Abstract
This Commentary reports on the effects of photoaging in an experimental model of ultraviolet radiation B on human skin.
Photoaging is a term coined by Kligman and Kligman1 in 1986 to describe changes that develop after many years of cutaneous exposure to UV radiation (UVR). It consists in part of deep furrows (wrinkles), dryness, and alterations in pigmentation. Unlike sunburn and tanning responses that take hours to days to develop, photoaging takes many decades; therefore it most often is superimposed on chronologically aged skin. Although the pathophysiological basis for human cutaneous photoaging is not well understood, a major industry has emerged focused on its prevention or reversal. By the year 2010, the anti-aging market is anticipated to account for more than $16.5 billion dollars in sales.2 Experimental model systems to better understand the cellular and molecular alterations that account for photoaging, as well as to evaluate the efficacy of products designed to prevent or reverse the related clinical effects and cellular pathology, are therefore of obvious importance.
Experimental studies of photoaging have been difficult to design and conduct. Notably, there has been confusion with regard to separating the attributes of photoaging from those that characterize chronological aging. For example, although both photoaging and chronological aging are associated with wrinkles, photoaging-induced wrinkles are regarded by some to be deeper and more coarse, whereas those associated with chronological aging are generally more superficial and delicate.3 Interestingly, in at least one seminal study of the human wrinkle, it was concluded that there was no significant correlative histopathological abnormality associated with the thin linear cutaneous indentation that incites such societal pathos.4
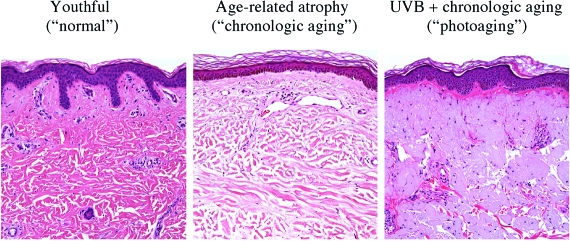
One key histological hallmark of photoaged skin is the presence of chronic photodamage, usually superimposed on alterations associated with chronological aging. Photodamage in its most basic form consists of the deposition of abnormal elastin as amorphous, blue-gray aggregates within the superficial dermis, so-called elastosis. Figure 1 represents a comparison between youthful skin, skin from an older individual, and photodamaged skin. Purely chronologically aged skin (eg, the buttock skin of most octogenarians), on the other hand, shows dermal and epidermal atrophy without significant elastosis.5,6 Nonetheless, one reason why these two processes are not clearly separable relates to the frequent superimposition of changes associated with photoaging onto those of chronological aging, with the former augmenting the clinical phenotype of the latter (ie, photodamaged skin tends to look more chronologically aged than mere passage of time should produce). To make matters more complex, there are known variations in pathology induction that relate to the affected skin site (eg, face versus trunk), constitutive skin pigmentation, and genetic background.7
Figure 1.
Comparison between youthful skin (regarded by society as normal), skin from an older individual showing loss of rete ridges and thinning of dermal collagen bundles, and photodamaged skin with superimposed aggregates of amorphous, pale blue-gray elastin in the superficial dermis. H&E stain. Original magnifications, ×200.
An additional and critical problem in evaluating the effects of photoaging has been paucity of in vivo models with structural relevance to the human integument. For example, although rodent skin has been studied extensively as a surrogate for human skin, it must be remembered that the skin of furred animals did not evolve to deal with the effects of sun exposure, nor is it entirely analogous to human skin with regard to structure, function, or molecular and genomic composition.
In this issue of The American Journal of Pathology Hachiya and co-workers8 attempt to circumvent this last experimental hurdle by using a human skin xenograft-severe combined immunodeficiency (SCID) mouse chimeric model to study the photoaging effects of UV radiation B (UVB). The SCID mouse-xenograft model has been used for several decades to investigate aspects of human cutaneous disease in grafted sites that importantly retain most of the critical architectural and cellular components of human skin.9
The UVR spectrum of sunlight is divided into three wavelengths: UVA (315 to 400 nm), UVB (280 to 315 nm), and UVC (100 to 280 nm). UVB is predominantly responsible for the deleterious effects of sunlight in nucleic acids and proteins, causing the formation of cyclobutane pyrimidine dimers and pyrimidyne-(6-4)-pyrimidone photoproducts, the defective repair of which is photocarcinogenic in humans.10,11 Although the effects of UVA are less well characterized, there is growing evidence suggesting that UVA induces production of reactive oxygen species (ROS), which in turn cause DNA damage.12 UVC is generally regarded as physiologically inconsequential given that it is primarily absorbed by the ozone layer in the atmosphere, but such environmental shields may be a subject of future concern.12 UVB is generally selected for photoaging studies in view of its established role in biological processes as diverse as sunburn, the tanning response, immunomodulation, skin cancer, and the collective alterations that constitute photoaging.10
In their study, Hachiya and colleagues8 exposed human xenografts derived from Caucasian abdominal skin to UVB starting at a 1 minimal erythemogenic dose and escalating thereafter in intermittent doses to a total of 1.65 J/cm2 throughout a 6-week period (five exposures per week). These doses would be anticipated to produce clinical erythema in humans (the beginning of sunburn) and were administered for a small fraction of the total duration (30 exposures throughout 6 weeks) that normally would account for clinical photoaging due to life-long environmental exposure. It has been estimated that American workers that spend most of their time indoors receive an annual dose of UVR between 20,000 and 30,000 J/m2 (or 2 to 3 J/cm2). This estimate excludes vacation time, which may increase the above doses by up to 30%.11 Thus, photoaging in humans interestingly appears to require considerably higher cumulative exposure to UVB throughout a much longer time than is reproduced in this animal model that used human abdominal skin xenografts.
On evaluation of the skin xenografts, however, Hachiya and co-workers8 found a number of abnormalities of potential relevance to human photoaging. The first consisted of increased skin roughness and furrow formation that lasted at least 4 weeks and that was quantifiable in replicas using image analysis technology. Because wrinkles resulting from both human chronological aging and photoaging tend to be permanent if untreated, long-term follow-up of xenografts for persistence of such furrow formation will be important in the future evaluation of this model. It also will be informative to determine how these surface alterations, as measured by identical imaging technology, actually compare with environmentally photodamaged human skin (eg, the wrinkled facial skin from a middle-aged commercial fisherman) as well as site- and age-matched human skin acutely exposed to erythemogenic doses of UVB.
In addition, they found an alteration in xenograft elasticity, as assessed by calculations of elastic recovery, viscoelastic properties and intermediate distention, and the ability to regain shape after deformation. Although Hachiya and colleagues8 show a number of provocative abnormalities in the relevant pathways that relate to collagen and elastin synthesis and degradation, these must also be interpreted in the context of treated xenografts displaying alterations (eg, overt dermal scarring; see their Figure 2, C–F) not characteristically identified in skin biopsies from patients with photoaging. Furthermore, it must be considered that in their model, the elastic tissue stains fail to show significant accumulation of relatively amorphous and clumped elastin deposits (elastosis), as is typical of clinically photodamaged skin. As with the data concerning furrow formation (discussed above), rigorous side-by-side controls evaluating clinical samples of chronically photodamaged skin using the same sophisticated techniques would add further credibility to the observations.
There are a number of additional findings reported by Hachiya and colleagues8 not further detailed in this Commentary that will require serious scrutiny as the legitimacy and potential utility of this model is now further evaluated by others. The authors should be congratulated, however, on embarking on a model system that obviates the relevance issues inherent in exposing rodent skin to UVB as an experimental surrogate for human photoaging. Certainly, the development and perfection of such a translationally relevant model for human cutaneous photoaging would be useful to photobiologists and those interested in cosmeceuticals alike. If the data of Hachiya and co-workers8 withstands the scrutiny of further studies, then the ability to produce authentic wrinkles and attendant cellular and molecular alterations in a xenograft model after only 30 UVB treatments administered throughout a 6-week period will also raise intriguing questions as to why mother nature takes so long to produce a similar effect!
As we learn more about photoaging through novel and translationally-relevant approaches like those presented in this issue of the AJP, we will undoubtedly begin to solve the mysteries that relate to this ubiquitous and important phenomenon at molecular and genomic levels of inquiry, as emphasized by Yaar and Gilchrest.7 Experimental evidence suggests that UVR induces production of ROS. These ROS can inhibit the enzyme protein-tyrosine phosphatase-κ, which hypophosphorylates cell surface receptors, rendering them inactive. Therefore, this inhibition results in constitutive activation of cell surface receptors such as the epidermal growth factor receptor, interleukin-1 receptor, and tumor necrosis factor receptor,13 which trigger intracellular signaling via stress-associated mitogen-activated protein kinases (eg, p38 and JNK)14 and ultimately results in the nuclear transcription of a complex known as activator protein-1, which is composed by proteins c-Jun and c-Fos.15
Increased activator protein-1 transcription results in decreased synthesis of collagens I and III and blocks the effects of transforming growth factor-β, a known enhancer of collagen gene transcription and negative regulator of keratinocyte proliferation.14,15 Transforming growth factor-β exerts its effects through activation of the intracellular signaling proteins SMAD2 and SMAD3, and its effects are antagonized by the SMAD7 protein, which interferes with transforming growth factor-β-SMAD2 and -3 signaling.16,17 Furthermore, induction of activator protein-1 transcription increases the levels and activity of metalloproteinases (MMP-1, MMP-3, and MMP-9), with consequent increased breakdown of collagen and extracellular matrix proteins, important steps in wrinkle formation.15
UVR also has deleterious effects on mitochondria. The electron transport chain (respiratory chain) generates ROS that may damage mitochondrial DNA. It is estimated that the constitutive mutation frequency in mitochondrial DNA is ∼50-fold higher than in nuclear DNA.18 Of relevance, it has been shown that a segment of mitochondrial DNA coding for electron transport chain components is consistently deleted in tissues from older patients. This deletion, known as the common deletion, has been reported to be up to 10-fold more common in photodamaged skin than in nonchronically sun-damaged skin.19 Interestingly, there does not appear to be a correlation between the extent of the common deletion in photodamaged skin with age, but rather with the severity of photodamage.20 This has lead some to propose that the common deletion may be a useful molecular biomarker of photodamage.20 Decreased mitochondrial function in photodamaged skin is thought to further contribute to ROS accumulation and imbalance on cellular energy production.
Proteins may also be altered as a consequence of oxidative damage from generation of ROS in photoaged skin. It has been shown that certain amino acids (ie, cysteine, methionine, proline) are more vulnerable to oxidative damage than others.21 UVR may also result in cross-linking of dermal proteins such as collagens and elastin. Oxidative modifications to proteins may result in loss of function and increased susceptibility to degradation. Cellular accumulation of oxidized proteins inhibits proteasomal function, which impedes the cell’s ability to successfully degrade additional damaged proteins.22
Telomeres, a tandem repeat of short sequence (TTAGGG), cover the terminal part of chromosomes, preventing their fusion. Because the terminal portion of DNA cannot be replicated, the last 100 to 200 bases of telomeres are lost after each cell division. Eventually, when telomeres become of a critically short length, cells no longer divide and enter a state of senescence.23 It has been proposed that the normal loop configuration of telomeres may also be disrupted as a result of UVR-induced damage, exposing the single-stranded overhang on their 3′ end. This allows interaction between the overhang and the Werner protein, which results in activation of the p53 tumor suppressor protein and other DNA damage response proteins to induce senescence or apoptosis.24 This conceptual model may explain some of the commonalities between chronological aging and photoaging.7,23
Using clinically relevant models, such as the one proposed by Hachiya and colleagues,8 it is envisioned that future studies may not only serve to validate their model, but that they will be useful to uncover the molecular pathogenic mechanisms of cutaneous photoaging. And as we progress in this regard, the definition of photoaging is likely to expand to include pathways not directly involved in the production of the cosmetically alarming wrinkle. For example, chronically-photodamaged facial skin shows dramatic depletion of interfollicular Langerhans cells responsible in part for intact immunosurveillance and thus potential sentinels that guard against UVB-induced procarcinogenic mutations.25 Interestingly, agents such as topical retinoids that reduce the cosmetic effects of photoaging also may result in dramatic replenishment of Langerhans cells.25 Procarcinogenic mutations that are specifically harbored in long-lived epithelial and neural crest-derived stem cells may also be viewed as a form of photoaging, and we are presently on the cusp of better identifying these cells to study this facet of cellular pathology induced by chronic sun damage and thus a likely molecular component of photoaged skin. Only by identifying methods for experimental evaluation of intact human skin will this important area of research be fully exploited, and the study by Hachiya and colleagues8 is an important step in this direction.
Footnotes
Address reprint requests to George F. Murphy, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School,75 Francis St., Boston, Massachusetts 02115.E-mail: gmurphy@rics.bwh.harvard.edu.
See related article on page 401
References
- Kligman LH, Kligman AM. The nature of photoageing: its prevention and repair. Photodermatology. 1986;3:215–227. [PubMed] [Google Scholar]
- Choi CM, Berson DS. Cosmeceuticals. Semin Cutan Med Surg. 2006;25:163–168. doi: 10.1016/j.sder.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51–58. doi: 10.1046/j.1523-1747.2002.19636.x. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Zheng P, Lavker RM. The anatomy and pathogenesis of wrinkles. Br J Dermatol. 1985;113:37–42. doi: 10.1111/j.1365-2133.1985.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Kurban RS, Bhawan J. Histologic changes in skin associated with aging. J Dermatol Surg Oncol. 1990;16:908–914. doi: 10.1111/j.1524-4725.1990.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Lavker RM. Cutaneous aging: chronologic versus photoaging. Gilchrest BA, editor. Cambridge: Blackwell Science; 1995:pp 123–135. [Google Scholar]
- Yaar M, Gilchrest BA. Photoageing: mechanism, prevention, and therapy. Br J Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Sriwiriyanont P, Fujimura T, Ohuchi A, Kitahara T, Takema Y, Kitzmiller WJ, Visscher MO, Tsuboi R, Boissy RE. Mechanistic effects of long-term ultraviolet B irradiation induces epidermal and dermal changes in human skin xenografts. Am J Pathol. 2008;174:401–413. doi: 10.2353/ajpath.2009.070500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GF. A short of mice and men: SCID mouse-human skin chimeras in immunology research. J Invest Dermatol. 1996;107:529–530. doi: 10.1111/1523-1747.ep12582780. [DOI] [PubMed] [Google Scholar]
- Lund LP, Timmins GS. Melanoma, long wavelength ultraviolet and sunscreens: controversies and potential resolutions. Pharmacol Ther. 2007;114:198–207. doi: 10.1016/j.pharmthera.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Godar DE. UV doses worldwide. Photochem Photobiol. 2005;81:736–749. doi: 10.1562/2004-09-07-ir-308r.1. [DOI] [PubMed] [Google Scholar]
- Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/s1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J Biol Chem. 2006;281:27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhee JJ. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGF-beta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Grasbon-Frodl E, Eitzen UV, Kosel S. Neurodegeneration and aging: role of the second genome. J Neurosci Res. 1998;52:1–6. doi: 10.1002/(SICI)1097-4547(19980401)52:1<1::AID-JNR1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Berneburg M, Gattermann N, Stege H, Grewe M, Vogelsang K, Ruzicka T, Krutmann J. Chronically ultraviolet-exposed human skin shows a higher mutation frequency of mitochondrial DNA as compared to unexposed skin and the hematopoietic system. Photochem Photobiol. 1997;66:271–275. doi: 10.1111/j.1751-1097.1997.tb08654.x. [DOI] [PubMed] [Google Scholar]
- Koch H, Wittern KP, Bergemann J. In human keratinocytes the common deletion reflects donor variabilities rather than chronologic aging and can be induced by ultraviolet A radiation. J Invest Dermatol. 2001;117:892–897. doi: 10.1046/j.0022-202x.2001.01513.x. [DOI] [PubMed] [Google Scholar]
- Shacter E. Protein oxidative damage. Methods Enzymol. 2000;319:428–436. doi: 10.1016/s0076-6879(00)19040-8. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Moreau M, Nizard C, Friguet B. Proteasome and photoaging. Ann NY Acad Sci. 2007;1100:280–290. doi: 10.1196/annals.1395.029. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Kosmadaki MG, Gilchrest BA. The role of telomeres in skin aging/photoaging. Micron. 2004;35:155–159. doi: 10.1016/j.micron.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Murphy GF, Katz S, Kligman AM. Topical tretinoin replenishes CD1a-positive Langerhans cells in chronically photodamaged human skin. J Cutan Pathol. 1998;25:30–34. doi: 10.1111/j.1600-0560.1998.tb01686.x. [DOI] [PubMed] [Google Scholar]