Abstract
Injured skeletal muscle has the capacity to regenerate through a highly coordinated sequence of events that involves both myoblast migration and differentiation into myofibers. Fibrosis may impede muscle regeneration by posing as a mechanical barrier to cell migration and fusion, providing inappropriate signals for cell differentiation, and limiting vascular perfusion of the injury site, subsequently leading to incomplete functional recovery. Our previous studies demonstrated that matrix metalloproteinase-1 (MMP-1) is able to digest fibrous scar tissue and improve muscle healing after injury. The goal of this study is to investigate whether MMP-1 could further enhance muscle regeneration by improving myoblast migration and differentiation. In vitro wound healing assays, flow cytometry, reverse transcriptase-polymerase chain reaction (RT-PCR), and Western blot analyses demonstrated that MMP-1 enhances myoblast migration but is not chemoattractive. We discovered that MMP-1 also enhances myoblast differentiation, which is a critical step in the sequence of muscle regeneration. In addition, RT-PCR and Western blot analyses demonstrated the up-regulation of myogenic factors after MMP-1 treatment. In vivo, we observed that myoblast transplantation was greatly improved after MMP-1 treatment within the dystrophic skeletal muscles of MDX mice. MMP-1 may therefore be able to improve muscle function recovery after injury or disease by increasing both the number of myofibers that are generated by activated myoblasts and the size of myoblast coverage area by promoting migration, thus fostering a greater degree of engraftment.
Muscle injuries are among the most common injuries seen in orthopaedic clinics and present a challenging problem in traumatology. Fibrosis is a consequence of the local overgrowth of extracellular matrix (ECM) at the site of injury. It poses a significant barrier in the prevention of complete muscle regeneration, thus leading to incomplete recovery, pain, and functional deficits. Accelerated ECM deposition may impede muscle regeneration by creating mechanical barriers against cell migration and fusion, providing inappropriate signals for cell differentiation, and limiting vascular perfusion of the injury site; this leads to incomplete functional recovery and a propensity for re-injury.1,2,3
Experimental studies have demonstrated the efficacy of preventing fibrosis after muscle injury by blocking key factors in the fibrotic cascade, such as transforming growth factor-β.4,5,6,7,8 However, treating patients before the onset of fibrosis is often unlikely; most persons with muscle injuries seek treatment only after the onset of fibrosis and the concomitant pain and functional deficits it produces. Additionally, pervasive skeletal muscle fibrosis can be caused by neuromuscular dysfunction and various musculoskeletal diseases such as Duchenne muscular dystrophy (DMD). DMD is an X-linked muscle disorder characterized by progressive muscle weakness caused by a lack of dystrophin expression in the sarcolemma of muscle fibers. Patients diagnosed with DMD begin to experience fibrous scar tissue formation in their muscles during their teenage years.9,10 This anomalous generation of matrix protein is thought to be driven by the repeated degeneration, inflammation, and regeneration of muscle in DMD patients.11,12
Myoblast transplantation has been considered as a potential therapeutic method for DMD.10,13,14 However, poor cell survival and low dispersion of grafted cells outside of the injection site after transplantation have hindered the overall success of this technology.15,16,17 Fibrous scar tissue continues to impede muscle cell migration, fusion, and muscle regeneration, even when myogenic cells are transplanted into the region. Significant improvements in cell survival have been obtained after immunosuppressive therapy,18,19 but few studies have been centered on muscle cell migration, especially after myogenic cell transplantation.
Our research has indicated that it is this secondary pathological process of DMD, namely fibrous scar tissue formation, which poses the most significant obstacle to myogenic cell migration, fusion, and regeneration. Digesting fibrous scar tissue could remodel the microenvironment to make it more hospitable to migration, fusion, and myogenic cell regeneration.20,21 Subsequently, it would enhance the myogenic transplantation process and improve muscle healing, not only in injured skeletal muscle, but also in patients suffering from DMD.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteolytic enzymes with the ability to digest specific components of the ECM.22 They present a promising approach to treat fibrosis after skeletal muscle injury or as a consequence of neuromuscular disease. MMP-1, in particular, has the ability to digest the main constitutive proteins in fibrous scar tissue, native fibrillar collagens type I and III, while sparing collagen type IV, which is a component of the basement membrane.23,24,25 MMP-1 may also play important roles in ECM remodeling and cell signaling with its ability to act on the cell surface, matrix, and nonmatrix substrates, such as insulin growth factor binding proteins, L-selectin, and tumor necrosis factor-α.26,27 Previous work in our laboratory has indicated that MMP-1 can help remove the fibrous blockade to enhance muscle healing.20,28 We hypothesize that MMP-1 can further enhance muscle regeneration by directly improving myoblast migration and differentiation capability. This may ultimately enhance muscle regeneration by improving the myoblasts’ ability to increase the number of regenerating myofibers within an area of injury as well as increasing the effective range of transplanted myoblast-enhanced muscle regeneration can occur. We tested this hypothesis by studying the effects of MMP-1 on myoblasts in vitro and myoblast transplantation in vivo.
Materials and Methods
In Vitro
In Vitro Wound Healing Assay
C2C12 cells were purchased from the American Type Culture Collection (Rockville, MD). The cells were cultured in a complete medium containing Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 10% horse serum, 0.5% chicken embryo extract, and 1% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere in 12-well plates until 70% confluent. The 12-well plates were either uncoated or coated with type I collagen or fibronectin. Cells were then placed in serum-free DMEM supplemented with 1% penicillin/streptomycin and treated with 0, 1.0, 10, or 100 ng/ml of MMP-1 (M1802; Sigma, St. Louis, MO). An artificial wound was created by disrupting the monolayer with a sterile plastic pipette tip. Cells were incubated for 1, 4, 6, and 12 hours to allow for migration back into the wound area. Cells were then fixed in cold methanol, washed with phosphate-buffered saline (PBS), and then stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) to help visualize cell migration. Northern Eclipse software (Empix Imaging Inc., Mississauga, Canada) was used to quantify the average migration distance of C2C12 cells that traveled past the original wound demarcation.
Flow Cytometry
C2C12 cells were cultured in a complete media (described above). Cells were then placed in serum-free DMEM supplemented with 1% penicillin/streptomycin and treated with 0, 1.0, 10, or 100 ng/ml of MMP-1 for 18 hours. Cells were incubated with either polyclonal N-cadherin (sc-31031; Santa Cruz Biotechnology, Santa Cruz, CA) or β-catenin (sc-1496, Santa Cruz Biotechnology) primary antibodies, and subsequently with a PE-conjugated secondary antibody. Marked cell samples were analyzed with a FACS Caliber flow cytometer (BD Biosciences, Sparks, MD) and CellQuest software (BD Biosciences).
Chemotaxis Assay
The assay was performed using a multiwell insert system (BD Biosciences) with an 8.0-μm pore size polyethylene terephthalate membrane. C2C12 cells (5 × 104) were placed in the top well of the system. Serum-free DMEM or serum-free DMEM supplemented with 10 and 100 ng/ml of MMP-1 were placed in the bottom well. Cells were incubated at 37°C for 3 hours to permit migration across the membrane in response to MMP-1. The top wells were then treated with 0.5% trypsin-ethylenediaminetetraacetic acid (Invitrogen) for 5 minutes to detach cells remaining on the top surface of the membrane and washed with PBS. Cells remaining on the bottom surface of the membrane were then fixed in cold methanol, washed with PBS, and then stained with DAPI (Sigma) to help visualization under fluorescent microscopy. The migration of cells was quantified by averaging the number of cells counted in five high-power fields and compared between control and MMP-1 treatment.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
C2C12 cells were seeded onto six-well plates and treated with MMP-1 for 3 and 12 hours. A monophasic solution of phenol and guanidine isothiocyanate (TRIzol, 10 cm2/ml; Life Technologies, Inc., Grand Island, NY) was used to extract total RNA from these cells. Reverse transcription was performed with the RETROscript kit (Ambion, Applied Biosystems, Austin, Texas) and cDNA was prepared. Primers used for gene detection were designed using Oligo software (OligoPerfect Designer, Invitrogen). The primers included: myogenin 5′-CCAGTGAATGCAACTCCCACAGC-3′ and 5′-AGACATATCCTCCACCGTGA-3′; MyoD 5′-GGCTACGACACCGCCTACTA-3′ and 5′-GTTCTGTGTCGCTTAGGGAT-3′; muscle regulatory factor-4 (MRF4) 5′-GCACCGGCTGGATCAGCAAGAG-3′ and 5′-CTGAGGCATCCACGTTTGCTCC-3′; desmin 5′-AACCTGATAGACGACCTGCAG-3′ and 5′-GCTTGGACATGTCCATCTCC-3′; insulin-like growth factor-1 receptor (IGF1R) 5′-GGTGGATGCTCTTCAGTTCG-3′ and 5′-GACTTGGCAGGCTTGAGGG-3′; and β-actin 5′-GGGTCAGAAGGACTCCTATGTGG-3′ and 5′-CCTGGATGGCTACGTACAT-3′. The conditions for IGF1R and β-actin amplification were the following: 93°C for 1 minute, 54°C for 1 minute, and 72°C for 2 minutes for 31 cycles. The conditions for desmin amplification were the following: 93°C for 1 minute, 54°C for 1 minute, and 72°C for 2 minutes for 34 cycles. The conditions for MyoD were the following: 93°C for 1 minute, 54°C for 1 minute, and 72°C for 2 minutes for 28 cycles. The conditions for MRF4 were the following: 93°C for 1 minute, 53°C for 1 minute, and 72°C for 2 minutes for 31 cycles. The conditions for myogenin were the following: 93°C for 1 minute, 53°C for 1 minute, and 72°C for 2 minutes for 33 cycles. Products were separated by size on 1% agarose gel.
Western Blot Analysis
C2C12 cells were harvested after 48 hours of incubation with or without treatment (0, 1.0, 10, 100 ng/ml) in serum-free DMEM. After lysing, the samples were separated by 12% sodium dodecyl-sulfate-polyacrylamide electrophoresis gel and transferred to nitrocellulose membranes. Anti-PreMMP-2 antibodies (Sigma) at a dilution of 1:1000, anti-TIMP-1 (sc-5538, Santa Cruz Biotechnology) at a dilution of 1:1000, and anti-myogenin (Sigma) at a dilution of 1:2000 were used as primary antibodies. Mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Sigma) at a dilution of 1:2000 was used for protein quantification. Anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Pierce, Rockford, IL) was applied at a dilution of 1:5000. Blots were developed using SuperSignal West Pico chemiluminescent substrate (Amersham Pharmacia Biotech, Piscataway, NJ), and positive bands were visualized on X-ray film. Northern Eclipse software (Empix Imaging) was used to evaluate these results.
Cell Differentiation Assay
C2C12 cells were cultured in 12-well plates to 75% confluency. Cells were subsequently incubated in differentiation media containing serum-free DMEM supplemented with 1% penicillin/streptomycin and treated with 0, 1.0, 10, or 100 ng/ml of MMP-1 (M1802, Sigma) for 3, 5, and 7 days. Cells were fixed in cold methanol for 1 minute and washed with PBS. The fixed cells were immunostained for myosin heavy chain (MyHC, Sigma) and DAPI to visualize mature myotubes. Cell differentiation was quantified by averaging the number of myotubes counted in five high-power fields and compared among different concentrations of MMP-1 treatment.
In Vivo
Myoblast Transplantation in Dystrophic Skeletal Muscle
Nine-week-old MDX/SCID mice (C57BL/10ScSn-Dmdmdx crossed with C57BL/6J-Prkdcscid/SzJ) were injected with C2C12 cells with MMP-1 (M1802, Sigma). LacZ-positive C2C12 cells (1 × 105) were mixed with 200 ng of MMP-1 in a volume of 5 μl of PBS, which was then injected into the left gastrocnemius muscles (GMs) or tibialis anterior (TAs) of MDX/SCID mice. The same number of LacZ-positive C2C12 cells was diluted with 5 μl of PBS and was injected into the right GMs or TAs of the mouse to serve as a control. Muscle tissues were harvested for histological analysis at 2 and 4 weeks after transplantation. GMs and TAs were isolated, mounted, and frozen in 2-methylbutane cooled in liquid nitrogen. Each muscle specimen was cryostat-sectioned at 10 μm for histological analysis. LacZ staining with eosin and immunohistochemistry for dystrophin (Sigma) and β-galactosidase (Abcam, Cambridge, MA) were performed.6,7 The dystrophin-positive myofibers were counted and their diameters were measured to evaluate the enhancement of MMP-1 on myoblast differentiation and fusion capacities in vivo. Myoblast migration distances from the initial site of injection were also quantified using Northern Eclipse software (Empix Imaging Inc.).
Statistics
Statistical significance was assessed by analysis of variance and two-tailed Student’s t-tests; P < 0.05 was considered significant.
Results
MMP-1 Stimulated Myoblast Migration in Vitro
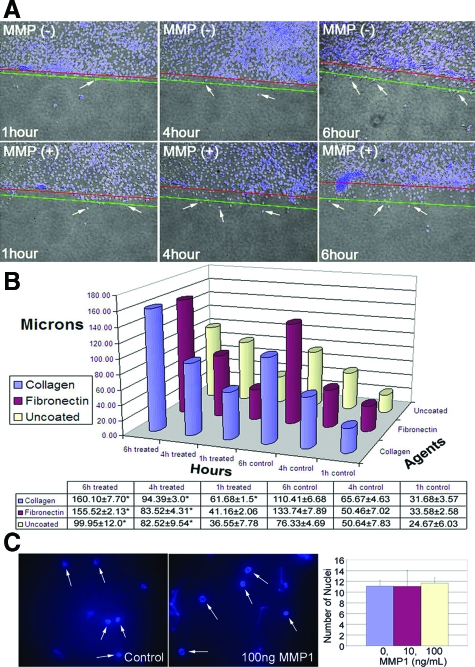
Our previous studies have not shown that MMP-1 actually stimulated myoblast migration directly. We used an in vitro wound-healing assay, flow cytometry, and Western blot analysis to determine whether MMP-1 could alter C2C12 migration. After culturing C2C12 cells to 70% confluence, we created artificial wounds as described previously and measured migration distances of C2C12 cells back into the wounded area using various conditions. Our results indicate that MMP-1 can enhance C2C12 myoblast migration throughout time in uncoated plates (Figure 1A) as well as under conditions that more closely represent in vivo ECM content, eg, type I collagen or fibronectin-coated plates (Figure 1B). We observed a significant difference in migration distances for C2C12 cells treated with 10 ng/ml of MMP-1 at 4 and 6 hours after wounding when compared with control cells in uncoated and fibronectin-coated plates (Figure 1B). Using type I collagen-coated plates, there were significant differences in migration distances for C2C12 cells treated with MMP-1 (10 ng/ml) at 1, 4, and 6 hours after wounding when compared with control cells (Figure 1B; *P < 0.05).
Figure 1.
Myoblasts (C2C12) were grown to confluence and artificially wounded by disrupting the monolayer with a sterile pipette. A: C2C12 (white arrows) show an enhanced ability to migrate into the wound area after 4 and 6 hours of treatment with MMP-1 (10 ng/ml) in noncoated dishes. Red lines represent original line demarcating the wound area. Green lines represent the approximate migration area. B: However, in conditions more accurately representing in vivo ECM content, eg, type I collagen or fibronectin-coated plates, C2C12 present more aggressive movement compared with noncoated conditions. C: The Boyden system was used and MMP-1 has no chemoattractive effect on C2C12 myoblasts at 10 and 100 ng/ml within 3 hours of tracking (arrows point to nuclei that stain with DAPi).
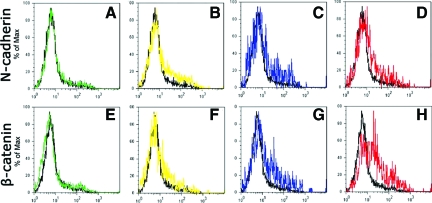
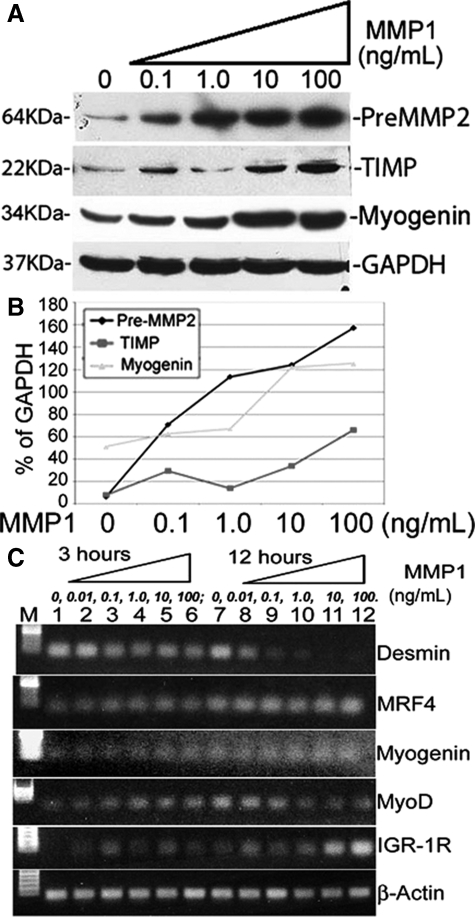
N-cadherin and β-catenin are two proteins expressed with myoblast migration29,30 and were used as markers for C2C12 cell migration analysis. Flow cytometry results demonstrated that MMP-1 promoted the up-regulation of N-cadherin and β-catenin in C2C12 cells when treated with 10 and 100 ng/ml of MMP-1 (Figure 2, C and D, G and H). Treatment with 0.1 and 1.0 ng/ml of MMP-1 did not yield significant results (Figure 2, A and B, E and F). Western blot analysis further demonstrates that MMP-1 treatment enhances the expression of migration-related proteins. It has been reported in the literature that pre-MMP-2 and TIMP are up-regulated with myoblast migration.18,31,32,33,34,35 Our Western blot results indicate that these two proteins are up-regulated with MMP-1 stimulation in myoblasts, especially with higher doses of MMP-1 (Figure 3A). Northern Eclipse software (Empix Imaging, Inc.) was used to analyze positive bands, which demonstrated that the expression of pre-MMP-2 increased mostly in a dose-dependent manner when treated with 0.1, 1.0, 10, and 100 ng/ml of MMP-1 (Figure 3B). TIMP1 expression increased mainly with 10 and 100 ng/ml of MMP-1 treatment; C2C12 cells treated with 0.1 and 1.0 ng/ml of MMP-1 demonstrated minor increases in the expression of TIMP1 when compared with nontreated control cells (Figure 3B).
Figure 2.
Myoblasts (C2C12) treated with MMP-1 for 18 hours display a dose-dependent up-regulation of two migration-related proteins: N-cadherin (A–D) and β-catenin (E–H). Flow cytometry results for 0 (black lines, A–H), 0.1 (green lines, A and E), 1.0 (yellow lines, B and F), 10 (blue lines, C and G), and 100 (red lines, D and H) ng/ml MMP-1-treated cells.
Figure 3.
Myoblasts (C2C12) were treated with 0, 1.0, 10, or 100 ng/ml of MMP-1 for 7 hours. A: Western blot analysis showed an increase in the expression of migration-related proteins, PreMMP-2 and TIMP as well as a myogenic protein, myogenin. GAPDH staining served as the control. The positive bands were evaluated and compared with standard GAPDH. B: Results showed the dose-dependent increase of protein expression with MMP-1 treatment. C: RT-PCR results indicated some myogenic genes, eg, MRF4, myogenin, and MyoD were initially increased within 3 hours of stimulation in a dose-dependent response with MMP-1 stimulation. With 12 hours of stimulation, myogenin, IGF1R, and MRF4 were promoted, thus increasing the level of mRNA. Desmin, however, was decreased in a dose-dependent response with MMP-1 treatment in 3-hour and 12-hour time periods; MyoD also decreased, but only at the 12-hour time point.
MMP-1 Has No Chemotactic Effect on Myoblast Migration
Results from the chemotaxis assay demonstrated that 10 and 100 ng/ml of MMP-1 treatment did not increase the number of C2C12 that migrated in the polyethylene terephthalate membrane compared with control, on visualization under fluorescent microscopy (Figure 1C). This suggests that MMP-1 may not have a chemoattractive effect on myoblast (C2C12) migration in vitro.
RT-PCR
We have tested the mRNA level of different genes within C2C12 myoblasts after treating with MMP-1. Results indicated that MMP-1 increases MRF4, myogenin, and MyoD initially within 3 hours of stimulation in a dose-dependent manner. In the following 12 hours of stimulation with MMP-1, myogenin, IGF1R, and MRF4 were promoted, thus increasing the mRNA level. Desmin, however, was decreased in a dose-dependent response with MMP-1 treatment in both 3-hour and 12-hour time periods; MyoD also decreased, but only at the 12-hour time point (Figure 3C). Results indicate MMP-1 somehow accelerates myogenic gene activation, with the exception of desmin, which mainly relates to mature myoblasts during development.36
MMP-1 Stimulated C2C12 Differentiation in Vitro
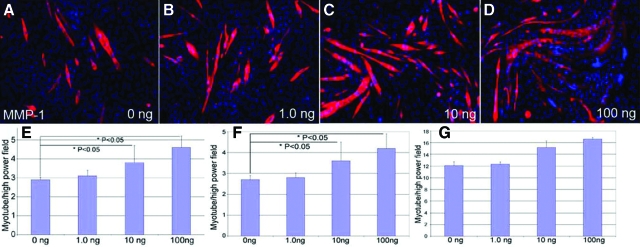
C2C12 cells proliferate when maintained in growth media containing serum, but differentiate into multinucleated myotubes when grown in serum-free media. This progression of differentiation is similar to that observed for myogenesis in vivo.37 We investigated whether MMP-1 could enhance C2C12 differentiation in vitro using a differentiation assay as well as Western blot analysis. When cultured in differentiation media, C2C12 cells displayed a dose-dependent increase in differentiation capacity when treated with 10 and 100 ng/ml of MMP-1 compared with control groups at 5 days (Figure 4, A–D). Treatment with 10 and 100 ng/ml of MMP-1 produced significantly more myotubes compared with the control group at 3 and 5 days (Figure 4, E and F) incubation, but not at 7 days (Figure 4G). To further demonstrate that MMP-1 enhances cell differentiation, we used Western blot analysis to detect myogenin, which has previously been shown to be a key protein controlling myofiber formation.32 When cultured in differentiation media, C2C12 cells displayed a dose-dependent increase in the expression of myogenin when treated with 0.1, 1.0, 10, and 100 ng/ml of MMP-1 (Figure 3, A and B).
Figure 4.
C2C12 cells were cultured until 75% confluent. They were subsequently treated with 0, 1.0, 10, or 100 ng/ml of MMP-1 in differentiation medium for 3, 5, and 7 days. A–D: Myotubes were immunostained with MyHC (red) and nuclei were stained with DAPI (blue) as shown at 3 days after treatment. The numbers of myotubes were quantified by averaging the count from five high-powered fields using a fluorescent microscope. C2C12 displayed a dose-dependent increase in ability to differentiate into myotubes at 10 and 100 ng/ml of MMP-1 compared with control at 3 (E) and 5 days (F) after treatment. G: At 7 days, the myotube formation remained similar among different concentrations of MMP-1. *P < 0.05.
MMP-1 Enhances Myoblast Migration and Differentiation in Vivo
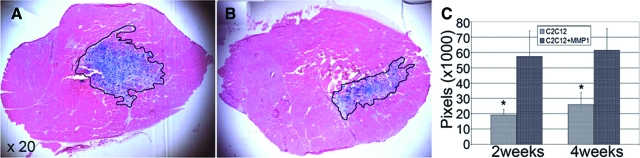
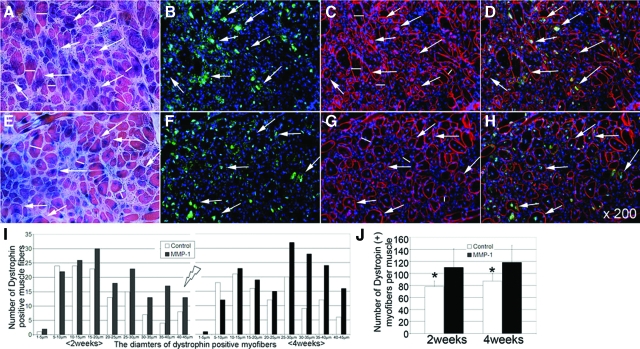
We elected to test the effects of MMP-1 on myoblast migration and differentiation by transplanting C2C12 cells in MDX mice. The MDX mouse contains a nonsense mutation in the dystrophin gene, which leads to the absence of full-length dystrophin protein in skeletal muscle, thus providing a suitable model for human DMD.9,38 By transplanting C2C12 cells into the GMs or TAs of MDX/SCID mice, we were able to assess whether MMP-1 was able to enhance myoblast migration based on migration distance and engraftment from the initial injection area through histological analysis. Concomitant injection of MMP-1 along with C2C12 cells significantly increased the migration area and graft coverage by the C2C12 cells (Figure 5, A and B). The measured C2C12 migration distance versus fused muscle grafts indicates that MMP-1-treated muscles have a significantly greater degree of engraftment (LacZ-positive muscle grafts) compared with control nontreated muscle at 2 and 4 weeks after injections (Figure 5C). We were also able to assess the effects of MMP-1 on myoblast differentiation and fusion with host myofibers using immunohistochemical analysis. Because MDX/SCID mice are deficient in dystrophin, dystrophin-positive myofibers with nuclei β-galactosidase (reflecting LacZ protein expression)-positive (dystrophin+/β-galactosidase+) represent transplanted C2C12 cells that have differentiated and fused with host myofibers. When C2C12 cells were injected with MMP-1, significantly more dystrophin+/β-galactosidase+ muscle grafts were visualized (Figure 6, A–D) compared with control without MMP-1 treatment (Figure 6, E–H) at 2 weeks after transplantation. The diameters of these dystrophin+/β-galactosidase+ myofibers were significantly increased in MMP-1-treated muscle compared with control at 2 and 4 weeks after transplantation (Figure 6I); similar results also indicated MMP-1-treated myoblasts fused a larger number of LacZ and dystrophin+/β-galactosidase+-positive myofibers compared with nontreated myoblasts after transplantation in vivo (Figure 6J).
Figure 5.
LacZ-positive C2C12 cells (1 × 105) were injected into the GMs or TAs of MDX/SCID mice along with 200 ng of MMP-1 in the left GMs and PBS in the right GMs or TA muscles. GMs and TAs were harvested at different time points after injection. LacZ staining with eosin, along with immunohistochemistry for dystrophin, were performed. MMP-1-treated C2C12 fused to a greater degree (A) compared with control nontreated C2C12 within TA muscles (B) at 2 weeks after transplantation. C: The measured LacZ-positive muscle grafts indicated MMP-1-treated C2C12 cells activated migration and fused to a greater degree of engraftment at 2 and 4 weeks after transplantation compared with control. *P < 0.05.
Figure 6.
Combining immunohistochemistry, higher numbers of LacZ, and dystrophin-positive, β-galactosidase-positive (dystrophin+/β-galactosidase+) myofibers were detected within MMP-1-treated groups (A–D) compared with control nontreated groups (E–H) at 2 weeks after C2C12 myoblast transplantation. I: The diameters of these dystrophin+/β-galactosidase+ myofibers were also measured; results indicate MMP-1-treated C2C12 enlarge these myofibers by either differentiation or fusion with host muscle compared with control nontreated C2C12 at 2 and 4 weeks after transplantation. J: The MMP-1-treated C2C12 also formed a larger number of LacZ and dystrophin+/β-galactosidase+ myofibers compared with nontreated control C2C12 at 2 and 4 weeks after myoblast transplantation. Red: dystrophin; green: β-galactosidase; blue: nuclei. White arrows: dystrophin+/β-galactosidase+; white lines: diameters of newly fused myofibers. *P < 0.05.
Discussion
Injured skeletal muscle has an enormous capacity to heal itself in a process that is dependent on the activation and differentiation of myoblasts.2,21,39,40 Disruption of the basal lamina and plasma membrane of skeletal muscle cells leads to the release of satellite cells, which follow a highly coordinated sequence of steps in which they migrate toward the injured muscle; subsequently, they differentiate and fuse together to form myoblasts, multinucleated myotubes, and ultimately, mature muscle fibers.2,21,36 Unfortunately, excess deposition of the ECM in the form of fibrotic scar tissue often leads to incomplete recovery. A persistent imbalance between collagen biosynthesis and degradation contributes to scar formation and fibrosis in tissues; high levels of collagens have been detected in injured skeletal muscle4,8,41 and inhibition of collagen deposition has been able to reduce scar tissue formation in injured skeletal muscle.42 Additionally, muscle injuries usually result in hematomas that are gradually replaced by granulation tissue that fosters fibrosis.1,2,3,21,43
The clinical applications of anti-fibrotic therapies, such as suramin and decorin, are limited in scope because most persons with muscle injuries seek treatment for muscle injuries only after the onset of fibrosis.21,44 Furthermore, large quantities of fibrotic tissue are already present in the skeletal muscles of patients who suffer from neuromuscular disorders such as DMD. This fibrous scar tissue presents a major limitation in the success of myogenic cell transplantation, a potential therapeutic modality for DMD. For patients suffering from DMD, increases in myoblast migration and fusion with host fibers or fusion with one another after muscle cell implantation would greatly improve the success of myoblast transplantation.16,45,46 To date, limited myoblast migration distances from the sites of transplantation have complicated therapy.15,16,45,47 As a result of this limitation, repeated injections of myoblasts are required to obtain sustainable engraftment.47,48 Unfortunately, repeating the injections not only causes pain and discomfort for patients, but also fosters additional fibrosis via direct trauma to the skeletal muscle. Previous studies have demonstrated that MMP collagenases, such as MMP-1, are capable of removing fibrotic tissue within skeletal muscle,20,28 which is essential for muscle regeneration in vivo.45,49,50 They also appear to be involved in activation of satellite cells, which function as myoblast precursors capable of differentiating and fusing with muscle fibers to repair damage.45,49,51,52 Treatment with MMP-1 may augment the success of muscle cell transplantation by improving the efficacy of transplanted cells as well as reduce the number of injections required to sustain engraftment for DMD patients.
We have previously demonstrated that MMP-1 can help remove the fibrous blockade against migrating myoblasts, thereby improving myoblast differentiation/fusion in vivo.20,28 We were able to replicate these results in dystrophin-deficient MDX/SCID mice by demonstrating that transplanted myoblasts covered a significantly larger area from the initial injection site. Our results demonstrate that MMP-1 not only removes fibrous scar tissue from skeletal muscle, but can also activate myoblast migration and differentiation/fusion, which supports the results of previous research. We also observed MMP-1 has no chemoattractive effect on myoblasts. MMPs have been strongly implicated in the process of tumor cell migration and invasion during metastasis and in myoblast migration during development.53,54,55,56 It has also been reported that overexpression of MMP-1 and MMP-2 increased the migration and invasion of murine myoblasts in response to fibronectin in vitro.57 A recent study has shown that MMP-1 stimulates cell migration through epidermal growth factor receptor (EGFR) transactivation.58 MMP-1 and its inhibitors (eg, TIMP-1/2) also regulate the myogenic differentiation process.37,59,60 We were able to observe this directly by examining MMP-1’s effects on C2C12 cell migration into artificial wounds in vitro. This was further supported by the up-regulation of four migration-related proteins (N-cadherin, β-catenin, pre-MMP-2, and TIMP-1) after treatment with MMP-1, especially at higher doses (10 and 100 ng/ml). We had previously hypothesized that MMP-1 may be liberating growth factors and cell signaling molecules, or acting on cell adhesion sites located within the ECM.20 Given our in vitro experiments in the absence of ECM, MMP-1 may also be acting on binding proteins present on the myoblast cell surface to directly promote differentiation and migration, or may be involved in a more complex signaling cascade. These interesting phenomena will be investigated in our future studies.
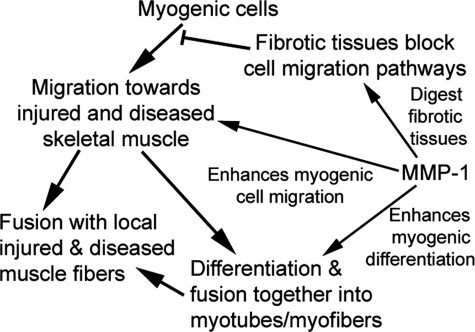
Myoblasts differentiate into multinucleated myotubes, which eventually fuse with mature muscle fibers to regenerate muscle.2,21 Our results indicate that MMP-1 can promote C2C12 myoblast differentiation in vitro as well as in vivo. RT-PCR and Western blot analysis showed that MMP-1 treatment increased the myogenic gene production from mRNA to proteins; the proteins with increased production include: MRF4, myogenin, and MyoD. We also detected that IGF1R was activated by MMP-1 treatment; however, desmin was somehow inhibited during MMP-1 stimulation. In fact, C2C12 cells treated with MMP-1 produce significantly more myotubes when cultured in vitro. Myoblasts transplanted into dystrophic skeletal muscles of MDX/SCID mice showed a significant increase in the number and diameter of regenerating myofibers when treated with MMP-1. We were also able to support these results on a molecular level; C2C12 cells treated with MMP-1 up-regulated their expression of myogenin, which is a checkpoint protein involved in myofiber formation.20 Because myoblast differentiation is a critical step in the process of muscle regeneration, MMP-1 may improve the recovery of muscle function after injury by increasing the number of myofibers generated by activated myoblasts. Therefore, the benefits of MMP-1 on muscle healing may include remodeling the microenvironment to facilitate myoblast migration, fusion, and regeneration (Figure 7). The healing of repetitively injured skeletal muscle generates the pathological repair process in congenitally diseased skeletal muscle. Results of this study indicate that MMP-1 enhances muscle cell migration and differentiation/fusion in vitro as well as accelerating muscle regeneration and grafting in vivo. Thus, MMP-1 could be beneficial in the treatment of diseased muscles in neuromuscular diseases such as DMD, particularly as an adjunctive therapy to myoblast transplantation.
Figure 7.
A schematic of the potential effect of MMP-1 on muscle healing within injured or diseased skeletal muscle.
Acknowledgments
We thank Alison Logar, Maria Branca, and Bin Sun for their technical assistance.
Footnotes
Address reprint requests to Yong Li, M.D., Ph.D., Director, The Laboratory of Molecular Pathology, Stem Cell Research Center, 3312 Rangos Research Center, 3460 Fifth Ave., Pittsburgh, PA 15213-2583. E-mail: yongli@pitt.edu.
Supported by the Department of Defense (grant W81XWH-06-01-0406 to Y.L.) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1R21AR055725 to Y.L.).
Award for Outstanding Research in Biochemistry/Cell Biology, and the Award for Overall Excellence in Research at the 2006 AMA-MSS and Resident and Fellow Section Joint Research Poster Symposium.
References
- Garrett WE., Jr Muscle strain injuries: clinical and basic aspects. Med Sci Sports Exerc. 1990;22:436–443. [PubMed] [Google Scholar]
- Li Y, Cummins J, Huard J. Muscle injury and repair. Curr Opin Orthop. 2001;12:409–415. [Google Scholar]
- Lehto MU, Jarvinen MJ. Muscle injuries, their healing process and treatment. Ann Chir Gynaecol. 1991;80:102–108. [PubMed] [Google Scholar]
- Chan YS, Li Y, Foster W, Horaguchi T, Somogyi G, Fu FH, Huard J. Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol. 2003;95:771–780. doi: 10.1152/japplphysiol.00915.2002. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Li Y, Foster W, Fukushima K, Badlani N, Adachi N, Usas A, Fu FH, Huard J. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Partridge TA. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve. 1991;14:197–212. doi: 10.1002/mus.880140302. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- Karpati G, Holland P, Worton RG. Myoblast transfer in DMD: problems in the interpretation of efficiency. Muscle Nerve. 1992;15:1209–1210. doi: 10.1002/mus.880151016. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Huard J, Bouchard JP, Roy R, Labrecque C, Dansereau G, Lemieux B, Tremblay JP. Myoblast transplantation produced dystrophin-positive muscle fibres in a 16-year-old patient with Duchenne muscular dystrophy. Clin Sci (Lond) 1991;81:287–288. doi: 10.1042/cs0810287. [DOI] [PubMed] [Google Scholar]
- Skuk D. Myoblast transplantation for inherited myopathies: a clinical approach. Expert Opin Biol Ther. 2004;4:1871–1885. doi: 10.1517/14712598.4.12.1871. [DOI] [PubMed] [Google Scholar]
- Caron NJ, Asselin I, Morel G, Tremblay JP. Increased myogenic potential and fusion of matrilysin-expressing myoblasts transplanted in mice. Cell Transplant. 1999;8:465–476. doi: 10.1177/096368979900800502. [DOI] [PubMed] [Google Scholar]
- Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Sato H, Okada Y, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in cell invasion. Thromb Haemost. 1997;78:497–500. [PubMed] [Google Scholar]
- Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann NY Acad Sci. 1998;857:110–118. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- Bedair H, Liu TT, Kaar JL, Badlani S, Russell AJ, Li Y, Huard J. Matrix metalloproteinase-1 therapy improves muscle healing. J Appl Physiol. 2007;102:2338–2345. doi: 10.1152/japplphysiol.00670.2006. [DOI] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84A:822–832. [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Sakaida I, Hironaka K, Kimura T, Terai S, Yamasaki T, Okita K. Herbal medicine Sho-saiko-to (TJ-9) increases expression matrix metalloproteinases (MMPs) with reduced expression of tissue inhibitor of metalloproteinases (TIMPs) in rat stellate cell. Life Sci. 2004;74:2251–2263. doi: 10.1016/j.lfs.2003.09.059. [DOI] [PubMed] [Google Scholar]
- Roach DM, Fitridge RA, Laws PE, Millard SH, Varelias A, Cowled PA. Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur J Vasc Endovasc Surg. 2002;23:260–269. doi: 10.1053/ejvs.2002.1598. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–288. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Kaar JL, Li Y, Blair HC, Asche G, Koepsel RR, Huard J, Russell AJ. Matrix metalloproteinase-1 treatment of muscle fibrosis. Acta Biomater. 2008;4:1411–1420. doi: 10.1016/j.actbio.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Gamel AJ, Krenn V, Muller TS, Wilting J, Christ B. N-cadherin is involved in myoblast migration and muscle differentiation in the avian limb bud. Dev Biol. 1996;178:160–173. doi: 10.1006/dbio.1996.0206. [DOI] [PubMed] [Google Scholar]
- Woodfield RJ, Hodgkin MN, Akhtar N, Morse MA, Fuller KJ, Saqib K, Thompson NT, Wakelam MJ. The p85 subunit of phosphoinositide 3-kinase is associated with beta-catenin in the cadherin-based adhesion complex. Biochem J. 2001;360:335–344. doi: 10.1042/0264-6021:3600335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino T, Watanabe Y, Matsui M, Miyamori H, Kudo T, Seiki M, Sato H. Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res. 2006;312:1381–1389. doi: 10.1016/j.yexcr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci. 2006;119:3822–3832. doi: 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- Mendes O, Kim HT, Lungu G, Stoica G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 2007;24:341–351. doi: 10.1007/s10585-007-9071-0. [DOI] [PubMed] [Google Scholar]
- Gong YL, Xu GM, Huang WD, Chen LB. Expression of matrix metalloproteinases and the tissue inhibitors of metalloproteinases and their local invasiveness and metastasis in Chinese human pancreatic cancer. J Surg Oncol. 2000;73:95–99. doi: 10.1002/(sici)1096-9098(200002)73:2<95::aid-jso7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park CI, Park BW, Lee HD, Jung WH. Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in ductal carcinoma in situ and invasive ductal carcinoma of the breast. Yonsei Med J. 2006;47:333–342. doi: 10.3349/ymj.2006.47.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. The Satellite Cell and Muscle Regeneration. Engel AG, Franzini-Armstrong C, editors. New York: McGraw-Hill,; 1994:pp 97–118. [Google Scholar]
- Lluri G, Jaworski DM. Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve. 2005;32:492–499. doi: 10.1002/mus.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Jokl P, Heinen GT, Connell MD, Panjabi MM. A muscle contusion injury model. Biomechanics, physiology, and histology. Am J Sports Med. 1994;22:702–710. doi: 10.1177/036354659402200521. [DOI] [PubMed] [Google Scholar]
- Foster W, Li Y, Usas A, Somogyi G, Huard J. Gamma interferon as an antifibrosis agent in skeletal muscle. J Orthop Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Velleman SG. The role of the extracellular matrix in skeletal muscle development. Poult Sci. 1999;78:778–784. doi: 10.1093/ps/78.5.778. [DOI] [PubMed] [Google Scholar]
- Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am J Sports Med. 1999;27:222–229. doi: 10.1177/03635465990270021801. [DOI] [PubMed] [Google Scholar]
- Li Y, Fu FH, Huard J. Cutting-edge muscle recovery: using anti fibrosis agents to improve healing. J Physician and Sports Medicine. 2005;33:44–50. doi: 10.3810/psm.2005.05.91. [DOI] [PubMed] [Google Scholar]
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258:279–287. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- Lewis MP, Tippett HL, Sinanan AC, Morgan MJ, Hunt NP. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J Muscle Res Cell Motil. 2000;21:223–233. doi: 10.1023/a:1005670507906. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugre FJ, Sylvain M, Lachance JG, Deschenes L, Senay H, Tremblay JP. Dystrophin expression in muscles of Duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, Lachance JG, Tremblay JP. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Kikuiri T, Okamoto S, Nonoshita S, Mizunoya W, Ikeuchi Y, Shimokawa H, Sunagawa K, Allen RE. Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve. 2006;34:313–319. doi: 10.1002/mus.20601. [DOI] [PubMed] [Google Scholar]
- Bandow K, Ohnishi T, Tamura M, Semba I, Daikuhara Y. Hepatocyte growth factor/scatter factor stimulates migration of muscle precursors in developing mouse tongue. J Cell Physiol. 2004;201:236–243. doi: 10.1002/jcp.20056. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- Chin JR, Werb Z. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development. 1997;124:1519–1530. doi: 10.1242/dev.124.8.1519. [DOI] [PubMed] [Google Scholar]
- Brauer PR. MMPs—role in cardiovascular development and disease. Front Biosci. 2006;11:447–478. doi: 10.2741/1810. [DOI] [PubMed] [Google Scholar]
- Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- Allen DL, Teitelbaum DH, Kurachi K. Growth factor stimulation of matrix metalloproteinase expression and myoblast migration and invasion in vitro. Am J Physiol. 2003;284:C805–C815. doi: 10.1152/ajpcell.00215.2002. [DOI] [PubMed] [Google Scholar]
- Langlois S, Nyalendo C, Di Tomasso G, Labrecque L, Roghi C, Murphy G, Gingras D, Beliveau R. Membrane-type 1 matrix metalloproteinase stimulates cell migration through epidermal growth factor receptor transactivation. Mol Cancer Res. 2007;5:569–583. doi: 10.1158/1541-7786.MCR-06-0267. [DOI] [PubMed] [Google Scholar]
- Lluri G, Langlois GD, Soloway PD, Jaworski DM. Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates myogenesis and beta1 integrin expression in vitro. Exp Cell Res. 2008;314:11–24. doi: 10.1016/j.yexcr.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol. 2008;52:307–314. doi: 10.1387/ijdb.072331mz. [DOI] [PubMed] [Google Scholar]