Abstract
Sarcoidosis is characterized by a disproportionate Th1 granulomatous immune response in involved organs. It is also associated with both peripheral and intratissular regulatory T cell (Treg) expansion. These cells exhibit powerful antiproliferative activity, yet do not completely inhibit the production of either tumor necrosis factor-α or interferon-γ. The origin of the observed Treg amplification and, more importantly, its impact on the evolution of sarcoidosis remain unresolved issues. Here, we show that CD4+CD45RA−FoxP3bright Tregs proliferate and accumulate within granulomas. However, circulating and tissue Treg numbers are neither correlated with the dissemination of the disease nor correlated locally with the extent of granulomatous inflammation. Rather, we found a positive correlation between the presence of Tregs in renal granulomas and the degree of interstitial fibrosis (r = 0.46, P = 0.03, n = 20). Furthermore, Treg depletion accelerates in vitro granuloma growth in mononuclear cell cultures of healthy controls, but not in those from patients with active sarcoidosis. The results of this study show that although healthy Tregs suppress the initial steps of granuloma formation, they have no positive influence on sarcoidosis lesions. Our findings argue for a more preventive than curative effect of Tregs on inflammatory processes.
Sarcoidosis is a multisystemic disorder of unknown etiology usually involving the respiratory tract, characterized by the formation of granulomas in involved organs.1,2 The clinical presentation of sarcoidosis can be extremely diverse, depending upon the involved organs and duration of inflammation. Although a benign course is observed in more than half of the cases, sarcoidosis can also be severe when extrapulmonary sites, such as heart, kidney, central nervous system, liver, larynx, and eye, are involved. Moreover, sarcoidosis can evolve into a chronic disease, possibly leading to pulmonary fibrosis, the main cause of morbidity and mortality.2 Unfortunately, sarcoidosis outcome cannot be predicted from the initial presentation, making it difficult to determine how to treat patients. Although corticosteroids may reverse the granulomatous process, their effects are just suppressive and it is still uncertain whether they can prevent the development of fibrotic lesions.1 Therefore, precise understanding of the immune processes that initiate the disease is required to predict the outcome, and thus to define the best specific treatment.
It has been shown that uncontrolled Th1 immune response occurring in diseased organs is a key mechanism in the initiation and the maintenance of inflammation.3,4 However, the nature of the antigen(s) accounting for the initiation of Th1 responses in sarcoidosis, and the mechanisms leading to the dissemination of granulomas and fibrosis, remain unknown.5,6,7 The occurrence of uncontrolled inflammatory responses in sarcoidosis suggests that immunoregulatory processes might be impaired.
FoxP3-expressing CD4+ regulatory T cells (Tregs) play a major role in the control of immune responses against self and exogenous antigens.8,9 Although the molecular mechanisms of Treg-mediated suppression of immune responses have not been completely elucidated,10 there is clear evidence that Treg impairment can be a cause of uncontrolled inflammatory processes.11
We have previously shown a global amplification of CD4+CD25bright Tregs in active sarcoidosis.12 Tregs were highly abundant in peripheral blood, bronchoalveolar lavage fluid and in lymph nodes. Although Tregs could potently suppress the proliferation and interleukin (IL)-2 production of conventional CD4+CD25− T cells in vitro, we observed that they could not completely abrogate the secretion of interferon (IFN)-γ and tumor necrosis factor (TNF)-α. Potent suppression of proliferation by amplified Tregs may account for the peripheral anergy usually observed in sarcoidosis, and impaired control of IFN-γ and TNF-α might be responsible for the uncontrolled growth of granulomas in sarcoidosis. However, it is still not clear whether Tregs can inhibit granuloma formation. Moreover, although Tregs were increased in almost all patients with active disease, whether amplitude of Tregs expansion is correlated with the severity of the disease remains unknown. Finally, although we have shown that Tregs were globally amplified, their origin still remains uncertain.
Here, we show that Tregs actively proliferate within sarcoidosis granulomas, defining the latter as a putative origin for the amplified Tregs observed in this condition. We also show that Treg amplification is neither correlated with clinical severity nor with the extent of tissue damage in extrathoracic organs. Rather, we found a positive correlation between the presence of Tregs in renal granulomas and the degree of interstitial fibrosis. Finally, we show that in contrast to Tregs from healthy controls, Tregs from sarcoidosis patients do not suppress granuloma formation in vitro.
Materials and Methods
Patients and Clinical Monitoring
Our study included 69 patients (40 women and 29 men; 40 Europeans and 29 Africans of whom 19 were North Africans) with a diagnosis of sarcoidosis established according to the criteria defined by the 1999 consensus conference of the American Thoracic Society, the European Respiratory Society, and the World Association of Sarcoidosis and Other Granulomatous Disorders.1 The 69 patients fell into the following groups:
1) Forty-nine patients with pulmonary involvement, of whom 18 (mean age 40.94 ± 11.36 years, range: 16 to 60 years) recruited from the Thoracic Surgery Department of the Hôpital Européen Georges Pompidou, Paris, had diagnostic mediastinal lymph node biopsies, while 31 patients (mean age 42.58 ± 13.12 years, range: 23 to 69 years) currently followed in the Pulmonary Department of the Hôpital Avicenne, Bobigny, were evaluated on the basis of peripheral blood samples only. Clinical patient characteristics are presented in Table 1.
Table 1.
Characteristics of Patients with Active Sarcoidosis
| Patients | Age (years) | Sex | Ethny | Chest X-ray staging* | Extrathoracic lesions | CVF (%)† | SACE (×N)‡ | Tregs (% of CD4+ T cells)
|
|
|---|---|---|---|---|---|---|---|---|---|
| Circulating tregs | Mediastinal tregs | ||||||||
| 1 | 46 | M | E4 | IV | Heart, skin, liver, spleen | 64 | 1 | 7.1 | — |
| 2 | 61 | F | E | IV | Nervous system, nose, larynx, bone, skin | 72 | 2.8 | 1 | — |
| 3 | 51 | F | E | I | Liver, kidney, skin | 82 | 3.3 | 4.2 | — |
| 4 | 56 | F | E | IV | Nervous system, nose, larynx, bone, liver | 83 | 1.4 | 1.4 | — |
| 5 | 35 | F | E | II | 0 | 116 | 1 | 3.03 | — |
| 6 | 56 | F | A5 | II | Nose, larynx, skin | 80 | 1.5 | 1.84 | — |
| 7 | 26 | M | E | IV | 0 | 96 | 1 | 2.89 | — |
| 8 | 69 | F | E | I | 0 | 123 | 2 | 6.81 | — |
| 9 | 23 | M | A | II | 0 | 102 | 2.2 | 2.96 | — |
| 10 | 53 | M | A | I | Nose, larynx | 113 | 3 | 6.71 | — |
| 11 | 31 | F | A | II | Nose, larynx, nervous system | 74 | 7 | 4.36 | — |
| 12 | 48 | M | E | I | Eyes, skin | 93 | 1 | 2.15 | — |
| 13 | 33 | M | A | I | Nervous system | 98 | 1.4 | 1.14 | — |
| 14 | 47 | M | A | IV | 0 | 69 | 4.1 | 5.12 | — |
| 15 | 25 | F | A | I | Erythema nodosum | 116 | 1.5 | 2.13 | — |
| 16 | 44 | F | E | I | 0 | 114 | 1.6 | 3.72 | — |
| 17 | 46 | M | E | I | Liver, spleen | 98 | 3.02 | 19.3 | — |
| 18 | 59 | F | E | II | Liver, bone, nose, larynx | 60 | 4.9 | 1.61 | — |
| 19 | 57 | F | E | I | Skin | 112 | 4 | 13.69 | — |
| 20 | 41 | M | A | IV | Liver, skin | 64 | 6 | 1.74 | — |
| 21 | 45 | F | A | IV | Erythema nodosum, eyes, heart, liver | 64 | 1 | 9.04 | — |
| 22 | 31 | M | A | II | 0 | 56 | 4.9 | 2.58 | — |
| 23 | 25 | M | E | II | 0 | 48 | 1 | 0.6 | — |
| 24 | 66 | F | E | I | 0 | 99 | 1 | 1.19 | — |
| 25 | 33 | M | E | I | 0 | 102 | 1 | 1 | — |
| 26 | 31 | F | E | III | 0 | 100 | 1.6 | 1 | — |
| 27 | 45 | M | A | II | 0 | 49 | 3 | 10.43 | — |
| 28 | 47 | F | A | I | Liver, erythema nodosum | 101 | 3 | 1.53 | — |
| 29 | 29 | M | A | II | 0 | 100 | 2.1 | 2.16 | — |
| 30 | 25 | F | A | I | Erythema nodosum | 102 | 5.5 | 17.98 | — |
| 31 | 36 | F | E | I | Nose, larynx, skin, heart, peripheral lymph nodes | 100 | 1.5 | 2.89 | — |
| 32 | 54 | F | E | I | 0 | 105 | 1 | — | 5 |
| 33 | 50 | M | E | I | 0 | 110 | 1.4 | — | 6.5 |
| 34 | 52 | F | E | I | Erythema nodosum | 106 | 1 | — | 13.3 |
| 35 | 29 | F | E | I | Erythema nodosum | 97 | 1 | — | 19 |
| 36 | 27 | M | E | I | 0 | 95 | 1.4 | — | 17.5 |
| 37 | 39 | M | E | I | Cervical lymph nodes | 100 | 1.2 | — | 22.75 |
| 38 | 40 | F | E | I | 0 | 102 | 1.3 | — | 17.9 |
| 39 | 40 | F | A | I | 0 | 94 | 1.1 | — | 40.9 |
| 40 | 40 | F | E | II | 0 | 81 | 1.4 | — | 13.2 |
| 41 | 16 | F | E | I | Nervous system | 97 | 3.2 | — | 18.8 |
| 42 | 37 | M | A | II | Eyes, heart | 64 | 2.6 | — | 27 |
| 43 | 37 | F | A | I | 0 | 86 | 1.2 | — | 36.4 |
| 44 | 44 | F | A | II | Kidney, liver | 45 | 1.6 | — | 30.8 |
| 45 | 43 | F | A | I | Erythema nodosum | 105 | 1.9 | — | 19.1 |
| 46 | 34 | M | E | II | 0 | 82 | 1.8 | — | 12.3 |
| 47 | 35 | M | A | II | Kidney, liver, parotid glands, nervous system, eyes | 55 | 1.7 | — | 34 |
| 48 | 60 | F | A | I | Kidney | 82 | 3 | — | 25.4 |
| 49 | 60 | F | A | II | Kidney | 58 | 2.5 | — | 29 |
Radiologic score using the modified criteria of Scadding.37
Percentage of forced vital capacity (FVC) compared with the theoretic predicted mean (% pred).
Value for serum angiotensis converting enzyme (SACE) compared with normal levels.
European (E).
African (A).
2) Twenty patients with renal involvement recruited from the Nephrology Department of the Hôpital Européen Georges Pompidou had a renal biopsy (mean age 54.55 ± 14.58 years, range: 32 to 78 years). In those patients, 13 (65%) had associated pulmonary involvement and nine (45%) had other extrathoracic lesions. None of the patients were under treatment at the time of recruitment.
Control specimens, for the different aspects of the study as appropriate, consisted of:
1) Six fresh lymph node biopsies obtained from mediastinal lymph node dissection during pulmonary surgery of control subjects explored for pulmonary cancer (n = 6, mean age 45.83 ± 6.33 years, range: 39 to 55 years). All lymph nodes were normal without any neoplastic or autoimmune disease.
2) Nine renal biopsies with nongranulomatous interstitial nephritis (mean age 52.67 ± 22.81 years, range: 21 to 84 years), two with systemic lupus erythematosus-related nephritis and seven of immuno-allergic origin.
3) Blood samples from 15 healthy volunteers (mean age 37.33 ± 7.88 years, range: 26 to 60 years).
There was no statistically significant age difference between patients and controls in the different groups evaluated in this study.
The study protocol was reviewed and approved by the local ethics committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale of Pitié-Salpêtrière Hospital).
Detection of CD4+FoxP3+ T Cells in Tissue Samples
Detection of CD4+FoxP3+ and CD4+FoxP3+Ki-67+ cells was performed on fixed, paraffin-embedded samples. Dewaxed slides were submitted to antigen retrieval by heating in 0.01 M/L Tris-EDTA pH 9.0 (Dako, Glostrup, Denmark). Before incubation with primary antibodies, the slides were treated with avidin/biotin blocker (Vector Laboratories, Burlingame, CA) and Fc receptor was blocked with human serum (5%, Biowest, Nuaillé, France). Slides were incubated for 1 hour with 10 μg/ml (diluted 1/10) human polyclonal goat anti-CD4 (R&D, Abingdon, UK) and 9 μg/ml (1/100) human monoclonal mouse anti-FoxP3 (clone 236A/E7, Abcam, Cambridge, UK). Slides were then incubated for 30 minutes with 14 μg/ml (1/100) FITC-donkey-anti-goat (Jackson Immunoresearch, West Grove, PA) and 2.8 μg/ml (1/500) biotinylated donkey anti-mouse (Jackson). Slides were finally incubated with 3 μmol/L (1/300) Cy3-streptavidin (Amersham, Piscataway, NJ) and mounted in Fluoromount (Amersham). For the triple immunofluorescence staining, slides were incubated for 1 hour with 1 μg/ml (1/200) of the human rabbit-anti-Ki-67 (clone SP6, Neomarker, Fremont, CA), followed by 6 μg/ml (1/250) of Cy5 donkey anti-rabbit (Jackson) for 30 minutes. Isotype-matched antibodies (Dako, Glostrup, Denmark) were used as negative controls in each case.
The double immunostaining CD4+FoxP3+ and triple immunostaining CD4+FoxP3+Ki-67+ specimens were analyzed with a Zeiss LSM 510 laser confocal microscope. All images were photographed with a ×60 objective. The CD4+FoxP3+ cells were counted, with the average percentage of CD4+FoxP3+ per field established on analysis of five fields with a ×60 objective. The area of each analyzed field was 1.33 mm2. Most CD4+FoxP3+ cells were detected in the interfollicular region of normal lymph nodes and were rarely observed in germinal centers. Presented data correspond to average numbers obtained after separate analysis of the complete set of slides by two authors (D.N., C.T.).
In Vitro Granuloma Formation
Granulomas were generated in vitro using the technique developed by F. Altare et al.13,14,15 Briefly, 2 × 106 peripheral blood mononuclear cells (PBMCs) were cultured in a medium composed of RPMI 1640 (Gibco, Paiseley, UK) supplemented with 8% human serum AB (Biowest), 1 mmol/L sodium pyruvate, 1% non essential amino acids, 100 U/ml of penicillin, and 100 μg/ml of streptomycin (all obtained from Gibco) in a 24-well plate (Falcon, BD bioscience) with 200 Bacille Calmette Guerin extract-coated 90-μm sepharose beads (SD91; Sigma-Aldrich) prepared as previously described,13 at 37°C in a 5% CO2 atmosphere for 10 days. CD25high T cells were removed from PBMCs by magnetic selection using bead-coated anti-CD25 antibodies (Clone 4E3; Miltenyi Biotec, Bergisch Gladbach, Germany). The evolution of the mean number of granulomas in four separate wells was evaluated at days 2, 3, 5, 7, and 8 after culture for each of the two groups. A granuloma was defined as a single bead totally covered with cells.13,14,15 Among aggregates, single granulomas were enumerated. Granuloma enumeration was performed using an inverted microscope and a ×4 objective (Olympus CK40). Pictures were taken using an inverted microscope with a ×10 objective (Nikon TE300 Eclipse).
Flow Cytometry
The PBMCs were stained with the following antibodies: anti-CD4-PB (eBioscience, San Diego, CA) or anti-CD4-APC, anti-CD45RA-PC7, anti-CD19-FITC, anti-CD11c-PE, and anti-CD14-APC (BD Biosciences, Franklin Lakes, NJ). The intranuclear detection of FoxP3 and Ki-67 with anti-FoxP3-PE (clone 236A/E7; eBioscience) and FITC-labeled anti-Ki-67 (BD Biosciences) was obtained on cells fixed and permeabilized using fixperm/washperm solution (eBioscience). For detection of intracellular cytokine production, PBMCs were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate and 1 μmol/L ionomycin in the presence of Brefeldin (Sigma-Aldrich) and Golgi-Stop (BD Biosciences) for 5 hours, and then stained with anti-FoxP3-PE, anti-IFN-γ-FITC (BD), anti-IL2-APC (BD), anti-TNF-α-FITC (BD), and anti-IL-17-Alexa Fluor 647 (clone eBio64DEC17, e-Bioscience) after fixation and permeabilization using Cytofix/Cytoperm (e-Bioscience). The data acquired with a FACSCalibur and a FACSCanto (BD Biosciences) were analyzed with the CellQuest Pro software (BD Biosciences) and WinMDI version 2.8 software (The Scripps Research Institute, La Jolla, CA) on 300,000 events.
Histopathology
Lymph node and renal biopsy specimens were stained for routine light microscopic study. The cellular infiltrates composing and surrounding the granulomas were analyzed systematically by immunoperoxidase with the following antibodies: anti-CD3 (Neomarker, Fremont, CA), anti-CD20, and anti-CD68 (Dako, Glostrup, Denmark). All of the mediastinal lymph nodes and renal biopsies showed granulomatous inflammation with giant cells, without caseous necrosis and diffuse lymphoplasmacytic inflammation. They were negative for Mycobacterium tuberculosis.
The following lesions were evaluated in kidney biopsies and expressed as percentage of the parenchyma: interstitial fibrosis, tubular necrosis, tubular atrophy, and lymphoplasmacytic inflammation. Histiocytic-giant cell granulomas were counted and expressed as absolute number in each biopsy. The borders between healthy tissue and that infiltrated by granulomas were established by the presence of the CD3+ lymphocytic halo or crown around them.
Statistical Analysis
Data are expressed as the mean ± SD. For analysis of clinical, morphological, and laboratory variables, Pearson’s product-moment correlations were used for comparisons of two continuous variables; Spearman’s rank-order correlations were used for the comparison of continuous with categorical variables, and for variables deviating from normal distribution Mann-Whitney U-tests were used. A P value less than 0.05 was considered significant. The program used was Statistica 6 (Statsoft, Tulsa, OK).
Results
Sarcoidosis Granulomas Are Enriched in Memory Phenotype Treg Cells
It is increasingly apparent that not all FoxP3+ human T cells correspond to Tregs.16 Flow cytometry analysis of human PBMCs revealed three subsets of CD4+FoxP3+ T cells: one naïve CD4+CD45RA+FoxP3+ (CD45RO−) corresponding to naïve Treg cells, and two memory subsets CD45RA− (CD45RO+), one non-regulatory population expressing intermediate levels of FoxP3 and one FoxP3bright subset corresponding to Treg cells with a memory phenotype (referred hereafter as Tregs; Figure 1A). We have previously shown an amplification of CD4+CD25bright Tregs in the peripheral blood of patients with active sarcoidosis.12 As shown in Figure 1A, most Tregs detected in sarcoidosis patients bear a CD45RA−FoxP3bright memory phenotype (4.62 ± 4.84% of CD4+ cells, n = 31 vs. 1.08 ± 0.39% in healthy subjects, n = 15, P < 0.0001, Figure 1A). We noted a modest but nevertheless significant decrease in the mean percentage of circulating naïve CD45RA+FoxP3+ Tregs (1.29 ± 0.61% of CD4+ cells, n = 31 vs. 1.76 ± 0.66% in healthy subjects, n = 15, P = 0.04).
Figure 1.
Increase in memory phenotype CD4+FoxP3bright Treg cells within peripheral blood, lymph nodes, and renal granulomas in sarcoidosis. A: FoxP3 expression by blood CD4+ T cells in a representative healthy donor (out of 15) and in a representative active sarcoidosis patient (out of 31). The CD4+CD45 RA−FoxP3bright gate (gate D) was adjusted to contain CD4+ T cells that display higher levels of FoxP3 than CD4+CD45RA+FoxP3+ cells (gate B), thus defining a CD4+CD45RA−FoxP3low gate (gate C). Gate A corresponds to effector CD4+FoxP3− T cells. Comparisons between sarcoidosis patients (S) and healthy subjects (H) were made using the nonparametric Mann-Whitney U-test. B: Production of inflammatory cytokines by effector non Treg CD4+ T cells in healthy donors and sarcoidosis patients. Flow cytometry of IFN-γ, IL-2, TNF-α, and IL-17 production by gated non Treg-cell subsets (gates A and C) from a sarcoidosis patient (red line) and a healthy subject (blue line) after stimulation with phorbol 12-myristate 13-acetate/ionomycin for 5 hours (top) and scattergram of the production of indicated cytokines (bottom). The black line in each histogram represents the relevant isotype control staining. C: Detection of FoxP3+CD4+ cells in two control lymph nodes using immunohistochemistry (IHC) and flow cytometry. CD4+ (green cytoplasmic and membrane staining) FoxP3+ (red nuclear staining) cells were enumerated in five independent areas in each paraffin section. D and E: Comparative confocal analysis of CD4+ FoxP3+ cell percentage among CD4+ cells in paraffin sections of a control lymph node (CLN) (one representative sample out of 6) and an active sarcoidosis lymph node (SLN, one representative sample out of 18) (D) and of a control nongranulomatous interstitial nephritis (CN, one representative sample out of 9) and a sarcoidosis granulomatous nephritis (SN, one representative sample out of 20) (E). The percentage of CD4+FoxP3+ cells within granuloma (G) and peripheral infiltrate (PI) is shown (E). Magnification: ×600. Comparisons were made using the nonparametric U Mann-Whitney test. *P value of <0.05.
The amplification of the memory phenotype Treg subset in sarcoidosis is associated with a higher production of the inflammatory cytokines IFN-γ, IL-2, TNF-α, and IL-17 by non Treg effector T cells including FoxP3− and CD45RA−FoxP3lowCD4+ T cells (Figure 1B).
CD4+FoxP3+ cells were studied in organs involved in sarcoidosis, by immunohistochemistry on fixed, paraffin-embedded tissue. In an effort to determine whether FoxP3+ T cells enumerated using immunohistochemistry correspond to bona fide CD4+CD25bright memory Treg cells,12,17 we compared immunochemistry and flow cytometry for the analysis of two control lymph nodes (Figure 1C). The proportion of CD4+FoxP3+ cells among CD4+ T cells, as calculated from slides, is consistent with the proportion of CD4+FoxP3bright T cells measured using flow cytometry. Therefore it appears that FoxP3 detection on fixed tissues mainly identifies FoxP3bright cells corresponding to memory phenotype Tregs, while FoxP3low cells (including cytokine-producing activated effector T cells and naïve Tregs) remain below the detection threshold of this technique.
We were able to confirm the increase of CD4+FoxP3+ cells in involved lymph nodes, as compared with those from nonsarcoidosis patients (21.6 ± 10.03% of CD4+ cells, n = 18 vs. 2.7 ± 1.25%, n = 6, P = 0.0004, Figure 1D). Granulomatous interstitial infiltrates in renal sarcoidosis also displayed a high prevalence of CD4+FoxP3+ cells (21.95 ± 10.41% of CD4+ cells, n = 20) in comparison with that observed in nongranulomatous interstitial nephritis (2.27 ± 2%, n = 9, P < 0.0001, Figure 1E). Study of the localization of CD4+FoxP3+ cells in infiltrates showed a greater accumulation within the granulomas than in the peripheral infiltrate (30.2 ± 11.58% vs 10.98 ± 7.32%, P < 0.0001).
These data confirm our previous findings that active sarcoidosis is characterized by an amplification of peripheral memory phenotype CD4+CD45RA−FoxP3bright Tregs12 and further show a high prevalence of memory Tregs within the granulomas present in nonlymphoid organs such as the kidney.
Treg Cells Actively Proliferate in Blood and in Granulomas
To identify the mechanisms involved in the global expansion of Tregs during sarcoidosis, we studied the expression of the proliferation marker Ki-67 antigen. As shown in Figure 2A, active in vivo proliferation is actually a typical feature of memory phenotype Tregs (in opposition to FoxP3low naïve Tregs), both in sarcoidosis patients and in healthy subjects. The peripheral Tregs express Ki-67 at the same frequency in sarcoidosis patients and in healthy subjects (29.4 ± 8.23% of CD4+FoxP3bright cells, n = 31 vs. 33.08 ± 8.91%, n = 11, P = 0.23, Figure 2A). However Tregs present in lymph nodes in sarcoidosis expressed Ki-67 more frequently (17.55 ± 8.05%, n = 8) than in control lymph nodes (6.76 ± 5.11%, n = 6, P = 0.01, Figure 2B). Similarly, as shown in Figure 2C, the Treg cells infiltrating renal granulomas expressed Ki-67 more frequently than those associated with nongranulomatous interstitial nephritis (6.89 ± 2.77% of Treg cells, n = 4 vs. 0%, n = 5, P = 0.01). These results indicate that Tregs are indeed proliferating within diseased organ granulomas during sarcoidosis.
Figure 2.
Intragranulomatous proliferation of memory phenotype Treg cells. A: Flow cytometry of Ki-67 and FoxP3 expression in CD4+ T cells in an active sarcoidosis patient (middle panel) and in a healthy subject (left panel). The percentage of Ki-67+ among FoxP3bright cells is indicated. A scattergram of 31 active patients and 11 healthy subjects is presented in the right panel. B and C: Comparative analysis of Ki-67 expression (blue nuclear staining) by CD4+FoxP3+ Treg cells (purple nuclear staining) assessed by immunochemistry of paraffin embedded sections of a control lymph node (out of 6) and an active sarcoidosis lymph node (out of 8) (B) and of a nongranulomatous interstitial nephritis (out of 5) and a sarcoidosis granulomatous nephritis (out of 4) (C). Magnification = original ×600. Comparisons were made using the nonparametric Mann-Whitney U-test.
Sarcoidosis Tregs Do Not Inhibit in Vitro Granuloma Formation
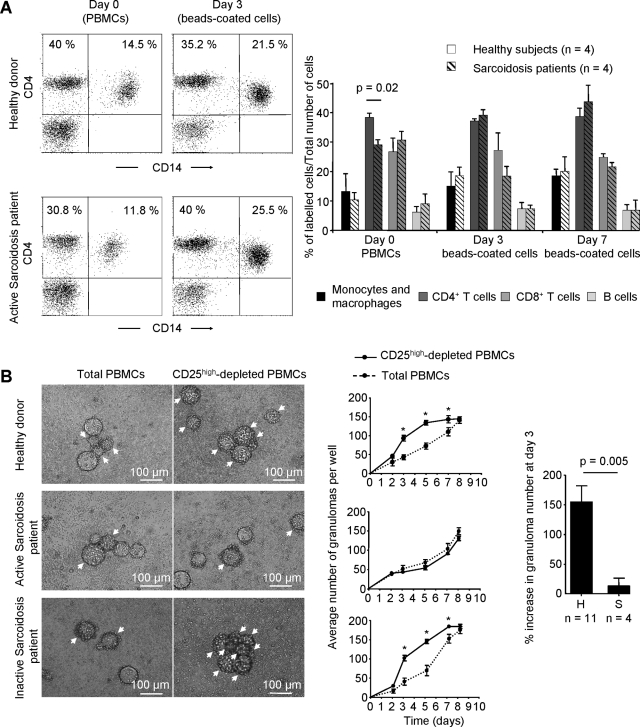
In previous studies, it has been demonstrated that cellular components of in vitro granulomas surrounding sepharose beads coated with Bacille Calmette Guerin extracts using healthy donor PBMCs were indeed similar to inflammatory cells observed in genuine granulomas.13,14,15 However, it is still unclear whether such granulomas can be observed in vitro using PBMCs isolated from patients with sarcoidosis. To ensure that cell accumulation around beads represents granulomas and not merely T cell proliferation centers at the site of antigen presentation, granuloma cell populations obtained from cultured sarcoid patient PBMCs were analyzed by flow cytometry. Although active sarcoidosis patients display a decrease in CD4+ T cells in the input cells as compared with healthy subjects (29.08 ± 3.38% of PBMCs, n = 4 vs. 38.33 ± 2.89, n = 4, P = 0.02, Figure 3A), we also observed fewer monocytes in those patients and the final lymphoid/monocytic ratio in the in vitro culture did not significantly vary between patients and controls (3.37 ± 1.89 vs. 4.5 ± 3, P = 0.48). The cellular composition of beads-driven granulomas after 3 and 7 days of culture reflected in both cases, all of the specific cell types found within natural human granulomas.
Figure 3.
Sarcoidosis Tregs do not suppress granuloma formation. A: CD4+ T cell and monocyte proportions were analyzed by flow cytometry among PBMCs at day 0 and within beads-coated cells at day 3 from a representative sarcoidosis patient (bottom panels) and a healthy subject (top panels). Cell type proportions were analyzed with the surface expression of monocyte and macrophage markers CD11c and CD14, T cells subsets markers CD4 and CD8, and CD19 for B cells. The mean ± SD of the proportions of indicated cell subsets in in vitro granulomas obtained from four healthy subjects (plain bars) and four patients with sarcoidosis (hatched bars) at days 3 (D3) and 7 (D7), and the initial proportions of the different cell types in PBMCs from the corresponding donors (D0) are presented in the right panel. B: In vitro day 3 granulomatous reactions in total or CD25high depleted PBMCs (left) and kinetics of in vitro granuloma formation (middle) from a representative healthy control (out of 11 analyzed, top), an active sarcoidosis patient (out of four analyzed, middle), and an inactive sarcoidosis patient (bottom). A granuloma was defined as a single bead totally covered with cells. Among aggregates, single granulomas were enumerated. Single granulomas are indicated by arrows. Magnification = original ×100. The mean relative percentage increase and SD of granuloma number in CD25high depleted PBMCs compared with total PBMCs from 11 healthy control subjects and four patients with active sarcoidosis at day 3 are indicated in the right panel. *P < 0.05.
To study the role of Tregs on granuloma formation, we compared the time course of granuloma formation on cultures of Bacille Calmette Guerin extracts with whole PBMCs or with PBMCs after removal of CD25high cells (Tregs) in active sarcoidosis patients and healthy subjects. In a representative healthy donor, depletion of CD25high cells accelerated granuloma formation measured after 3 days of culture (93.25 ± 15.13 vs. 42.75 ± 6.5 granulomas/well, P = 0.02, Figure 3B). This augmentation remained significant until day 7 in this individual, after which time the number of granulomas became equal by day 8 in the two conditions. In contrast, in a patient with active sarcoidosis, depletion of CD25high cells did not modify granuloma formation after 3 days of culture (41.25 ± 7.5 vs. 49.75 ± 8.65 granulomas/well, NS, Figure 3B). When we compared the percentage of increase in granuloma number after depletion of CD25high cells at day 3 in four patients with active sarcoidosis (mean age 35.25 ± 8.77 years, range: 29 to 48 years) versus eleven healthy age-matched donors (mean age 36.18 ± 8.96 years, range: 26 to 60 years), we observed a significant augmentation in healthy donors (153.5 ± 82.81%, n = 11 vs. 12.07 ± 21.76%, n = 4, P = 0.005, Figure 3B, right panel) persistent until day 7 of culture (data not shown).
To study the disease dependence of changes in Tregs activity, we compared granuloma formation in vitro in patients, during active disease and remission. In an inactive sarcoidosis patient, depletion of CD25high cells accelerated granuloma formation at day 3 (100.5 ± 14.8 vs. 39.5 ± 9.11 granulomas/well, P = 0.02, Figure 3B), and this acceleration remained persistent until day 7 of culture, as observed in healthy controls.
These data indicate that Tregs suppress early stages of autologous granuloma formation and that suppression of granuloma formation by Tregs is impaired in vitro in active sarcoidosis.
Neither Circulating nor Lymphoid Treg Expansion Correlates with Disease Severity
We sought next to determine whether the peripheral and in situ augmentation of Tregs in sarcoidosis had an influence on the control of the progression and dissemination of the disease. Hence, we attempted to correlate the amplitude of circulating and/or lymphoid Treg expansion with the severity of sarcoidosis. The measure used was the percentage of CD4+ T cells marked as Tregs (% Tregs), whether in the peripheral blood or in tissue. We found no correlation between the % Tregs in the peripheral blood and extrathoracic involvement, % predicted forced vital capacity, serum angiotensin converting enzyme level, or chest X-ray staging (Table 2). In examining the Tregs infiltrating mediastinal lymph nodes, we observed that there was a significantly higher % Tregs in African patients compared with European patients (30.3 ± 6.8%, n = 8, vs 14.6 ± 5.7%, n = 10, P = 0.00006). Because of this difference, we have separated these two populations in correlations between % Tregs and the different criteria of severity of disease. No correlation was found in the two populations between the percentage of Tregs and the different clinical parameters cited above (Table 2).
Table 2.
Correlation of Disease Severity with Both Circulating and Lymphoid Treg Cells
| Peripheral tregs percentage
|
Mediastinal lymph node tregs percentage
|
|||||
|---|---|---|---|---|---|---|
| r | P value* | Europeans r | P value* | Africans r | P value* | |
| Chest X-ray stage | −0.26 | 0.16 | −0.17 | 0.63 | −0.02 | 0.96 |
| FVC (% predicted) | 0.19 | 0.31 | −0.21 | 0.56 | −0.13 | 0.76 |
| Extrathoracic involvement | −0.01 | 0.95 | 0.57 | 0.08 | −0.02 | 0.97 |
| SACE | 0.34 | 0.06 | 0.21 | 0.54 | −0.42 | 0.29 |
P-level using Spearman’s rank-order correlations for percentage of Tregs in peripheral and mediastinal lymph nodes.
Tregs Are Abundant in Extensively Fibrotic Diseased Kidneys
The impaired suppression of granuloma formation by autologous Tregs observed in vitro in sarcoidosis suggests that they would not properly control the granulomatous responses in vivo. Therefore, we sought to determine the relationship of Tregs to the severity of tissue lesions during the course of sarcoidosis and particularly to the extent of granuloma lesions. We thus correlated their presence in renal granulomas with the severity of histological lesions. Sarcoid granulomas were particularly abundant in five patients (25%), involving more than 60% of the parenchyma. Interstitial fibrosis was present in all of the biopsies, but particularly diffuse and severe (> 40%) in 10 patients (50%).
No correlation was found between the percentage of renal Tregs and the severity of granulomatous inflammation (r = −0.07, P = 0.74, Figure 4). This result indicates that Tregs do not seem able to effectively control the extent of granulomas and tissue damage, as they are found at all disease stages, irrespective of histological severity. However, there was a positive correlation between the percentage of Tregs and the extent of interstitial fibrosis (r = 0.46, P = 0.03). Altogether our data indicate that, far from being able to prevent tissue damage, Tregs might even be associated with the extension of interstitial fibrosis.
Figure 4.
Treg infiltration is abundant at all lesional stages and particularly in extensively fibrotic kidneys. The relationship between renal Treg infiltration and granulomatous inflammation or interstitial fibrosis is shown, with their Pearson product-moment correlations. A significant positive relationship between the percentage of renal Treg cells and interstitial fibrosis at the time of biopsy was found (n = 20). There was no such relation between the percentage of renal Treg cells and the number of granulomas. Regression line (solid line) is shown with 95% confidence interval (dotted lines).
Discussion
Several studies have shown the role of Tregs not only in the suppression of response to self-antigens, thus preventing autoimmunity, but also in the regulation of immune responses to foreign antigens.18 In sarcoidosis it has been suggested that a defect in immunoregulation may be at the origin of the disproportionate Th1 response seen in involved organs.3,19 The target of this regulatory response and the mechanisms leading to its apparent deficit remain undetermined. We previously reported the amplification of blood, bronchoalveolar lavage fluid and lymphoid Tregs in active sarcoidosis.12 Here, we show an in situ amplification of CD4+FoxP3+ cells within renal granulomas. Importantly, we compared immunohistochemistry and flow cytometry for the detection of CD4+FoxP3+ cells and it appears that FoxP3 detection on fixed tissues mainly identifies FoxP3bright cells corresponding to immunosuppressive Tregs12,17 while FoxP3low cells (including cytokine-producing activated effector T cells [Figure 1B] and naïve Tregs) remain below the detection threshold of this technique.
Ki-67 analysis suggests that CD4+FoxP3+ Tregs are indeed proliferating within the granuloma, thus being at the origin of their increase in the periphery. These results are consistent with observations in the mouse and in humans showing a strong proliferative ability of Tregs in vivo,20 contrasting with their hyporesponsiveness in vitro.21 The marked local production of IL-2 observed in involved organs in sarcoidosis22 might be the cause of the proliferation in situ of Tregs followed by their redistribution to the periphery. Another potential mechanism that might explain this accumulation of Tregs in involved organs is their recruitment to the site of granulomatous inflammation. We have shown that CXCR3 is overexpressed on bronchoalveolar lavage fluid CD4+CD25bright cells during pulmonary sarcoidosis.12 In another study, using gene expression profiling, the chemokine CCL4 has also been implicated in the migration of CD4+CD25bright cells to sites of inflammation, possibly mediated through their expression of CCR5.23
The accumulation and proliferation in situ of Tregs raise the question of their role in the formation, persistence, and resolution of granulomas in involved organs. Recently, it has been shown in a murine model of tuberculosis that there is an accumulation and proliferation of fully functional Tregs at the sites of infection, suppressing the effector response to Mycobacterium tuberculosis, thus possibly contributing to the persistence of the infection.24 Moreover, several studies in mice have shown the beneficial role of Tregs in the course of the anti-infectious response in controlling the tissue lesions secondary to an intense inflammatory response.25,26,27,28,29 In the instance of sarcoidosis, it can be speculated that the accumulation of Tregs in the involved organs might be beneficial in suppressing granuloma formation, thus limiting the dissemination of the disease and the resulting tissue lesions. To directly assess this hypothesis, we used an in vitro granuloma culture model.13,14,15 In this system, culture of PBMCs with sepharose beads leads to formation of granulomas from day 2 onward. Although depletion of CD4+CD25high cells from PBMCs in healthy subjects accelerates granuloma formation, no changes in the time course of granuloma growth are observed using PBMCs from patients with sarcoidosis. These results suggest a defect in the suppressive function of Tregs on autologous granulomas in sarcoidosis. They are consistent with our previous results that show in vitro partially altered Treg suppressive function, the latter being able to inhibit the proliferation of effector T cells without completely inhibiting their secretion of IFN-γ and of TNF-α.12 These results, however, do not permit us to determine whether this suppression defect is a cause or consequence of the disease. Several murine studies have suggested that the suppressive function of Tregs depends both on their own state of activation and on the level of activation of effector T cells.30,31,32 Billiard et al33 have shown in vivo in the mouse that strong activation of effector cells renders them resistant to the suppressive action of Tregs. More generally, the inflammatory context seems to be determinant in the inhibition of the suppressive function of Tregs, notably through Toll-like receptor pathways. Sutmuller et al34 have shown an in vitro expansion of Tregs having altered suppressive function in response to the Toll-like receptor 2 ligand (Pam3cys) in a murine model. Finally, as shown in Figure 1A, sarcoidosis-associated Tregs are memory phenotype Tregs, naïve Tregs being virtually absent in these patients. Memory phenotype Tregs have undergone extensive cell cycling in vivo35 and are much more prone to apoptosis than naïve Tregs.36 Therefore, sarcoidosis-associated Tregs would not present any intrinsic functional defect, but would rather correspond to “exhausted” Tregs with reduced in vitro survival potential and Toll-like receptor-imprinted defects. The apparent defect in immunoregulation observed in sarcoidosis could result both from resistance of effector cells due to their strong activation status and from chronic inflammation-related Treg impairment.
Our in vitro results are supported by the absence of correlation between the presence in situ of Tregs in the renal parenchyma and the degree of granulomatous inflammation. Similarly, the percentage of Tregs in the blood and mediastinal lymph nodes of patients with pulmonary sarcoidosis is neither correlated with the severity of pulmonary involvement nor with the degree of dissemination of the disease. Thus, the accumulation of Tregs in the blood and different organs does not lead to the suppression of the granulomatous response, nor the associated tissue lesions. These results thus suggest that the defect in suppression of granuloma formation observed in vitro, is also present in vivo in the involved organs.
Moreover, we have found a positive correlation between the presence of Tregs in the renal granulomas and the degree of interstitial fibrosis. Fibrosis is an important cause of morbidity and mortality in sarcoidosis. In particular, pulmonary fibrosis is the principal cause of mortality in this disease.2 However, the correlation observed in our study between the percentage of renal Tregs and interstitial fibrosis does not imply that Tregs might be themselves implicated in fibrogenesis, but suggests that these cells have little protective effect on severe lesions.
In conclusion, we have shown that healthy human regulatory T cells are able to control, at least in vitro, the first steps of granuloma evolution. However, in the context of sarcoidosis, the same cells accumulate and proliferate within granulomas, but fail to suppress their growth. These in vitro results are well corroborated by clinical and histopathological studies showing that Tregs are not more abundant in less severely afflicted patients and vice-versa. Altogether, our results argue for a more preventive than curative effect of Tregs on inflammatory processes. We put forward the hypothesis that this defective function participates in the amplification and the persistence of the Th1 immune response that characterizes the disease. It remains to be shown whether Tregs would be primarily defective in sarcoidosis, or only “exhausted” by repetitive and chronic stimuli.
Acknowledgments
We are particularly grateful to all patients and control subjects who participated in this study. We thank Eric Tartour for support and discussion, Eva Comperat and Morgan Roupret for providing fresh lymph nodes samples, and Christophe Klein for expert assistance with the confocal microscope.
Footnotes
Address reprint requests to Pr. Guy Gorochov, M.D., Ph.D., INSERM UMR-S 945, Hôpital Pitié-Salpêtrière, 83 bv. De l’Hôpital, 75013 Paris, France, E-mail: guy.gorochov@psl.aphp.fr.
Supported by the Institut National et de la Recherche Médicale, by the Centre d’Investigation Biologiques (C.I.B.) Pitié-Salpêtrière, by Subvention of the CNMR 2006 (Comité National contre les Maladies respiratoires) and by the European FP6 “ATTACK” program (Contract: LSHC-CT-2005-018914). C.T. was supported by the Fonds d’Etude et de Recherche du Corps Médical des Hôpitaux de Paris and was the recipient of a price hosted by l’Association Amicale des Anciens Internes en Médecine des Hôpitaux et Hospices Civils de Paris.
C.T. and M.M. contributed equally to the work.
References
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- Nunes H, Soler P, Valeyre D. Pulmonary sarcoidosis. Allergy. 2005;60:565–582. doi: 10.1111/j.1398-9995.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: A disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981;305:429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Agostini C, Meneghin A, Semenzato G. T-lymphocytes and cytokines in sarcoidosis. Curr Opin Pulm Med. 2002;8:435–440. doi: 10.1097/00063198-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–1118. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- Wahlstrom J, Dengjel J, Persson B, Duyar H, Rammensee HG, Stevanovideltac S, Eklund A, Weissert R, Grunewald J. Identification of HLA-DR-bound peptides presented by human bronchoalveolar lavage cells in sarcoidosis. J Clin Invest. 2007;117:3576–3582. doi: 10.1172/JCI32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Kambouchner M, Valeyre D, Chapelon-Abric C, Debre P, Piette JC, Gorochov G. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissegur MP, Botanch C, Duteyrat JL, Delsol G, Caratero C, Altare F. An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell Microbiol. 2004;6:423–433. doi: 10.1111/j.1462-5822.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- Puissegur MP, Lay G, Gilleron M, Botella L, Nigou J, Marrakchi H, Mari B, Duteyrat JL, Guerardel Y, Kremer L, Barbry P, Puzo G, Altare F. Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent. ADAM9- and beta1 integrin-mediated pathway. J Immunol. 2007;178:3161–3169. doi: 10.4049/jimmunol.178.5.3161. [DOI] [PubMed] [Google Scholar]
- Lay G, Poquet Y, Salek-Peyron P, Puissegur MP, Botanch C, Bon H, Levillain F, Duteyrat JL, Emile JF, Altare F. Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol. 2007;211:76–85. doi: 10.1002/path.2092. [DOI] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- Ho LP, Urban BC, Thickett DR, Davies RJ, McMichael AJ. Deficiency of a subset of T-cells with immunoregulatory properties in sarcoidosis. Lancet. 2005;365:1062–1072. doi: 10.1016/S0140-6736(05)71143-0. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: More questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983;308:793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98. discussion 98–105, 106–114. [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Fredriksson M, Svennerholm AM, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin Exp Immunol. 2003;132:393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehervari Z, Sakaguchi S. Control of Foxp3+CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;16:1769–1780. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- Billiard F, Litvinova E, Saadoun D, Djelti F, Klatzmann D, Cohen JL, Marodon G, Salomon BL. Regulatory and effector T cell activation levels are prime determinants of in vivo immune regulation. J Immunol. 2006;177:2167–2174. doi: 10.4049/jimmunol.177.4.2167. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, Krammer P, Linderkamp O, Suri-Payer E. Naive regulatory T cells: A novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood. 2006;108:3371–3378. doi: 10.1182/blood-2006-02-005660. [DOI] [PubMed] [Google Scholar]
- Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation, Br Med J. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]