Abstract
Microglia are the immune cells of the central nervous system (CNS) that become activated in response to pathological situations such as cerebral ischemia. Tissue-type plasminogen activator (tPA) is a serine proteinase that is found in the intravascular space and the CNS. The low-density lipoprotein receptor-related protein 1 (LRP1) is a member of the low-density lipoprotein receptor gene family found in neurons, astrocytes, and microglia. The present study investigated whether the interaction between tPA and microglial LRP1 plays a role in cerebral ischemia-induced microglial activation. We found that middle cerebral artery occlusion (MCAO) induces microglial activation in both wild-type and plasminogen-deficient (Plg−/−) mice. In contrast, MCAO-induced microglial activation is significantly decreased in tPA-deficient (tPA−/−) mice and in mice that lack LRP1 in microglial cells (macLRP−). We observed a significant increase in microglial activation when tPA−/− mice received treatment with murine tPA after MCAO. In contrast, treatment of macLRP− mice with tPA did not have an effect on the extent of microglial activation. Finally, both the volume of the ischemic lesion as well as inducible nitric oxide synthase production were significantly decreased in macLRP− mice and macLRP− microglia. In summary, our results indicate that the interaction between tPA and LRP1 induces microglial activation with the generation of an inflammatory response in the ischemic brain, suggesting a cytokine-like role for tPA in the CNS.
Microglia are the immune cells of the central nervous system (CNS) initially described by del Rio-Hortega1 as a unique cell type that comprises ∼12% of the brain. Microglia become activated in response to changes in the microenvironment induced by multiple pathological situations such as cerebral ischemia.2 This process is characterized by a number of features including morphological changes, the acquisition of a phagocytic phenotype, and the release of free radicals and nitric oxide.3 The onset of cerebral ischemia induces the activation of microglial cells, which results in the generation of a local inflammatory reaction mediated, including the induction of nuclear factor (NF)-κB-regulated pro-inflammatory molecules such as inducible nitric oxide synthase (iNOS).4,5
In vitro studies have indicated that one pathway leading to microglial activation is initiated by tissue-type plasminogen activator (tPA).6 tPA, which is produced by endothelial cells, astrocytes, microglia, and neurons,7,8,9 is a highly specific serine proteinase and one of the two main plasminogen activators.10 In the intravascular space tPA functions as a thrombolytic enzyme in which its main substrate is plasminogen. Based on these properties, recombinant tPA is the only Food and Drug Administration-approved medication for the treatment of patients with acute ischemic stroke.11 In contrast, in the CNS tPA initiates multiple physiological and pathological processes via plasminogen-independent pathways, including learning,12 synaptic plasticity,7,8,12,13 cell death,14,15,16,17,18 regulation of the permeability of the neurovascular unit,19,20 and microglial activation.6,21
The low-density lipoprotein (LDL) receptor-related protein 1 (LRP1) is a member of the LDL receptor gene family that interacts with multiple ligands including plasminogen activators.22,23 In the CNS, LRP1 is found in perivascular astrocytes, neurons, and microglia19,24 where it has been implicated in cellular signal transduction pathways.25 After middle cerebral artery occlusion (MCAO) there is an increase in endogenous tPA activity within the ischemic tissue,17,18,20 and the association of this tPA with LRP1 has an effect on cerebrovascular tone,26 NF-κB activation,27 Akt phosphorylation,28 and regulation of the permeability of the neurovascular unit.19,20
To gain insight into mechanisms leading to microglial activation during cerebral ischemia in vivo, we have generated a mouse model in which LRP1 has selectively been deleted in macrophages and microglia. Our results reveal that after MCAO, an interaction between tPA and microglial LRP1 is required for microglial activation with induction of iNOS and accumulation of nitrotyrosine. This novel pathway for cerebral ischemia-induced microglial activation during cerebral ischemia represents a potential target for the treatment of patients with acute ischemic stroke.
Materials and Methods
Animal Model of Cerebral Ischemia
Murine strains were wild-type C57BL/6J, tPA-deficient (tPA−/−), and plasminogen-deficient (Plg−/−).29 LRP floxed mice30 on a LDL receptor (LDLR)-deficient background were kindly provided by Dr. Joachim Herz (University of Texas Southwestern, Dallas, TX). These mice were crossed with LysMCre mice as described,31,32 and then backcrossed with wild-type C57BL/6J mice to generate LRPflox+/−Cre+/− mice on a LDLR wild-type background. These mice were crossed with each other to generate LRP flox+/+ Cre+ (designated macLRP−) or LRP floxed+/+ Cre− (designated wild-type), which were used as the littermate controls. Transient occlusion of the middle cerebral artery (tMCAO) was induced with a 6-0 silk suture advanced from the external carotid artery into the internal carotid artery until the origin of the middle cerebral artery as described elsewhere.33 Briefly, after a midline skin incision, the external carotid artery was isolated and ligated proximally with a 6-0 silk suture. A nylon monofilament (6-0; Ethicon, Issy Les Moulineaux, France), coated with a mixture of silicone resin (Xantopren Mucosa; Heraeus Kulzer, Hanau, Grunerweg, Germany) and a hardener (Universal activator; Heraeus Kulzer) was introduced through the incision in the external carotid artery and advanced gently up to the origin of the middle cerebral artery. The suture was tightly fixed at the final position and withdrawn after 60 minutes of cerebral ischemia. Cerebral perfusion in the distribution of the middle cerebral artery was monitored throughout the surgical procedure and after reperfusion with a laser Doppler (Perimed Inc., North Royalton, OH), and only animals with a >70% decrease in cerebral perfusion after occlusion and complete recovery after suture withdrawal were included in this study. The rectal and masseter muscle temperatures were controlled at 37°C with a homoeothermic blanket. Immediately after tMCAO, animals were intracortically injected at bregma, −1 mm; mediolateral, 3 mm; and dorsoventral, 3 mm with 2 μl of either phosphate-buffered saline (PBS) (in wild-type, tPA−/−, Plg−/−, and macLRP− mice) or murine tPA (1 μmol/L, in tPA−/− and macLRP− mice; Molecular Innovations Inc., Royal Oak, MI). A subgroup of tPA−/− mice was intracortically injected with inactive tPA with an alanine for serine substitution at the active site Ser481 (S481A, 1 μmol/L; Molecular Innovations Inc.). Twenty-four hours later the brains of a second subgroup of wild-type and macLRP− mice were harvested and the volumen of the ischemic lesion was measured in brain sections stained with 2,3,5-triphenyltetrazolium chloride as described elsewhere.34 Statistical analysis was performed with the Student’s t-test.
Definition of Areas of Interest (AOI) and Immunohistochemistry
Three AOI were previously defined in the ischemic hemisphere by magnetic resonance imaging parameters as described elsewhere.19,27 In brief, each coronal section of the brain was divided into 16 square areas (150 mm2 each one) that involved the necrotic core and the area of ischemic penumbra, and comparable areas in the nonischemic hemisphere.19,27 AOI-1 and AOI-2 were localized in the area of ischemic penumbra in the fronto- and temporo-parietal lobes, whereas AOI-3 corresponded to the necrotic zone in the parietal lobe. For the immunohistochemistry studies, 20 frozen brain sections 10 μm each, were obtained 24 hours after reperfusion in wild-type, tPA−/−, Plg−/−, and macLRP− mice and co-stained with the nuclear marker 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA), the glial cell marker β-isolectin (Sigma-Aldrich, St. Louis, MO), and antibodies against F4/80 (Serotec, Raleigh, NC). A subset of brains was stained with DAPI and antibodies against Mac-1 and nitrotyrosine (1:200; Cayman Chemical, Ann Arbor, MI). Activated microglia was identified by the simultaneous presence of ameboid morphology and immunoreactivity for β-isolectin and F4/80. Images were digitized in a Zeiss Axioplan 2 microscope (20-fold objective) with a Zeiss AxioCam and imported into AxioVision (Carl Zeiss Microimaging, Thornwood, NY). Images were then viewed at 150% of the original ×20 images with an Image MetaMorph Software (Molecular Devices, Sunnyvale, CA). The number of cells with ameboid morphology and positive for both, β-isolectin and F4/80, was expressed as a percentage of the total number of cells in each field in each AOI. Each observation was repeated 10 times. Statistical analysis was performed with a one-way analysis of variance test.
Cell Cultures and Laser Confocal Microscopy Studies
Microglial cells were cultured from 1-day-old wild-type and macLRP− C57BL/6J mice as described elsewhere.35,36 Briefly, cells were dissociated into a single-cell suspension by tritration through a Pasteur pipette and plated onto either 12-mm glass coverslips or six-well plates coated with 0.05 mg/ml poly-d-lysine and grown in Dulbecco’s modified Eagle’s medium media (Life Technologies, Inc., Grand Island, NY) supplemented with 25 mmol/L glucose, 10% heat-inactivated horse serum, 10% heat-inactivated fetal bovine serum, 2 μmol/L glutamine, 10 U/ml penicillin, and 10 μg/ml streptomycin. At the end of day 12, microglia were microscopically identified floating in the medium of the stationary cultures and centrifuged at 80 × g for 5 minutes to obtain a pellet of nearly pure microglia, which was then plated directly into poly-d-lysine-coated coverslips and stained with DAPI and antibodies against Mac-1 (Serotec, Oxford, UK) and LRP1. As controls, a separate set of coverslips was incubated with an IgG isotype control or with the secondary antibody only. The determination of the co-expression of LRP1 and Mac-1 was performed with a laser confocal microscope (Carl Zeiss Microimaging).
Western Blot Analysis
Polyclonal antibodies to nitrotyrosine were purchased from Cayman Chemical. Polyclonal antibodies to β-actin were obtained from Sigma-Aldrich. Wild-type and macLRP− mice underwent tMCAO and brains were extracted 24 hours later. Tissue was processed and gels were loaded as described.37 A total of three observations were made for each time point.
Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Wild-type and macLRP− mice underwent tMCAO and brains were extracted 24 hours later. A subset of macLRP− mice was injected directly into the ischemic area immediately after MCAO with murine tPA as described above. Wild-type and macLRP− microglial cultures were maintained under oxygen-glucose deprivation conditions for 3 hours as described elsewhere.19 Briefly, cultures were incubated with serum-free media and washed with PBS three times. The culture medium was then replaced by glucose-free Earle’s balanced salt solution previously saturated with 95% N2/5% CO2 at 37°C. Cultures were placed in an anaerobic chamber (Billups-Rothenberg, Inc., Del Mar, CA) equipped with inlet and outlet valves, and equilibrated for 15 minutes with a continuous flux of gas (5% CO2/95% N2). With this setting the concentration of oxygen in the media drops to <1%. The chamber was then sealed and placed in an incubator at 37°C for 3 hours. As a control, a similar group of cells was kept under normoxic conditions. For quantitative measurement of mRNA, 2 μg of DNase I-treated total RNA was used for cDNA synthesis. Reverse transcription was performed with a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA) with random oligonucleotide primers. TaqMan Gene Express assays of TaqMan probes and primers for iNOS (Mm00440485-m1) and LRP1 (Mm00464608-m1) were purchased from Applied Biosystems. Polymerase chain reactions were performed in an ABI Prism 7000 system (Applied Biosystems) under the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute. Each observation was repeated six times. PCR results were analyzed as described elsewhere38 and statistical analysis was performed with the Student’s t-test.
Results
Tissue-Type Plasminogen Activator Mediates Cerebral Ischemia-Induced Microglial Activation via a Plasminogen-Independent Mechanism
To study the role of tPA on cerebral ischemia-induced microglial activation in vivo, we investigated the presence of cells with ameboid morphology positive for both F4/80 and β-isolectin (activated microglial cells) in each AOI in wild-type and tPA−/− mice 24 hours after MCAO. The contralateral, nonischemic hemisphere was used as a negative control (Figure 1A, d, h, j, and p). We observed that compared with wild-type mice (Figure 1A, a–c), genetic deficiency of tPA resulted in a significant decrease in the number of activated microglial cells in the ischemic tissue (Figure 1A, e–g). In contrast, the extent of microglial activation in tPA−/− mice treated with tPA (Figure 1A, i–k) and Plg−/− animals (Figure 1A, m–o) was indistinguishable from that observed in wild-type mice. To quantify these results we counted the number of β-isolectin-positive cells with ameboid morphology and immunoreactive for F4/80 in each AOI in wild-type, tPA−/− and Plg−/− mice. These data reveal that the average percentage of activated microglial cells in each AOI was 45.99 ± 2.43% in wild-type mice, 11.2 ± 3.53% in tPA−/− mice, and 33.32 ± 4.92% in Plg−/− animals. Additionally, when tPA−/− mice were injected with either active or inactive tPA directly into the ischemic area immediately after the onset of the ischemic insult, the average percentage of activated microglial cells in each AOI increased to 36.67 ± 3.80% and 29 ± 2.50%, respectively (Figure 1B; n = 10, P < 0.001 when wild-type mice were compared with sham and untreated tPA−/− animals, and not significant when wild-type mice are compared with either tPA−/− mice treated with tPA or with Plg−/− animals).
Figure 1.
TPA mediates cerebral ischemia-induced microglial activation via a plasminogen-independent mechanism. A: Representative micrographs corresponding to immunohistochemical analysis of microglial activation in the AOI-2 24 hours after transient middle cerebral artery occlusion (tMCAO) in wild-type (a–d), and tPA−/− (e–h) mice, as well as in tPA−/− mice treated with tPA (i–l), and in Plg−/− mice (m–p). c, g, k, and o represent the merged images corresponding to the ipsilateral, ischemic hemisphere, whereas d, h, l, and p are the merged images for the corresponding area in the contralateral, nonischemic hemisphere. Blue is DAPI, red is F4/80, and green is β-isolectin. The insets in a–c correspond to a higher magnification of examples of cells with ameboid morphology and positive for β-isolectin and F4/80. Images were visualized using a Leica DMRBE microscope (Leica, Houston, TX) equipped with a 100×/1.30 numerical aperture (NA) and a LeicaDC500 camera. B: Average percentage of active microglial cells in each AOI 24 hours after tMCAO in wild-type (Wt, white bars), tPA−/− (black bars), and Plg−/− (gray bar) mice. atPA denotes treatment with active tPA whereas itPA denotes treatment with inactive tPA immediately after tMCAO. NT, no treatment. n = 10. Bars indicate SEM. NS, nonsignificant. Original magnifications: ×20 (a–p); ×40 (insets in a–c).
LRP1 Is Required for tPA-Mediated Microglial Activation during Cerebral Ischemia
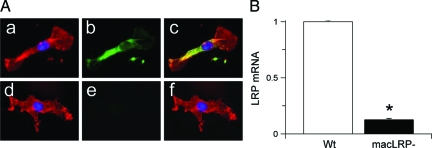
Previous studies confirmed an effective deletion of LRP1 in macrophages by crossing into the LysMCre mice.39 Because microglia and macrophages share the same mesenchymal origin,3,40,41,42,43 we suspected that the macLRP− mice would also have an LRP1 deletion within microglia. To determine this, we performed laser confocal microscopy analysis for Mac-1 and LRP1 (Figure 2A) and quantitative real-time RT-PCR analysis for LRP1 mRNA (Figure 2B) in microglial cultures from wild-type and macLRP− mice. Our results indicate that the macLRP− mice have no detectable LRP1 expression in microglial cells (Figure 2, A and B), confirming effective deletion of LRP1 in these cells.
Figure 2.
Microglial LRP1 is effectively deleted in macLRP− mice. A: Representative micrograph of confocal microscopy analysis of microglial cultures from wild-type (a–c) and macLRP− mice (d–f). Blue is DAPI, red is Mac-1, and green is LRP. c and f correspond to merged images. B: Quantitative RT-PCR analysis in microglial cultures from wild-type (Wt, white bar) and macLRP− (black bar) mice. n = 4. Bars denote SD. *P < 0.001. Original magnifications, ×100.
In earlier studies we demonstrated that the interaction between tPA and LRP1 in the ischemic brain induces an inflammatory response in glial cells.27 Thus, we decided to investigate whether the observed effect of tPA on microglial activation was mediated by LRP1. We performed immunohistochemical analysis for F4/80 and β-isolectin and DAPI in wild-type and macLRP− mice 24 hours after MCAO (Figure 3A, a–f). To determine whether the effect of tPA on microglial activation is mediated by microglial LRP, a subgroup of macLRP− mice was injected with tPA directly into the ischemic area immediately after MCAO (Figure 3A, g–i). We found that the average percentage of activated microglial cells in each AOI was 45.08 ± 7.23% in wild-type mice, 20.33 ± 4.80% in macLRP− mice, and 17.45 ± 2.58% in macLRP− mice treated with tPA (n = 10, P < 0.001 when wild-type mice are compared with either sham or macLRP− mice either left untreated or injected with tPA). In absolute numbers, we found a 54.9% reduction in the percentage of activated microglial cells in macLRP− mice. Interestingly, in contrast to our earlier observation with tPA−/− mice (Figure 1), the percentage of activated microglial cells in macLRP− mice did not increase after the injection of tPA into the ischemic tissue (Figure 3B).
Figure 3.
LRP1 mediates tPA-induced microglial activation after MCAO. A: Representative micrographs corresponding to immunohistochemical analysis for β-isolectin and F4/80 in the AOI-2 24 hours after transient middle cerebral artery in wild-type (a–c) and macLRP− (d–i) mice. g–i correspond to macLRP− mice treated with tPA immediately after MCAO. c, f, and i represent the merged images. Blue is DAPI, red is F4/80, and green is β-isolectin. Images were visualized using a Leica DMRBE microscope equipped with a 100×/1.30 numerical aperture (NA) and a Leica DC500 camera. B: Average percentage of activated microglial cells in each AOI 24 hours after tMCAO in Wt (white bars) and macLRP− (black bars) mice, either left untreated (NT) or intracerebrally injected with tPA immediately after MCAO (+tPA). n = 10. NS, nonsignificant. Original magnifications, ×20.
Volume of the Ischemic Lesion in macLRP− Mice
To study whether microglial LRP1 has an effect on the final outcome of the ischemic insult, we measured the volume of the ischemic lesion in wild-type and macLRP− mice 24 hours after MCAO (Figure 4A). We found that the volume of the ischemic lesion decreased from 78.8 ± 11 mm3 in wild-type mice to 40.56 ± 13 mm3 in macLRP− mice (Figure 4B; n = 10, P < 0.001).
Figure 4.
Effect of microglial LRP1 on the volume of the ischemic lesion. A: Example of 2,3,5-triphenyltetrazolium chloride staining in wild-type (Wt) and macLRP− mice 24 hours after MCAO. The black arrows depict the infracted area. B: Volume of the ischemic lesion 24 hours after tMCAO in Wt and macLRP− mice. n = 10; *P < 0.001. Bars depict SEM.
Microglial LRP1 Increases iNOS mRNA Levels and the Production of NO during Cerebral Ischemia
In response to ischemic insult, there is a sharp increase in the production of iNOS by activated microglial cells.44,45 Therefore, we studied the effect of microglial LRP1 deficiency on hypoxia-induced iNOS expression by performing quantitative real-time RT-PCR analysis for iNOS in wild-type and macLRP− microglial cultures exposed to oxygen-glucose deprivation conditions for 3 hours. Our results indicate that hypoxia induces a 41.2 ± 3.1-fold and 5.8 ± 1.8-fold increase in iNOS expression in wild-type and LRP1-deficient microglia, respectively (Figure 5A, *P < 0.001).
Figure 5.
Role of microglial LRP1 on cerebral ischemia-induced iNOS expression. A and B: Quantitative real-time RT-PCR analysis of iNOS expression in microglial cultures 3 hours after exposure to oxygen-glucose deprivation conditions (A) and brain extracts 24 hours after MCAO (B). NT, no treatment; +tPA, animals treated with tPA immediately after MCAO; Wt, wild-type mice; macLRP−, mice deficient in microglial LRP1. Error bars described SEM. n = 6. *P < 0.05 when compared with either macLRP− microglia (A) or with untreated (NT) tPA mice or untreated (NT) and treated (+tPA) macLRP mice (B).
To study the effect of microglial LRP1 deficiency on cerebral ischemia-induced iNOS expression we performed quantitative real-time RT-PCR analysis for iNOS in the ischemic hemisphere of wild-type, tPA−/−, and macLRP− mice 24 hours after MCAO. Immediately after MCAO, a subgroup of macLRP− and tPA−/− mice was injected directly into the ischemic tissue with tPA. Our data indicate that cerebral ischemia induces a 13.1 ± 1.8-fold increase in the expression of iNOS in wild-type mice, and that this augmentation is significantly decreased in both tPA−/− and macLRP− mice (1.1 ± 0.66-fold and 3.5 ± 0.88-fold increase, respectively; Figure 5B; *P < 0.001 when compared with untreated tPA−/− mice and to treated and untreated macLRP− mice). Importantly, in contrast to macLRP− mice, treatment with tPA induced a significant increase in iNOS expression in tPA−/− mice (11.8 ± 2.5-fold increase in tPA−/− mice and 4.8 ± 0.33-fold in macLRP− mice; Figure 5B).
One of the consequences of increased NO production is the accumulation of nitrotyrosine, which has a toxic effect on the ischemic tissue. Indeed, our earlier work indicates that after MCAO there is accumulation of nitrotyrosine in the ischemic tissue of wild-type mice, and that this effect is significantly attenuated in both tPA−/− mice and in wild-type animals treated with the receptor-associated protein.27 Thus, to investigate the role of microglial LRP1 on peroxynitrate (ONOO−) production and nitrotyrosine accumulation during cerebral ischemia we performed Western blot analysis and immunohistochemical staining for nitrotyrosine in both wild-type and macLRP− mice 24 hours after MCAO. A subgroup of macLRP− mice was treated with tPA immediately after the onset of the ischemic insult. Our results indicate that MCAO induces the accumulation of nitrotyrosine in the ischemic tissue of wild-type mice and that this effect is significantly attenuated in macLRP− mice. Importantly, in contrast to tPA−/− mice,27 treatment of macLRP− mice with tPA immediately after MCAO failed to increase the expression of nitrotyrosine (Figure 6, A and B).
Figure 6.
Role of microglial LRP1 on cerebral ischemia-induced nitrotyrosine accumulation. A: Representative pictures of immunohistochemical analysis for nitrotyrosine formation in the AOI-2 in wild-type (a–c) and macLRP− (d–i) mice. g–i correspond to macLRP− mice treated with tPA immediately after MCAO. c, f, and i are merged images. Blue is DAPI, red is F4/80, and green is nitrotyrosine. B: Western blot analysis of nitrotyrosine formation 24 hours after MCAO in wild-type (Wt) and macLRP− mice either left untreated (NT) or intracerebrally injected with tPA (+tPA) immediately after MCAO. Original magnifications, ×20.
Discussion
LRP1 is a member of the LDL receptor gene family that interacts with multiple ligands including plasminogen activators.22 LRP1 is expressed in multiple sites, and specific deletion of the LRP1 gene from vascular smooth muscle cells on a background of LDL receptor deficiency causes vascular smooth muscle cell proliferation-increased susceptibility to cholesterol-induced atherosclerosis and aneurysm formation.46 In the CNS LRP1 is expressed in neurons, perivascular astrocytes, and microglia. In neurons LRP1 mediates events such as long-term potentiation47 and calcium influx via NMDA receptors.25,48 In perivascular astrocytes the interaction between tPA and LRP1 has been demonstrated to increase the permeability of the blood-brain barrier.19,20,27 In contrast, to this date the role of microglial LRP1 has not been investigated. The data presented here indicate that after MCAO the interaction between tPA and LRP1 on microglial cells induces microglial activation and the generation of an inflammatory response with deleterious effects on the ischemic brain.
TPA is a highly specific serine proteinase and one of the two main plasminogen activators.10 In the intravascular space tPA is primarily a thrombolytic enzyme, and based on this property recombinant tPA is the only Food and Drug Administration-approved medication for the treatment of patients with acute ischemic stroke.11 In contrast, there is a growing body of evidence indicating that tPA also has a deleterious effect in the ischemic brain. Indeed, animal studies demonstrate that after MCAO there is an increase in endogenous tPA activity within the ischemic tissue,17,18,20 and that either genetic deficiency of tPA17,34 or its inhibition with neuroserpin18,49 are associated with neuronal survival, decrease in the volume of the ischemic lesion, and preservation of the barrier function of the blood-brain barrier.19,36
Cerebral ischemia triggers an inflammatory reaction that is mediated by the infiltration of leukocytes and the activation of microglia in the ischemic tissue.44,45,50 The inflammatory response of the ischemic brain is a dynamic process, modulated by a variety of pro-inflammatory genes, including those regulated by activation of the NF-κB pathway.51 A growing body of evidence indicates that the interaction between tPA and LRP1 has a pro-inflammatory effect. Indeed, in vitro studies have demonstrated that binding of tPA to LRP1 increases the synthesis of MMP-9.52,53 Additionally, in earlier studies we demonstrated that tPA induces NF-κB activation in the ischemic brain, with increased expression of iNOS27 and MMP-9.20 The results presented herein indicate that tPA has a direct effect on microglial cell activation and that this process, as previously reported by others, is independent of its proteolytic activity.6
Microglia are resident brain macrophages that are considered the immune cells of the CNS.2 In response to changes in the microenvironment and pathological processes such as cerebral ischemia, microglia become activated. This process is characterized by morphological transformation, expression of myeloid markers, free radicals and iNOS, and by the acquisition of a phagocytic phenotype.3 There is considerable debate about the origin of microglia. However, currently it is accepted that microglia are myeloid lineage-derived cells that invade the CNS in the late prenatal and early postnatal periods.41,42,43 Therefore, microglia and peripheral macrophages share many immunological markers.
Immunoreactivity against F4/80, a 160-kDa glycoprotein expressed by murine macrophages, has been classically used to detect activated microglia. However, this marker is also found in blood-borne macrophages, which infiltrate the ischemic tissue after the onset of the ischemic insult.44,45 In contrast, β-isolectin is a marker of glial cells not found in macrophages. In our study, we counted the number of cells positive for both markers, F4/80 and β-isolectin, which helps to exclude the possibility of counting infiltrating macrophages instead of activated microglia. This point is of utmost relevance because the interaction between tPA and LRP1 also induces macrophage migration,54 which would raise the possibility that the decrease in the number of F4/80 cells in tPA−/− mice is attributable to attenuation of the infiltration of macrophages into the CNS and not to inhibition of microglial activation. Recent data indicate that the resident microglial cell population is supplemented by recruited bone marrow-derived cells, which migrate into the brain parenchyma where they differentiate into microglia.55,56 In our study, although most of the F4/80 cells present in the ischemic tissue were also β-isolectin-positive, a reduced number of F4/80 cells were also β-isolectin-negative, suggesting that the interaction between tPA and LRP1 may also have an effect on the migration and infiltration of peripheral macrophages into the ischemic tissue. The process of microglial activation is also characterized by morphological changes. Indeed, whereas resting microglia are characterized by multiple ramifications, activated microglia have large round bodies, which make these cells morphologically indistinguishable from blood-borne macrophages. In the present study, we counted only those F4/80 and β-isolectin-positive cells with ameboid morphology, therefore making it highly unlikely to confuse activated microglia with blood-borne macrophages.
It has been previously demonstrated that tPA mediates kainic acid-induced microglial activation in the hippocampus.9 However, this is the first report of tPA-induced microglial activation during ischemic conditions. Importantly, our results agree with those reported by others indicating that both active and inactive tPA restore microglial activation in tPA−/− mice.6 However, in contrast to microglial activation induced by the injection of kainic acid into the hippocampus, the effect of tPA on cerebral ischemia-induced microglial activation occurs via a plasminogen-independent mechanism. This discrepancy may reflect the fact that the pathways leading to microglial activation differ according to the nature of the insult. Our data indicate that cerebral ischemia-induced microglial activation is decreased in macLRP− mice. However, in contrast to tPA−/− mice treated with tPA, we did not observe an increase in microglial activation in macLRP− mice treated with tPA. Together, our results indicate not only that, as reported by others, the effect of tPA on microglial activation is independent of its proteolytic activity,6 but also that this effect is mediated by its interaction with microglial LRP1. Parallel to their morphological transformation, activated microglia also synthesize iNOS and pro-inflammatory cytokines.57 In earlier work, we showed that treatment with tPA induces iNOS expression and accumulation of nitrotyrosine in the ischemic brain, and that genetic deficiency of tPA resulted in a significant reduction in cerebral ischemia-induced iNOS expression.27 In the present study, we show that absence of microglial LRP1 inhibits the expression of iNOS in response to the ischemic insult, and that in contrast to our previous observation with tPA−/− mice,27 this effect is not reversed by treatment with tPA. Because activated microglia are the main source of iNOS in the ischemic brain, we then postulated that the interaction between tPA and LRP1 in microglial cells induces a pro-inflammatory response characterized by the induction of iNOS and the subsequent synthesis of nitric oxide and accumulation of nitrotyrosine. This finding has an especially significant clinical relevance because recombinant tPA, used for the treatment of acute stroke patients,11,58 may cross from the intravascular space into the ischemic tissue,59,60 inducing microglial activation and the generation of a pro-inflammatory process. This inflammation may have an impact on cell survival and blood-brain barrier permeability. This hypothesis is supported by recent data demonstrating that treatment of acute stroke patients with tPA increases the permeability of the blood-brain barrier.61
The role of microglial activation during cerebral ischemia has been controversial. Indeed, whereas some reports indicate that activation of microglial cells during cerebral ischemia has a deleterious effect after MCAO,62 others have informed of a neuroprotective role.63,64 This suggests that the net effect of microglial activation on the ischemic brain may depend on the mechanism of activation. Our data demonstrate that tPA-induced LRP1-dependent microglial activation has a deleterious effect on the ischemic brain because the macLRP− mice have a significant decrease in the volume of the ischemic lesion when compared with wild-type mice. It has been postulated that resting microglia play a dynamic surveillance role in the CNS.2,65 However, at this moment we do not know if the interaction between tPA and microglial LRP plays a role in the function of inactive, resting microglia.
In summary, our data identify a novel pathway for microglial activation after the onset of the ischemic insult that involves the binding of tPA to its receptor LRP1. This association results in microglial activation and the induction of iNOS expression and nitrotyrosine accumulation. The mechanism whereby this occurs remains to be fully investigated but likely involves the initiation of cell signaling events. The identification of this pathway may reveal new targets for ischemic stroke therapy.
Footnotes
Address reprint requests to Manuel Yepes, Department of Neurology and Center for Neurodegenerative Disease, Whitehead Biomedical Research Building, 615 Michael St., Suite 505J, Atlanta, GA 30322. E-mail: myepes@emory.edu.
Supported in part by the National Institutes of Health (grants NS49478 to M.Y., HL54710 to D.K.S., and HL50784 to D.K.S.).
C.Z. and J.A. contributed equally to this study.
References
- del Rio-Hortega P. Microglia. Penfield W, editor. New York: Hoeber,; Cytology and Cellular Pathology of the Nervous System. 1932:pp 482–534. [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Siao C, Keyt B, Strickland S, Tsirka SE. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J Cell Sci. 1999;112:4007–4016. doi: 10.1242/jcs.112.22.4007. [DOI] [PubMed] [Google Scholar]
- Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Krystosek A, Seeds NW. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci USA. 1981;78:7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siao CJ, Fernandez SR, Tsirka SE. Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J Neurosci. 2003;23:3234–3242. doi: 10.1523/JNEUROSCI.23-08-03234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003;23:7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka SE, Bugge TH, Degen JL, Strickland S. Neuronal death in the central nervous system demonstrates a non-fibrin substrate for plasmin. Proc Natl Acad Sci USA. 1997;94:9779–9781. doi: 10.1073/pnas.94.18.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf BB, Lopes MB, VandenBerg SR, Gonias SL. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Nassar T, Haj-Yehia A, Akkawi S, Kuo A, Bdeir K, Mazar A, Cines DB, Higazi AA. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem. 2002;277:40499–40504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-{kappa}B pathway activation. Am J Pathol. 2007;171:1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Zhang C, Polavarapu R, Zhang X, Zhang X, Yepes M. Tissue-type plasminogen activator and the low density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood. 2008;112:2787–2794. doi: 10.1182/blood-2008-02-141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J Immunol. 2008;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- Fedoroff S, Richardson A. Cultures of astroglia and microglia from primary cultures of mouse neopallium. Fedoroff S, Richardson A, editors. New Jersey: Humana Press,; Protocols for Neuronal Cell Culture. 2001:pp 139–148. [Google Scholar]
- Yepes M, Moore E, Brown SA, Hanscom HN, Smith EP, Lawrence DA, Winkles JA. Progressive ankylosis (Ank) protein is expressed by neurons and Ank immunohistochemical reactivity is increased by limbic seizures. Lab Invest. 2003;83:1025–1032. doi: 10.1097/01.lab.0000075640.49586.e6. [DOI] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Winkles JA, Yepes M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappaB pathway activation. J Neurosci. 2005;25:10094–10100. doi: 10.1523/JNEUROSCI.3382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J Immunol. 2008;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Tonder N, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the prenatal rat hippocampus. J Comp Neurol. 1997;377:70–84. [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B. Dynamics of microglia in the developing rat brain. J Comp Neurol. 2003;458:144–157. doi: 10.1002/cne.10572. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28:382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli P, Madani R, Tsuzuki N, Vallet P, Arras M, Zhao CN, Osterwalder T, Rulicke T, Sonderegger P. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol Cell Neurosci. 2001;18:443–457. doi: 10.1006/mcne.2001.1028. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Cao C, Lawrence DA, Li Y, von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Ingall TJ, O'Fallon WM, Asplund K, Goldfrank LR, Hertzberg VS, Louis TA, Christianson TJ. Findings from the reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke. 2004;35:2418–2424. doi: 10.1161/01.STR.0000140891.70547.56. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Fernandez-Monreal M, Brillault J, Valable S, Dehouck MP, Cecchelli R, Vivien D, Touzani O, Ali C. Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke. 2005;36:1065–1070. doi: 10.1161/01.STR.0000163050.39122.4f. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, Jahan R, Vinuela F, Kang DW, Warach S. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25:338–343. doi: 10.1159/000118379. [DOI] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]