Abstract
Inhalation of asbestos and oxidant-generating pollutants causes injury and compensatory proliferation of lung epithelium, but the signaling mechanisms that lead to these responses are unclear. We hypothesized that a protein kinase (PK)Cδ-dependent PKD pathway was able to regulate downstream mitogen-activated protein kinases, affecting pro- and anti-apoptotic responses to asbestos. Elevated levels of phosphorylated PKD (p-PKD) were observed in distal bronchiolar epithelial cells of mice inhaling asbestos. In contrast, PKCδ−/− mice showed significantly lower levels of p-PKD in lung homogenates and in situ after asbestos inhalation. In a murine lung epithelial cell line, asbestos caused significant increases in the phosphorylation of PKCδ-dependent PKD, ERK1/2, and JNK1/2/c-Jun that occurred with decreases in the BH3-only pro-apoptotic protein, Bim. Silencing of PKCδ, PKD, and use of small molecule inhibitors linked the ERK1/2 pathway to the prevention of Bim-associated apoptosis as well as the JNK1/2/c-Jun pathway to the induction of apoptosis. Our studies are the first to show that asbestos induces PKD phosphorylation in lung epithelial cells both in vivo and in vitro. PKCδ-dependent PKD phosphorylation by asbestos is causally linked to a cellular pathway that involves the phosphorylation of both ERK1/2 and JNK1/2, which play opposing roles in the apoptotic response induced by asbestos.
Asbestos is a group of naturally occurring mineral fibers that are linked to the development of lung cancer, mesothelioma, and pleural and pulmonary fibrosis, ie, asbestosis.1,2 The mechanisms leading to asbestos-related diseases are still unclear, but oxidative stress due to phagocytosis of longer fibers, iron-driven generation of oxidants from fiber surfaces, and depletion of cellular antioxidants are linked to cell injury and inflammation.3,4,5,6
Bronchiolar and alveolar type II epithelial cells, which first encounter asbestos fibers after inhalation, are key cell types in asbestos-associated inflammation and fibroproliferation.2 Initial cell reactions to asbestos include epithelial cell injury, ie, apoptosis and necrosis,5,6 which may lead to compensatory cell proliferation7,8 and the production of inflammatory and fibrogenic cytokines.8,9,10 Asbestos-induced signaling mechanisms governing these cell responses appear to involve a broad variety of cascades including the mitogen-activated protein kinases (MAPK),3,7,11,12 nuclear factor-κB (NF-κB),9,13,14 and the protein kinase (PK)C10,12,15,16 and A families.17
A critical signaling protein involved in asbestos signaling is PKCδ, which is known to be activated in bronchiolar and alveolar epithelial cells in vivo and in vitro10,12,16 via increased formation of diacylglycerol.18 We have shown that PKCδ governs apoptosis via an oxidant-dependent mitochondrial pathway after exposure of lung epithelial cells to asbestos fibers.16 Recent studies comparing PKCδ +/+ and PKCδ −/− mice also reveal an important role of PKCδ in metalloproteinase expression as well as cytokine production in vitro and in vivo.10,15 A variety of other studies also link PKCδ to either pro-apoptotic or anti-apoptotic events depending on the stimulus and cell type.19,20
In this study, we focused on PKD as a potential link between PKCδ, activation of MAPKs and downstream repercussions such as expression of fos/jun proto-oncogenes and apoptosis in asbestos-exposed lung epithelium. PKD is a serine/threonine protein kinase classified as a subfamily of the Ca2+/calmodulin-dependent kinase superfamily.21 PKD1, which includes mouse PKD and its human homolog PKCμ, is the most extensively studied PKD.22 The other two members of this family include PKD223 and PKD3, (originally PKCν).24 Conserved regions of PKDs include a phosphorylation-dependent catalytic domain, a pleckstrin-homology domain that inhibits the catalytic activity, and cysteine-rich motifs that recruit PKD to the plasma membrane. PKCδ is proposed to interact with the pleckstrin-homology domain of PKD, transphosphorylating its activation loop at Ser744 and Ser748, and leading to PKD activation.25 In addition, PKD can be activated through the Src-Abl pathway by tyrosine phosphorylation of Tyr463 (T463) in the pleckstrin-homology domain after oxidative stress,26 as well as by caspase-mediated proteolytic cleavage 27 and by bone morphogenetic protein 2.28 Downstream targets of PKD signaling include several important signaling molecules such as ERK1/2, JNK1/2, and NF-κB,21,26,29,30 but how these affect functional ramifications of carcinogens, such as asbestos, are unclear.
The BH3-only protein, Bim, is a pro-apoptotic member of the Bcl-2 family that links stress-induced signals to the core apoptotic machinery.31,32 There are three different splice variants of the Bim gene encoding short, long, and extra-long Bim proteins (BimS, BimL, and BimEL).33 BimS-induced apoptosis requires mitochondrial localization but not interaction with anti-apoptosis proteins,34 whereas BimL is bound to microtubules and is less cytotoxic.35 Disruption of BimL binding to microtubules via JNK-dependent phosphorylation can cause its redistribution to the mitochondria and induction of pro-apoptotic machinery.36 BimEL is post-translationally regulated by ERK1/2, which promotes its phosphorylation and rapid dissociation from Mcl-1 and Bcl-x(L)37 and proteasomal degradation.38
We reveal here that PKD is involved in multiple signaling events after asbestos inhalation and in vitro. Specifically, PKD is a downstream effector of PKCδ and modulates phosphorylation of both ERK1/2 and JNK1/2 in lung epithelial cells after asbestos exposure. Our data also suggest that PKD inhibits apoptosis through an ERK1/2-mediated destabilization of the pro-apoptotic BH3-only protein, BimEL. The fact that PKD is an important signaling molecule in MAPK signaling and survival after cell injury by asbestos may have important therapeutic implications in asbestos-related diseases.
Materials and Methods
Cell Cultures and Inhibitors
A contact inhibited, non-transformed murine alveolar type II epithelial cell line (C10),39 was grown in CMRL 1066 medium supplemented with l-glutamine (2 mmol/L), penicillin/streptomycin (100 μg/ml), and 10% fetal bovine serum (GIBCO BRL, Rockville, MD). Cells were grown to near confluence; then complete medium was replaced with serum-free (maintenance) medium for 16 hours before exposure to asbestos or the phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (100 ng/ml; Calbiochem, La Jolla, CA). Crocidolite asbestos fibers [Na2(Fe3+)2(Fe2+)3(OH)2(Si8O22)], National Institute of Environmental Health Sciences reference sample) were suspended in Hanks’ balanced salt solution (GIBCO BRL, Rockville, MD) at a concentration of 1 mg/ml, sonicated, triturated 10X through a 22-gauge needle to obtain a homogenous suspension, and added directly to medium at a final concentration of 5 μg/cm2 area culture dish for the times indicated. This concentration was selected because it causes apoptosis in C10 cells at 24 hours followed by compensatory proliferation at 72 hours.7
In some experiments, the NF-κB inhibitor, Bay 11-7082 (5 and 10 μmol/L; Sigma, St. Louis, MO), the JNK inhibitor SP600125 (10 and 20 μmol/L; Calbiochem, La Jolla, CA), or the ERK1/2 inhibitor U0126 (10 μmol/L; Sigma, St. Louis, MO) were added to C10 cells 1 hour before asbestos at effective concentrations of inhibitors reported in the literature.40,41,42 Control cultures received medium without agents and were treated identically. All experiments were performed in triplicate or more.
Inhalation Experiments
Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals following protocols approved by the University of Vermont Institutional Animal Care and Use Committee. PKCδ−/− mice bred into the C57Bl/6 background were characterized as PKCδ+/+ or PKCδ−/− as described previously.10,43 In brief, mice (8 to 12 weeks of age) were exposed to ambient air or the National Institute of Environmental Health Sciences reference sample of chrysotile asbestos (7 mg/m3 air, 6 hours/day, 5 days/week) for 9 or 40 days as described previously.9,10,12 The mice were euthanized using an intraperitoneal injection of pentobarbital (Abbott Laboratories, Abbott Park, IL), the lungs instilled with PBS, and lobes tied off using sutures. The left lung lobe was immersed in optimal cutting temperature embedding compound (Tissue-Tek, Torrance, CA) before snap-freezing in liquid-cooled isopentane and storage at −80°C until sectioning. Other lobes were used for Western blot analyses.
Transient Transfections with Small Interfering RNA
The siControl Non-Targeting small interfering (si)RNA #2 (scrambled control), Smartpool mouse PKD, or PKCδ siRNA (50 nmol/L, Dharmacon, Lafayette, CO) were transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), following the manufacturer’s protocol. The efficiency of PKD and PKCδ knockdown was determined by Western blot analysis after 48 and 72 hours.
Immunofluorescence Techniques
To determine whether phosphorylated PKD was expressed in lung after inhalation of asbestos, 10 μm thick sections of lung tissue were fixed in 4% paraformaldehyde for 7 minutes, washed in PBS, and permeabilized with methanol for 10 minutes at −20°C. After washing in PBS, sections were treated for antigen retrieval in 1% SDS for 5 minutes, washed with PBS, and then incubated with a blocking solution containing 10% normal goat serum in 2% bovine serum albumin (BSA)/0.1% Triton X-100/PBS solution for 1 hour at room temperature. After aspiration of blocking solution, primary antibody (rabbit polyclonal phospho-Ser744/Ser748 PKD, Cell Signaling Technology, Danvers, MA; 1:1000) diluted in 2% BSA plus 0.1% Triton X-100 in PBS (BSA/PBS-T) was added, and sections were incubated overnight at 4°C. Sections were then washed with PBS, and incubated with secondary antibody (AlexaFluor 568 goat-anti-rabbit IgG, Molecular Probes, Carlsbad, CA; 1:400 in PBS) for 1 hour at room temperature. Controls consisted of sections incubated with secondary antibody alone. Sections then were washed in PBS followed by incubation with the nuclear counterstain, YOYO-1 iodide (Molecular Probes; 1:10000), 1 unit/ml RNase, and 0.1% sodium azide in BSA/PBS-T for 30 minutes at room temperature. After washing in PBS, coverslips were mounted onto slides using AquaPolyMount (Polysciences, Inc. Warrington, PA). For each section, confocal images were collected in fluorescence modes using a Bio-Rad MRC1024ES confocal scanning laser microscope (Bio-Rad, Hercules, CA).
To determine whether PKD-driven signaling pathways occurred in the same cell, confluent C10 cells were exposed to H2O2, a mediator of asbestos-induced lung injury4,44 at concentrations (0.5 and 5.0 mmol/L for 30 minutes) sufficient to activate JNK1/2 and induce cell death.45 C10 cells were grown to confluency in 4 well-chambered slides (BD Biosciences, San Jose, CA), and untreated and H2O2-exposed cells were fixed for 20 minutes in 100% methanol at −20°C. After a wash in PBS and blocking for 1 hour in 10% goat serum (Jackson Immunoresearch, West Grove, PA) and 3.7% mouse on mouse IgG blocking reagent (Vector Labs, Burlingame, CA) in PBS, cells were incubated overnight at 4°C with primary antibodies [mouse anti-pERK1/2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and rabbit anti-pJNK1/2 (Cell Signaling Technologies, Danvers, MA)] at 1:50 dilutions in 1% BSA in PBS. Controls were incubated with 1% BSA in PBS alone. Cells were washed in PBS before incubation with the secondary antibodies, Alexa Fluor 568 goat anti-mouse IgG and Alexa Flour 647 goat anti-rabbit IgG (Invitrogen, Carslbad, CA), that were diluted 1:400 in PBS at room temperature for 1 hour in the dark. After washing in PBS, a 1:20,000 dilution of Sytox Green (Invitrogen, Carlsbad, CA) was added for 10 minutes at room temperature in the dark. Cells were then washed in distilled water, allowed to air dry, coverslips mounted with Aqua Poly/Mount (Polysciences Inc., Warrington, PA), and stored at 4°C until examination using a Bio-Rad MRC1024ES confocal scanning laser microscope (Bio-Rad, Hercules, CA).
Immunoprecipitation Experiments
To test the hypothesis that physical interactions between: 1) PKCδ, PKD, and ERK1/2 proteins, and 2) PKD, JNK1/2, and ERK1/2 occurred, C10 cells grown in 100-mm culture dishes were washed three times with ice-cold PBS and collected in lysis buffer (20 mmol/L Tris pH 7.6, 1% Triton X-100, 137 mmol/L NaCl, 2 mmol/L EDTA, 1 mmol/L Na3O4V, 10 mmol/L NaF, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) on ice for 30 minutes. Cells were then sonicated and centrifuged at 14,000 × g for 15 minutes at 4°C. Supernatants were collected, and protein concentrations were determined using the Bradford assay (Bio-Rad, Richmond, CA). We first used 300 μg of protein that was immunoprecipitated for 2 hours at 4°C with PKD antibody (1:100, Cell Signaling), and the antigen-antibody complexes collected by incubation with Agarose protein A (Life Technologies, Inc.) for 1 hour at 4°C. After pellets were washed three times with lysis buffer, remaining immunocomplexes were collected in SDS sample buffer. After boiling the immunocomplexes for 5 minutes at 95°C, Western blots with antibodies to ERK1/2, PKCδ, and PKD were then performed (see below). We then used an identical procedure to immunoprecipitate protein with a JNK1/2 antibody (Cell Signaling) and probed Western blots with antibodies to ERK1/2 and PKD.
Western Blot Analyses
Cells were exposed to agents as described above, the medium aspirated, and cells washed twice with ice-cold PBS prior collection in 4X sample buffer (200 μmol/L Tris, pH6.8, 4% SDS, 4 mg/ml β-mercaptoethanol, 40% glycerol, 2 μmol/L pyronin-Y). The amount of protein was determined using the RC DC protein assay (Bio-Rad, Hercules, CA). A total of 30–60 μg protein was separated by 10% SDS-PAGE and transferred to nitrocellulose. Western blots were performed as described previously,7 using antibodies specific to total and phosphorylated PKD (rabbit polyclonal anti-PKD, 1:1000, rabbit polyclonal anti-phosphor-Ser 744/748 PKD 1:1000, Cell Signaling Technology, Danvers, MA), total and phosphorylated ERK1/2 (rabbit polyclonal anti-ERK1/2, 1:1000; rabbit polyclonal phosphor-ERK1/2 1:500, and mouse monoclonal anti-ERKp42 1:1000; Cell Signaling Technology, Danvers, MA), total and phosphorylated JNK1/2 (rabbit polyclonal JNK1/2, 1:200, rabbit polyclonal phospho JNK1/2, 1:1000, Cell Signaling Technology, Danvers, MA), total and phosphorylated c-Jun (rabbit polyclonal c-Jun at 1:1000, rabbit polyclonal phospho c-Jun at 1:500, Cell Signaling Technology, Danvers, MA), total PKCδ (rabbit polyclonal PKCδ at 1:1000, Santa Cruz, Santa Cruz, CA), total β-Actin 1:2000 (Abcam, Cambridge, MA), and total Bim (rabbit polyclonal anti-Bim at 1:500, Cell Signaling Technology, Danvers, MA). QuantityOne was used to quantify band density, and values were normalized to respective total kinase. Blots are representative of three different experiments. In some experiments, TPA was added for 30 minutes as a positive control for phosphorylation of PKCδ or PKD.
Detection and Quantitation of Apoptosis
To determine whether modulation of PKD levels altered cell death in lung epithelial cells, detection of apoptosis was performed as previously described.7 Briefly, C10 cells grown on glass coverslips were fixed in methanol for 24 hours at −20°C, boiled for 5 minutes in PBS containing 5 mmol/L MgCl2, then immersed in ice-cold PBS for 10 minutes followed by blocking with 40% fetal bovine serum for 15 minutes. Cells were then incubated with Apostain F7–26 (10 μg/ml; Alexis Biochemicals, San Diego, CA) for 30 minutes at room temperature, followed by peroxidase-conjugated secondary antibody (goat anti-mouse IgM, 15 μg/ml; Jackson Laboratories, West Grove, PA) for 30 minutes at room temperature. To visualize secondary antibody binding, the peroxidase substrate diaminobenzidine (DakoCytomation, Carpinteria, CA) was used. Nuclei were counterstained with hematoxylin, and coverslips were mounted onto slides with AquaPolyMount (Polysciences, Warrington, PA) for subsequent examination using bright field light microscopy. To determine the numbers of apoptotic cells and total cell numbers per field, five random fields were evaluated at original magnification ×400, on each coverslip.
Statistical Analysis
Results were evaluated by one-way analysis of variance using the Student-Newman-Keul’s procedure for adjustment of multiple comparisons. Differences with P values ≤0.05 were considered statistically significant. Data from in vivo experiments were evaluated by the Student’s t-test using paired comparisons.
Results
Asbestos-Mediated PKD Activation is PKCδ-Dependent in Vivo and in Vitro
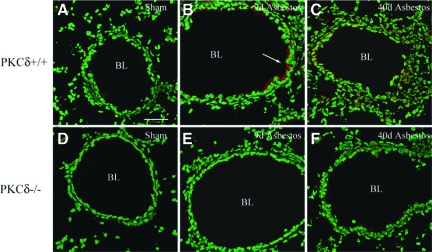
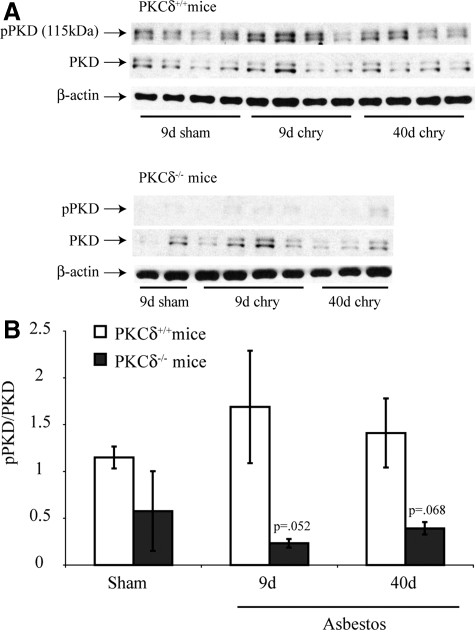
We first used immunostaining and Western blot analyses to determine whether increased amounts of phosphorylated PKD in lung were observed after inhalation of asbestos. In comparison with wild-type mice (PKCδ+/+) inhaling clean air (shams; Figure 1A), PKCδ+/+ mice inhaling asbestos showed increased immunostaining for phosphorylated PKD (p-PKD) that was more extensive after exposure to asbestos for 9 days (Figure 1B). p-PKD was localized predominantly in the apical cytoplasm of bronchiolar epithelial cells and was still increased after 40 days of exposure to asbestos, where some staining was also observed in alveoli and associated inflammation (Figure 1C). In contrast, sham and asbestos-exposed PKCδ−/− mice that develop altered inflammation and less fibrogenesis after exposure to chrysotile asbestos10,15 did not show increased p-PKD expression (Figure 1, D–F). No fluorescence was observed in the absence of a primary antibody (data not shown). Western blot analyses using lung lysates also exhibited p-PKD in PKCδ+/+ wild-type mice that was reduced in lungs of PKCδ−/− mice (Figure 2, A and B).
Figure 1.
PKD activation in bronchiolar epithelial cells occurs after inhalation of chrysotile asbestos by wild-type (PKCδ+/+), but not PKCδ knockout (PKCδ−/−) mice. An antibody recognizing the PKD activation loop (anti-pSer 744/748) was used to detect p-PKD (red; arrow), and nuclei (green) were stained with YOYO-1 iodide before examination of lung sections (N = 4 mice/group/time point) using a confocal scanning laser microscope. Note red fluorescence predominantly in bronchiolar epithelial cells lining the bronchiolar lumen (BL) in wild-type (PKCδ+/+) mice (B and C) exposed to asbestos, which was decreased in PKCδ−/− mice (E and F). (A) and (D) depict lungs from sham, clean air exposed mice. All micrographs at original magnification ×400; scale bar = 50 μm.
Figure 2.
PKD is activated by asbestos in whole lung homogenates of PKCδ+/+ and PKCδ−/− mice as revealed by Western blot analyses. Lungs of sham air exposed (9 days) and mice exposed to chrysotile asbestos for 9 or 40 days were examined by Western blot analysis using antibodies to p-PKD and total PKD as described in Materials and Methods. A: Representative Western blots. Blots in (A) were probed with an antibody to β-Actin to verify equal amounts of protein loaded in lanes; B: Quantitation of data. Bars = Mean ± SEM.
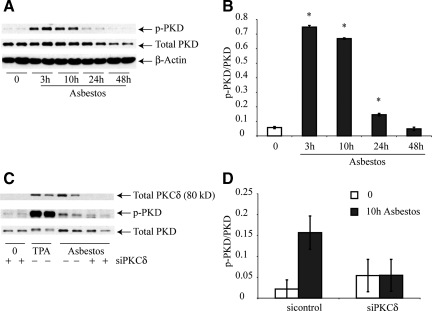
We next used a murine lung epithelial cell line (C10) to further confirm that phosphorylation of PKD was induced by asbestos in vitro as suggested by our in vivo immunohistochemistry and Western blot experiments. Western blot analyses confirmed that asbestos-induced PKD phosphorylation in epithelial cells peaked from 3 to 10 hours, but levels returned to those observed in control cells at 48 hours (Figure 3, A and B). We also used a siPKCδ construct to determine whether p-PKD levels induced by asbestos would be diminished. In these experiments, TPA-treated cells were used as a positive control for p-PKD. As shown in Figure 3C, transfection of cells with siPKCδ decreased PKCδ. Moreover, as quantitated in Figure 3D, levels of p-PKD were markedly reduced in C10 cells transfected with siPKCδ in comparison with the scrambled control vector.
Figure 3.
PKD is phosphorylated in C10 lung epithelial cells via a PKCδ-dependent pathway. Cells were homogenized and Western blot analyses were performed using antibodies to PKCδ, p-PKD and total PKD as described in Materials and Methods. A and B: Time course study. Blot in (A) was probed with an antibody to β-Actin to verify equal amounts of protein loaded in lanes. C and D: Use of siPKD construct. TPA (100 ng/ml) was used as a positive control for p-PKD. All results are typical of three independent experiments. *P ≤ 0.05 in comparison with sham (0) group. Bars = Mean ± SEM.
PKD Signaling by Asbestos is Upstream of ERK1/2 and JNK1/2 Phosphorylation
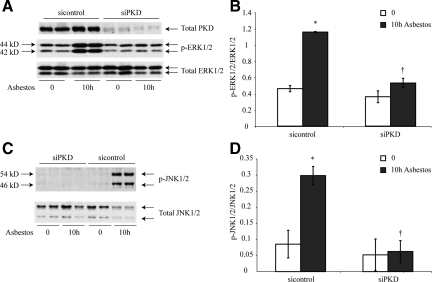
To determine whether PKD activation was causally related to phosphorylation of ERK1/2 or JNK1/2, we next transfected C10 lung epithelial cells with a siRNA to inhibit PKD expression (siPKD). Western blot analyses confirmed that total PKD levels were negligible in C10 cells transfected with the siPKD but not with a scrambled RNA control (Figure 4A). Moreover, siPKD-transfected cells exhibited significantly decreased ERK1/2 (Figure 4, A and B) and JNK1/2 phosphorylation (Figure 4, C and D) in response to asbestos.
Figure 4.
Phosphorylation of ERK1/2 and JNK1/2 is decreased after silencing of PKD in C10 lung epithelial cells. C10 cells were transfected with siPKD or scramble control construct before addition of asbestos for 10 hours. Cells were homogenized and Western blot analyses were performed using antibodies to p-ERK1/2 and total ERK1/2 (A and B); p-JNK1/2 and total JNK1/2 (C and D). A and C: Representative Western blots. B and D: Quantitation of data, as described in Materials and Methods. Results are typical of three independent experiments.*P ≤ 0.05 in comparison with sham group (0). †P ≤ 0.05 in comparison with siControl group. Bars = Mean ± SEM.
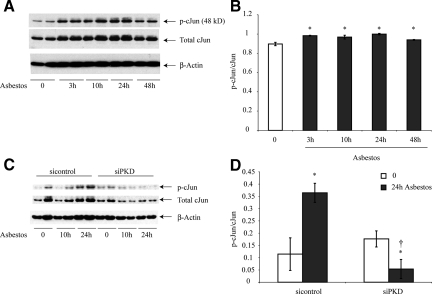
PKD is Critical to Increased Expression of c-Jun by Asbestos in C10 Lung Epithelial Cells
We have reported previously that mRNA expression of members of the fos/jun family is increased in rodent lung epithelial cells exposed to asbestos in vitro46,47 as well as in lungs of mice after inhalation of asbestos 48. Use of laser capture microdissection in these studies48 showed that increases in c-jun occurred in distal bronchiolar epithelial cells, sites of impaction of asbestos fibers after inhalation. Based on these observations and because silencing of PKD in C10 cells resulted in dramatic decreases in phosphorylation of JNK1/2 (see Figure 4, C and D), we next focused on whether PKD was causally linked to phosphorylation of c-Jun. Exposure to asbestos resulted in increases in c-Jun phosphorylation and protein levels that peaked at 24 hours (Figure 5, A and B). However, C10 cells transfected with siPKD showed decreased amounts of c-Jun and c-Jun phosphorylation (Figure 5, C and D).
Figure 5.
Asbestos-induced c-Jun phosphorylation is inhibited in C10 lung epithelial cells transfected with siPKD. Cells were homogenized, and Western blot analyses were performed using antibodies to p-c-Jun, total c-Jun, and β-Actin as described in Materials and Methods. A: Western blots of representative time course study. B: Quantitation of data. C: Representative Western blots after using the siPKD construct or a scrambled control construct (sicontrol) D: Quantitation of assays using siPKD or sicontrol transfected C10 cells. Results are typical of three independent experiments. *P ≤ 0.05 in comparison with unexposed cells (0), †P ≤ 0.05 in comparison with sicontrol group at the same time. Bars = Mean ± SEM.
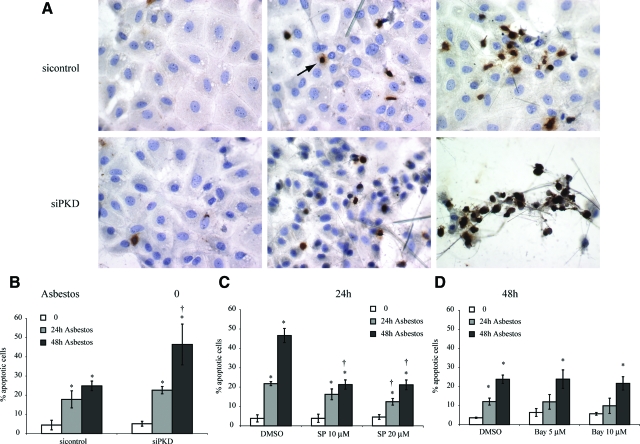
Silencing PKD Increases Asbestos-Induced Apoptosis of Lung Epithelial Cells
Asbestos-induced apoptosis in mesothelial and epithelial cells is causally related to activation and translocation of PKCδ via a mitochondrial pathway.16 We therefore tested whether inhibition of PKD also modified epithelial cell apoptosis. As shown in Figure 6A using the Apostain technique, addition of asbestos fibers to si control-transfected cells caused focal apoptosis at 24 hours that increased at 48 hours. Numbers of asbestos-induced apoptotic cells were significantly increased in C10 cells transfected with the siPKD construct at 48 hours (Figure 6B). In contrast, inhibition of JNK1/2 with the JNK inhibitor SP600125 resulted in inhibition of asbestos-associated apoptosis that was most striking at 48 hours (Figure 6C). Since activation of the NF-κB pathway in other cell types is regulated by PKCδ and PKD,26 we also examined effects of inhibiting the NF-κB pathway on asbestos-induced apoptosis using the IκB-inhibitor, Bay11-7082.40 Results showed that increased apoptosis by asbestos was unaffected by Bay11-7082 at 5 or 10 μmol/L (Figure 6D). Since we have previously used ERK1/2 small molecule inhibitors, including U0126, to show that the duration of ERK1/2 signaling by asbestos determines proliferative versus apoptopic responses in C10 cells,17,42 experiments using UO126 were not repeated here.
Figure 6.
Asbestos-induced apoptosis is increased after silencing of PKD in part through a JNK-dependent, but NF-κB independent pathway. C10 epithelial cells were transfected with siPKD or a si scramble control before exposure to asbestos, and the Apostain procedure was performed as described in Materials and Methods. A: Immunochemistry using light microscopy. Arrow shows cell staining positively for apoptosis. B: Quantitation of data from three independent experiments. C: Quantitation of data in studies using the JNK inhibitor SP600125 (SP) at 10 and 20 μmol/L. D: Quantitation of data in studies using the NF-κB inhibitor, Bay 11–7082 (Bay) 5 μmol/L and 10 μmol/L. *P ≤ 0.05 in comparison with unexposed cells (0), †P ≤ 0.05 in comparison with sicontrol or solvent (dimethyl sulfoxide) control group at same time point. Bars = Mean ± SEM.
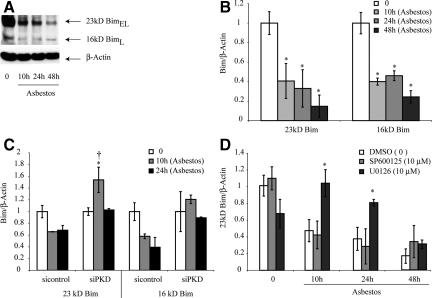
Bim is Decreased after Asbestos Exposure and Increased after Inhibition of PKD or ERK1/2
To examine a possible mechanism to explain the interplay between ERK1/2 and JNK1/2 pathways in the development of asbestos-induced apoptosis, we focused on the pro-apoptotic BH3-only protein Bim that can be stabilized by JNK1/249 and degraded after activation of ERK1/2.50,51 Western blots in Figure 7, A and B show that protein levels of both BimL (16KD) and BimEL (23KD) decreased in C10 cells after asbestos exposure from 10 to 48 hours. No BimS was detected in C10 lung epithelial cells. As shown in Figure 7C, silencing of PKD significantly increased BimEL levels. Pretreatment of cells with the JNK inhibitor, SP600125 (10 μmol/L), failed to alter asbestos-associated decreases in BimEL protein, whereas inhibition of ERK1/2 using U0126 (10 μmol/L) increased amounts of BimEL at both 10 and 24 hours post asbestos exposure (Figure 7D). These data suggest that activation of ERK1/2 is in part responsible for the inhibition of Bim-associated apoptosis by asbestos, whereas JNK is pro-apoptopic, presumably by many mechanisms described in the literature.52
Figure 7.
BimEL (23kD) and BimL (16kD) proteins are decreased after addition of asbestos to C10 lung epithelial cells (A, B) and up-regulated after silencing of PKD (C) or inhibition of ERK1/2 using U0126 (D). A: Representative Western blot and (B) quantitation of data. C: Quantitative data showing that BimEL (23kD) is increased in siPKD transfected cells. D: Quantitative data showing that U0126, but not SP600125, prevents asbestos-associated decreases in BimEL. The ERK1/2 inhibitor, U0126 (10 μmol/L), and JNK1/2 inhibitor, SP600125 (SP; 10 μmol/L) were added before asbestos as described in Materials and Methods. *P ≤ 0.05 in comparison with unexposed cells (0); †P ≤ 0.05 in comparison with siControl at same time point. Bars = Mean ± SEM.
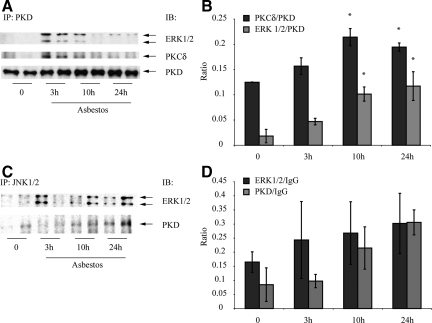
PKD Interacts with PKCδ, ERK1/2, and JNK1/2 Proteins in C10 Lung Epithelial Cells
To determine whether PKD physically interacted with PKCδ and MAPK proteins in C10 cells, we performed immunoprecipitation studies using an antibody to PKD (Figure 8, A and B) or JNK1/2 (Figure 8, C and D), as described in Materials and Methods. As shown in Figure 8, A and B, both PKCδ and ERK1/2 associated with PKD protein (P ≤ 0.05) after exposure to asbestos for 10 and 24 hours. Although similar trends were noted (Figure 8D) after use of an antibody to precipitate JNK1/2, variability between groups precluded statistically significant differences.
Figure 8.
Immunoprecipitation (IP) experiments showing association of PKCδ, PKD, and ERK1/2 (A, B) and PKD, JNK1/2, and ERK1/2 (C, D) after immunoprecipitation of protein using antibodies to PKD (A, B) or JNK1/2 (C, D) respectively, in C10 lung epithelial cells. A and C show representative Western blots; and B and D show quantitation of data from duplicate experiments. *P ≤ 0.05 in comparison with respective groups at 0 time. IgG was used as a loading control in C and D. IB = Immunoblotting. Bars = Mean ± SEM.
p-ERK1/2 and p-JNK1/2 Signaling Events Occur in the Same C10 Lung Epithelial Cell
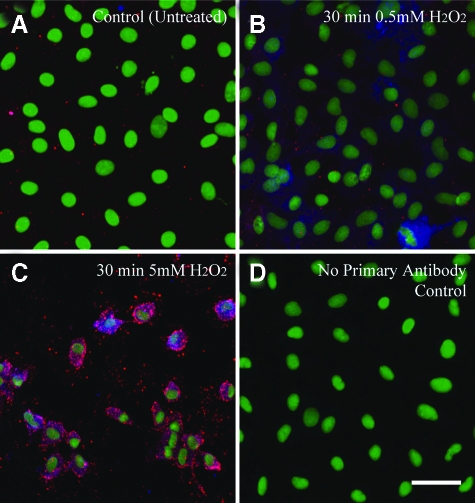
Data in Figure 8 show interaction of PKD with PKCδ and MAPK proteins. However, it is unclear whether pro- and anti-apoptic signaling events caused by activation of JNK1/245 and ERK1/2,7,12,42 respectively, occur in C10 cells simultaneously in the same cell. Since asbestos-induced phosphorylation of ERK1/2 is either transient or sustained depending on the cell outcome, ie, proliferation or apoptosis, and difficult to detect in situ because of the focal presence of fibers on cells, we used H2O2, a soluble mediator of asbestos-induced cell and lung injury,4,44 to address this question. As shown in Figure 9A, untreated C10 cells show punctate p-ERK1/2 (red) in the cytoplasm. Cells exposed to 0.5 mmol/L H2O2 for 30 minutes exhibit occasional JNK1/2 phosphorylation (blue) that is both cytoplasmic and nuclear (Figure 9B). In contrast, cells exposed to lethal concentrations of H2O2 (5 mmol/L) exhibit both cytoplasmic and nuclear translocated pJNK1/2 and pERK1/2 as suggested by Western blot analyses in rat lung epithelial49 and mesothelial53 cell homogenates (Figure 9C). Figure 9D shows the lack of p-JNK1/2 and p-ERK1/2 reactivity in cells stained with secondary antibody alone.
Figure 9.
Immunofluorescence experiments showing simultaneous induction of H2O2-induced pJNK1/2 and pERK1/2 signaling in C10 lung epithelial cells. (A) shows untreated confluent C10 cells. B and C show C10 cells exposed to 0.5 mmol/L and 5 mmol/L H2O2 for 30 minutes. (D) illustrates the lack of p-JNK1/2 or p-ERK1/2 staining in cells stained with secondary antibody alone. Nuclei are stained with Sytox Green. Red dots indicate p-ERK1/2 phosphorylation. All micrographs at original magnification ×400. Bar = 50 mmol/L.
Discussion
MAPK signaling via oxidative stress is critical to the development of inflammation, carcinogenesis and fibrogenesis by asbestos or cigarette smoke,3 although asbestos stimulates a number of signaling pathways in target cells (epithelial, fibroblasts, etc) that may be linked cooperatively or antagonistically to disease development or lung repair. In this study we focused on PKD as a key regulatory molecule affecting asbestos-induced MAPK signaling responses. Moreover, we hypothesized that PKD might be linked to activation of MAPK and downstream events such as apoptosis. Here we demonstrate that PKD is an important molecule in asbestos signaling in vivo and in vitro and is linked to activation of both JNK1/2 and ERK1/2 in asbestos-induced cell injury. By initially using an animal inhalation model, we showed that asbestos exposure activated PKD in lungs of wild-type, but not PKCδ−/− mice. Although others have shown that PKD is activated by PKCδ in vitro,26 to our knowledge, ours is the first study confirming this link in a model of lung injury in vivo. Based on the observation that p-PKD was expressed at the apical surface of bronchiolar epithelial cells after inhalation of asbestos, as is p-ERK1/2 in our murine model of fibrogenesis,11,12 we explored the hypothesis that asbestos activated PKD in murine lung epithelial cells in vitro. Although we reported previously that ERK1/2 is a key signaling molecule induced after inhalation of asbestos or its addition to mesothelial and epithelial cells in vitro,7,11,12 we show here that both ERK1/2 and JNK1/2 are phosphorylated by asbestos and decreased after silencing of PKD.
In support of our observations, a role for PKD activation of individual MAPKs has been indicated in some studies. For example, overexpression of PKD activates p42 ERK1 that is mediated by consecutive activation of Raf and MEK.54 Constitutively active PKD suppresses epidermal growth factor-associated c-Jun phosphorylation in human embryonic kidney cells55 whereas constitutively active PKD or PKC inhibition in hepatocytes leads to sustained overactivation of JNK1/2 and c-Jun.56 These findings rely on data derived from PKD overexpression studies where its physiological significance is unknown. Moreover, in vitro phosphorylation studies show that PKCη (another member of the PKC family)-mediated PKD triggers the p42 MAPK cascade while JNK activation is reduced, providing evidence for dual regulation of MAPK.57
Silencing approaches used here reveal the biological role of PKD in control of apoptotic cell death via phosphorylation of both ERK1/2 and JNK1/2 pathways that play opposing roles in this process. Our results also may explain disparate results on the involvement of PKD in anti-apoptotic or pro-apoptotic pathways as reported by others. For example, in a model of mouse skin carcinoma, increased PKD expression is related to cell proliferation.58 Likewise, in pancreatic tissue, PKD overexpression leads to strongly enhanced cell growth and up-regulated anti-apoptotic proteins in pancreatic tumor cells.59 In mouse fibroblasts, PKD enhances the expression of genes encoding apoptosis-inhibiting proteins (eg, cIAP-2, inhibitor of apoptosis protein 2).60 Conversely, other studies show that PKD is cleaved by caspases after its induction by TNFα and chemotherapeutic drugs, thus sensitizing cells to apoptosis-inducing effects of genotoxic agents.61 Our data using a concentration of asbestos that induces apoptosis followed by compensation proliferation of C10 cells suggest that a dose responsive, JNK-mediated pathway predominates after exposure to toxic amounts of asbestos. In contrast, as supported by reports showing that PKD induction of ERK1/2 is a survival response in tumor cells,59,62 ERK1/2-related anti-apoptotic pathways may predominate at lower concentrations of asbestos.
To explore possible downstream targets of MAPK pathways, we next focused on the pro-apoptotic protein Bim that is known to be regulated by ERK1/2. We show here that Bim levels decrease over time in response to asbestos, a phenomenon dependent on ERK1/2 signaling. Bim is known to be pro-apoptic because of its ability to activate Bax, and a recent report shows that Bim upregulation is critical to anoikis, a Bax-dependent apoptosis triggered by detachment from the extracellular matrix.63
Several reports implicate ERK1/2 in Bim regulation. For example, after serum withdrawal, Bim is increased through rapid protein stabilization and de novo synthesis that can be repressed by activation of ERK1/2 in fibroblasts51 or epithelial cells,50 most likely via a destabilizing process involving the proteosome.38,64 Inhibition of ERK1/2 activation also increases the amount of BimEL present in the mitochondrial cellular fraction.65 The ability of JNK to phosphorylate BimEL is controversial, but has been reported in neuronal cells.49 Other studies show a relationship between forkhead transcription factor activation and Bim-mediated apoptosis66 suggesting other pathways involved in regulation or stabilization of Bim, and a recent paper reports that sensitivity to Adriamycin correlates with apoptosis and levels of Bim protein in renal cell carcinomas.67
Our work here suggests a chain of signaling events that are initiated by asbestos and/or oxidative stress in individual lung epithelial cells and regulate the development of apoptosis or survival. Asbestos fibers initially interact with the plasma membrane to activate PKCδ via increased hydrolysis of inositol phospholipids and diacylglycerol formation.18 Asbestos also causes translocation of PKCδ to mitochondria as well as mitochondrial generation of ROS.16 Immunoprecipitation studies here show that PKCδ, PKD, and ERK1/2 physically interact, consistent with prior observations that these proteins occur in mitochondria.16,68,69 The accumulation of mitochondrial ROS is known to lead to mitochondrial activation of PKD,68 and tyrosine phosphorylation by Src, which occurs in C10 cells exposed to asbestos,70 may allow further activation of PKD by PKCδ. PKCδ-PKD-mediated phosphorylation of ERK1/2 and JNK1/2 may also occur by mitochondrial ROS, resulting in a predominantly apoptotic response at high concentrations of asbestos and cell homeostasis or proliferation at lower concentrations of fibers. These two MAPK pathways might also interact at the level of the Bim promoter, which contains an AP-1 site.71 Our observations are consistent with the fact that proliferation and apoptosis are dynamic dose-related phenomena in epithelial and mesothelial cells exposed to asbestos.72 Taken together, our results demonstrate that PKCδ-dependent PKD functions as a regulatory pathway affecting the downstream outcomes of MAPK activation by asbestos.
Acknowledgments
We appreciate the assistance of Dr. Pamela Vacek with statistical analyses and Jennifer Díaz in manuscript formatting.
Footnotes
Address reprint requests to Brooke T. Mossman, PhD, University of Vermont College of Medicine, 89 Beaumont Avenue, Given E203, Burlington, VT 05405. E-mail: brooke.mossman@uvm.edu.
Supported by NHLBI Program Project Grant P0I HL67004 (BTM).
References
- Mossman BT, Bignon J, Corn M, Seaton A, Gee JB. Asbestos: scientific developments and implications for public policy. Science. 1990;247:294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med. 1998;157:1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Lounsbury KM, Reddy SP. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am J Respir Cell Mol Biol. 2006;34:666–669. doi: 10.1165/rcmb.2006-0047SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med. 2003;34:1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Jung M, Davis WP, Taatjes DJ, Churg A, Mossman BT. Asbestos and cigarette smoke cause increased DNA strand breaks and necrosis in bronchiolar epithelial cells in vivo. Free Radic Biol Med. 2000;28:1295–1299. doi: 10.1016/s0891-5849(00)00211-2. [DOI] [PubMed] [Google Scholar]
- Panduri V, Surapureddi S, Soberanes S, Weitzman SA, Chandel N, Kamp DW. p53 mediates amosite asbestos-induced alveolar epithelial cell mitochondria-regulated apoptosis. Am J Respir Cell Mol Biol. 2006;34:443–452. doi: 10.1165/rcmb.2005-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- Sabo-Attwood T, Ramos-Nino M, Bond J, Butnor K, Heintz N, Gruber A, Steele C, Taatjes D, Vacek P, Mossman B. Gene expression profiles reveal increased mClca3 (Gob5) expression and mucin production in a murine model of asbestos-induced fibrogenesis. Am J Pathol. 2005;167:1243–1256. doi: 10.1016/S0002-9440(10)61212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens A, Barrett TF, Gell J, Shukla A, Macpherson M, Vacek P, Poynter ME, Butnor KJ, Janssen-Heininger YM, Steele C, Mossman BT. Airway epithelial NF-{kappa}B activation modulates asbestos-induced inflammation and mucin production in vivo. J Immunol. 2007;178:1800–1808. doi: 10.4049/jimmunol.178.3.1800. [DOI] [PubMed] [Google Scholar]
- Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, Taatjes DJ, Davis GS, Vacek P, Nakayama KI, Nakayama K, Steele C, Mossman BT. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-{delta} knockout mice. Am J Pathol. 2007;170:140–151. doi: 10.2353/ajpath.2007.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins AB, Palmer C, Mossman BT, Taatjes DJ. Persistent localization of activated extracellular signal-regulated kinases (ERK1/2) is epithelial cell-specific in an inhalation model of asbestosis. Am J Pathol. 2003;162:713–720. doi: 10.1016/S0002-9440(10)63867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo RF, Buder-Hoffmann SA, Cummins AB, Walsh ES, Taatjes DJ, Mossman BT. Increased phosphorylated extracellular signal-regulated kinase immunoreactivity associated with proliferative and morphologic lung alterations after chrysotile asbestos inhalation in mice. Am J Pathol. 2000;156:1307–1316. doi: 10.1016/S0002-9440(10)65001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen YM, Barchowsky A, Treadwell M, Driscoll KE, Mossman BT. Asbestos induces nuclear factor kappa B (NF-kappa B) DNA-binding activity and NF-kappa B-dependent gene expression in tracheal epithelial cells. Proc Natl Acad Sci USA. 1995;92:8458–8462. doi: 10.1073/pnas.92.18.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, Franzoso G, Carbone M. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci USA. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Barrett TF, Nakayama KI, Nakayama K, Mossman BT, Lounsbury KM. Transcriptional up-regulation of MMP12 and MMP13 by asbestos occurs via a PKCdelta-dependent pathway in murine lung. FASEB J. 2006;20:997–999. doi: 10.1096/fj.05-4554fje. [DOI] [PubMed] [Google Scholar]
- Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C delta-dependent. Am J Respir Cell Mol Biol. 2003;29:198–205. doi: 10.1165/rcmb.2002-0248OC. [DOI] [PubMed] [Google Scholar]
- Barlow CA, Barrett TF, Shukla A, Mossman BT, Lounsbury KM. Asbestos-mediated Creb phosphorylation is regulated by protein kinase A and extracellular signal-regulated kinases 1/2. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1361–L1369. doi: 10.1152/ajplung.00279.2006. [DOI] [PubMed] [Google Scholar]
- Sesko A, Cabot M, Mossman B. Hydrolysis of inositol phospholipids precedes cellular proliferation in asbestos-stimulated tracheobronchial epithelial cells. Proc Natl Acad Sci USA. 1990;87:7385–7389. doi: 10.1073/pnas.87.19.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Sanchez-Margalet V. Protein kinase C involvement in apoptosis. Gen Pharmacol. 1995;26:881–887. doi: 10.1016/0306-3623(94)00295-x. [DOI] [PubMed] [Google Scholar]
- Whelan RD, Parker PJ. Loss of protein kinase C function induces an apoptotic response. Oncogene. 1998;16:1939–1944. doi: 10.1038/sj.onc.1201725. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturany S, Van Lint J, Muller F, Wilda M, Hameister H, Hocker M, Brey A, Gern U, Vandenheede J, Gress T, Adler G, Seufferlein T. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J Biol Chem. 2001;276:3310–3318. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Seki N, Hattori A, Kozuma S, Saito T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim Biophys Acta. 1999;1450:99–106. doi: 10.1016/s0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Rozengurt E. Oxidative stress induces protein kinase D activation in intact cells. Involvement of Src and dependence on protein kinase C. J Biol Chem. 2000;275:17114–17121. doi: 10.1074/jbc.M908959199. [DOI] [PubMed] [Google Scholar]
- Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantus T, Vertommen D, Saelens X, Rykx A, De Kimpe L, Vancauwenbergh S, Mikhalap S, Waelkens E, Keri G, Seufferlein T, Vandenabeele P, Rider MH, Vandenheede JR, Van Lint J. Doxorubicin-induced activation of protein kinase D1 through caspase-mediated proteolytic cleavage: identification of two cleavage sites by microsequencing. Cell Signal. 2004;16:703–709. doi: 10.1016/j.cellsig.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Lemonnier J, Ghayor C, Guicheux J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- Storz P, Doppler H, Toker A. Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor kappaB. Mol Pharmacol. 2004;66:870–879. doi: 10.1124/mol.104.000687. [DOI] [PubMed] [Google Scholar]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Paschen SA, Heger K, Wilfling F, Frankenberg T, Bauerschmitt H, Seiffert BM, Kirschnek S, Wagner H, Hacker G. BimS-induced apoptosis requires mitochondrial localization but not interaction with anti-apoptotic Bcl-2 proteins. J Cell Biol. 2007;177:625–636. doi: 10.1083/jcb.200610148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines–tools for the study of differentiation and the neoplastic phenotype. Toxicology. 1997;123:53–100. doi: 10.1016/s0300-483x(97)00108-x. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Taatjes DJ, Mossman BT, Heintz NH. The duration of nuclear extracellular signal-regulated kinase 1 and 2 signaling during cell cycle reentry distinguishes proliferation from apoptosis in response to asbestos. Cancer Res. 2004;64:6530–6536. doi: 10.1158/0008-5472.CAN-04-0946. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Marsh JP, Sesko A, Hill S, Shatos MA, Doherty J, Petruska J, Adler KB, Hemenway D, Mickey R, Vacek P, Kagan E. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis. 1990;141:1266–1271. doi: 10.1164/ajrccm/141.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- Pantano C, Shrivastava P, McElhinney B, Janssen-Heininger Y. Hydrogen peroxide signaling through tumor necrosis factor receptor 1 leads to selective activation of c-Jun N-terminal kinase. J Biol Chem. 2003;278:44091–44096. doi: 10.1074/jbc.M308487200. [DOI] [PubMed] [Google Scholar]
- Heintz NH, Janssen YM, Mossman BT. Persistent induction of c-fos and c-jun expression by asbestos. Proc Natl Acad Sci USA. 1993;90:3299–3303. doi: 10.1073/pnas.90.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Timblin CR, Hubbard AK, Bravman J, Mossman BT. Silica-induced activation of c-Jun-NH2-terminal amino kinases, protracted expression of the activator protein-1 proto-oncogene, fra-1, and S-phase alterations are mediated via oxidative stress. Cancer Res. 2001;61:1791–1795. [PubMed] [Google Scholar]
- Manning CB, Cummins AB, Jung MW, Berlanger I, Timblin CR, Palmer C, Taatjes DJ, Hemenway D, Vacek P, Mossman BT. A mutant epidermal growth factor receptor targeted to lung epithelium inhibits asbestos-induced proliferation and proto-oncogene expression. Cancer Res. 2002;62:4169–4175. [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated Bim phosphorylation potentiates Bax-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Marani M, Hancock D, Lopes R, Tenev T, Downward J, Lemoine NR. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene. 2004;23:2431–2441. doi: 10.1038/sj.onc.1207364. [DOI] [PubMed] [Google Scholar]
- Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wagner EF, Cook SJ. Activation of ERK1/2 by deltaRaf-1: ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene. 2003;22:1281–1293. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Jimenez LA, Zanella C, Fung H, Janssen YM, Vacek P, Charland C, Goldberg J, Mossman BT. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol. 1997;273:L1029–L1035. doi: 10.1152/ajplung.1997.273.5.L1029. [DOI] [PubMed] [Google Scholar]
- Hausser A, Storz P, Hubner S, Braendlin I, Martinez-Moya M, Link G, Johannes FJ. Protein kinase C mu selectively activates the mitogen-activated protein kinase (MAPK) p42 pathway. FEBS Lett. 2001;492:39–44. doi: 10.1016/s0014-5793(01)02219-0. [DOI] [PubMed] [Google Scholar]
- Hurd C, Rozengurt E. Protein kinase D is sufficient to suppress EGF-induced c-Jun Ser 63 phosphorylation. Biochem Biophys Res Commun. 2001;282:404–408. doi: 10.1006/bbrc.2001.4591. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem. 2004;279:31089–31097. doi: 10.1074/jbc.M404170200. [DOI] [PubMed] [Google Scholar]
- Brandlin I, Hubner S, Eiseler T, Martinez-Moya M, Horschinek A, Hausser A, Link G, Rupp S, Storz P, Pfizenmaier K, Johannes FJ. Protein kinase C (PKC)eta-mediated PKC mu activation modulates ERK and JNK signal pathways. J Biol Chem. 2002;277:6490–6496. doi: 10.1074/jbc.M106083200. [DOI] [PubMed] [Google Scholar]
- Rennecke J, Rehberger PA, Furstenberger G, Johannes FJ, Stohr M, Marks F, Richter KH. Protein-kinase-Cmu expression correlates with enhanced keratinocyte proliferation in normal and neoplastic mouse epidermis and in cell culture. Int J Cancer. 1999;80:98–103. doi: 10.1002/(sici)1097-0215(19990105)80:1<98::aid-ijc19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Schmiedel S, Sipos B, Wermann H, Westphal S, Roder C, Klapper W, Arlt A, Lehnert L, Ungefroren H, Johannes FJ, Kalthoff H. PKCmu prevents CD95-mediated apoptosis and enhances proliferation in pancreatic tumour cells. Oncogene. 2003;22:8939–8947. doi: 10.1038/sj.onc.1207001. [DOI] [PubMed] [Google Scholar]
- Johannes FJ, Horn J, Link G, Haas E, Siemienski K, Wajant H, Pfizenmaier K. Protein kinase Cmu downregulation of tumor-necrosis-factor-induced apoptosis correlates with enhanced expression of nuclear-factor-kappaB-dependent protective genes. Eur J Biochem. 1998;257:47–54. doi: 10.1046/j.1432-1327.1998.2570047.x. [DOI] [PubMed] [Google Scholar]
- Endo K, Oki E, Biedermann V, Kojima H, Yoshida K, Johannes FJ, Kufe D, Datta R. Proteolytic cleavage and activation of protein kinase C [micro] by caspase-3 in the apoptotic response of cells to 1-beta- D-arabinofuranosylcytosine and other genotoxic agents. J Biol Chem. 2000;275:18476–18481. doi: 10.1074/jbc.M002266200. [DOI] [PubMed] [Google Scholar]
- Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, Vandenheede JR, Seufferlein T. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. doi: 10.1016/s0962-8924(02)02262-6. [DOI] [PubMed] [Google Scholar]
- Woods NT, Yamaguchi H, Lee FY, Bhalla KN, Wang HG. Anoikis, initiated by Mcl-1 degradation and Bim induction, is deregulated during oncogenesis. Cancer Res. 2007;67:10744–10752. doi: 10.1158/0008-5472.CAN-07-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- van Gorp AG, Pomeranz KM, Birkenkamp KU, Hui RC, Lam EW, Coffer PJ. Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res. 2006;66:10760–10769. doi: 10.1158/0008-5472.CAN-06-1111. [DOI] [PubMed] [Google Scholar]
- Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- Zantl N, Weirich G, Zall H, Seiffert BM, Fischer SF, Kirschnek S, Hartmann C, Fritsch RM, Gillissen B, Daniel PT, Hacker G. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 2007;26:7038–7048. doi: 10.1038/sj.onc.1210510. [DOI] [PubMed] [Google Scholar]
- Storz P. Mitochondrial ROS–radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23:805–813. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Liu DX, Greene LA. Bim is a direct target of a neuronal E2F-dependent apoptotic pathway. J Neurosci. 2005;25:8349–8358. doi: 10.1523/JNEUROSCI.1570-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Zanella CL, Janssen YM, Timblin CR, Jimenez LA, Vacek P, Taatjes DJ, Mossman BT. Novel cell imaging techniques show induction of apoptosis and proliferation in mesothelial cells by asbestos. Am J Respir Cell Mol Biol. 1997;17:265–271. doi: 10.1165/ajrcmb.17.3.2991. [DOI] [PubMed] [Google Scholar]