Abstract
Background
A reduction in interhemispheric connectivity is thought to contribute to the etiology of schizophrenia. Diffusion Tensor Imaging (DTI) measures the diffusion of water and can be used to describe the integrity of the corpus callosum white matter tracts, thereby providing information concerning possible interhemispheric connectivity abnormalities. Previous DTI studies in schizophrenia are inconsistent in reporting decreased Fractional Anisotropy (FA), a measure of anisotropic diffusion, within different portions of the corpus callosum. Moreover, none of these studies has investigated corpus callosum systematically, using anatomical subdivisions.
Methods
DTI and structural MRI scans were obtained from 32 chronic schizophrenic subjects and 42 controls. Corpus callosum cross sectional area and its probabilistic subdivisions were determined automatically from structural MRI scans using a model based deformable contour segmentation. These subdivisions employ a previously generated probabilistic subdivision atlas, based on fiber tractography and anatomical lobe subdivision. The structural scan was then co-registered with the DTI scan and the anatomical corpus callosum subdivisions were propagated to the associated FA map.
Results
Results revealed decreased FA within parts of the corpus interconnecting frontal regions in schizophrenia compared with controls, but no significant changes for callosal fibers interconnecting parietal and temporo-occipital brain regions. In addition, integrity of the anterior corpus was statistically significantly correlated with negative as well as positive symptoms, while posterior measures correlated with positive symptoms only.
Conclusions
This study provides quantitative evidence for a reduction of interhemispheric brain connectivity in schizophrenia, involving corpus callosum, and further points to frontal connections as possibly disrupted in schizophrenia.
INTRODUCTION
The corpus callosum (CC) is a midline brain structure that is the largest white matter fiber tract in the brain. This structure interconnects left and right hemispheres, and plays a primary role in sensory, as well as high-level cognitive integration(Gazzaniga, 2000). Neuropsychological schizophrenia studies provide evidence of interhemispheric information transfer impairment(Coger and Serafetinides, 1990;Bruder et al., 1995;Mohr et al., 2001;Seymour et al., 1994;Gruzelier, 1999). Additional evidence regarding interhemispheric connectivity abnormalities comes from electrophysiological studies demonstrating differences in latency and coherence between patients with schizophrenia and healthy controls(Norman et al., 1997).
One of the first morphological studies of CC in schizophrenia was a post-mortem study by Bigelow and Rosenthal(Bigelow and Rosenthal, 1972) that showed a thicker CC in patients compared with controls. A meta-analysis by Woodruff(Woodruff et al., 1995), however, demonstrated a decrease in CC size of about 0.5 cm2. Also, several subsequent post-mortem studies reported trends towards a total, or local decrease in CC size, although statistical significance was reached only in a few studies e.g.(Highley et al., 1999;Downhill et al., 2000). In a recent review of CC investigations in schizophrenia, Innocenti and colleagues(Innocenti et al., 2003) point to several limitations of post-mortem morphological studies, including variability of sampling, small sample sizes, methodological problems with precision and reproducibility of midsagittal sectioning, and the influence of brain size and CC shape on cross sectional measurements.
Structural MR investigations of CC in schizophrenia are free from some of these limitations, allowing for better controlled placement of the midsagittal plane and larger and more homogeneous populations. However, due to relatively low resolution and low specificity of measurements in comparison to histopathological studies (abnormalities in either axonal density, thickness or myelination, suggested in schizophrenia, might not necessarily lead to a volume loss), MRI studies have also reported inconsistent findings. In a review of 27 structural MRI studies of the CC, 17 report positive findings and 10 report negative findings(Shenton et al., 2001).
Thus in recent years, in order to increase specificity and increase power of in vivo investigations, studies of CC in schizophrenia have gradually migrated from rough estimates of CC volumes to separating the CC into geometric, anatomically or functionally relevant subdivisions (e.g.,(Witelson, 1989)). In parallel, additional in vivo methods, such as Magnetization Transfer Imaging (MTR), MR spectroscopy, and DTI, are being employed to study CC, as well as other cortico-cortical connections in schizophrenia. For example, MTR, a method sensitive to myelin disruptions in the brain, has been used to study CC in schizophrenia, where decreased myelin content has been reported in the genu(Foong et al., 2001), and in the body of the CC(Kubicki et al., 2005). In addition, DTI, an MR method capable of investigating properties of water diffusion within the brain and sensitive to fiber tract integrity disruptions, has been applied to schizophrenia. More specifically, Foong and coworkers(Foong et al., 2000) conducted the first DTI study of the CC in schizophrenia, and reported increased mean diffusivity (a measure of axonal density) and decreased fractional anisotropy (a measure of coherence, and integrity of axonal fibers) in the splenium of the CC. More recently, CC integrity abnormalities have been reported in most CC segments, including the genu(Kanaan et al., 2006;Price et al., 2007), the isthmus(Ardekani et al., 2003), the body(Ardekani et al., 2003;Kubicki et al., 2005;Hubl et al., 2004;Buchsbaum et al., 2006) and the splenium(Ardekani et al., 2003;Foong et al., 2000;Agartz et al., 2001;Price et al., 2007). There have also been negative findings reported(e.g.,(Foong et al., 2002;Price et al., 2005;Sun et al., 2003;Kumra et al., 2004)). Inconsistencies in DTI investigations in schizophrenia mirror those of post-mortem as well as structural MR studies, and point to the need for precise, sensitive methods of measurement.
Unfortunately, similar to structural MRI, DTI methodology has several limitations. The most popular analytic method, Voxel Based Morphometry (VBM), turns out to be sensitive to large intensity variations, rather than to subtle, well localized abnormalities, in addition to not correcting very well for shape, or for overall size differences. Region of Interest (ROI) analyses, on the other hand, might be confounded by the type of diffusion measure (e.g., FA, T1W, directional map), and/or by slice thickness or slice orientation of the image used for their definition.
A recent DTI post-processing technique -fiber tractography- shows promise, and two schizophrenia investigations utilizing this method show its higher sensitivity and specificity, compared to both VBM and ROI approaches(Jones et al., 2005;Kanaan et al., 2006). Since fiber tractography is capable of visualizing entire fiber tracts, thus following them from one hemisphere to another, it might be well suited for defining anatomical divisions of the CC(Huang et al., 2005);(Hofer and Frahm, 2006). In fact it has been demonstrated(Hofer and Frahm, 2006) that DTI based CC subdivisions reflect anatomy of the callosal connections much better than the traditional geometric subdivisions of CC proposed by Witelson(Witelson, 1989).
Diffusion quantification, however, along the entire tracts defined by DTI may nonetheless still be problematic, due to the fact that interhemispheric callosal fibers are crossed by intrahemispheric longitudinal fiber bundles in multiple areas of the human brain, which affects both the shape and appearance of callosal tracts, making the measurements less reliable. In the current study we try to minimize some of the aforementioned confounding factors. We use a combination of MRI automatic lobar parcellation, and DTI tractography, to segment CC more precisely, and we include a template warping procedure for better intersubject reproducibility. Finally, in order to minimize the influence of other fiber bundles on CC, we project our tractography based parcellation onto the midsagittal plane of the high resolution DTI scans, where all measurements are performed. We predict that CC, particularly in regions connected to the frontal lobe (regions most frequently indicated in post mortem and genetic studies), will be more affected in schizophrenia compared with controls, and that these abnormalities will be related to clinical symptoms of schizophrenia.
METHODS
Subject Population and Inclusion-Exclusion Criteria
32 patients with chronic schizophrenia were recruited from in-patient, day treatment, out-patient, and foster care programs at the VA Boston Healthcare System, Brockton, MA. SCID-P interviews were administered by professional staff to make DSM-IV diagnoses. SCID-NP interviews were completed for the 42 normal comparison subjects. Comparison subjects were recruited through advertisements, and group-matched to patients on age, sex, handedness, and parental social economic-status (PSES).
Inclusion criteria for all subjects were: right-handedness, ages between 18 and 55 years, no history of electroconvulsive shock treatment, no history of neurological illness, no alcohol or drug dependence in the last 5 years and no abuse in the past year, verbal IQ above 80, no medication affecting neurological or cognitive functions. In addition, control subjects were screened to exclude individuals who had a first degree relative with an Axis I disorder. The study was approved by the local IRB committees at the VA and BWH. All the subjects signed informed consent prior to study participation.
MRI Protocol
For the DTI data acquisition, we used a 1.5 Tesla GE scanner, with a quadrature head coil. We used a line scan diffusion imaging protocol (LSDI), which decreases the number of artifacts and distortions compared to other techniques(Gudbartsson et al., 1996; Kubicki et al., 2005; Kubicki et al., 2002). In order to obtain high resolution corpus scans, we acquired 5 sagittal oblique (aligned to the interhemispheric fissure) 1.7 x 1.7 mm in plane, 4 mm thick slices, with Echo Time=70 ms; Repetition Time=2500 ms. We acquired 6 independent diffusion directions(B=1000) and 2 baseline images(B=5), and one NEX (number of excitations, with LSDI sequence gaining signal-to-noise ratios comparable to 16 NEX when single shot EPI is used, but is free from EPI related geometric distortions (Kubicki et al, 2003). In the same session, high resolution structural scans were also acquired, and later co-registered to DTI scans. The acquisition parameters were as follows: contiguous spoiled gradient-recalled acquisition (SPGR), TR=35ms, TE=5ms, 45 degree flip angle, resulting in 0.9375x0.9375x1.5 mm voxels.
Image Analysis
We utilized a novel method for the computation of a probabilistic subdivision of CC, which is described in detail in Styner et al. (Styner et al., 2005) and in Cascio et al. (Cascio et al., 2006).
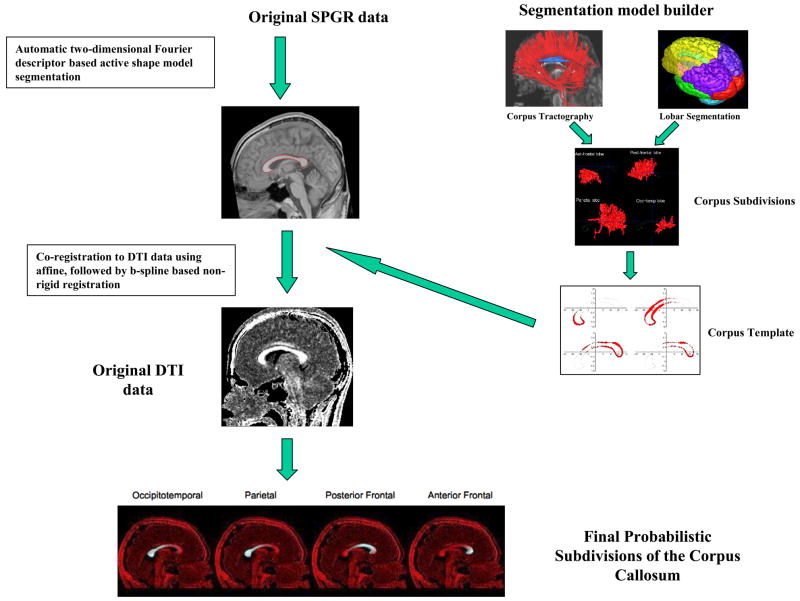
Here, we provide only a brief description, also schematically illustrated in Figure 1. The procedure involved four separate steps: 1) Automatic segmentation of the entire corpus callosum using SPGR data and a statistical, deformable model approach, described in detail in Styner et al. (Styner et al., 2005) (top left of Figure 1); 2) Further separation of the corpus ROI (still using structural scan) into 4 separate segments using a previously created atlas (right side of Figure 1); 3) Co-registration of the structural scan (along with corpus segments) to DTI was performed via a routine non-rigid b-spline, mutual information based registration method (The Image Registration Toolkit, Rueckert, 1999), which accounts for DTI distortions (although minimal in this brain region and with this pulse sequence), patient motion and FOV differences. (left middle part of Figure 1) and 4) After diffusion tensors estimation (using a standard linear square fit, which seems adequate since the principal direction of diffusion in midsagittal plane is quite uniform), the area and mean FA for each corpus segment were calculated.
Figure 1.
Schematic Description of processing steps, described in detail in “Styner et al., 2005 and Cisco et al., 2006), involving automatic segmentation of the corpus callosum into probabilistic segments using previously generated atlas, co-registration of the corpus segmentation with DTI data, and extraction of FA values for each segment separately.
The segmentation of CC into 4 smaller anatomical sections(step 3) was performed using a probabilistic atlas/model(Styner et al., 2005;Cascio et al., 2006), which was generated in a separate study(Cascio et al., 2006). Its creation is schematically described on the right side of the Figure 1, and involved tractography of the entire corpus callosum, its further division using automatic lobar segmentation, and back-mapping of these divisions onto midsagittal plane. Last step of this process resulted in midsagittal plane labels of pre-frontal, frontal, parietal and temporo-occipital callosal segments corresponding to the probability of callosal fibers projecting to each of these four brain regions. The entire process was automatic and fully reproducible, i.e., no manual measurements were conducted.
Volume and mean FA for each callosal segment were subjected to group comparison, as well as Pearson product correlations, where relationship was tested between FA, age, and clinical symptoms (derived from Scale for the Assessment of Negative and Positive Symptoms(Andreasen, 1990)).
RESULTS
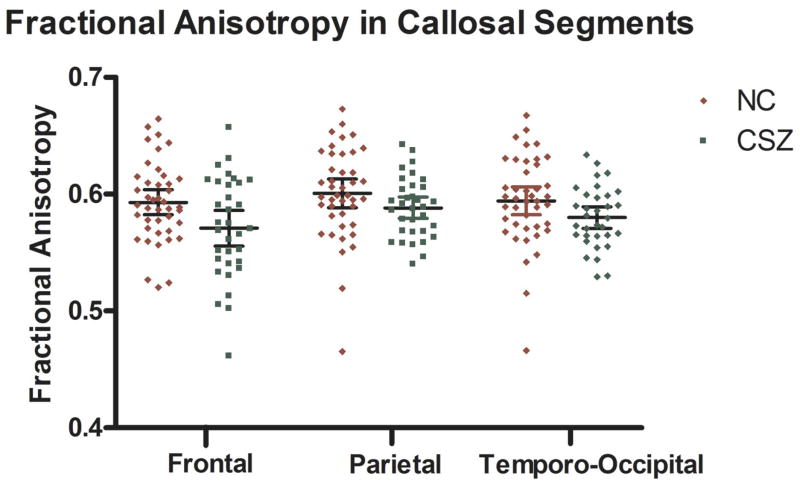
Groups did not differ in age [mean age 40.5±8.8 for controls, and 39.8±9.3 for schizophrenics; P(1,73)=0.78)], parental socioeconomic status P(1.73)=0.33), or gender (all males). Since age of healthy subjects has been shown to be correlated with FA of anterior CC (Pfefferbaum et al., 2003), we entered age as a covariate into a General Linear Model investigating group effects for FA and volume of the four CC subdivisions. When CC volume was analyzed, ICC (intra-cranial content) volume was also entered as a covariate. ANCOVA revealed no significant overall group effect for area of the CC (F=2.99;P=0.09), but did reveal a significant group effect for FA (F=5.77;P=0.02). In addition, we found group by region interaction (F=5.23; P=0.03), with individual ANOVAs showing FA decrease in the entire frontal (F=5.68; P=0.02), but not parietal (F=2.39; P=0.13), or temporo-occipital (F=3.36; P=0.07) callosal connections (see Figure 2). Also, controls, but not schizophrenics, showed a relationship between age and CC integrity in anterior, but not posterior CC regions (rho=−0.35;P=0.02 for frontal segment; and rho=−0.13;P=0.42; rho=−0.16;P=0.31 for parietal and ocipito-temporal segments respectively). Finally, FA for CC regions was statistically significantly correlated with negative (anterior portion), and positive (whole corpus) clinical symptoms (see Table 2 for details).
Figure 2.
Results of group comparison, indicating largest group differences for the anterior parts of the corpus callosum.
DISCUSSION
Our investigation revealed decreased fiber tract integrity in patients with schizophrenia, compared with controls, in the corpus callosum, the largest white matter fiber tract of the human brain. We did not, however, find volume differences between groups for this structure, which is consistent with the view that structural MRI might not be sensitive enough to detect subtle white matter changes present in schizophrenia(Innocenti et al., 2003;Rossell et al., 2001). In fact, Rossell and colleagues calculated that in order to find statistically significant 0.2 cm2 volume differences between schizophrenics and controls in the corpus callosum, one would need 308 schizophrenia patients. Indeed, our present investigation, as well as other DTI studies conducted to date, confirms the increased sensitivity of DTI methods to detect subtle microstructural changes within the white matter in schizophrenia.
Post-hoc analyses suggested that the callosal tracts providing interhemispheric communication between frontal cortices were the regions strongest affected in schizophrenia. This is in line with previous studies reporting decreased size of pyramidal neurons in layer 3 of the prefrontal cortex (i.e.(Rajkowska et al., 1998), which is also the region known for its involvement in cortico-cortical interhemispheric connections.
Additionally, since regions that we found to be abnormal topographically roughly correspond to the genu and anterior part of the body of the corpus callosum, our results are in line with some findings obtained recently by other groups that use ROIs and fiber tractography approach(Kanaan et al., 2006;Price et al., 2007). We did not find group differences within the tracts inter-connecting parietal, temporal and occipital lobes, that is fibers projecting into the isthmus and splenium of the CC. Some previous DTI schizophrenia investigations, however, describe abnormalities within these regions. While we can not rule out the possibility that these regions are still affected, we note that most studies reporting positive findings in this region have used methods based on the whole brain registration(Ardekani et al., 2003;Foong et al., 2000;Agartz et al., 2001), which could, in part, be confounded by registration error (affecting controls, but perhaps more profoundly patients with schizophrenia), due to higher inter subject anatomical variability existing in posterior parts of the CC(Ozdemir et al., 2007).
FA, in general, is considered to be a measure of axonal integrity, density, thickness and myelination. Beaulieau (Beaulieu and Allen, 1994) demonstrated that myelination is one of the major contributors to diffusion anisotropy. In addition, several recent post-mortem studies(Uranova et al., 2004), as well as genetic(Hakak et al., 2001) studies, point to myelin, and oligodendrocytes forming the myelin sheath, as possibly being affected in schizophrenia. Also, since the anterior parts of the CC mature earlier than the rest of the CC, and developmental studies show size of CC increasing until the late twenties(Giedd et al., 1999;Keshavan et al., 2002), maturation of anterior portions falls roughly into the time period when most patients develop schizophrenia, i.e., early adulthood. Thus the observed CC abnormalities could reflect a breakdown in the developmental process at this particular point of maturation. Additionally, insufficient communication between hemispheres might further affect the developmental process of each individual hemisphere, resulting in the relative lack of asymmetry observed in schizophrenic brains(Innocenti et al., 2003). In addition, our findings regarding the correlations between the integrity within the anterior part of the corpus callosum and age in control subjects confirms previously published results by Sullivan and coworkers(Sullivan et al., 2006), which were attributed to the fact that the frontal lobes contain thinner and less myelinated fibers, which are more vulnerable to age related breakdown(Aboitiz et al., 1996;Bartzokis et al., 2004). Similar results were also reported by Woodruff et al (Woodruff at el., 1997), who reported correlations between corpus volume and age in controls, but similar to our results, did not see these relationships in schizophrenia.
Correlations between FA values and clinical symptoms found here, also confirm the role of interhemispheric communication in schizophrenia symptomatology, further suggesting involvement of the entire CC in positive symptoms, but only the anterior part in negative symptomatology. We note, however, that the role of interhemipsheric connectivity provided by the corpus callosum in schizophrenia, and its correlation with clinical symptoms is poorly understood, although we note that similar correlations between CC integrity and clinical symptoms have been reported previously in the literature. For example, Downhill (Downhill et al., 2000) reported negative correlations between whole CC size and severity of positive symptoms, while several other studies suggest the involvement of the anterior part of the CC in negative symptomatology (e.g., Woodruff et al., 1997; Foong et al., 2001). The anterior portion of the CC is functionally associated with frontal lobe, with its ventral regions heavily involved in emotional and attentional processing and this may, in part, explain why negative symptom correlations predominate although further investigation is needed to determine the nature of this association.
Our study also included chronic, medicated subjects only. Thus even though we, as well as many others, did not observe any statistically significant correlations between medication dosage and diffusion measures, our results could still be confounded by the cumulative effect of medication, or other environmental factors not accounted for in this study.
Methodological issues
Our investigation is one of the first to utilize fiber tractography to subdivide and measure corpus callosum integrity in schizophrenia. Recent investigations highlight the advantages of tractography over more traditional analytic approaches to DTI data, and show diffusion tractography to be more sensitive than more popular ROI or VBM DTI approaches, in finding abnormalities in schizophrenia(Kanaan et al., 2006;Jones et al., 2006). Tractography based segmentation of callosal fibers, has also already been shown to be more precise, and better reflect the anatomy than the traditional geometric segmentation(Hofer and Frahm, 2006). Thus by using tractography based segmentation in our investigation, we hoped to increase both sensitivity as well as specificity of our measurements. One of the limitations of our approach is the fact that our DTI data was acquired in only 6 diffusion directions. This limitation, however, affects our results only minimally, since we do not have to use our DTI data to generate tractography, but instead are mapping an already existing tractography based atlas onto our DTI data. On the other hand, precision of our measurements should be increased thanks to the sagittal acquisition and high in-plane resolution of the data (1.7 x 1.7 mm in the midsagittal plane), by decreasing partial volume effects associated with thick axial slices used in most investigations. Finally, our subjects were all medicated males, thus additional studies need to be performed on a first episode and mixed gender populations in order to understand fully the meaning of our findings.
CONCLUSIONS
DTI is powerful and sensitive tool that demonstrates white matter abnormalities in schizophrenia. Our study demonstrates the utility of using DTI and tractography-based atlas in segmenting and measuring corpus callosum subdivisions in schizophrenia, and points to interhemispheric communication between frontal lobes as most affected in schizophrenia, and associated with negative symptoms.
ROLE OF FUNDING SOURCE
This work was funded by the National Alliance for Research on Schizophrenia and Depression (MK), the National Institute of Health (R03 MH068464-01 to MK, K05 MH070047 and R01 MH 50747 to MES and R01 MH 40799 to RWM), the Department of Veterans Affairs Merit Awards (MES, RWM), and the VA Schizophrenia Center Grant (RWM/MES), and Harvard Medical School (Milton Award to MK). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (RK, GG, MES, MS, MK), P50MH080272 (RWM MES, MK,SB). The funding sources had no further role in the study design, in the collection, analysis, and interpretation of data; in the writing of the report, and in the decision to submit the paper for publication.
Table 1.
Table of clinical correlations, indicating significant correlations between segments of the corpus callosum and clinical scores.
| Clinical measure | Region of CC | |||
|---|---|---|---|---|
| Number of subjects | Frontal | Parietal | Occipito-temporal | |
| Global SANS | 33 | −.463 (0.007) | ns | ns |
| Global Attention | 33 | −.421 (0.015) | ns | ns |
| Social Attention | 29 | −.446 (0.015) | ns | ns |
| Global SAPS | 32 | −.400 (0.023) | −.401 (0.023) | −.403 (0.022) |
| Global TDS | 32 | −.382 (0.031) | −.405 (0.022) | −.369 (0.037) |
| Distracted speech | 31 | −.492 (0.005) | −.420 (0.019) | −.449 (0.011) |
Acknowledgments
We would like to thank Nancy Maxwell and Jennifer Goodrich for their administrative assistance. The Image Registration Toolkit was used under Licence from Ixico Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. Neuroreport. 1996;7:1761–4. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- Agartz I, Andersson JL, Skare S. Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport. 2001;12:2251–4. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsychiatry. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–9. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Quantifying age-related myelin breakdown with MRI: novel therapeutic targets for preventing cognitive decline and Alzheimer's disease. J Alzheimers Dis. 2004;6:S53–9. doi: 10.3233/jad-2004-6s604. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen P. Determinants of Anisotropic Water Diffusion in Nerves. MRM. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- Bigelow L, Rosenthal R. Schizophrenia and the corpus callosum. Lancet. 1972;1:694. doi: 10.1016/s0140-6736(72)90503-x. [DOI] [PubMed] [Google Scholar]
- Bruder G, Rabinowicz E, Towey J, Brown A, Kaufmann CA, Amador X, Malaspina D, Gorman JM. Smaller right ear (left hemisphere) advantage for dichotic fused words in patients with schizophrenia. Am J Psychiatry. 1995;152:932–5. doi: 10.1176/ajp.152.6.932. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, Schneiderman JS, Torosjan Y, Tang C, Hof PR, Stewart D, Davis KL, Gorman J. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2006;60:1181–7. doi: 10.1016/j.biopsych.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Cascio C, Styner M, Smith RG, Poe MD, Gerig G, Hazlett HC, Jomier M, Bammer R, Piven J. Reduced relationship to cortical white matter volume revealed by tractography-based segmentation of the corpus callosum in young children with developmental delay. Am J Psychiatry. 2006;163:2157–63. doi: 10.1176/ajp.2006.163.12.2157. [DOI] [PubMed] [Google Scholar]
- Coger RW, Serafetinides EA. Schizophrenia, corpus callosum, and interhemispheric communication: a review. Psychiatry Res. 1990;34:163–84. doi: 10.1016/0165-1781(90)90017-y. [DOI] [PubMed] [Google Scholar]
- Downhill JE, Jr, Buchsbaum MS, Wei T, Spiegel-Cohen J, Hazlett EA, Haznedar MM, Silverman J, Siever LJ. Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res. 2000;42:193–208. doi: 10.1016/s0920-9964(99)00123-1. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–4. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA. Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport. 2002;13:333–6. doi: 10.1097/00001756-200203040-00017. [DOI] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, Ron MA. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124:882–92. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–88. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. Functional neuropsychophysiological asymmetry in schizophrenia: a review and reorientation. Schizophr Bull. 1999;25:91–120. doi: 10.1093/oxfordjournals.schbul.a033370. [DOI] [PubMed] [Google Scholar]
- Gudbartsson H, Maier S, Mulkern R. Line scan diffusion imaging. Magn Reson Med. 1996;36:509–519. doi: 10.1002/mrm.1910360403. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999;122(Pt 1):99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–68. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8:261–74. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, Golesworthy P, McGuire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–8. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, Maguire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry. 2005;13:1092–9. doi: 10.1176/appi.ajgp.13.12.1092. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Shergill SS, Barker GJ, Catani M, Ng VW, Howard R, McGuire PK, Jones DK. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, Sweeney JA, Minshew N, Pettegrew JW. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci. 2002;70:1909–22. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–20. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, McMeniman M, Vogel J, Augustin R, Becker DE, Nakayama E, Gyato K, Kane JM, Lim K, Szeszko P. Reduced frontal white matter integrity in early-onset schizophrenia: a preliminary study. Biol Psychiatry. 2004;55:1138–45. doi: 10.1016/j.biopsych.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Mohr B, Heim S, Pulvermuller F, Rockstroh B. Functional asymmetry in schizophrenic patients during auditory speech processing. Schizophr Res. 2001;52:69–78. doi: 10.1016/s0920-9964(00)00183-3. [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK, Williamson PC, Morrison-Stewart SL, Helmes E, Cortese L. EEG coherence and syndromes in schizophrenia. Br J Psychiatry. 1997;170:411–5. doi: 10.1192/bjp.170.5.411. [DOI] [PubMed] [Google Scholar]
- Ozdemir ST, Ercan I, Sevinc O, Guney I, Ocakoglu G, Aslan E, Barut C. Statistical shape analysis of differences in the shape of the corpus callosum between genders. Anat Rec (Hoboken) 2007;290:825–30. doi: 10.1002/ar.20558. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. J Magn Reson Imaging. 2003;18:427–33. doi: 10.1002/jmri.10377. [DOI] [PubMed] [Google Scholar]
- Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2005;76:585–7. doi: 10.1136/jnnp.2004.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007;35:458–66. doi: 10.1016/j.neuroimage.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–24. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Shapleske J, Fukuda R, Woodruff PW, Simmons A, David AS. Corpus callosum area and functioning in schizophrenic patients with auditory--verbal hallucinations. Schizophr Res. 2001;50:9–17. doi: 10.1016/s0920-9964(00)00070-0. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Non-rigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Seymour SE, Reuter-Lorenz PA, Gazzaniga MS. The disconnection syndrome. Basic findings reaffirmed. Brain. 1994;117(Pt 1):105–15. doi: 10.1093/brain/117.1.105. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner MA, Oguz I, Smith RG, Cascio C, Jomier M. Corpus callosum subdivision based on a probabilistic model of inter-hemispheric connectivity. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2005;8:765–72. doi: 10.1007/11566489_94. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–9. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, Cong Z, Zhang H, Li B, Hong N, Zhang D. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14:1833–6. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:457–61. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]