bulaj G. Synthesis, Folding and Deconvolution of Combinatorial Peptide Libraries Based on alpha-Conotoxins: Generation of a Novel Ligand for α-7 Nicotinic Acetylcholine Receptors. In: Chorev M, Sawyer TK, editors. 18th American Peptide Symposium; Boston. American Peptide Society; 2003. pp. 841–842. [Google Scholar]
- 21.Kaas Q, Westermann JC, Halai R, Wang CK, Craik DJ. ConoServer, a database for conopeptide sequences and structures. Bioinformatics. 2008;24:445–446. doi: 10.1093/bioinformatics/btm596. [DOI] [PubMed] [Google Scholar]
- 22.Jungo F, Bairoch A. Tox-Prot, the toxin protein annotation program of the Swiss-Prot protein knowledgebase. Toxicon. 2005;45:293–301. doi: 10.1016/j.toxicon.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 23.He QY, He QZ, Deng XC, Yao L, Meng E, Liu ZH, Liang SP. ATDB: a uni-database platform for animal toxins. Nucleic Acids Res. 2008;36:D293–D297. doi: 10.1093/nar/gkm832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulaj G, Olivera BM. Folding of conotoxins: formation of the native disulfide bridges during chemical synthesis and biosynthesis of conus peptides. Antioxid Redox Signal. 2008;10:141–156. doi: 10.1089/ars.2007.1856. [DOI] [PubMed] [Google Scholar]
- 25.Altamirano MM, Garcia C, Possani LD, Fersht AR. Oxidative refolding chromatography: folding of the scorpion toxin Cn5. Nat Biotechnol. 1999;17:187–191. doi: 10.1038/6192. [DOI] [PubMed] [Google Scholar]
- 26.Darlak K, Wiegandt Long D, Czerwinski A, Darlak M, Valenzuela F, Spatola AF, Barany G. Facile preparation of disulfide-bridged peptides using the polymer-supported oxidant CLEAR-OX. J Pept Res. 2004;63:303–312. doi: 10.1111/j.1399-3011.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 27.Green BR, Bulaj G. Oxidative folding of conotoxins in immobilized systems. Protein Pept Lett. 2006;13:67–70. doi: 10.2174/092986606774502162. [DOI] [PubMed] [Google Scholar]
- 28. Armishaw CJ, Daly NL, Nevin ST, Adams DJ, Craik DJ, Alewood PF. α-Selenoconotoxins, a new class of potent α7 neuronal nicotinic receptor antagonists. J Biol Chem. 2006;281:14136–14143. doi: 10.1074/jbc.M512419200. This is the first example of a successful application of diselenide bridges into conotoxins. Structural and functional data support using this strategy to engineer novel analogs of various peptidic toxins.
- 29.Moroder L. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J Pept Sci. 2005;11:187–214. doi: 10.1002/psc.654. [DOI] [PubMed] [Google Scholar]
- 30.Harris KM, Flemer S, Jr, Hondal RJ. Studies on deprotection of cysteine and selenocysteine side-chain protecting groups. J Pept Sci. 2007;13:81–93. doi: 10.1002/psc.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bondebjerg J, Grunnet M, Jespersen T, Meldal M. Solid-phase synthesis and biological activity of a thioether analogue of conotoxin G1. Chembiochem. 2003;4:186–194. doi: 10.1002/cbic.200390030. [DOI] [PubMed] [Google Scholar]
- 32.Robinson AJ, Elaridi J, Van Lierop BJ, Mujcinovic S, Jackson WR. Microwave-assisted RCM for the synthesis of carbocyclic peptides. J Pept Sci. 2007;13:280–285. doi: 10.1002/psc.840. [DOI] [PubMed] [Google Scholar]
- 33.Derksen DJ, Stymiest JL, Vederas JC. Antimicrobial leucocin analogues with a disulfide bridge replaced by a carbocycle or by noncovalent interactions of allyl glycine residues. J Am Chem Soc. 2006;128:14252–14253. doi: 10.1021/ja066203q. [DOI] [PubMed] [Google Scholar]
- 34.Gottler LM, de la Salud-Bea R, Marsh EN. The Fluorous Effect in Proteins: Properties of alpha4F6, a 4-alpha-Helix Bundle Protein with a Fluorocarbon Core. Biochemistry. 2008;47:4484–4490. doi: 10.1021/bi702476f. [DOI] [PubMed] [Google Scholar]
- 35.Lee HY, Lee KH, Al-Hashimi HM, Marsh EN. Modulating protein structure with fluorous amino acids: increased stability and native-like structure conferred on a 4-helix bundle protein by hexafluoroleucine. J Am Chem Soc. 2006;128:337–343. doi: 10.1021/ja0563410. [DOI] [PubMed] [Google Scholar]
- 36.Blanchfield JT, Dutton JL, Hogg RC, Gallagher OP, Craik DJ, Jones A, Adams DJ, Lewis RJ, Alewood PF, Toth I. Synthesis, structure elucidation, in vitro biological activity, toxicity, and Caco-2 cell permeability of lipophilic analogues of α-conotoxin MII. J Med Chem. 2003;46:1266–1272. doi: 10.1021/jm020426j. [DOI] [PubMed] [Google Scholar]
- 37.Blanchfield JT, Gallagher OP, Cros C, Lewis RJ, Alewood PF, Toth I. Oral absorption and in vivo biodistribution of α-conotoxin MII and a lipidic analogue. Biochem Biophys Res Commun. 2007;361:97–102. doi: 10.1016/j.bbrc.2007.06.138. [DOI] [PubMed] [Google Scholar]
- 38.Craik DJ, Cemazar M, Daly NL. The chemistry and biology of cyclotides. Curr Opin Drug Discov Devel. 2007;10:176–184. [PubMed] [Google Scholar]
- 39.Clark RJ, Fischer H, Dempster L, Daly NL, Rosengren KJ, Nevin ST, Meunier FA, Adams DJ, Craik DJ. Engineering stable peptide toxins by means of backbone cyclization: stabilization of the α-conotoxin MII. Proc Natl Acad Sci U S A. 2005;102:13767–13772. doi: 10.1073/pnas.0504613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovelace ES, Armishaw CJ, Colgrave ML, Wahlstrom ME, Alewood PF, Daly NL, Craik DJ. Cyclic MrIA: a stable and potent cyclic conotoxin with a novel topological fold that targets the norepinephrine transporter. J Med Chem. 2006;49:6561–6568. doi: 10.1021/jm060299h. [DOI] [PubMed] [Google Scholar]
- 41.Craik DJ, Adams DJ. Chemical modification of conotoxins to improve stability and activity. ACS Chem Biol. 2007;2:457–468. doi: 10.1021/cb700091j. [DOI] [PubMed] [Google Scholar]
- 42. Green BR, Catlin P, Zhang MM, Fiedler B, Bayudan W, Morrison A, Norton RS, Smith BJ, Yoshikami D, Olivera BM, et al. Conotoxins containing nonnatural backbone spacers: cladistic-based design, chemical synthesis, and improved analgesic activity. Chem Biol. 2007;14:399–407. doi: 10.1016/j.chembiol.2007.02.009. This is the first example of replacing conformationally constrained, but nonessential parts of cysteine-rich peptides with flexible isostere backbone spacers. Resulting conotoxin-based polytides appeared better sodium channel blockers and analgesic compounds.
- 43.Bulaj G, DeLaCruz R, Azimi-Zonooz A, West P, Watkins M, Yoshikami D, Olivera BM. δ-Conotoxin structure/function through a cladistic analysis. Biochemistry. 2001;40:13201–13208. doi: 10.1021/bi010683a. [DOI] [PubMed] [Google Scholar]
- 44. Beeton C, Smith BJ, Sabo JK, Crossley G, Nugent D, Khaytin I, Chi V, Chandy KG, Pennington MW, Norton RS. The D-diastereomer of ShK toxin selectively blocks voltage-gated K+ channels and inhibits T lymphocyte proliferation. J Biol Chem. 2008;283:988–997. doi: 10.1074/jbc.M706008200. By replacing all 35 amino acid residues with D-isomers, D-allo-ShK toxin appeared to block Kv1.3 potassium channels with nanomolar potency and maintained immunosupressive acitivty in the T-cell proliferation assay. This is the first example of a bioactive all-D analog of a cysteine-rich peptide.
- 45.Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, Gerwick WH. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem Biol. 2004;11:817–833. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Donia MS, Hathaway BJ, Sudek S, Haygood MG, Rosovitz MJ, Ravel J, Schmidt EW. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nat Chem Biol. 2006;2:729–735. doi: 10.1038/nchembio829. [DOI] [PubMed] [Google Scholar]
- 47.Donia MS, Ravel J, Schmidt EW. A global assembly line for cyanobactins. Nat Chem Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating CaV channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- 49.Banghart MR, Volgraf M, Trauner D. Engineering light-gated ion channels. Biochemistry. 2006;45:15129–15141. doi: 10.1021/bi0618058. [DOI] [PubMed] [Google Scholar]
- 50.Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci U S A. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buczek O, Bulaj G, Olivera BM. Conotoxins and the posttranslational modification of secreted gene products. Cell Mol Life Sci. 2005;62:3067–3079. doi: 10.1007/s00018-005-5283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]