Despite the development and progress of Pd-catalyzed C-H activation/C-C coupling reactions with organometallic reagents,1-2 improving the practicality of Pd(II)/Pd(0) catalysis3 and expanding substrate scope remain significant challenges. In this regard, the highly versatile Pd-catalyzed cross-coupling reactions using organohalides and other surrogates have two principal advantages:4-5 1) an exogenous oxidant is not required, and 2) rationally designed ligands that promote these reactions and modulate the reactivity with wide range of substrates are available. Herein we disclose a new catalytic system for C-H activation/C-C coupling that offers greatly improved scope and practicality by using aryltrifluoroborates6 as the coupling partners and O2 or air as the oxidant. These new conditions made possible, for the first time, the ortho-C-H coupling of highly electron-deficient arenes and phenyl acetic acids, a class of broadly useful starting materials. Importantly, the presence of acidic α-hydrogens is tolerated. This method provides a general preparative route for compounds that are not accessible via the widely used ortho-metalation/iodination/cross-coupling sequence (Fig. 1-2).7
Fig. 1.
Directed ortho-Metalation (DOM)
Fig. 2.
C-H Activation/Aryl-Aryl Coupling
We have previously reported Pd-catalyzed ortho-coupling of benzoic acids with phenylboronate using Ag2CO3 as the oxidant.1c This arylation protocol is limited to only a handful of benzoic acids. Poor yields (<40%) are obtained with electron deficient arenes. No coupling products are observed with phenyl acetic acid substrates due to the sluggish six-membered cyclopalladation directed by the carboxyl group. From the viewpoint of synthetic applications, development of C-H activation reactions for phenyl acetic acids would substantially improve the versatility and usefulness of this reaction. Therefore, we extensively screened reaction parameters and coupling partners, using benzoic acids as substrates, in an effort to accelerate the C-H activation step. We found the replacement of the Ag2CO3 oxidant by air or O2 and the use of aryltrifluoroborates together greatly improves the yield and scope of the ortho-coupling of benzoic acids (Table 1). Since the κ2 coordination of carboxylate with the K+ is essential for C-H activation,1c Ag+ or Cu2+ apparently retards the reaction by binding to the carboxyate. The influence of ArBF3K on C-H activation reaction deserves further study.
Table 1.
Coupling of Benzoic Acids with Potassium Aryltrifluoroboratea
 |
3b-3d, 3f-3p were isolated as their methyl esters formed by reacting with CH2N2.
Treatment of the coupling products with oxalyl chloride afforded 3q and 3r.
Although, the use of 20 atm of air or O2 was needed to shorten the reaction time, we were pleased to find that use of 1 atm O2 or air gave the desired products in 60-70% yields after 72 h (3c). Especially noteworthy are the excellent yields obtained with the highly electron-deficient substrates containing fluoride, bromide, trifluoromethyl, cyano and acetyl groups (3f, 3g, 3i, 3j, 3k), the presence of which is crucial for further synthetic manipulations. Excellent regioselectivity in favor of the less hindered C-H bonds was observed with meta-substituted substrates (3a, 3f, 3g). The isolation of the coupling products 3o, 3p, and 3q also demonstrated the compatibility of this reaction with other aryltrifluoroborates. Ketones 3q and 3r were readily formed by treating the initially formed coupling products with oxalyl chloride, illustrating potential applications for the synthesis of biologically interesting fluoren-9-ones.8
The efficiency of this reaction protocol encouraged us to test the coupling of phenyl acetic acids. Notably, our previous approach using an oxazoline as the auxiliary for the ortho-coupling failed with substrates containing α-hydrogens.1a Another existing protocol using O-methyl hydroxamic acids as a directing group, unfortunately, is not applicable to sp2 C-H activation/C-C coupling.1e We were pleased to find that the coupling of phenyl acetic acid with PhBF3K using O2 as the oxidant proceeded effectively to give the diarylated product 5a in 69% yield. Interestingly, the presence of Ag+ oxidant results in a complete loss of the reactivity. The presence of an α-substituent provides sufficient steric hindrance to induce the mono-selectivity (5b, 5c). Both strongly electron-withdrawing (5d-5g) and electron-donating (5j) groups are compatible with this reaction. Aryltrifluoroborates containing either electron-donating or electron-withdrawing groups were also effective (5 h-j).
In general, phenyl acetic acids are a class of broadly useful starting materials for synthetic chemistry. For example, the reduction of the nitro group in the methyl ester of 5g to the corresponding amine simultaneously triggers lactamization to give a synthetically useful lactam. Treating the coupling products with oxalyl chloride readily gave the cyclized ketone (5k), and the coupling product from a drug, ibuprofen, was also converted to a tricyclic enoether 5l by treatment with oxalyl chloride in the presence of trace ethanol.
Considering the importance of heterocycles in medicinal chemistry, we attempted to introduce a pyridyl group into benzoic acid via this newly developed coupling protocol. While coupling of benzoic acids with 3-pyridyltrifluoroborate gave the desired products in only approximately 10% yield, pyridyltrifluoroborate bearing 2-substitution proved to be effective. Surprisingly, the coupling products underwent a further intramolecular fluoro-displacement by the carboxyl groups to give the tricyclic lactones in an one-pot process.
In summary, we have developed a versatile protocol for C-H activation/aryl-aryl coupling using aryltrifluoroborates. This new protocol substantially expands the scope of reactions involving benzoic acids and made possible, for the first time, the ortho-C-H coupling of phenyl acetic acids containing α-hydrogens, and electron-deficient arenes. We are currently investigating a possible effect of ArBF3K on C-H activation reactions, and improving the efficiency of this reaction under 1 atm air.
Supplementary Material
Table 2.
Coupling of Arylacetic Acids with Potassium Aryltrifluoroboratesa
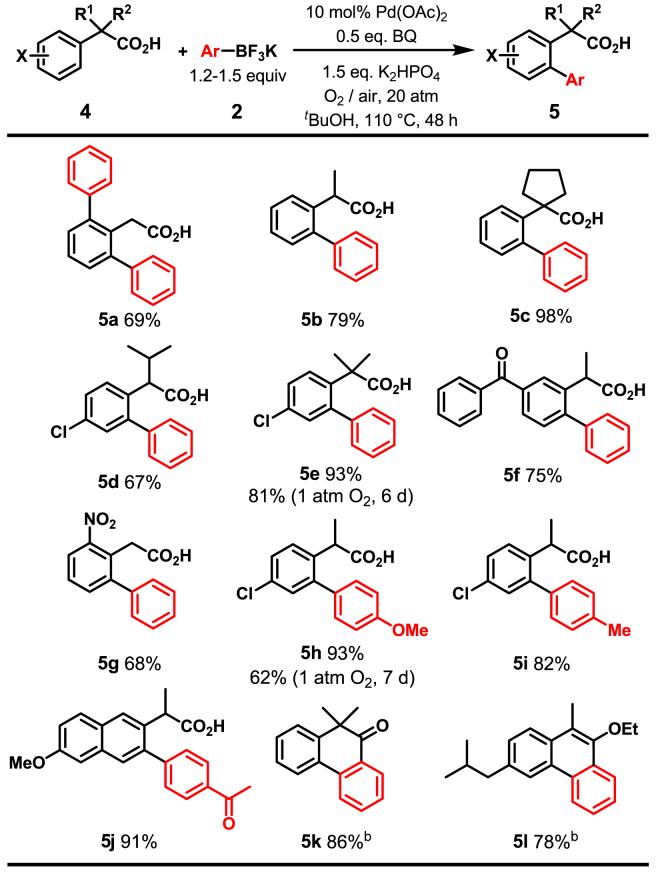 |
5a, 5c, 5e-5j were isolated as their methyl esters formed by treating with CH2N2.
Treatment of the coupling products with oxalyl chloride afforded 5k and 5l.
Table 3.
Coupling with Potassium Pyridyltrifluoroboratea
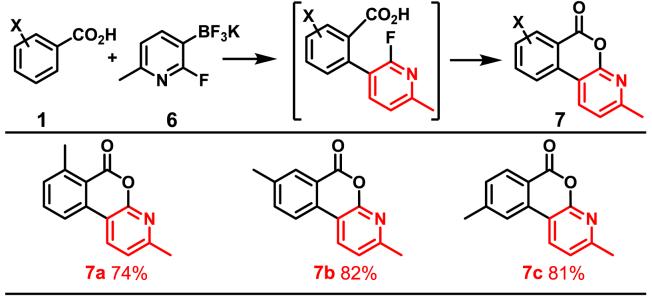 |
Reaction conditions: 1 (0.5 mmol), 6 (0.7 mmol), Pd(OAc)2 (0.05 mmol), BQ (0.25 mmol), K2HPO4 (1 mmol), O2 or air (20 atm), t-BuOH (2 mL), 110 °C, 36 h.
Acknowledgement
We gratefully acknowledge the financial support of the National Institutes of Health (NIGMS, 1 R01 GM084019-01A1) and A. P. Sloan Foundation for a Fellowship (J.-Q. Y).
Footnotes
Supporting Information Available: Experimental procedure and characterization of all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Chen X, Li J-J, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:78. doi: 10.1021/ja0570943. [DOI] [PubMed] [Google Scholar]; (b) Chen X, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:12634. doi: 10.1021/ja0646747. [DOI] [PubMed] [Google Scholar]; (c) Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; (d) Yang S, Li B, Wan X, Shi Z. J. Am. Chem. Soc. 2007;129:6066. doi: 10.1021/ja070767s. [DOI] [PubMed] [Google Scholar]; (e) Wang D-H, Wasa M, Giri R, Yu J-Q. J. Am. Chem. Soc. 2008;130:7190. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- (2).For recent examples of Pd-catalyzed arylation of C-H bonds involving other types of catalysis see: Kalyani D, Deprez NR, Desai LV, Sanford MS. J. Am. Chem. Soc. 2005;127:7330. doi: 10.1021/ja051402f.. Daugulis O, Zaitsev VG. Angew. Chem., Int. Ed. 2005;44:4046. doi: 10.1002/anie.200500589.. Campeau L-C, Rousseaux S, Fagnou K. J. Am. Chem. Soc. 2005;127:18020. doi: 10.1021/ja056800x..
- (3).For recent reviews on Pd(II)/Pd(0) catalysis see: Stahl SS. Angew. Chem. Int. Ed. 2004;43:3400. doi: 10.1002/anie.200300630.. Stoltz B. Chem. Lett. 2004;33:362.. Gligorich KM, Sigman MS. Angew. Chem. Int. Ed. 2006;45:6612. doi: 10.1002/anie.200602138.. Piera J, Backvall J-E. Angew. Chem., Int. Ed. 2008;47:3506. doi: 10.1002/anie.200700604.
- (4).For reviews see: Diederich F, Stang PJ, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; New York: 1998. . Hiyama T. J. Organomet. Chem. 2002;653:58.. Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368.. Negishi E, Hu Q, Huang Z, Qian M, Wang G. Aldrichimica Acta. 2005;38:71.
- (5).(a) Old DW, Wolf JP, Buchwald SL. J. Am. Chem. Soc. 1998;120:9722. [Google Scholar]; (b) Littke AF, Fu GC. Angew. Chem., Int. Ed. 1998;37:3387. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3387::AID-ANIE3387>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; (c) Herrmann WA, Reisinger C-P, Spiegler M. J. Organomet. Chem. 1998;557:93. [Google Scholar]; (f) Beller M, Riermeier TH. Eur. J. Inorg. Chem. 1998;29 [Google Scholar]; (e) Shaughnessy KH, Kim P, Hartwig JF. J. Am. Chem. Soc. 1999;121:2123. [Google Scholar]; (d) Zhang C, Huang J, Trudell ML, Nolan SP. J. Org. Chem. 1999;64:3804. [Google Scholar]
- (6).(a) Vedejs E, Chapman RW, Fields SC, Lin S, Schrimpf MR. J. Org. Chem. 1995;60:3020. [Google Scholar]; (b) Molander GA, Ito T. Org. Lett. 2001;3:393. doi: 10.1021/ol006896u. [DOI] [PubMed] [Google Scholar]; (c) Molander GA, Ellis N. Acc. Chem. Res. 2007;40:275. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; (d) Darses S, Genet J-P. Chem. Rev. 2008;108:288. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]
- (7).Beak P, Snieckus V. Acc. Chem. Res. 1982;15:306. [Google Scholar]
- (8).Zhao J, Yue D, Campo MA, Larock RC. J. Am. Chem. Soc. 2007;129:5288. doi: 10.1021/ja070657l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




