Abstract
Recent evidence suggests that bone marrow-derived fibroblasts are involved in airway remodeling in asthma, but the role and mechanism of recruitment of these fibroblasts remains unclear. Stem cell factor (SCF), a key factor in the propagation of hematopoietic stem cells, is important in the process of airway remodeling as well. To test the hypothesis that SCF is involved in the recruitment and differentiation of bone marrow-derived progenitor cells, GFP-bone marrow chimeric mice were created. These mice were then sensitized and chronically challenged with cockroach antigen to induce chronic airway disease. Fluorescence microscopy revealed an influx of significant numbers of GFP-expressing fibroblasts in the airways of these mice, which was confirmed by flow cytometric analysis of cells co-expressing both GFP and collagen I. These cells preferentially expressed c-kit, interleukin-31 receptor, and telomerase reverse transcriptase when compared with control lung-derived fibroblasts. Interestingly, SCF stimulated interleukin-31 receptor expression in bone marrow cells, whereas interleukin-31 strongly induced telomerase reverse transcriptase expression in fibroblasts. Treatment with neutralizing antibodies to SCF significantly reduced airway remodeling and suppressed the recruitment of these bone marrow-derived cells to the lung. Thus SCF in conjunction with interleukin-31 may play a significant role in airway remodeling by promoting the recruitment of bone marrow-derived fibroblast precursors into the lung with the capacity to promote lung myofibroblast differentiation.
Airway inflammation, airflow obstruction, and bronchial hyperresponsiveness are characteristic phenotypic features of asthma. Clinically, airflow obstruction in asthma often is not fully reversible, and many asthmatic patients experience an accelerated and progressive loss of lung function throughout time.1 This is accompanied by peribronchial eosinophil accumulation with mucus hypersecretion and structural changes characterized by an increase in smooth muscle mass and subepithelial fibrosis. These changes correlate with airway hyperresponsiveness, reduced lung function, and an increase in fibroblast and myofibroblast numbers. Moreover, there is mounting evidence that bone marrow-derived fibroblasts or fibroblast-like cells may participate in this airway remodeling,2,3,4 although the role of these cells and the mechanism of their recruitment remain poorly defined.
Stem cell factor (SCF) is a critical factor for hematopoiesis and hematopoietic stem survival.5,6 It also has a role in mobilization of bone marrow stem cells, as well as in cell differentiation.7 SCF is also involved in pathogenesis of allergic airway disease and can directly induce a dose-depended increase in airway hyperreactivity8 via mast cell activation.9 Additionally, mutant mice deficient in SCF and pulmonary mast cells demonstrate significant alteration in the allergen-induced airway hyperreactive responses.8 In murine models of asthma, neutralization of SCF in vivo attenuates Th2 responses, eosinophilia, mucus production, airway remodeling, and collagen deposition.8,10,11,12 However, the mechanism by which SCF mediates the airway remodeling responses in asthma is not fully understood, although this may be dependent on fibrogenic factor production by SCF-responsive cells, such as eosinophils and mast cells.13,14,15,16,17 In one study, suppression of SCF expression using antisense oligonucleotides reduces production of interleukin (IL)-4, a well-known promoter of fibrosis.18 However a direct role for SCF in recruitment and activation of fibroblasts has not been excluded and is of particular interest in light of recent discoveries suggesting a potential role for bone marrow-derived fibroblast progenitor cells in airway remodeling and pulmonary fibrosis.4,19
Clinical evaluation of SCF in allergic asthma recently revealed another cytokine closely associated with allergic airway disease—IL-31. The authors indicated that evaluation of plasma levels of SCF and IL-31 or expression in peripheral blood mononuclear cells will be valuable parameters for the diagnosis of allergic asthma.20 IL-31 is a four-helix bundle cytokine belonging to the gp130-IL-6 family and is preferentially produced by activated T cells.21 Its activity on target cells is mediated through binding to a heterodimeric receptor composed of the IL-31 receptor A (IL-31RA) and the oncostatin M receptor (OSMR).21 In human alveolar epithelial cells transfected with IL-31RA, IL-31 activates the signal transducer and activator of transcription factor 3 (STAT-3), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and Akt signaling pathways.22 Interleukin-31RA and OSMR are expressed in a variety of tissues including intestine, testis, skin, thymus, brain, and bone marrow.22,24,25 IL31RA deficiency exacerbates Schistosoma mansoni egg-induced lung granulomatous inflammation.23 Known target cells for IL-31 include keratinocytes, subepithelial myofibroblasts, activated monocytes, macrophages, myeloid progenitor cells, eosinophils, and bronchial epithelial cells.21,22,24,25,26,27,28,29,30,31,32
With regard to biological activity, IL-31 is known to induce dermal inflammation, pruritus, and severe dermatitis when overexpressed in transgenic mice.21 Bronchial epithelial cells expressed higher levels of chemokines and cytokines in response to IL-31.29 Given the recent interest on bone marrow progenitor cells in pulmonary disease, it is noteworthy that IL-31 is recently reported to significantly promote survival of both bone marrow and spleen-derived hematopoietic progenitor cells.30 Finally, and most germane to this study, elevated levels of both SCF and IL-31 proteins in peripheral blood, and their mRNAs in peripheral blood mononuclear cells have been reported in patients with asthma.33 However the precise role of IL-31 in airway remodeling (if any) remains unclear.
Thus, the present study was developed to investigate whether i) fibroblasts involved in the airway remodeling in the cockroach antigen (CRA)-induced model could originate from bone marrow; ii) the previously observed beneficial effect of anti-SCF treatment could be related to suppression of the recruitment of these bone marrow-derived cells into the sites of airway remodeling; and iii) whether these cells are regulated by IL-31. Using the chronic CRA model in GFP bone marrow chimera mice, our results showed that a significant proportion of fibroblasts in remodeling airways were derived from bone marrow. Furthermore, a significant proportion of these cells expressed c-kit, IL-31RA, and telomerase reverse transcriptase (TERT), but did not express α-smooth muscle actin, a marker of myofibroblast differentiation. Although SCF induced IL-31RA expression in bone marrow cells, IL-31 significantly induced TERT in fibroblasts. Treatment with neutralizing antibodies to SCF caused a marked reduction in the recruitment of these cells, along with significant reduction in airway remodeling. The role of these bone marrow-derived fibroblasts might be to secrete factor(s) that promote endogenous lung myofibroblast differentiation.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml fungizone) were from Invitrogen Corp. (Carlsbad, CA). Rat biotin-conjugated anti-mSCF R/c-kit (CD117) antibody was from R&D Systems Inc. (Minneapolis, MN). Rabbit biotin-conjugated anti-collagen type I antibody was from Rockland (Gilbertsville, PA). Mouse biotin-conjugated anti-α-smooth muscle actin antibody (clone 1A4) was from Lab Vision Corp. (Fremont, CA). Rabbit polyclonal anti-TERT antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse streptavidin-APC-Cy7, streptavidin-PE-Cy5, Fc block (clone 2.4G2), and the BD Cytofix/Cytoperm kit were from BD Biosciences (San Diego, CA).
Mice
FVB.Cg-Tg(GFPU)5Nagy or FVB/NJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained under specific pathogen-free conditions. We obtained approval for experimental protocols and mouse use from The University of Michigan Committee on Use and Care of Animals.
Bone Marrow Chimera Mice
Bone marrow chimeras were prepared as previously described.19 Bone marrow cells were collected from femurs and tibias of donor GFP Tg or wild-type FVB mice by aspiration and flushing. Recipient FVB mice were exposed to two doses of 5 Gy given 3 hours apart using a 137Cs irradiator, and then maintained on acidified water and autoclaved feed ad libitum. After irradiation, 4 × 106 bone marrow cells from GFP FVB mice in a volume of 200 μl of sterile phosphate-buffered saline (PBS) were injected retro-orbitally under anesthesia.
CRA Challenge
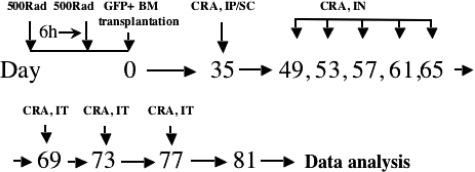
Five weeks after bone marrow transfection mice were sensitized intraperitoneally and subcutaneously with 1000 protein nitrogen units (PNUs) of cockroach Ag (CRA; Holliser Stier, Toronto, Canada) 1/1 in incomplete Freunds adjuvant (Sigma-Aldrich, St. Louis, MO) as we described before.10,11,12,14,15,34,35,36,37,38,39 Then mice were challenged intranasally with 150 PNUs of CRA every 4th day after initial sensitization to localize the response to the lung (Figure 1). The final two allergen challenges (500 PNUs) were given 4 days apart by intratracheal injection. Then animals were euthanized and lungs were removed. In some experiments along with the intratracheal injections of cockroach allergen, mice were given either anti-SCF (50 μg, rabbit anti-murine SCF Abs) or control antibody treatment (50 μg, total IgG).
Figure 1.
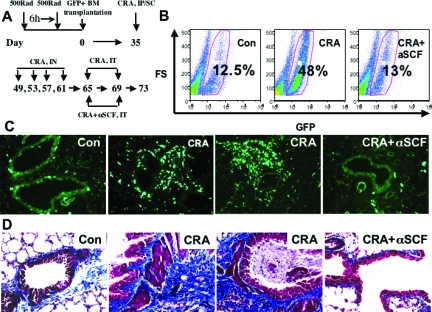
Development of GFP bone marrow chimera mouse and CRA asthma model. The experimental protocol for the model is summarized.
Production of Anti-SCF Antibodies
Rabbit anti-murine SCF Abs was prepared by multiple-site immunization of New Zealand White rabbits with recombinant murine SCF (R&D Systems Inc., Minneapolis, MN) in CFA. Polyclonal Abs were titered by direct enzyme-linked immunosorbent assay and specifically verified by the failure to cross-react to mIL-3, mIL-1β, mTNF-α, mMIP-1α, IL-6, mJE, mMIP-1β, mMCP-1, mIL-8, mRANTES, mMIP-1α, mTNF-β, and mMIP-1α. The IgG portion of the serum was purified over a protein A column.
Assaying of SCF Level in Plasma
Anesthetized animals were bled by aortic puncture into titrated plastic syringes (50 μl of 0.2 mol/L Tris-citrate/ml of blood). The syringes were placed in ice and all subsequent manipulations (except as stated below) were done in plasticware at 4°C. Samples were centrifuged for 18 minutes at 850 × g and the supernatant recentrifuged at 26,800 × g for 30 minutes. The resulting platelet-depleted plasma was transferred into glass tubes and clotted for 1 hour at 56°C in a presence of 5 mmol/L of CaCl2. Then the samples were centrifuged at 60,000 × g for 2 hours and the resulting plasma was used to determine SCF levels. SCF capturing and detection antibodies were from R&D Systems Inc.
Quantitative Polymerase Chain Reaction (PCR) Analysis
RNA was isolated using Trizol reagent (Invitrogen) and each sample was reverse-transcribed into cDNA. QPCR analysis was performed using TaqMan reagents and predeveloped reagent kits (PE Biosystems, Foster City, CA) on 2 μl of cDNA in a total reaction volume of 25 μl.
Morphological Fluorescence Analysis
The lungs were fully inflated by the intratracheal perfusion with 4% paraformaldehyde. Lungs were then dissected and placed in fresh paraformaldehyde on ice for 6 hours, infused with graded sucrose washes for 24 hours, and finally fixed and frozen in OCT compound (Miles Inc., Elkhart, IN). Serial cryostat sections (4 μm thick) were then analyzed by fluorescence microscopy within 1 hour to evaluate distribution or localization of GFP+ cells. Some tissue sections were stained to visualize collagen I, GFP, and α-smooth muscle actin. Tissue sections were washed in PBS, blocked with Fc block, 1:200 in DPBS plus 7% bovine serum albumin plus 1% fetal calf serum plus 1% normal goat serum and permeabilized with 0.25% Triton X-100 in DPBS for 5 minutes at room temperature in the dark. Then specimens were stained with i) anti-collagen I primary antibodies (AbCam, ab34710) and goat anti-rabbit IgG Alexa Fluor-568-conjugated secondary antibodies (Molecular Probes/ Invitrogen), 1:400 (all in PBS plus 2% bovine serum albumin plus 0.05% sodium azide plus 2% normal goat serum); ii) anti-α-smooth muscle actin fluorescein isothiocyanate-conjugated antibodies; iii) anti-GFP Alexa Fluor-647-conjugated antibodies (Molecular Probes/Invitrogen). Images were taken using a confocal microscope (Zeiss, Thornwood, NY).
Whole Lung Histological Analysis
The lungs were fully inflated by the intratracheal perfusion with 4% paraformaldehyde. Lungs were then dissected and placed in fresh paraformaldehyde for 24 hours. Routine histological techniques were used to paraffin-embed this tissue, and 4-μm sections of whole lung were stained with Masson Trichrome or hematoxylin and eosin. Inflammatory infiltrates and other histological changes were examined around bronchioles and larger airways, using light microscopy.
Flow Cytometry
Whole lungs from mice were dispersed using type IV collagenase; red blood cells were then lysed (using RBC lysis buffer: 388 mmol/L NH4Cl, 29.7 mmol/L NaHCO3, 25 μmol/L Na2EDTA) for 3 minutes on ice, and total number of cells per lung counted. After appropriate washing and blocking with Fc block (CD16/32), 1 × 106 cells were surface-stained with biotin-conjugated c-kit antibody. Secondary antibodies for c-kit were streptavidin-PE-Cy5-conjugated. Then cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit and stained with rabbit biotin-conjugated anti-collagen I or isotype-matched control IgG. The staining was detected with streptavidin-APC-Cy7-conjugated antibody. For some experiments cells also were stained with rabbit anti-TERT antibody and adequate PE-conjugated secondary detection antibody or PE-Cy7-conjugated CD11b antibody. Flow-cytometric analysis was undertaken using a BD LSR II and flow sorting was done using FACS ARIA (both BD Biosciences). Data collected were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Mouse Lung Fibroblast Culture
Mouse lung fibroblasts were isolated from lung tissue by mincing and enzymatic digestion with collagenase IV as previously described.19 After filtration, released cells were washed and then used for sorting. After flow-sorting, GFP-positive and CD11b-negative cells were cultured for 12 hours to obtain conditioned media (culture media: STEMPROR MSC SFM serum-free medium for human mesenchymal stem cells; Invitrogen). Control aged media were also placed in empty (without cells) wells under similar incubation conditions. Where indicated, control aged media alone or conditioned media from GFP+ fibroblasts were mixed 50:50 with control aged media and used to treat GFP− fibroblasts. Samples were collected after 6 hours in culture.
Analysis of Cultured Cells by Flow Cytometry
Cells were washed and detached on ice using Versene mix. Cell staining was performed as described for whole lung mince.
Statistics
Statistical significance was determined using analysis of variance with P values less than 0.05 followed by Student-Neuman-Keuls posttest when appropriate.
Results
Analysis of Cultured Bone Marrow-Derived Fibroblasts
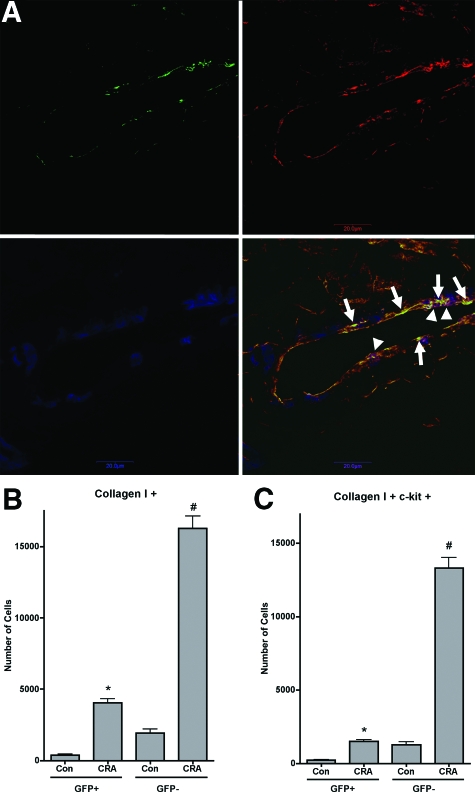
GFP bone marrow chimera mice have been used previously to document recruitment of bone marrow-derived fibroblasts to the lung in response to lung injury and fibrosis.4,19 Based on previous studies and the ready availability of GFP transgenic mice in the FVB/NJ background, initial experiments used this background strain for donor and recipient mice. After stable engraftment, the animals were chronically challenged with CRA (Figure 1) to induce an airway remodeling as we described before.10,11,12,14,15,34,35,36,37,38,39 The presence of GFP-positive and collagen-positive cells in the tissue, as well as α-smooth muscle actin-positive but GFP-negative cells was illustrated by immunofluorescence (Figure 2A). The representative lung tissue section from a chronic CRA-treated animal was immunostained for GFP (green), collagen I (red), and α-smooth muscle actin (blue). The GFP-positive and collagen I-positive cells appear yellowish-green and are indicated by arrows, whereas the α-smooth muscle actin-positive and collagen I-positive, but GFP-negative cells appear purplish and are indicated by arrowheads.
Figure 2.
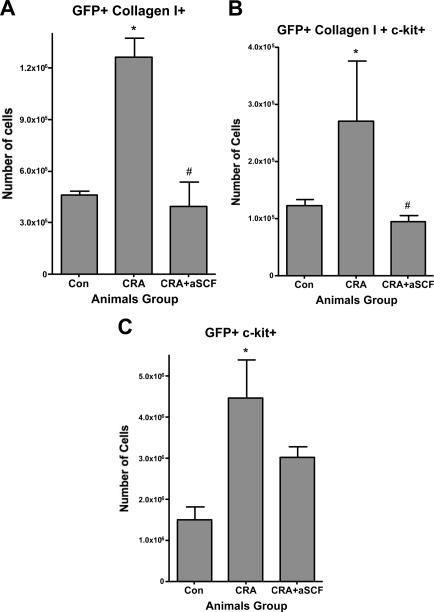
Phenotypic analysis of lung fibroblast cultures. A: A representative lung tissue section from a chronic CRA-treated animal was immunostained for GFP (green), collagen I (red), and α-smooth muscle actin (blue). The single color images for each antigen are shown at the top left (GFP), top right (collagen I), and bottom left (α-smooth muscle actin) panels. The bottom right panel shows the merged image. The GFP-positive and collagen I-positive cells appear yellowish-green and are indicated by arrows; whereas the α-smooth muscle actin-positive and collagen I-positive, but GFP-negative cells appear purplish and are indicated by arrowheads. B and C: Whole lung mince was digested with collagenase IV from control unchallenged animals or chronic CRA allergen-challenged animals, was cultured in six-well plates for 48 hours, and was analyzed by flow cytometry for expression of the indicated collagen I (B) and c-kit (C) as described. Significance at *#P < 0.05 compared with untreated control. Con, control unchallenged animals; CRA, chronic CRA-challenged animals. N = 12 animals per group.
In view of the fibroblast-like morphology of the GFP-positive cells in remodeling airways in CRA-challenged lung tissue sections, lung fibroblasts were isolated and cultured from these and control lungs. After the second passage, the cells were analyzed by flow cytometry for expression of GFP, type I collagen, and progenitor cell marker c-kit expression. At this early passage, the number of cells that were of bone marrow origin (ie, GFP-positive) represented 15% of the total cells cultured from CRA-challenged lungs. Virtually all of the cells were positive for type I collagen, consistent with their fibroblast-like morphology (Figure 2B). A significant number of GFP-positive and collagen I-producing cells also expressed c-kit (Figure 2C).
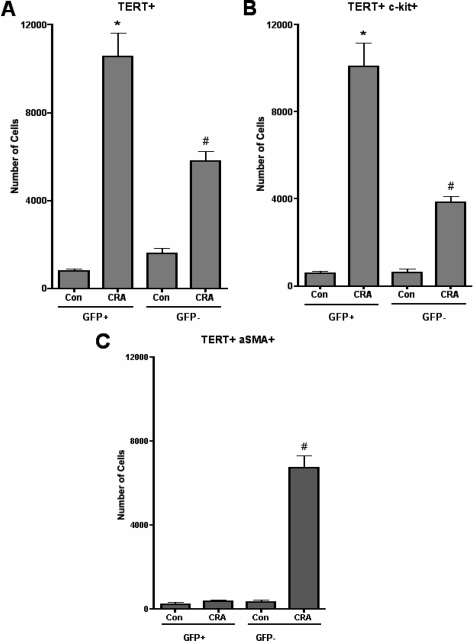
TERT expression is induced in lung injury and fibrosis, and associated with some of the bone marrow-derived fibroblasts.13,40,41,42,43,44 Figure 3A showed induction of TERT in CRA-challenged lung-derived cells, with most (>70%) being derived from bone marrow. Comparable numbers of bone marrow-derived cells were c-kit-positive (Figure 3B). In contrast, virtually all of the bone marrow-derived fibroblasts did not express α-smooth muscle actin, which was expressed only in the GFP-negative population (Figure 3C). Thus a significant proportion of fibroblasts in the CRA-challenged lung were derived from bone marrow precursors, many of which still expressed progenitor markers but not α-smooth muscle actin, a marker of myofibroblast differentiation.
Figure 3.
Phenotypic analysis of lung fibroblast cultures. Whole lung mince digested with collagenase IV from control unchallenged animals or chronic CRA allergen-challenged animals was cultured in six-well plates for 48 hours and analyzed by flow cytometry for expression of TERT (A), TERT and c-kit (B), or α-smooth muscle actin (α-SMA) (C) as described. Significance at *#P < 0.05 compared with untreated control within GFP+ or GFP− samples. Con, control unchallenged animals; CRA, chronic CRA-challenged animals. N = 6 animals per group.
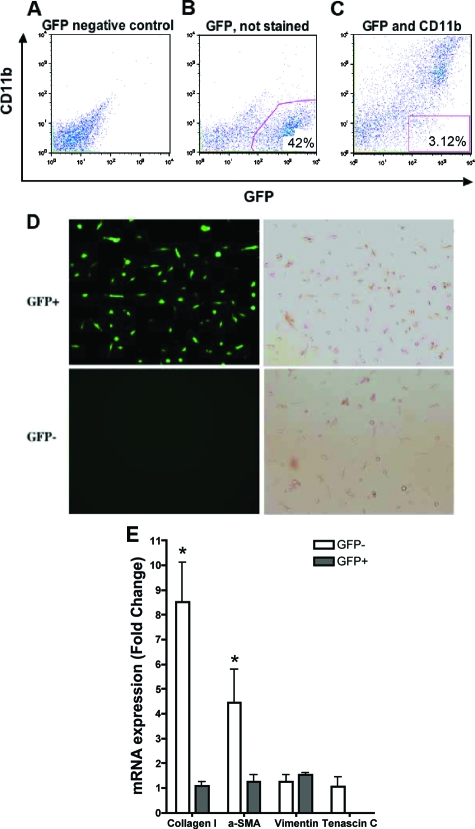
Phenotypic Analysis of Purified Bone Marrow-Derived Lung Fibroblasts
To more definitively analyze the phenotype of the recruited bone marrow-derived lung fibroblasts, whole lung digests were purified for GFP-positive and CD11b-negative cells by flow cytometry. Normal wild-type lung digests were used as a GFP-negative control (Figure 4A) to set up the appropriate sorting gates as shown in Figure 4, B and C. To avoid macrophages, the GFP-positive and CD11b-negative population was flow-sorted (Figure 4C). Most of the sorted GFP-positive and GFP-negative cells have typical fibroblast-like morphology (Figure 4D). For further analysis of phenotype, mRNA from these cells was analyzed for several marker gene expressions. The results (Figure 4E) showed that both GFP-positive and GFP-negative cell cultures expressed vimentin, an intermediate intracytoplasmic filament expressed in fibroblasts.45 Expression of collagen I, although readily detectable in GFP-positive cells, was significantly lower than that in GFP-negative cells, consistent with the flow cytometric analysis (Figure 2). Expression of α-smooth muscle actin was readily detectable in GFP-negative cells, but was dramatically lower in GFP-positive cells, where it was close to the limit of detection. Interestingly GFP-negative fibroblasts also expressed tenascin C (Figure 4E), which is expressed in fibroblast foci, areas consisting of fibroblasts and myofibroblasts found in lung biopsies of patients with idiopathic pulmonary fibrosis.46 Tenascin C is an oligomeric extracellular matrix glycoprotein suggested to play a structural role and to modulate the adhesive and migratory functions of cells. In contrast, bone marrow-derived fibroblasts expressed much lower and barely detectable levels of tenascin C (Figure 4E). Freshly isolated murine eosinophil mRNA was used as a negative control for collagen I, α-smooth muscle actin, vimentin, and tenascin C expression, and did not exhibit any amplification products for these genes. These findings confirmed that although bone marrow-derived fibroblasts were recruited to the lung in this CRA model, they were phenotypically distinct from fibroblasts that were of local or pulmonary origin.
Figure 4.
Phenotypic analysis of purified GFP-positive lung fibroblasts. A: Wild-type (GFP-negative) control lung fibroblasts were sorted and used for gating purposes. B: GFP-positive cells were distinguished based on the calibration developed in A. C: Using the calibration developed in A and B, sorting of CD11b-negative lung fibroblasts. D: Microscopic analysis (fluorescence, left; phase contrast, right) revealed morphology consistent with fibroblasts in both the GFP-positive (top) and GFP-negative (bottom) cell populations. E: RNA from the purified GFP-positive and -negative cells was analyzed by real-time PCR for the following mRNA species: collagen I, α-smooth muscle actin, vimentin, and tenascin-C. Except for vimentin, which was equally expressed in both cell populations, expression of these genes was lower in the GFP-positive cells relative to the GFP-negative cells. Data are expressed as 2−ΔΔCT, using GAPDH as the reference and the GFP-negative cell signal as calibrator. N = 12 animals per group. *P < 0.05.
Effect of Anti-SCF on Lung Recruitment of Bone Marrow-Derived Fibroblasts
Previous studies have shown the importance of SCF in the pathogenesis of murine models of asthma,6,9,10,11,12,13,14,47 but the precise role(s) of SCF remains incompletely understood. In view of evidence in the current study showing bone marrow origin of c-kit-expressing fibroblasts in remodeling airways of mice exposed to chronic CRA challenge, the possibility that SCF might be important in their recruitment was examined. Whole lung mince from naïve mice and mice challenged with CRA without or with treatment with neutralizing antibody to SCF, were digested to obtain single cell suspensions for flow cytometric analysis. The results showed a significant increase (2.7-fold) in GFP and collagen I-positive cells after chronic CRA challenge (Figure 5A) relative to that in unchallenged animals. Anti-SCF antibody treatment given with the two final intratracheal CRA challenges caused significant reduction in the number of cells expressing both GFP and collagen I, to essentially control levels (3.2-fold decrease, CRA + aSCF over CRA) (Figure 5A). Additionally, 21.4% of GFP- and collagen I-positive cells from chronic CRA animals were also c-kit-positive (Figure 5B) and this population was also reduced to control levels on treatment with anti-SCF antibodies. The total population of GFP-positive and c-kit-positive cells were also elevated (2.98-fold increase over controls) after chronic CRA treatment (Figure 5C). Neutralization of SCF caused significant reduction in this (1.49-fold decrease) GFP and c-kit-positive population, but not completely to control levels as was observed with bone marrow-derived collagen-producing cells. Thus, neutralization of SCF in vivo caused a significant reduction in the recruitment of bone marrow-derived fibroblast progenitor cells to the remodeling airway.
Figure 5.
Effects of anti-SCF antibodies on lung recruitment of bone marrow-derived collagen I-positive cells. Whole lungs from mice in the indicated treatment groups were minced, digested with collagenase IV, and processed for flow cytometry as described. Cells were stained for GFP and collagen I (A), GFP, collagen I and c-kit (B), or GFP and c-kit (C). Total numbers of cells expressing the indicated antigen or antigens were calculated by multiplying the percentage of positive cells by the total number of cells recovered per lung. Significance at *P < 0.05 compared with untreated control. Significance at #P < 0.05 compared with CRA. Con, control unchallenged animals; CRA, chronic CRA-challenged animals; CRA + aSCF, chronic CRA and anti-SCF antibody-treated animals. N = 12 animals per group.
Effect of Anti-SCF on Lung Remodeling in GFP Bone Marrow Chimera Mice Exposed to Chronic Allergen Challenge
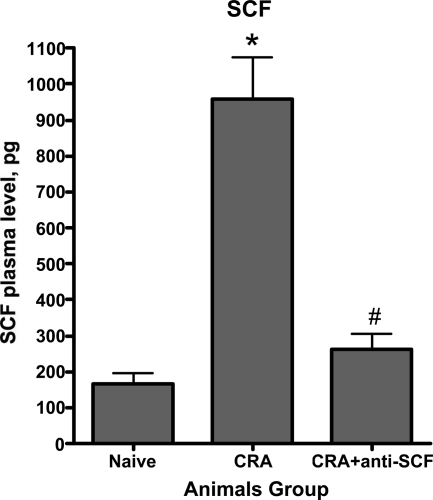
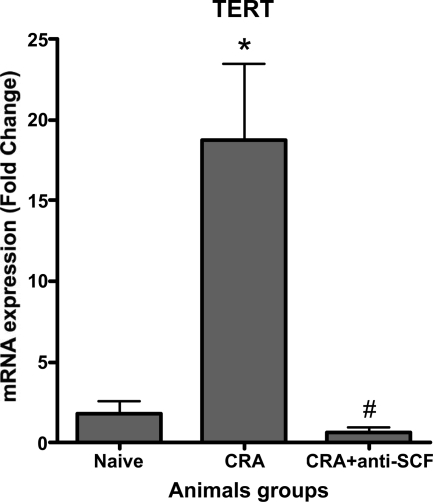
Neutralization of SCF significantly reduced peribronchial remodeling and collagen deposition in the CRA model of asthma.10 To extend this observation to remodeling in the GFP bone chimera mice and assess the role of bone marrow-derived cells, anti-SCF antibodies were administered with the two final allergen challenges as diagrammed in Figure 6A. Neutralization of SCF significantly reduced the number of CRA-induced GFP-positive cells in the lung back to essentially control levels (Figure 6B). To confirm the flow cytometric analysis, and the associated impact on airway remodeling, GFP bone marrow chimera mice were chronically challenged with cockroach allergen to develop asthma and the effects of SCF neutralization examined by immunohistopathology. The results showed that although control lungs exhibited normal architecture with occasional GFP-positive cells (Figure 6C, Con panel), CRA-challenged lungs showed marked infiltration by GFP-positive cells (Figure 6C, CRA panels). In tissue sections from CRA-challenged mice treated with anti-SCF antibodies, there was a marked reduction in the number of GFP-positive cells (Figure 6C, CRA + αSCF panel) with only a scattering of mostly mononuclear cells in the interstitial areas. These findings were consistent with the flow cytometric analysis showing the marked reduction in GFP-positive cell recruitment by the anti-SCF treatment. Moreover, when the tissue sections were analyzed for extent of remodeling and presence of myofibroblasts, the efficacy of anti-SCF was also confirmed (Figure 6D). Masson Trichrome staining of the lung tissue sections for evaluation of collagen deposition revealed significant fibrosis of the airway by CRA challenge in these FVB mice, which was markedly reduced by anti-SCF treatment. Significant numbers of myofibroblasts were also detected by immunostaining for α-smooth muscle actin, which were also reduced by the anti-SCF treatment. Reduction in mucus plugging also accompanied the reduction in airway remodeling. These findings were comparable to those previously studied using Balb/C mice.10,11,12 Thus neutralization of SCF resulted in diminished recruitment of bone marrow-derived cells, including fibroblasts, which correlated with reduction in airway remodeling. We also assayed plasma level of SCF in chronic allergen-challenged and anti-SCF antibody-treated animals. We found that neutralization of SCF significantly suppressed CRA-induced SCF in plasma (Figure 7). The neutralization of SCF also had an interesting effect on lung-derived fibroblasts, cultured as we described before. Lung fibroblasts derived from chronic allergen-challenged animals displayed significantly up-regulated TERT expression (Figure 8). Whereas in fibroblasts derived from lungs of anti-SCF-treated animals TERT expressions were significantly suppressed. As we already mentioned before, TERT expression is induced in lung injury and fibrosis, and associated with some of the bone marrow-derived fibroblasts.13,40,41,42,43,44
Figure 6.
Effects of anti-SCF antibody treatment on airway remodeling. A: Shown are the days of anti-SCF antibody administration in the GFP bone marrow chimera and CRA asthma mice model. B: Administration of anti-SCF antibodies abrogates CRA-induced influx of bone marrow-originated cells into lungs. C: Lungs of mice from the indicated treatment groups were inflated and processed as described. Serial cryostat sections were analyzed within 1 hour for presence of GFP-positive cells in the lung tissue sections. Representative sections from control (Con panel), CRA-challenged (CRA panels), and CRA plus anti-SCF antibody-treated mice (CRA + αSCF panel) are shown. D: Representative lung tissue sections from mice in the indicated treatment groups were stained for collagen using the Masson trichrome stain. Significant remodeling in CRA-challenged mice with mucus plugs was significantly reduced in mice also treated with anti-SCF antibodies. N = 12 animals per group.
Figure 7.
Effects of anti-SCF antibodies on SCF plasma level. The blood was collected from anesthetized animals and processed to obtain plasma. Significance at *P < 0.05 compared with naive animals. Significance at #P < 0.05 compared with CRA animals. Naïve, control unchallenged animals; CRA, chronic CRA-challenged animals; CRA + aSCF, chronic CRA and anti-SCF antibody-treated animals. N = 10 animals per group.
Figure 8.
Effects of anti-SCF antibodies on TERT expression in purified CD11b-negative lung fibroblasts. Flow-sorted CD11b-negative lung fibroblasts were cultured for 24 hours and assayed for TERT expression. Significance at *P < 0.05 compared with naive animals. Significance at #P < 0.05 compared with CRA animals. Naïve, control unchallenged animals; CRA, chronic CRA-challenged animals; CRA + SCF, chronic CRA and anti-SCF antibody-treated animals. N = 10 animals per group.
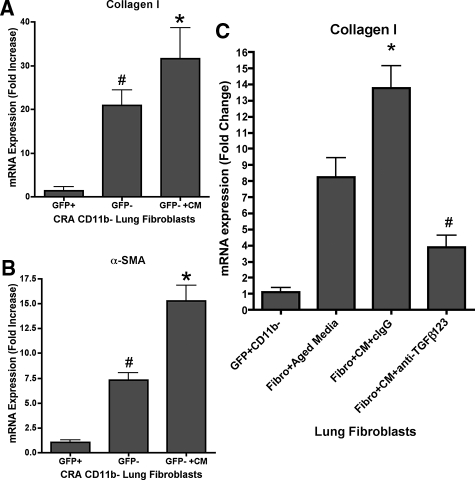
The findings thus far suggested a significant role for the bone marrow-derived fibroblasts, but not by direct differentiation to myofibroblasts. To investigate further the role of these bone marrow-derived fibroblasts, conditioned media from these cells were tested for effects on lung-derived fibroblasts, specifically to see if they could promote myofibroblast differentiation. GFP-positive fibroblasts from CRA-challenged GFP bone marrow chimera mice were purified by flow sorting and depleted of CD11b-positive cells. Conditioned media from these cells were collected and added to GFP-negative lung fibroblasts that had also been depleted of CD11b-positive cells. The results showed that GFP-negative lung fibroblasts treated with these conditioned media had significant augmentation of both α-smooth muscle actin and procollagen I gene expression (Figure 9, A and B). Thus, although the bone marrow-derived (GFP-positive) fibroblasts themselves did not undergo myofibroblast differentiation, they did secrete factor(s) that could promote myofibroblast differentiation in endogenous lung fibroblasts (GFP-negative). Transforming growth factor (TGF)-β was highly expressed in the bone marrow-derived fibroblasts (data not shown), and thus could represent the key factor in the conditioned media responsible for the promotion of endogenous lung myofibroblast differentiation. To address the role of TGF-β in that experiment, we used anti-TGF-β1, -2, and -3 together with conditioned media. Neutralization of TGF-β1, -2, and -3 partially inhibited conditioned media-induced collagen I expression (Figure 9C).
Figure 9.
Effects of bone marrow-derived fibroblast-conditioned media on lung fibroblast procollagen I and α-smooth muscle actin gene expression. Conditioned media were prepared from purified GFP-positive/CD11b-negative fibroblasts from lungs of CRA-challenged mice, and added to media of cultured GFP-negative lung fibroblasts. The effects of this treatment with conditioned media (GFP− + CM) on procollagen I (A, C) and α-smooth muscle actin (B) mRNA levels were compared with the levels in untreated GFP-positive (GFP+) and GFP-negative (GFP−) cells. Data are expressed as 2−ΔΔCT, using GAPDH as the reference and the GFP-positive cell signal as calibrator. A and B: Significance (#P < 0.05) compared with mean signal in GFP-positive cells. Significance compared with mean signal in untreated GFP-negative cells. C: Significance (*P < 0.05) compared with mean signal in fibro + aged media samples. Significance compared with mean signal in fibro + CM + IgG samples. N = 10 animals per group.
GFP+CD11b− Fibroblasts Express High Levels of IL-31RA
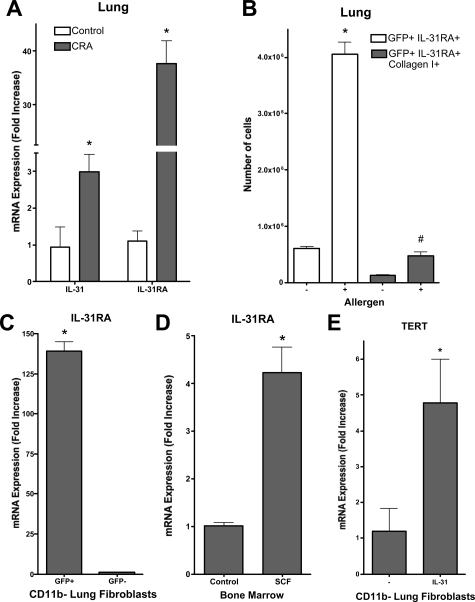
Allergic airway disease is characterized by elevated lung and blood IL-31 expression.21,33 We confirmed that lung IL-31 expression was significantly elevated in the CRA model 6 hours after allergen challenge (Figure 10A). Given that hematopoietic progenitor cells express IL-31 RA and are able to respond to IL-31 with increased survival,30 the possibility that bone marrow-derived fibroblasts could express this receptor and also respond to IL-31 was examined by real-time PCR. Initial evaluation of lung cell IL-31RA expression by flow cytometry revealed a significant increase (more than fourfold) in the number of cells expressing this receptor in antigen-challenged mice relative to that in control mice (Figure 10B). When this was combined with analysis for collagen I expression as a marker for fibroblasts, the results showed a comparable increase in the number of fibroblasts expressing IL-31RA in the antigen-challenged murine samples. To confirm that expression was in fibroblasts and to distinguish between bone marrow-derived versus lung endogenous fibroblasts, the fibroblast population from CRA-challenged GFP bone marrow chimeric mice were isolated and purified by removal of CD11b-positive cells by magnetic bead separation. These CD11b-negative fibroblasts were then flow sorted into GFP-positive (bone marrow-derived) and GFP-negative (endogenous lung origin) populations and analyzed for IL-31RA expression by real time PCR. The results showed a 140-fold higher level of IL-31RA expression in GFP-positive fibroblasts relative to that in GFP-negative fibroblasts, indicating that virtually all of the fibroblast IL-31RA expression in CRA-challenged lungs was from bone marrow-derived fibroblasts (Figure 10C). OSMR, the heterodimeric partner of IL-31RA was also expressed in the GFP-positive-CD11b-negative fibroblasts (data not shown), thus suggesting their myeloid progenitor origin. Indeed, isolated bone marrow cells expressed IL-31RA, which was markedly enhanced (more than fourfold) by SCF treatment (Figure 10D). Finally IL-31 was found to be a potent inducer of TERT expression in lung fibroblasts expressing IL-31RA (Figure 10E), which was not suppressed by TGF-β (data not shown) a known inhibitor of TERT expression.48 Thus, stimulation of lung fibroblasts in vitro with IL-31 leads to increased TERT expression, which is associated with increased survival of lung fibroblasts and favors the development of fibrosis instead of injury resolution.48 Taken together the data indicated the importance of bone marrow-derived c-kit+collagenI+IL-31RA+CD11b− cells that could provide an interesting link between SCF and IL-31 roles in allergic airway disease.
Figure 10.
IL-31 and IL-31RA expression in the CRA model and their role in TERT induction. A: Lung IL-31 and IL-31RA mRNA levels were measured by real-time PCR at 6 hours after final allergen challenge. Results are expressed as described in the legend to Figure 9. B: CD11b-negative lung fibroblasts isolated from control or CRA (allergen)-challenged mice were analyzed also for IL-31RA or IL-31RA plus collagen I expression by flow cytometry. C: IL-31RA mRNA levels were measured by real-time PCR in GFP+ or GFP− CD11b-negative lung fibroblasts isolated from CRA-challenged GFP bone marrow chimera mice. D: The effect of SCF treatment on bone marrow cell IL-31RA mRNA levels was examined using real-time PCR. E: Finally the effect of IL-31 stimulation on lung fibroblast TERT mRNA levels was analyzed using real-time PCR. A and B: Significance (*#P < 0.05) compared with whole lung cells from naïve animals. C: Significance compared with mean signal in untreated GFP-negative cells. D and E: Significance (*P < 0.05) compared with untreated control. N = 6 animals per group.
Discussion
Airway remodeling is a key aspect of asthma that is characterized by subepithelial fibrosis with prominence of myofibroblasts. Despite intensive research, the mechanism underlying this fibrotic response is not completely understood. There is evidence for Th2-type responses, including mediation by the eosinophil, in driving this remodeling process.17,50,51 In addition to these Th2 factors, SCF, a cytokine with well-documented properties vis-à-vis regulation of stem cell function, is shown to be essential for airway remodeling in animal models and human asthmatic airways.6,10,11,12,13,14,47,52 Moreover recent evidence suggests that SCF may be important also in human asthma and is highly expressed after lung transplantation.52,53,54 However the precise mechanism of how these factors could recruit and activate fibroblasts in the remodeling airway in vivo remains poorly understood. Recent reports on the plasticity of adult bone marrow stem cells and the presence of circulating fibroblast-like cells known as fibrocytes, have engendered considerable interest on the potential extrapulmonary origin of fibroblasts and myofibroblasts in the remodeling airway. Additionally the known importance of SCF in bone marrow stem cell function and recruitment6,7,55,56 may implicate its role in bone marrow-derived fibroblast recruitment as well. This study was designed to evaluate if bone marrow progenitors represented a significant source of lung fibroblasts in airway remodeling in the CRA model, and that SCF played a prominent role in their recruitment.
Previously GFP bone marrow chimera mice have been used to show significant contribution of bone marrow progenitors to the lung fibroblast population in animal models of pulmonary fibrosis.3 Based on the data shown here, this observation can be extended to recruitment of fibroblasts to the remodeling airway. Significant numbers of GFP-positive (indicative of bone marrow origin) fibroblasts were seen in the CRA-challenged lung and could be isolated and analyzed in vitro. These bone marrow-derived fibroblasts did not express α-smooth muscle actin indicating that myofibroblasts present in the remodeling airway in CRA-challenged mice were not derived from bone marrow progenitors. However a significant proportion (>20%) of these bone marrow-derived fibroblasts expressed markers that are more closely associated with stem cells, namely c-kit and TERT, indicating perhaps their relative immaturity or less differentiated state. Supporting this possibility was the finding that these cells expressed high levels of IL-31RA, which is expressed by hematopoietic progenitor cells.30 This population might represent more recently migrated or recruited progenitor cells that had not differentiated to assume the mature fibroblast phenotype. Expression of these two markers was not restricted to bone marrow-derived cells, suggesting some contribution by potential progenitor cells of intrapulmonary origin. Recruitment of c-kit-positive cells to sites of tissue injury is not unprecedented, and has been recently reported in myocardial injury.57
In view of expression of these markers by the bone marrow-derived cells, especially c-kit, the possibility that SCF may play a role in their recruitment was examined. The findings confirmed the efficacy of neutralizing anti-SCF antibodies to suppress airway remodeling, but more interestingly they also inhibited recruitment of the bone marrow-derived fibroblasts, including those that expressed c-kit. The ability of SCF to recruit stem cells is well documented, perhaps because of its chemotactic properties and ability to promote survival of these cells.5,58,59 Interestingly stem cells with long-term engraftment capabilities can be mobilized by s-kit (soluble c-kit), and that s-kit combined with G-CSF treatment leads to significant enhancement of engraftment efficiency, suggesting mobilization via disruption of c-kit and SCF interaction as the mechanism.7 The bone marrow progenitor cell population that gave rise to the lung fibroblast in this CRA model remains to be identified. An additional role for SCF is suggested by the finding that it could up-regulate expression of IL-31RA, although the importance of IL-31 in airway disease and remodeling requires further clarification. A significantly elevated level of IL-31 in plasma of allergic asthma patients was recently documented.20 It may potentially be a key factor in the induction of TERT in the bone marrow-derived fibroblasts, which previously has been shown to promote survival of these cells.49 This could result in the promotion of fibrosis by prolonged or enhanced production of fibrogenic factors from these cells to enhance myofibroblast differentiation. Further studies are necessary to further elucidate the role of IL-31, but the findings from this study reveal a potential novel link between SCF and IL-31 in the regulation of bone marrow-derived fibroblast recruitment and function that are germane to the pathogenesis of allergic airway disease.
Studies relying on immunostaining for fibrocyte markers suggest that these circulating cells are a source of myofibroblasts in airway remodeling.3,4 Because these cells are assumed to be derived from bone marrow progenitors, the findings in this study using the GFP bone marrow chimera mice appear to be at variance with the implication of the fibrocyte studies. This discrepancy is also noted in other studies using these two different approaches of monitoring extrapulmonary origin of fibroblasts in response to lung injury.2,46,60 Using similar bone marrow chimera mice, the origin of myofibroblasts from bone marrow progenitors could not be demonstrated in wound healing and a model of liver fibrosis, despite the presence of bone marrow-derived collagen I-producing cells.61,62 In another study, transgenic mice expressing GFP under control of the α-smooth muscle actin promoter are used as bone marrow donors for transplantation into wild-type mice, creating a bone marrow chimera that only has this transgene in the bone marrow cells.63 Using these chimera mice, the study reveals that the bone marrow is not a source for α-smooth muscle actin expressing cells in distal organs. In vitro culture of bone marrow cells however can yield α-smooth muscle actin-expressing cells, but on transplantation, they apparently cannot give rise to myofibroblasts or smooth muscle cells in distal organs in vivo. The possibility that fibrocytes may originate from tissues (eg, endothelium) other than the bone marrow has not been excluded. In this regard, it is noteworthy that endothelial cells have been reported to have the capacity to transition to an α-smooth muscle-expressing cell phenotype.64
In summary, the studies reported herein revealed significant recruitment of bone marrow-derived fibroblasts to remodeling airway in the CRA model characterized by induced expression of SCF. The recruited cells did not express α-smooth muscle actin, a marker of myofibroblast differentiation. However a significant subpopulation of these recruited cells expressed markers that are more characteristic of progenitor cells, namely c-kit, IL-31RA, and TERT. Neutralization of SCF resulted in reduced remodeling and a marked reduction in recruitment of these bone marrow-derived cells. Taken together these findings for the first time revealed a novel role for SCF, perhaps in conjunction with IL-31, in airway remodeling that is related to its involvement in the recruitment of bone marrow-derived fibroblasts with the capacity to promote myofibroblast differentiation in endogenous lung fibroblasts.
Footnotes
Address reprint requests to Vladislav A. Dolgachev, Ph.D., University of Michigan, Department of Pathology, 109 Zina Pitcher Pl., Rm. 4618, Ann Arbor, MI 48109-2200. E-mail: vdolgach@med.umich.edu.
Supported by the National Institutes of Health (grants HL28737, HL31963, HL52285, and HL77297 to S.H.P. and HL59178 to N.W.L.) and the Sandler Program in Asthma Research (award to S.H.P.).
References
- Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116:477–487. doi: 10.1016/j.jaci.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Nihlberg K, Larsen K, Hultgardh-Nilsson A, Malmstrom A, Bjermer L, Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama VN, Phan SH. The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Erlandsson A, Larsson J, Forsberg-Nilsson K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp Cell Res. 2004;301:201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Kunkel SL, Strieter RM, Evanoff HL, Kunkel RG, Key ML, Taub DD. The role of stem cell factor (c-kit ligand) and inflammatory cytokines in pulmonary mast cell activation. Blood. 1996;87:2262–2268. [PubMed] [Google Scholar]
- Nakamura Y, Tajima F, Ishiga K, Yamazaki H, Oshimura M, Shiota G, Murawaki Y. Soluble c-kit receptor mobilizes hematopoietic stem cells to peripheral blood in mice. Exp Hematol. 2004;32:390–396. doi: 10.1016/j.exphem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Campbell E, Hogaboam C, Lincoln P, Lukacs NW. Stem cell factor-induced airway hyperreactivity in allergic and normal mice. Am J Pathol. 1999;154:1259–1265. doi: 10.1016/S0002-9440(10)65377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SH, Hogaboam CM, Berlin A, Lukacs NW. SCF-induced airway hyperreactivity is dependent on leukotriene production. Am J Physiol. 2001;280:L1242–L1249. doi: 10.1152/ajplung.2001.280.6.L1242. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest. 2006;86:557–565. doi: 10.1038/labinvest.3700419. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Lincoln P, Tomkinson A, Lukacs NW. Inhibition of stem cell factor reduces pulmonary cytokine levels during allergic airway responses. Clin Exp Immunol. 2004;136:15–20. doi: 10.1111/j.1365-2249.2004.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin AA, Lukacs NW. Treatment of cockroach allergen asthma model with imatinib attenuates airway responses. Am J Respir Crit Care Med. 2005;171:35–39. doi: 10.1164/rccm.200403-385OC. [DOI] [PubMed] [Google Scholar]
- Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J Immunol. 1998;160:6166–6171. [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Lincoln PM, Brownell E, Pullen DM, Schock HJ, Chensue SW, Taub DD, Kunkel SL. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. J Immunol. 1996;156:3945–3951. [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Shaklee CL, Chensue SW, Kunkel SL. Macrophage inflammatory protein-1 alpha influences eosinophil recruitment in antigen-specific airway inflammation. Eur J Immunol. 1995;25:245–251. doi: 10.1002/eji.1830250140. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Taub DD, Nagel J, Smith R, Hogaboam CM, Berlin A, Lukacs NW. Stem cell factor induces eosinophil activation and degranulation: mediator release and gene array analysis. Blood. 2002;100:4291–4297. doi: 10.1182/blood.V100.13.4291. [DOI] [PubMed] [Google Scholar]
- Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796–804. doi: 10.1016/j.jaci.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Finotto S, Buerke M, Lingnau K, Schmitt E, Galle PR, Neurath MF. Local administration of antisense phosphorothioate oligonucleotides to the c-kit ligand, stem cell factor, suppresses airway inflammation and IL-4 production in a murine model of asthma. J Allergy Clin Immunol. 2001;107:279–286. doi: 10.1067/mai.2001.113049. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang GM, Feng ZH, Huang B. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Tracy E, Liang P, Robledo O, Rose-John S, Baumann H. Interleukin-31 and oncostatin-M mediate distinct signaling reactions and response patterns in lung epithelial cells. J Biol Chem. 2007;282:3014–3026. doi: 10.1074/jbc.M609655200. [DOI] [PubMed] [Google Scholar]
- Perrigoue JG, Li J, Zaph C, Goldschmidt M, Scott P, de Sauvage FJ, Pearce EJ, Ghilardi N, Artis D. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007;204:481–487. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreuw A, Radtke S, Pflanz S, Lippok BE, Heinrich PC, Hermanns HM. Characterization of the signaling capacities of the novel gp130-like cytokine receptor. J Biol Chem. 2004;279:36112–36120. doi: 10.1074/jbc.M401122200. [DOI] [PubMed] [Google Scholar]
- Diveu C, Lak-Hal AH, Froger J, Ravon E, Grimaud L, Barbier F, Hermann J, Gascan H, Chevalier S. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15:291–302. [PubMed] [Google Scholar]
- Yagi Y, Andoh A, Nishida A, Shioya M, Nishimura T, Hashimoto T, Tsujikawa T, Saito Y, Fujiyama Y. Interleukin-31 stimulates production of inflammatory mediators from human colonic subepithelial myofibroblasts. Int J Mol Med. 2007;19:941–946. [PubMed] [Google Scholar]
- Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, Krieg T, Stanzel S, Heinrich PC, Merk HF, Bosio A, Baron JM, Hermanns HM. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ip WK, Wong CK, Li ML, Li PW, Cheung PF, Lam CW. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. 2007;122:532–541. doi: 10.1111/j.1365-2567.2007.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE, Li J, Hangoc G, Cooper S, Tao W, Mantel C, Graham-Evans B, Ghilardi N, de Sauvage FJ. Regulation of myeloid progenitor cell proliferation/survival by IL-31 receptor and IL-31. Exp Hematol. 2007;35:78–86. doi: 10.1016/j.exphem.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, Storey H, LeCiel C, Harder B, Gross JA. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142:1263–1271. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang GM, Feng ZH, Huang B. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Standiford TJ, Chensue SW, Kunkel RG, Strieter RM, Kunkel SL. C-C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J Leukoc Biol. 1996;60:573–578. doi: 10.1002/jlb.60.5.573. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Interleukin-4-dependent pulmonary eosinophil infiltration in a murine model of asthma. Am J Respir Cell Mol Biol. 1994;10:526–532. doi: 10.1165/ajrcmb.10.5.8179915. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Activation and regulation of chemokines in allergic airway inflammation. J Leukoc Biol. 1996;59:13–17. doi: 10.1002/jlb.59.1.13. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995;154:5411–5417. [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995;13:1–6. doi: 10.1165/ajrcmb.13.1.7598934. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–4404. [PubMed] [Google Scholar]
- Liu T, Hu B, Chung MJ, Ullenbruch M, Jin H, Phan SH. Telomerase regulation of myofibroblast differentiation. Am J Respir Cell Mol Biol. 2006;34:625–633. doi: 10.1165/rcmb.2005-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai M, Tamaki T, Yonekawa M, Kawamura A, Sawada N. Transient increase in telomerase activity of proliferating fibroblasts and endothelial cells in granulation tissue of the human skin. Wound Repair Regen. 2002;10:59–66. doi: 10.1046/j.1524-475x.2002.10506.x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Lim Y, Kim KA, Seo MS, Kim JD, Lee KH, Park CY. Activation of telomerase by silica in rat lung. Toxicol Lett. 2000;111:263–270. doi: 10.1016/s0378-4274(99)00195-2. [DOI] [PubMed] [Google Scholar]
- Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- Liu T, Nozaki Y, Phan SH. Regulation of telomerase activity in rat lung fibroblasts. Am J Respir Cell Mol Biol. 2002;26:534–540. doi: 10.1165/ajrcmb.26.5.4668. [DOI] [PubMed] [Google Scholar]
- Finotto S, Hausding M, Doganci A, Maxeiner JH, Lehr HA, Luft C, Galle PR, Glimcher LH. Asthmatic changes in mice lacking T-bet are mediated by IL-13. Int Immunol. 2005;17:993–1007. doi: 10.1093/intimm/dxh281. [DOI] [PubMed] [Google Scholar]
- Pääkkö P, Kaarteenaho-Wiik R, Pollanen R, Soini Y. Tenascin mRNA expression at the foci of recent injury in usual interstitial pneumonia. Am J Respir Crit Care Med. 2000;161:967–972. doi: 10.1164/ajrccm.161.3.9809115. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Lukacs NW. Stem cell factor: a hemopoietic cytokine with important targets in asthma. Curr Drug Targets Inflamm Allergy. 2003;2:313–318. doi: 10.2174/1568010033483990. [DOI] [PubMed] [Google Scholar]
- Hu B, Tack DC, Liu T, Wu Z, Ullenbruch MR, Phan SH. Role of Smad3 in the regulation of rat telomerase reverse transcriptase by TGFbeta. Oncogene. 2006;25:1030–1041. doi: 10.1038/sj.onc.1209140. [DOI] [PubMed] [Google Scholar]
- Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, Choi YY, Ishikawa F, Phan SH. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117:3800–3809. doi: 10.1172/JCI32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, Emonard H, Nagler A, Maquart FA. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta). Proc Natl Acad Sci USA. 1999;96:9660–9665. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Muhsen SZ, Shablovsky G, Olivenstein R, Mazer B, Hamid Q. The expression of stem cell factor and c-kit receptor in human asthmatic airways. Clin Exp Allergy. 2004;34:911–916. doi: 10.1111/j.1365-2222.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- Da Silva CA, Adda M, Stern M, de Blay F, Frossard N, Israel-Biet D. Marked stem cell factor expression in the airways of lung transplant recipients. Respir Res. 2006;7:90. doi: 10.1186/1465-9921-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CA, Frossard N. Regulation of stem cell factor expression in inflammation and asthma. Mem Inst Oswaldo Cruz. 2005;1:145–151. doi: 10.1590/s0074-02762005000900025. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Kaur D, Berger P, Duffy SM, Brightling CE, Bradding P. Co-cultivation of mast cells and Fc epsilon RI alpha+ dendritic-like cells from human hip bone marrow. Clin Exp Allergy. 2005;35:226–233. doi: 10.1111/j.1365-2222.2005.02161.x. [DOI] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Tsuji K, Ebihara Y, Tanaka I, Sawai N, Koike K, Komiyama A, Nakahata T. Chemotactic and chemokinetic activities of stem cell factor on murine hematopoietic progenitor cells. Blood. 1996;87:4100–4108. [PubMed] [Google Scholar]
- Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo KM, Westermark B, Heldin CH. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991;10:4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol. 2005;98:715–721. doi: 10.1152/japplphysiol.00840.2004. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Kawakami Y, Nagai Y, Ma JX, Tsai JY, Kincade PW, Sato S. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells. 2006;24:13–22. doi: 10.1634/stemcells.2004-0346. [DOI] [PubMed] [Google Scholar]
- Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]