Abstract
Pseudoxanthoma elasticum (PXE) is a multisystem disorder characterized by ectopic mineralization of connective tissues with primary manifestations in the skin, eyes, and cardiovascular system. The classic forms of PXE are due to mutations in the ABCC6 gene that encodes the ABCC6 protein, a putative transmembrane transporter expressed primarily in the liver and the kidneys. PXE-like clinical findings have been encountered in association with vitamin K-dependent coagulation factor deficiency, an autosomal recessive disorder that is due to mutations in either the GGCX or VKORC1 genes. In this study, we investigated a family with two siblings with characteristic features of PXE and vitamin K-dependent coagulation factor deficiency. Mutation analysis identified two GGCX mutations in the affected individuals (p. R83W and p.Q374X); however, no mutations in either ABCC6 or VKORC1 could be found. GGCX encodes a γ-glutamyl carboxylase necessary for activation of both coagulation factors in the liver and matrix gla protein, which, in fully carboxylated form, is able to prevent ectopic mineralization. Analysis of skin by specific antibodies demonstrated that matrix gla protein was found predominantly in undercarboxylated form and was associated with the mineralized areas in the patients’ lesional skin. These observations pathomechanistically suggest that, in our patients, reduced carboxylase activity results in a reduction of matrix gla protein carboxylation, thus allowing peripheral mineralization to occur. Our findings also confirm GGCX as the second gene locus causing PXE.
Pseudoxanthoma elasticum (PXE; OMIM #264800) is an autosomal recessive multisystem disorder with primary manifestations in the skin, the eyes, and the arterial blood vessels.1,2 The histopathological hallmark of PXE is dystrophic mineralization of soft connective tissues, particularly the elastic structures. In the skin, the primary lesions are small, yellowish papules with predilection for flexural areas, and these lesions progressively coalesce into larger plaques of inelastic, leathery, and loose skin with yellowish hue. The skin histopathology reveals accumulation of pleiomorphic elastotic material in the upper and mid-dermis, which becomes progressively mineralized. The characteristic eye manifestations consist of angioid streaks and peau d’orange, and mineralization of the elastin-containing retinal layer, the Bruch’s membrane, causes fractures, neovascularization, and retinal bleeding. This may cause progressive loss of visual acuity and lead to primarily central blindness. Cardiovascular complications arise from the progressive mineralization of the elastin-rich arterial blood vessels, and clinical sequelae include intermittent claudication, internal bleeding from the gastric arterial blood vessels, arteriosclerosis that often leads to hypertension, and, rarely, myocardial infarction at a relatively early age. Although PXE can be associated with considerable morbidity and significant mortality, the phenotypic spectrum is highly variable with both inter- and intrafamilial heterogeneity.
Classic PXE is caused by mutations in the ABCC6 gene, which encodes an efflux transporter protein, ABCC6 (also known as multidrug resistance-associated protein 6-MRP6).2,3 The ABCC6 gene is comprised of 31 exons spanning approximately 73 kb of genomic DNA on chromosomal region 16p13.1. The ABCC6 protein consists of 1503 amino acids, with three predicted transmembrane-spanning domains (TMSD1–3) and two evolutionarily conserved intracellular nucleotide-binding folds (NBF1 and NBF2), which are critical for the binding and hydrolysis of ATP and for the function of the protein as a transmembrane transporter.2 ABCC6 is expressed predominantly on the basolateral surface of hepatocytes in the liver and in proximal tubules of the kidneys, but to a lesser extent, if at all, in tissues clinically affected by PXE.4,5 The precise physiological function of ABCC6 and its ligands in vivo are currently unknown. However, PXE is thought to be a metabolic disease in which critical, yet-to-be-identified metabolite(s) are not present in circulation due to nonfunctional ABCC6 transporter activity, consequently allowing mineralization of the peripheral tissue to occur.2,6
In addition to classic PXE, a number of genetically distinct clinical conditions display PXE-like clinical features, with aberrant mineralization of elastic structures in the skin. One phenotype, with important potential pathomechanistic implications, involves features of PXE in association with vitamin K-dependent multiple coagulation factor deficiency.7,8,9 These patients demonstrate cutaneous lesions that are very similar, and, in some cases, indistinguishable from those in classic PXE, ie, small yellowish papules, which tend to coalesce and which by histopathological and ultrastructural examination show profound mineralization. In addition to skin findings, some of these patients demonstrate retinal angioid streaks.
In this study, we examined a family with combined features of PXE and vitamin K-dependent coagulation factor deficiency. This family consists of two siblings with a rare coagulation deficiency that was originally reported in 1982.10 At the time of that publication, the skin findings were not described. However, extensive cutaneous involvement with features characteristic of PXE developed over the ensuing years, leading to a presumptive PXE diagnosis in both siblings. We now report that the proband and her brother are compound heterozygotes for a nonsense and a missense mutation in the GGCX gene that encodes γ-glutamyl carboxylase, required for activation of both vitamin K-dependent coagulation factors and also matrix gla protein (MGP), an inhibitor of ectopic mineralization. These observations potentially explain both the cutaneous and hematological findings in this family.
Materials and Methods
Patients
For histopathology, skin biopsies were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with H&E, Alizarin Red, and von Kossa stains using standard techniques.
For transmission electron microscopy, skin biopsy fragments were double-fixed in PBS-buffered glutaraldehyde (2.5%) and osmium tetroxide (0.5%), dehydrated, and embedded into Spurr’s epoxy resin. Ultrathin sections (90 nm) were made and double-stained with uranyl acetate and lead citrate, and viewed in a Philips CM10 transmission electron microscope.
Informed consent was obtained from all patients.
Molecular Analysis
Genomic DNA was isolated from either peripheral blood samples (QIAamp Blood Mini Kit; Qiagen Inc., Valencia, CA) or buccal swabs (Isohelix DNA Isolation Kit; Isohelix, Boca Raton, FL). PCR was performed using 1.5 U Taq polymerase (Qiagen) mixed with 5 U Optimase Taq polymerase (Transgenomic, Gaithersburg, MD) and Q buffer (Qiagen), according to the manufacturers’ instructions. PCR reactions contained 200 ng DNA as template and 20 ng of each primer in a final volume of 50 μl.
The GGCX and ABCC6 genes were analyzed using primers and PCR conditions as previously described.3,11 For the detection of the recurrent deletion of exons 23–29 in ABCC6, the primers described by Le Saux et al12 were used. The VKORC1 coding region was analyzed using primers described previously.13
The entire coding region and intron/exon boundaries of GGCX, ABCC6, and VKORC1 were analyzed via direct sequencing using an Applied Biosystems 3730 Sequencer (Applied Biosystems, Foster City, CA). For GGCX sequences the nucleotide numbers are derived from cDNA (GenBank accession no. NM000821); the +1 corresponds to the −28 with respect to the translation initiation codon. The alignments of the GGCX protein sequences in different species were generated with the Clustal W program (http://www.ebi.ac.uk/tools/clustalw2).
Immunohistochemistry
Immunohistochemistry was performed on tissues embedded in paraffin. Sections of 5 μm were stained with moAb-cMGP (4.5 μg/ml; reactive with the carboxylated forms of human matrix gla protein; cMGP) and moAb-ucMGP (4.5 μg/ml; reactive with the undercarboxylated forms of human matrix gla protein; ucMGP),14 respectively. Immunostaining was performed using biotinylated rabbit anti-mouse IgG (DAKO, Carpinteria, CA) as a secondary antibody (60 minutes at room temperature), followed by incubation with avidin-linked alkaline phosphatase complex (30 minutes at room temperature; DAKO); staining was performed using the Vector Red Alkaline Phosphatase Substrate Kit I (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin and mounted with Permount (Fisher, Fair Lawn, NJ). Controls for the immunoreactions were performed by omitting the primary antibody or by incubating the sections with nonimmune sera instead of the primary antibody.
Results
Clinical Findings
The proband (II-2 in Figure 1) is a 48-year-old female who initially presented with menorrhagia requiring multiple blood transfusions at age 13. Her bleeding disorder was extensively studied and combined functional deficiencies of multiple vitamin K-dependent coagulation factors were identified and have been reported previously.10 At the time of her initial hematological evaluation, both prothrombin and factor X activities were severely reduced. Immuno-electrophoretic examination suggested that the amounts of these affected coagulation factors were normal, and crossed immuno-electrophoresis of the plasma suggested the presence of a relatively homogeneous species of dysfunctional prothrombin. These studies were interpreted to be most consistent with a posttranslational defect in hepatic carboxylation of vitamin K-dependent factors. The proband has a younger brother, currently 46 years of age, who also had a similar bleeding diathesis with similar laboratory findings. Both parents had normal levels of all coagulation factors, and the family was diagnosed with an autosomal recessive disorder of vitamin K-dependent coagulation factor deficiency. The proband’s functional deficiencies in these clotting factors responded to 5 mg vitamin K1, which has been continuously administered daily without further significant bleeding problems. Her laboratory values at the present are as follows: proliferation time 14.9 (normal limits 12.3 to 14.6), partial thromboplastin time 25.9 (normal limits 27.3 to 35.3), and thrombin time 17.9 (normal limits 14.4 to 19.3). Her brother did not initiate oral or parenteral vitamin K1.
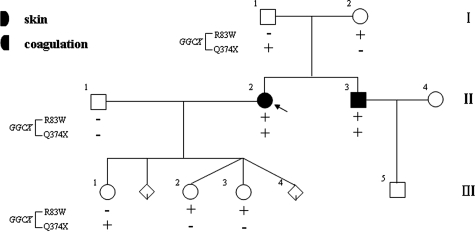
Figure 1.
Nuclear pedigree of the family with PXE-like skin findings and vitamin K-dependent multiple coagulation factor deficiency. The proband (II-2) is indicated by an arrow. On the left, mutations were identified in the GGCX gene.
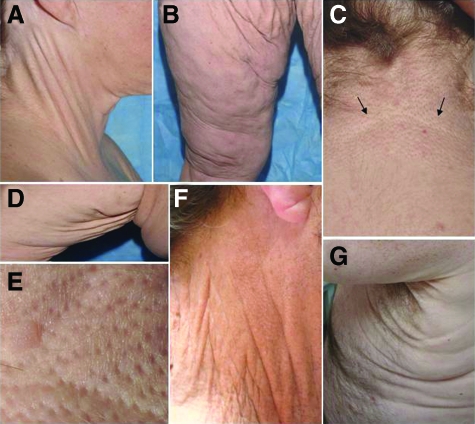
At age 19, the proband developed joint pains, and she was diagnosed with systemic lupus erythematosus at age 23, for which she eventually received treatment with intravenous immunoglobulin, cyclophosphamide, and prednisone for renal and central nervous system involvement. Subsequent to the report describing her bleeding disorder (at age 20), she was evaluated by other physicians for skin laxity and yellow papules in the axillae (at age 27). A skin biopsy was performed that was consistent with PXE. Progressive facial skin laxity led the patient to undergo a face lift procedure at age 39. Similar progressive skin changes consistent with PXE were identified in her brother (Figure 2).
Figure 2.
Cutaneous findings in the proband (A–E) and her brother (F,G) with PXE-like phenotype. Note the yellowish papules and plaques in the lateral and posterior neck (A,C), which on close examination demonstrate characteristic features of PXE (E). Also note the loose and sagging skin primarily in the groin (B) and axillary (D) areas. The loose skin was also noted in the proband’s brother in the lateral neck (F) and axillary areas (G). (A, C) the lower border of the plaque is demarcated by arrows in C.
More recently (2008), the proband was seen for a follow-up study for her systemic lupus erythematosus at the National Institutes of Health, Bethesda, MD. During this visit, her extensive skin findings were noted and a dermatology consultation was requested. On physical examination, small yellowish papules coalescing into larger plaques were noted on the lateral and posterior neck (Figure 2, A and C), and on closer inspection, these lesions demonstrated characteristic features of PXE (Figure 2E). In addition, the patient exhibited loose, sagging and redundant skin, particularly affecting the groin and axillary areas (Figure 2, B and D). The brother of the proband, also with vitamin K-dependent multiple coagulation factor deficiency, was subsequently evaluated and noted to have similar skin changes (Figure 2, F and G). On ophthalmologic examination, the proband was found to have very early evidence of angioid streaks, with no loss of vision. Her brother had fundus changes suggestive of angioid streaks and peau d’orange without vision loss.
Examination of the members of the nuclear pedigree, including the parents of the proband and her three children, revealed no significant skin manifestations (Figure 1). However, one of the proband’s daughters (Figure 1, III-2) had been evaluated at age 17 for menorrhagia and was diagnosed with mild Type I von Willebrand disease based on reduced von Willebrand antigen in her plasma (31, normal range 55 to 200). Her vitamin K-dependent coagulation factors were all within normal limits.
Collectively, the clinical examination revealed two siblings with history of vitamin K-dependent multiple coagulation factor deficiency and extensive skin findings consistent with PXE. Both conditions are apparently autosomal recessive, as the parents are clinically normal.
Histopathology and Ultrastructural Features of the Skin
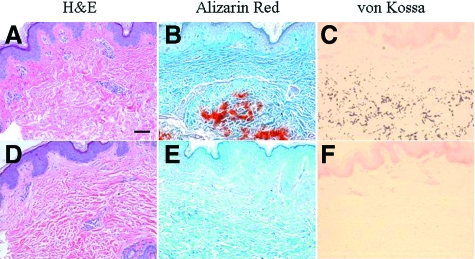
Histopathology of the skin lesions in the proband revealed accumulation of basophilic elastotic material in the mid-dermis, and this material was associated with ectopic mineralization, as demonstrated with H&E, Alizarin Red, and von Kossa special stains (Figure 3A–C). In comparison, control skin from an unrelated individual of similar age showed no evidence of mineralization (Figure 3, D–F). Thus, histopathology confirmed that these patients have features of PXE. Skin biopsies from the proband’s parents, husband, and three daughters revealed no elastotic material accumulation or mineralization (data not shown).
Figure 3.
Histopathology of the cutaneous lesions in the proband (A–C), in comparison with an unrelated healthy control (D–F). Staining with H&E (A,D) demonstrates basophilic abnormal elastic structures in the mid-dermis, and special stains (Alizarin Red and von Kossa) revealed that these elastotic structures are mineralized. Scale bar = 0.1 mm.
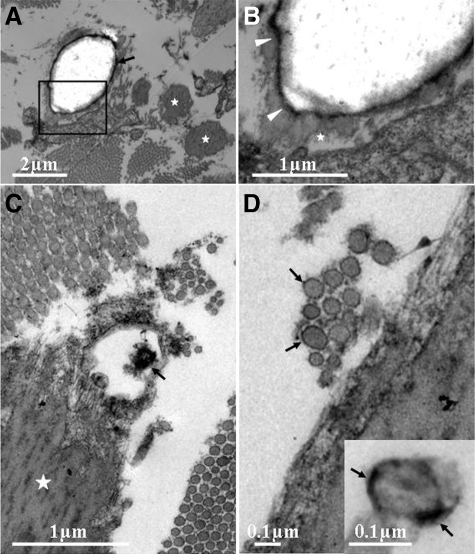
Transmission electron microscopy was further used to evaluate the mineralization of soft connective tissues in the skin. A number of mineralized elastic structures were noted, although not all elastic fibers showed evidence of mineralization (Figure 4A). In some areas, the elastic structures depicting mineralization revealed an electron-lucent central core surrounded by an electron-dense rim separating the core from the peripheral elastic structures, which appeared fairly normal (Figure 4B). The mineralized elastic structures were often in the proximity of collagen fibers, which appeared in most cases normal (Figure 4, B and C). However, in some areas the collagen fibers displayed considerable variability in their diameter, and some of them showed an electron-dense rim suggesting mineralization of the outer surface of the collagen fibers (Figure 4D).
Figure 4.
Transmission electron microscopy of the lesional skin in the proband. A: Electron microscopy reveals elastic structures with extensive mineralization with central electron lucency. Note that not all elastic structures are mineralized (asterisks). B: Higher magnification of the area boxed in (A) shows that the translucent mineralized core is separated from apparently normal looking elastic structure (asterisk) by an electron dense rim (arrowheads). C: Degeneration of part of the elastic fiber adjacent to normal appearing collagen fibers. D: In some areas, collagen fibers show considerable variability in their diameter and demonstrate electron dense rim (arrows), suggesting mineralization of the surface of the collagen fibers (inset).
Mutation Analyses
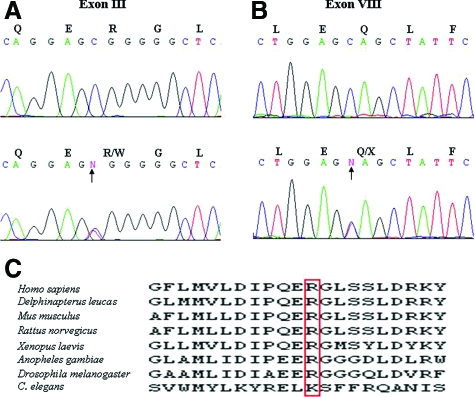
Considering the clinical presentation of the proband with vitamin K-dependent coagulation factor deficiency, we first sequenced the GGCX and VKORC1 genes known to underlie the bleeding tendency in some patients with this form of coagulopathy.15,16 Sequencing of the VKORC1 gene did not reveal pathogenic mutations (not shown). In contrast, sequencing of the GGCX gene revealed two putative pathogenic mutations in the proband and her brother. First, a single nucleotide transition mutation (c.247C→T) resulting in substitution of an arginine by tryptophan at amino acid position 83 (p.R83W) was detected in exon III (Figure 5A). This mutation was not present in 200 control alleles as determined by direct nucleotide sequencing. Furthermore, the arginine in this position is highly conserved through evolutionary sequence conservation, with the exception of a lysine substitution in Caenorhabditis elegans (Figure 5C). It should be noted that the amino acid sequences surrounding the arginine residue in position 83 of the human protein showed a high degree of conservation within a number of species (Figure 5C). Secondly, a single nucleotide substitution (c.1148C→T) resulting in substitution of a glutamine by a stop codon in amino acid position 374 (p.Q374X) was detected in exon VIII (Figure 5B). Thus, both the proband and her brother were compound heterozygous for a missense and a nonsense mutation in the GGCX gene. Further sequencing of DNA from the parents of the proband and her children, who were clinically normal, revealed that all these individuals were heterozygotes for one of the GGCX mutations (Figure 1). This inheritance is clearly consistent with autosomal recessive mode.
Figure 5.
Identification of GGCX mutations in the family. A: Identification of a heterozygous C→T transition substitution at nucleotide position 247 in the proband’s DNA (lower panel, arrow), as compared with a control sample (upper panel). B: Identification of a nonsense mutation p.Q374X due to a C→T transition mutation in exon VIII (lower panel, arrow), in comparison with the control (upper panel). C: Note the evolutionary conservation of the amino acid R83 in the γ-glutamyl carboxylase protein.
Sequencing of the ABCC6 gene, associated with the classic form of PXE, using primers that covered the entire coding sequence and flanking intronic sequences, failed to reveal mutations in this gene (not shown). No evidence of exonic deletions was noted, and in particular, the common deletion of exons 23 to 29 (del 23 to 29) was not present. Finally, the presence of seven heterozygous nucleotide polymorphisms in the exons 10 to 19 were detected, excluding hemizygosity of the ABCC6 gene.
Undercarboxylation of Matrix Gla Protein in the Skin
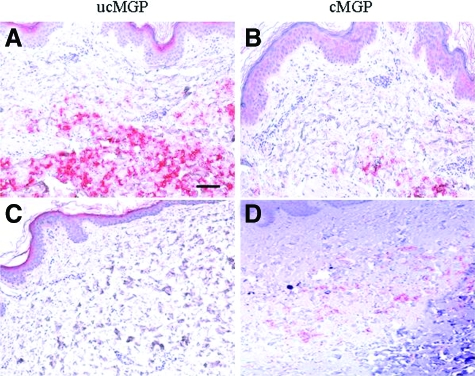
GGCX encodes γ-glutamyl carboxylase, the enzyme required for activation of the vitamin K-dependent clotting proteins.15 Furthermore, γ-glutamyl carboxylation is also required to activate matrix gla protein (MGP), a factor critical for prevention of premature mineralization.17 This protein can be detected both at the local site of mineralization and also in circulation. We assessed the degree of γ-glutamyl carboxylation of MGP by using antibodies that specifically recognize the undercarboxylated (ucMGP) and fully carboxylated (cMGP) forms of the protein.14 Immunohistochemistry of the proband’s skin revealed deposition of ucMGP in the mid-dermis but very little of the active carboxylated form of the protein (Figure 6, A and B). In contrast, immunohistochemistry of control skin stained in parallel with these two antibodies indicated essentially complete absence of ucMGP (Figure 6C), as well as very little cMGP (Figure 6D).
Figure 6.
Immunohistochemistry of lesional skin in the proband (A,B) as well as in normal control skin (C,D). Monoclonal antibodies recognized the undercarboxylated (ucMGP) and carboxylated (cMGP) forms of matrix gla protein. The secondary antibodies, biotin conjugated anti-IgG, were recognized by alkaline-phosphatase conjugates and visualized by incubation with an alkaline phosphatase substrate yielding red color. Scale bar = 0.1 mm.
Discussion
GGCX Mutations Cause Coagulation Disorder and PXE
This kindred exhibits two discrete phenotypic features, a deficiency of vitamin K-dependent coagulation factors, and cutaneous features of abnormal skin elasticity, skin laxity, and aberrant skin mineralization. Vitamin K-dependent coagulation factor deficiency is an autosomal recessive disorder caused either by mutations in the GGCX gene (type I:VKCFD1 – OMIM #277450) on chromosome 2p12, or in the VKORC1 gene (type II:VKCFD2 – OMIM #607473) on chromosome 16p11.2.15,16
In this study we identified two mutations in the GGCX gene, a missense mutation (p.R83W) and a nonsense mutation (p.Q374X). The missense mutation was not present in 200 control alleles, indicating that it is not a frequent polymorphism. Furthermore, the arginine residue encoded by the human GGCX is highly conserved during evolution. The second mutation (p.Q374X) truncates the polypeptide ∼51% downstream of its total length. These observations strongly imply, therefore, that the γ-glutamyl carboxylase is nonfunctional in these patients, explaining the vitamin K-dependent coagulation factor deficiency. The GGCX gene encodes a carboxylase required for γ-glutamyl carboxylation of Gla-proteins, including clotting factors, enabling them to attach to the phospholipid bilayer of membranes as a prerequisite for blood coagulation.18 Thus, γ-glutamyl carboxylase deficiency provides a pathomechanistic explanation for the coagulation disorder in this family. It should be noted that the targeted mutant Ggcx−/− mouse phenotype is prenatal or perinatal lethal due to complete absence of carboxylase activity, which results in severe bleeding diathesis.19 In our proband, the bleeding tendency was corrected by oral vitamin K, but the types of mutations in the GGCX gene may also influence these severity of the coagulopathy. Specifically, one of the genetic lesions is a missense mutation (p.R83W), which may demonstrate low levels of residual enzyme activity, as was previously shown in case of other missense mutations.20
The second phenotypic feature in this family was PXE-like cutaneous findings, including yellowish papules and plaques with dot-like depressions, typical of PXE. Histopathology of the skin from the proband revealed distinct mineralization of elastotic structures in the mid-dermis, findings consistent with PXE. The co-existence of vitamin K-dependent coagulation factor deficiency and PXE-like cutaneous features has been noted previously in the literature, and in some cases been associated with mutations in the GGCX gene.7,8,9,20 Similar to our patients, no evidence for mutations in the ABCC6 gene were noted in previously reported patients exhibiting PXE-like features in association with vitamin-K dependent coagulation factor deficiency.9,20
Putative Pathomechanisms and Clinical Implications
The putative pathomechanistic explanation for the development of mineralization in the skin in patients with GGCX deficiency relies on the ability of MGP, a low molecular weight protein, to prevent premature mineralization when modified by γ-glutamyl carboxylation.17 If the carboxylation reaction does not take place due to deficient carboxylase enzyme activity, the undercarboxylated form of MGP (which is inactive) cannot prevent aberrant mineralization of peripheral connective tissues, such as the elastic fibers in the skin. Antibodies that discriminate between the undercarboxylated and fully carboxylated forms of MGP in biopsy specimens from lesional skin from the proband and non-mineralized control skin, respectively, demonstrated an abundance of ucMGP14 in biopsy specimens from lesional skin from the proband, whereas this form of the protein was essentially absent in the control skin. Our findings are consistent with previous demonstrations that undercarboxylated MGP, as detected by a novel conformation-specific antibody, was localized in areas of calcification in human vascular tissue,20 and similar findings were represented in an animal model of warfarin-induced medial elastocalcinosis.21
Our findings have potential implications regarding the pathomechanisms of classic PXE due to mutations in ABCC6. Specifically, our results imply that undercarboxylation of MGP plays a critical role in aberrant mineralization of tissues in PXE, a suggestion supported by recent electron microscopic examination of the skin from patients with classic PXE,2,22 as well as by analysis of the carboxylation status of MGP in Abcc6−/− knock-out mice that recapitulate features of PXE.23 More specifically, the Abcc6−/− targeted mutant mice display the presence of ucMGP in the mineralized areas of lesional skin, thus recapitulating the findings in our patients with GGCX mutations.
How the absence of ABCC6, an efflux transporter expressed primarily in the basolateral surfaces of the hepatocytes, can elicit reduction of γ-glutamyl carboxylation of MGP remains to be explored. However, one possibility may relate to cellular transport and redistribution of vitamin K or its derivatives, which can serve as obligatory co-factors for γ-glutamyl carboxylase.2,24 The bulk of dietary vitamin K is transported from the gastrointestional tract to the liver where it undergoes modifications in its side chain or become conjugated for example with glutathione. Consequently, one could postulate that a form of vitamin K, modified or conjugated in the liver, could serve as a substrate for ABCC6 to be transported to the circulation. If correct, the concentration of critical vitamin K derivatives in the circulation, as well as in cells, such as dermal fibroblasts, in peripheral tissues would be reduced in the absence of ABCC6 transporter activity, leading to deficient γ-glutamyl carboxylation of MGP and allowing mineralization of adjacent connective tissues. This would be consistent with the absence of obvious coagulation defects in classic PXE due to ABCC6 mutations, since the concentration of critical vitamin K derivatives would be sufficient in hepatocytes to lead to full carboxylation of the coagulation factors allowing normal clotting to occur. If our hypothesis is correct, it would then raise the intriguing possibility that administration of appropriate forms of vitamin K or its derivates may counteract the progressive mineralization of connective tissues in PXE and might ameliorate, or even cure, this currently intractable disease in patients that have mutations in the ABCC6 gene. In patients with GGCX deficiency, vitamin K supplementation is able to correct the coagulation defect but is not sufficient to interfere with the pathways leading to aberrant connective tissue mineralization in the peripheral tissues.
Genetic Heterogeneity of PXE
The complementary nature of the ABCC6 and GGCX gene defects has been demonstrated by previous phenotypic observations of a family with features of PXE in association with vitamin K-dependent coagulation factor deficiency.20 While some of the members of that family were compound heterozygotes for missense mutations in the GGCX gene, two patients with characteristic PXE-like cutaneous findings were found to be heterozygous for a missense mutation in the GGCX gene (p.V255M) and a recurrent nonsense mutation (p.R1141X) in the ABCC6 gene in trans. These findings suggest that PXE-like skin findings can be due to digenic inheritance, and they re-emphasize the notion that the GGCX is the second gene locus for PXE, in addition to the initially described ABCC6 gene. Finally, it should be noted that not all patients with vitamin K-dependent coagulation factor deficiency due to GGCX mutations develop PXE-like cutaneous features. This paradoxical observation could be explained by the fact that distinct mutations in the GGCX gene can reduce the corresponding carboxylase activity to a different degree in different substrates.20 Consequently, the precise degree of carboxylation of MGP could be variable with different GGCX mutations, thus explaining the phenotypic variability or lack of skin findings associated with the coagulation deficiency.
Acknowledgments
We thank Jeffrey Travers, MD, PhD, Kathleen Coyle, MD, and Susan Booher, MS, RN, for clinical assistance in evaluation of the patients, and Carol Kelly for technical help.
Footnotes
Address reprint requests to Jouni Uitto, MD, PhD, Department of Dermatology and Cutaneous Biology, Jefferson Medical College, 233 South 10th Street, Suite 450 BLSB, Philadelphia, PA 19107. E-mail: jouni.uitto@jefferson.edu.
Supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR28450 and R01AR52627 to J.U.) and by the Intramural Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute and Medical Genetics Branch, and National Human Genome Research Institute.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the peer review process or final disposition for this article.
References
- Neldner KH, Struk B. Pseudoxanthoma elasticum. Royce PM, Steinmann B, editors. New York: Wiley-Liss, Inc.,; Connective tissue and its heritable disordersMolecular, genetic and medical aspects. 2002:pp 561–583. [Google Scholar]
- Li Q, Jiang Q, Pfendner EG, Váradi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2008;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfendner EG, Vanakker O, Terry SF, Vourthis S, McAndrew P, McLain MR, Fratta S, Marais AS, Hariri S, Coucke PJ, Ramsay M, Viljoen D, Terry PF, Paepe AD, Uitto J, Bercovitch LG. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky MG, Kruh GD. MOAT-E (ARA) is a full length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2008;PMID:18685618. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongioletti F, Bertamino R, Rebora A. Generalized pseudoxanthoma elasticum with deficiency of vitamin K-dependent clotting factors. J Am Acad Dermatol. 1989;21:1150–1152. doi: 10.1016/s0190-9622(89)70320-0. [DOI] [PubMed] [Google Scholar]
- Le Corvaisier-Pieto C, Joly P, Thomine E, Lair G, Lauret P. Generalized pseudoxanthoma elasticum combined with vitamin K dependent clotting factors deficiency. Ann Dermatol Venereol. 1996;123:555–558. [PubMed] [Google Scholar]
- Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, Paepe AD. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;27:581–587. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- George H, Goldsmith JR, Pence RE, Ratnoff OD, Adelstein DJ, Furie B. Studies on a family with combined functional deficiencies of vitamin K-dependent coagulation factors. J Clin Invest. 1982;69:1253–1260. doi: 10.1172/JCI110564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg J, von Brederlow B, Fregin A, Rost S, Wolz W, Eberl W, Eber S, Lenz E, Schwaab R, Brackmann HH, Effenberger W, Harbrecht U, Schurgers LJ, Vermeer C, Müller CR. Congenital deficiency of vitamin K dependent coagulation factors in two families presents as a genetic defect of the vitamin K-epoxidereductase- complex. Thromb Haemost. 2000;84:937–941. [PubMed] [Google Scholar]
- Le Saux O, Beck K, Sachsinger C, Silvestri C, Treiber C, Göring HH, Johnson EW, De Paepe A, Pope FM, Ronchetti IP, Bercovitch L, Marais AS, Viljoen DL, Terry SF, Boyd CD. A spectrum of ABCC6 mutations is responsible for pseudoxanthoma elasticum. Am J Hum Genet. 2001;69:749–764. doi: 10.1086/323704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darghouth D, Hallgren KW, Shtofman RL, Mrad A, Gharbi Y, Maherzi A, Kastally R, LeRicousse S, Berkner KL, Rosa JP. Compound heterozygosity of novel missense mutations in the gamma-glutamyl-carboxylase gene causes hereditary combined vitamin K–dependent coagulation factor deficiency. Blood. 2006;108:1925–1931. doi: 10.1182/blood-2005-12-010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C. Novel conformation specific antibodies against matrix γ-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–1633. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- Berkner KL. The vitamin K-dependent carboxylase. Ann Rev Nutr. 2005;25:127–149. doi: 10.1146/annurev.nutr.25.050304.092713. [DOI] [PubMed] [Google Scholar]
- Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG, Müller CR, Strom TM, Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Mtab Care. 2000;3:433–438. doi: 10.1097/00075197-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ginsburg D. Familial multiple coagulation factor deficiencies: new biologic insight from rare genetic bleeding disorders. J Thromb Haemost. 2004;2:1564–1572. doi: 10.1111/j.1538-7836.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- Zhu A, Sun H, Raymond RM, Jr, Furie BC, Furie B, Bronstein M, Kaufman RJ, Westrick R, Ginsburg D. Fatal hemorrhage in mice lacking γ-glutamyl carboxylase. Blood. 2007;109:5270–5275. doi: 10.1182/blood-2006-12-064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Grange DK, Armstrong NL, Whelan AJ, Hurley MY, Rishavy MA, Hallgren KW, Berkner KL, Schurgers LJ, Jiang Q and Uitto J: Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol 2008, doi:10.1038/jid.2008.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- Gheduzzi D, Boraldi F, Annovi G, DeVincenzi CP, Schurgers LJ, Vermeer C, Quaglino D, Ronchetti IP. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest. 2007;87:998–1008. doi: 10.1038/labinvest.3700667. [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Schurgers LJ, Uitto J. Pseudoxanthoma elasticum: reduced gamma-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6−/−). Biochem Biophys Res Commun. 2007;364:208–213. doi: 10.1016/j.bbrc.2007.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]