Abstract
Melanoma is the most aggressive skin cancer once metastasis begins; therefore, it is important to characterize the molecular players involved in melanoma dissemination. The chemokine receptor CXCR4 and the membrane-bound metalloproteinase MT1-MMP are expressed on melanoma cells and represent candidate molecules for the control of metastasis. Using human melanoma transfectants that either overexpress or silence CXCR4 or MT1-MMP, or that have a combination of overexpression and interference of these proteins, we show that CXCR4 and MT1-MMP coordinate their activities at different steps along melanoma cell metastasis into the lungs. Results from in vivo xenograft mouse models of melanoma lung colonization and mice survival and short-term, homing nested polymerase chain reaction experiments from lung samples indicated that CXCR4 is required at early phases of melanoma cell arrival in the lungs. In contrast, MT1-MMP is not needed for these initial steps but promotes subsequent invasion and dissemination of the tumor with CXCR4. Investigation of potential cross talk between CXCR4 and MT1-MMP revealed that MT1-MMP accumulates intracellularly after melanoma cell stimulation with the CXCR4 ligand CXCL12, and that this process involves the activation of the Rac-Erk1/2 pathway. Subsequent to cell contact with specific basement membrane proteins, MT1-MMP redistributes to the cell membrane in a phosphatidylinositol 3-kinase-dependent manner. These results suggest that combination therapies that target CXCR4 and MT1-MMP should improve the limitations of the current therapies for metastatic melanoma.
Trafficking of cancer cells from primary tumor sites via intravasation into blood circulation and later extravasation to colonize distant organs requires tightly regulated directional cues and cell migration and extracellular matrix (ECM) degradation that are mediated by chemokines, growth factors, integrins, and metalloproteinases.1 Solid tumor cells express chemokine receptors that provide guidance to these cells to organs where their ligands are expressed, constituting a homing model resembling the one used by immune cells to exert their immune surveillance functions.2
CXCR4 is a chemokine receptor expressed by tumor cells in melanoma, breast, prostate, and colon cancer.3,4,5,6 Its ligand CXCL12 (also called SDF-1) is expressed in lymph nodes, lungs, bone marrow, and liver.3 The importance of the CXCR4/CXCL12 axis in cancer is exemplified by the fact that blocking CXCR4 function leads to inhibition of metastasis in in vivo mouse models of breast carcinoma and pancreatic cancer.3,7,8
Melanoma incidence has been steadily growing in western populations. Although melanoma only accounts for less than 5% of skin cancers, current therapies are primarily refractory for metastatic melanoma. Therefore, melanoma is responsible for 80% of deaths from skin cancers.9 Expression of CXCR4 in human melanoma has been detected in the vertical growth phase and on regional lymph nodes and correlated with poor prognosis and increased mortality.10,11 We previously demonstrated that CXCL12 stimulates in vitro melanoma cell invasion involving Vav-Rho GTPase activation, as well as activation of the metalloproteinase MT1-MMP/MMP-2 ECM-degrading system.12,13
MT1-MMP is a key component of the pericellular proteolysis machinery involved in degradation of gelatin, laminin, and fibrillar collagens and is an activator of pro-MMP-2 in coordination with TIMP-2.14,15 Accordingly, its cell membrane expression must be tightly controlled to avoid excessive ECM pericellular degradation. Furthermore, MT1-MMP proteolytic activity controls cell adhesion and growth.14,15 MT1-MMP is expressed on melanoma, and breast and lung cancer, and its expression often correlates with tumor invasiveness across tissue barriers.16,17,18,19 MT1-MMP and MMP-2 are found in malignant melanoma often associated to the invading tumor front,20,21 suggesting that their proteolytic activity could be involved in melanoma cell dissemination.
Whereas the above data support an important role for CXCR4 and MT1-MMP in melanoma metastasis, the potential functional relationships and mechanistic coordination of these molecules in lung metastasis, as well as their roles at different steps of melanoma cell homing into lungs, have not been evaluated. In the present study we have generated stable transfectants of the highly metastatic human melanoma cell line BLM expressing combinations of overexpression and silencing of CXCR4 and MT1-MMP to investigate whether these proteins establish coordinated activities during in vivo melanoma metastasis. The results reveal that CXCR4 and MT1-MMP need each other’s activities during melanoma metastasis into lungs and provide mechanistic characterization of molecular cross-talks between these proteins. The data suggest that combinatorial therapies against these proteins might provide beneficial advantages to inhibit melanoma metastasis.
Materials and Methods
Cells, Antibodies, and Reagents
BLM human melanoma cells were cultured as previously described.4 Anti-CXCR4 antibodies were from R&D Systems (Minneapolis, MN), anti-MT1-MMP LEM-2/15 mAb was a gift from Dr. Alicia G. Arroyo (Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain), and control P3X63 and antibodies to β1 and α4 integrins (Lia 1/2.1 and HP1/2, respectively) were from Dr. Francisco Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain). Anti-Rac1 antibodies were from BD Biosciences (San Diego, CA), anti-RhoA from Santa Cruz Biotechnology (Santa Cruz, CA), anti-β-actin from Sigma-Aldrich (St. Louis, MO), anti-GFP from Molecular Probes (Eugene, OR), and anti-Erk1/2, anti-phospho-Erk1/2, anti-Akt, and anti-phospho-Akt (Ser473) antibodies were from Cell Signaling Technology (Danvers, MA). Type IV collagen and LY294002 were from Sigma-Aldrich Co., and U0126 from Calbiochem (Darmstadt, Germany). We used a laminin 1-rich extract from Sigma-Aldrich (catalog no. L2020) as source for laminin. Transforming growth factor-β1 and epidermal growth factor were obtained from R&D Systems.
Vectors, siRNA, Transfections, and Retroviral Gene Transfer
The pEGFP-C1 vector or GFP-fused forms of wild-type or activated V12-Rac1 were gifts from Dr. Francisco Sanchez-Madrid and were transfected into BLM cells following the described method.13 siRNA for Rac1 has been earlier reported22 and was transfected into BLM cells according to the published procedure.13 CXCR4 and MT1-MMP shRNA vectors were based on 19-mer sequences (5′-GCAGTCCATGTCATCTACA-3′, for CXCR4; 5′-TTGGCAGCCTCTCACTACT-3′, for MT1-MMP), which constitute targets for siRNA-mediated interference.13 Synthetic oligonucleotides (64-mer) that include these 19-mer sequences were synthesized, annealed, and ligated into pSuper vector as described.23 pSuper cassettes coding CXCR4 or MT1-MMP shRNAs were cloned into pRETRO-Hygro vector,24 generating pRETRO-Super-CXCR4 and pRETRO-Super-MT1-MMP. cDNAs for CXCR4 and MT1-MMP (from Dr. José Miguel Rodríguez-Frade, Centro Nacional de Biotecnología, Madrid, Spain, and Dr. Alicia G. Arroyo, respectively) were cloned into pRetro-Blast retroviral vector24 for overexpression of CXCR4 and MT1-MMP (pRetro-CXCR4 and pRetro-MT1-MMP, respectively). Retroviral particles were obtained by co-transfecting these vectors with plasmids coding for retroviral proteins gag.pol (pNGVL-MLV-gag-pol)25 and the vesicular stomatitis virus envelope glycoprotein (pNGVL-VSV-G),26 into 293FT packaging cells using lipofectamine (Invitrogen Corp., Carlsbad, CA). Conditioned media containing viral particles were used to infect BLM cells, which were selected with blasticidin (Invitrogen Corp.) or hygromicin (Calbiochem), obtaining sublines with interference (CXCR4lo and MT1lo), overexpression (CXCR4hi and MT1hi), or simultaneous overexpression and interference of CXCR4 and MT1-MMP (CXCR4hiMT1lo and CXCR4loMT1hi). As a control, we infected BLM cells with empty pRETRO-containing viral particles to generate the mock subline.
Flow Cytometry, Western Blotting, and Zymography
Melanoma cells were incubated with primary antibodies, followed by incubation with fluorescein isothiocyanate-conjugated secondary antibodies (DAKO A/S, Copenhagen, Denmark) and analysis in an Epics XL cytofluorometer (Beckman Coulter, Fullerton, CA). P3X63 was used as control primary antibody. For Western blotting, cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and proteins transferred to membranes that were incubated with primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL). For zymography we used the described method.13 Briefly, cells were lysed in Laemmli buffer, and samples resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels embedded with 1 mg/ml of fibrinogen (Calbiochem-Novabiochem Co., Darmstadt, Germany). Gels were rinsed in 2.5% Triton X-100, followed by incubation in 50 mmol/L Tris-HCl, pH 7.5, 10 mmol/L CaCl2, and 200 mmol/L NaCl. Gels were stained with Coomassie Blue, and areas of gelatinolytic activity were visualized as transparent bands.
Proliferation and Cell Cycle Assays
Cells (1.2 × 105) were cultured in Dulbecco’s modified Eagle’s medium/fetal bovine serum 1% with or without CXCL12 (R&D Systems) (200 ng/ml), and after detachment, cells were counted in a Neubauer chamber. For cell cycle assays, cells were pulsed with 5′-bromodeoxyuridine (BrdU) (Sigma-Aldrich Co) (15 μmol/L for 1 hour) and cultured for different times. Thereafter, cells were detached, fixed with 70% ethanol, permeabilized with 0.5% Triton X-100 in 2 mol/L HCl, and washed with 0.1 mol/L sodium tetraborate. Finally, cells were incubated with fluorescein isothiocyanate-conjugated anti-BrdU antibodies (BD Biosciences), followed by analysis in a cytofluorometer. Cell cycle time was estimated as the interval necessary to have a ratio of BrdU-labeled cells in S phase to BrdU-labeled cells in G2 phase equal as in 0 hours time.
Invasion and Adhesion Assays
Invasions across Matrigel were done as previously established.12 Briefly, cells (3.5 × 104) were loaded on the upper compartments of invasion chambers coated with Matrigel (BD Biosciences, Bedford, MA). The lower compartments were filled with invasion medium with or without CXCL12. Invasive cells were fixed, stained, and counted under a microscope. For adhesion to Matrigel, cells (6 × 105) were labeled for 20 minutes at 37°C with 2′,7′-bis(carboxyethyl)-5(6′)-carboxyfluorescein-acetoxymethyl ester (Molecular Probes), and after pre-incubation with antibodies, cells in triplicates (3 × 104) were loaded on 96-well dishes (Costar, Cambridge, MA) coated with Matrigel (1 μg/ml). Cells were allowed to adhere for 10 minutes at 37°C and, on washing to remove unbound cells, adhesion was quantified using a fluorescence analyzer (Polarstar Galaxy, Offenburg, Germany).
Animal Studies
BALB/c SCID mice (Harlan, Indianapolis, IN) bred and maintained under specific pathogen-free conditions were used for xenograft studies. The Consejo Superior de Investigaciones Cientificas Ethics Committee (Madrid, Spain) approved the protocols used for experiments with mice. Mice were injected subcutaneously in the lateral thoracic wall or intravenously into the tail vein with 2 × 106 cells resuspended in 0.2 ml of phosphate-buffered saline (PBS). Mice were daily inspected for local tumor growth and general condition, and were sacrificed when clear signs of respiratory stress were noted, or when subcutaneous tumors reached volumes of 2.5 cm3. For nested reverse transcriptase-polymerase chain reaction (RT-PCR), portions of lungs from mice inoculated with GFP-expressing melanoma transfectants were lysed in Tri-Reagent (Sigma-Aldrich Co.), and RNA was extracted and reverse-transcribed. PCR was performed using TaqDNA polymerase (Invitrogen Corp.) and outer primers for GFP: 5′-GACTGGGTGCTCAGGTAGTG-3′ and 5′-GTAAACGGCCACAAGTTCAG-3′. A 1-μl PCR product was further amplified using inner primers for GFP: 5′-TCGTGACCACCCTGACCTAC-3′ and 5′-TCACCTTGATGCCGTTCTTC-3′. The first PCR profile was 35 cycles of 45-second denaturation at 94°C, 45-second annealing at 52°C, and 1-minute polymerization at 72°C. The second PCR was performed in the same way, except that only 30 cycles were performed and annealing was executed at 56°C. For positron emission tomography (PET) analyses, mice were anesthetized with isoflurane, followed by administration of [18F]-fluorodeoxyglucose (624 ± 49 μCi) via the tail vein before analyses. Mice were scanned in a dedicated small animal PET scanner (rPET; Suinsa, Madrid, Spain), and tomographic images reconstructed using a three-dimensional FBP algorithm creating 55 55 × 55 images. Voxel size was 0.81 × 0.81 × 0.81 mm3, and the spatial resolution was 1.65 mm full width at half maximum isotropic. The energy window was 400 to 700 keV, and decay and dead-time corrections were applied. For computed tomography (CT) studies, mice were scanned using a CT scanner (HQT-Y15D, Suinsa) for small animals. CT acquisition was performed as follows: one bed position, 200 μA, 50 kV, 200 μm pixel size, and 360 projections. PET and CT images were co-registered using a marker-based rigid registration algorithm to obtain an initial realignment followed by a refinement step based on a mutual information registration algorithm.
Statistical Analyses
Data were analyzed by one-way analysis of variance followed by Tukey-Kramer multiple comparisons. In both analyses, the minimum acceptable level of significance was P < 0.05.
Results
In Vivo Melanoma Metastasis Requires CXCR4 and MT1-MMP Coordinated Activities
To investigate if functional coordination between CXCR4 and MT1-MMP is needed during melanoma cell metastasis, we made BLM melanoma stable transfectants with combined overexpression and silencing of these proteins to be subsequently tested in in vivo models of metastasis. To generate these transfectants, we used pRetro-Super and pRetro retroviral vectors as templates for stably silencing or for overexpression of CXCR4 and MT1-MMP, respectively (see Materials and Methods). After antibiotic selection, total transfectant populations were used for all experiments without subsequent cloning. Flow cytometry and Western blotting analyses of single CXCR4 or MT1-MMP transfectants revealed that CXCR4lo and MT1lo cells displayed minimal levels of CXCR4 and MT1-MMP expression, whereas CXCR4hi and MT1hi transfectants showed threefold and twofold higher expression of these proteins than mock counterparts, respectively (Figure 1, A and B). Accordingly, CXCR4hiMT1lo and CXCR4loMT1hi double transfectants displayed overexpression and silencing of CXCR4 or MT1-MMP.
Figure 1.
In vitro functional characterization of human melanoma transfectants with stable interference and overexpression of CXCR4 and MT1-MMP. A: Flow cytometry analyses of CXCR4 and MT1-MMP expression on BLM melanoma transfectants. Note the 3-decade log scale starting at 0 and ending at 103. B: Western blotting analyses of CXCR4 and MT1-MMP expression on BLM melanoma transfectants. Loading controls were assessed with anti-RhoA antibodies. Shown is a representative result of three independent experiments (top) and densitometer analyses in arbitrary units showing fold induction of expression related to mock transfectants (bottom). C: Transfectants were subjected to Matrigel invasion assays toward CXCL12 or medium alone. Invasion was significantly up-regulated, *P < 0.05, or significantly inhibited, ΔP < 0.05, with respect to mock transfectant invasion. D: Transfectants (1.2 × 105) were incubated in Dulbecco’s modified Eagle’s medium/1% fetal bovine serum, without (medium) or with CXCL12, and cell number was determined after the indicated times.
To in vitro characterize the invasive properties of transfectants, we subjected them to invasion assays across Matrigel, a basement membrane matrix extract rich in laminin, type IV collagen, heparan sulfate proteoglycans, and growth factors, which constitutes a valuable in vitro basis for assessing the invasive potential of tumor cells.27 CXCR4hi and MT1hi cells showed increased CXCL12-promoted invasion compared with mock counterparts, whereas CXCR4lo and MT1lo knockdown cells barely invaded (Figure 1C). Interestingly, CXCR4hiMT1lo and CXCR4loMT1hi transfectants displayed invasion levels similar to single knockdown CXCR4lo and MT1lo cells, suggesting that CXCR4 and MT1-MMP are mutually required for invasion of melanoma cells. In addition, CXCR4hi and MT1hi transfectants displayed higher degree of migration in wound-healing assays on Matrigel layers in the presence of CXCL12 than CXCR4lo and MT1lo counterparts (see Supplemental Figure S1A at http://ajp.amjpathol.org), together indicating that in vitro invasive and migratory properties of melanoma transfectants reflect CXCR4 and MT1-MMP expression levels.
CXCR4hi transfectants displayed a modest growth advantage in medium containing CXCL12, compared with CXCR4lo cells (Figure 1D). Cell cycle analyses with BrdU-pulsed cells showed that high CXCR4 expression was associated with shorter doubling times (22 hours) than CXCR4 knockdown cells (26 hours), or mock transfectants (24 hours) (Supplemental Figure S1B at http://ajp.amjpathol.org). Instead, changes in MT1-MMP expression did not influence cell cycle kinetics.
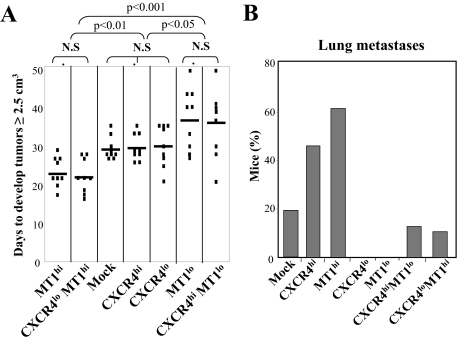
To investigate if functional mutual requirement between CXCR4 and MT1-MMP observed in in vitro invasion also takes place during in vivo metastasis, we first subcutaneously injected the melanoma transfectants into SCID mice and followed the growth and dissemination of the tumors. MT1hi and CXCR4loMT1hi tumors grew significantly faster than MT1lo or CXCR4hiMT1lo ones (Figure 2A). Instead, silencing or overexpression of CXCR4 did not affect the growth rate of melanoma cells, suggesting that MT1-MMP, but not CXCR4, influences the growth of subcutaneous melanoma tumors, in agreement with the reported MT1-MMP role in cell proliferation.19 None of the mice injected with CXCR4lo or MT1lo transfectants had invasive tumors. These tumors remained under the skin without crossing into thoracic or abdominal cavities (not shown), and no lung metastases were detected in these mice (Figure 2B). In contrast, subcutaneously-injected CXCR4hi and MT1hi melanoma cells reached the lungs and formed metastatic nodes (one to two tumor nodes: 45% and 60% of mice, respectively). Importantly, metastasis was impaired in CXCR4hiMT1lo and CXCR4loMT1hi tumors (Figure 2B), indicating that silencing CXCR4 or MT1-MMP reversed the invasive stimulation properties provided by overexpression of the counterpart protein. The pattern of CXCR4 and MT1-MMP expression and CXCL12-promoted invasiveness across Matrigel of melanoma cells derived from subcutaneous tumors or from lung metastases was maintained with respect to the originally inoculated transfectants (see Supplemental Figure S2, A and B, at http://ajp.amjpathol.org), confirming the functional characteristics of both proteins along the metastatic process.
Figure 2.
Growth and lung metastasis of subcutaneously-inoculated CXCR4 and MT1-MMP melanoma transfectants. Mice (n = 9) were inoculated into the lateral thoracic wall with the indicated melanoma transfectants. Plotted are the days when tumors reached 2.5 cm3, together with statistical significance (N.S., nonsignificant) (A), and percentage of mice displaying lung metastases (B). Shown is a representative result from three independent experiments.
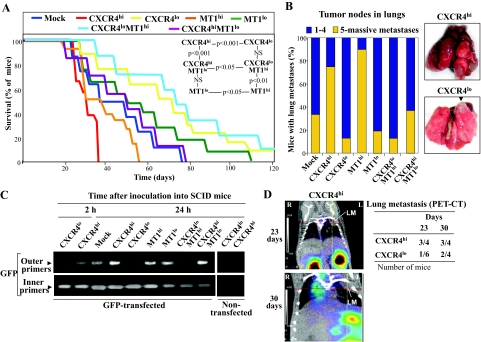
When melanoma transfectants were intravenously inoculated, we found that CXCR4lo and MT1lo mice displayed significantly longer survival than CXCR4hi and MT1hi ones, respectively (Figure 3A). Lung examinations revealed that 70 to 90% of CXCR4hi and MT1hi mice had from five tumor nodes to widespread tumor dissemination (Figure 3B). All CXCR4lo and MT1lo mice eventually developed lung metastasis, but only 10% had five or more tumor nodes, indicating that high CXCR4 expression on melanoma cells conferred strong lung invasiveness. Notably, CXCR4hiMT1lo and CXCR4loMT1hi mice showed a substantially prolonged survival compared with CXCR4hi and MT1hi counterparts, and high tumor node number or massive lung metastases were only observed in 15 to 35% of mice inoculated with the double transfectants (Figure 3B). Control experiments confirmed the expression of human GAPDH mRNA in addition to CXCR4 and MT1-MMP in the lung tumor samples (see Supplemental Figure S3 at http://ajp.amjpathol.org). These results indicate that CXCR4 and MT1-MMP on melanoma establish crucial coordinated activities for efficient lung colonization.
Figure 3.
CXCR4 and MT1-MMP are mutually required for in vivo melanoma metastasis. A: Survival curves of mice (n = 10) inoculated into the tail vein with the indicated transfectants, and statistics from the different curves (N.S., nonsignificant). B: Degree of lung colonization by melanoma transfectants (left), and representative lung metastases from CXCR4hi (34 days, massive metastasis) and CXCR4lo (70 days) mice. Arrow indicates a metastatic tumor node. C: Melanoma transfectants were transiently transfected with pEGFP-C1 vector, and subsequently intravenously inoculated into SCID mice. After the indicated times, GFP expression in lungs was determined by nested PCR. Negative controls with nontransfected cells are also shown. D, left: SCID mice were inoculated with CXCR4hi or CXCR4lo melanoma transfectants and subjected to PET-CT analyses. Co-registered PET and CT studies were superimposed. Coronal sections from a CXCR4hi mouse at days 23 and 30 are shown. Intersections between lines correspond to the center of lung metastases (LM) (R, right; L: left). D, right: Data indicate number of mice with lung metastases, mostly one to two tumor nodes.
The Role of CXCR4 and MT1-MMP in Early Events of Melanoma Cell Homing into Lungs
To investigate CXCR4 and MT1-MMP involvement at initial migratory events during melanoma cell homing into lungs, we first performed nested PCR using lung tissue from mice intravenously inoculated with melanoma transfectants that had been transiently transfected with pEGFP-C1 as a reporter. Before PCR analyses, we verified by flow cytometry that all transfectants expressed similar GFP levels (not shown). A GFP transcript in the lungs of CXCR4hi mice was consistently detected in the first round of nested PCR (outer primers) at 2 hours after inoculation, which further increased on 24 hours of inoculation, whereas no GFP transcript was seen in CXCR4lo or Mock mice at these times (Figure 3C). However, a GFP product using the inners primers was evident at 2 hours and 24 hours in lungs from CXCR4lo and Mock mice, in addition of CXCR4hi counterparts. In contrast with CXCR4, we did not observe significant differences in nested PCR products from MT1hi and MT1lo mice after 24 hours of inoculation. Moreover, GFP transcript levels in lungs from CXCR4hiMT1lo mice using the outer primers were similar to those of CXCR4hi counterparts, and CXCR4loMT1hi mice displayed only traces of GFP products in the first round of nested PCR. Together, these data indicate that high CXCR4 expression provides melanoma cells with greater homing efficiency during early steps of lung colonization than CXCR4 knockdown cells, and suggest that MT1-MMP does not play relevant roles at these initial phases. Furthermore, we found that the earlier accumulation of CXCR4hi melanoma cells in lungs led to a subsequent faster development of metastases in this organ because a higher frequency of CXCR4hi mice displayed tumors nodes (one to two) that were detectable by PET-CT imaging sooner than CXCR4lo mice (Figure 3D).
Firm attachment to endothelium is a required step for posterior tumor cell invasion to form additional metastatic sites.1 Immunohistochemistry analyses using antibodies against HMW-MAA (also known as melanoma chondroitin sulfate proteoglycan) revealed large clusters of melanoma cells around blood vessels in lungs from CXCR4hi mice (see Supplemental Figure S4A at http://ajp.amjpathol. org). In agreement with earlier results,28 we found that high CXCR4 expression provided melanoma cells with advantage to up-regulate their α4β1-dependent attachment to VCAM-1 through CXCL12-promoted inside-out signaling. Thus, in vitro adhesion assays under flow conditions to VCAM-1 co-immobilized with CXCL12 revealed that CXCR4hi cells had a fourfold higher resistance to detachment than CXCR4lo or mock transfectants at shear stresses up to 5 dyne/cm2 (see Supplemental Figure S4B at http://ajp.amjpathol.org). Control experiments indicated that pertussis toxin and anti-α4 mAb abolished attachment to VCAM-1 (not shown).
Erk1/2 MAP Kinase Regulates MT1-MMP Expression, Whereas Phosphatidylinositol 3-Kinase Controls MT1-MMP Cell Surface Redistribution
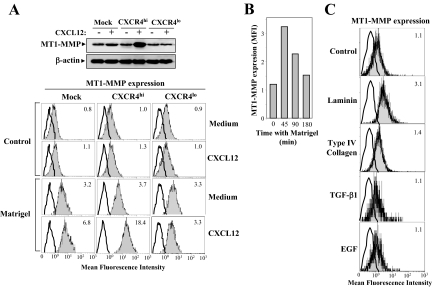
We previously showed that CXCL12 stimulates MT1-MMP expression in melanoma cells at mRNA and protein levels.12 However, enhancement in total cellular MT1-MMP protein levels was not translated into increased cell membrane protein expression on Mock and CXCR4hi transfectants (Figure 4A, top and bottom), indicating a predominant intracellular localization of this metalloproteinase on CXCL12 stimulation. As expected, no induction of MT1-MMP was detected in CXCR4lo transfectants. To address whether intracellular MT1-MMP might redistribute to the melanoma cell membrane after interaction with basement membranes and endothelial ligands, we analyzed its surface expression on CXCL12-stimulated cells plated on Matrigel or other substrates. Notably, nonstimulated transfectants displayed a significant increase in cell membrane MT1-MMP expression on plating them on Matrigel for 45 minutes, compared with cells incubated in invasion medium alone, and the increase was of higher magnitude on CXCL12-stimulated CXCR4hi and mock transfectants (Figure 4A, bottom). Correlating with CXCR4 silencing, no further up-regulation of cell membrane MT1-MMP levels was observed in CXCR4lo transfectants pre-incubated with the chemokine. Kinetic studies indicated that Matrigel-triggered increase in surface MT1-MMP expression had a maximum at ∼45 minutes, and then gradually decreased with longer incubations (Figure 4B). Because laminin and type IV collagen are main components of Matrigel, we tested their possible role in MT1-MMP redistribution to the melanoma cell membrane. We found that incubation on laminin led to an increase in cell surface MT1-MMP to levels similar to those achieved with Matrigel, whereas plating on type IV collagen did not induce such an effect (Figure 4C). In addition, incubation with transforming growth factor-β1 or epidermal growth factor, which are also Matrigel constituents, or with VCAM-1, fibronectin, or gelatin, did not influence melanoma cell MT1-MMP membrane levels (Figure 4C; and see Supplemental Table S1 at http://ajp.amjpathol.org). Control experiments indicated that 45-minute incubation with Matrigel, VCAM-1, fibronectin, or gelatin did not alter cell surface expression of CXCR4.
Figure 4.
MT1-MMP accumulates intracellularly on CXCL12 stimulation and redistributes to the cell surface after melanoma cell incubation with basement membrane proteins. A: Melanoma transfectants were incubated for 24 hours with CXCL12 and subjected to immunoblotting with anti-MT1-MMP mAb. Control loading was assessed with β-actin antibodies (top). Then cells were plated for 45 minutes on Matrigel (1.5 μg/mm2) or incubated in invasion medium alone (control), and after detachment with PBS/EDTA they were analyzed for MT1-MMP cell surface expression by flow cytometry (bottom). Mock transfectants were plated for the indicated times either on Matrigel (B), on the indicated ECM proteins, or incubated with transforming growth factor-β1 or epidermal growth factor (C), and subsequently analyzed by flow cytometry for MT1-MMP cell surface expression. Note the 3-decade log scale starting at 0 and ending at 103.
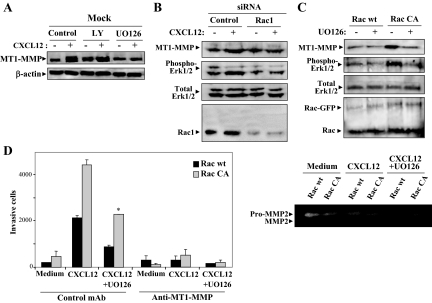
Stimulation by CXCL12 of total MT1-MMP expression involved Erk1/2 MAP kinase activity because treatment with the MEK inhibitor UO126 led to inhibition of increase in the metalloproteinase expression, whereas the phosphatidylinositol 3-kinase (PI3-K) inhibitor LY294002 (LY) did not affect the enhanced expression (Figure 5A), as also earlier reported.13 Because activation of Vav1-Rac1 signaling by CXCL12 controls up-regulation of MT1-MMP expression in melanoma,13 we next tested whether Erk1/2 was a required Rac1 downstream target to increase this expression. When BLM melanoma cells were transfected with Rac1 siRNA, CXCL12-promoted enhancement in MT1-MMP expression was abrogated, which was associated with blockade in the stimulation of Erk1/2 phosphorylation (Figure 5B). Moreover, expression of a constitutively active Rac1 form (Rac CA) triggered both an increase in MT1-MMP expression and in Erk1/2 phosphorylation, compared with Rac wild-type transfectants that were inhibited by UO126 (Figure 5C). Notably, CXCL12-stimulated Rac CA transfectant invasion was inhibited by UO126 and blocked by anti-MT1-MMP mAb (Figure 5D, left). In addition, zymography assays showed that supernatants from Rac CA transfectant invasions contained higher amounts of active MMP-2 than in Rac wild-type counterparts, whereas the presence of UO126 in the invasions resulted in inhibition of pro-MMP-2 activation (Figure 5D, right). No MMP-9 was detected in the invasion supernatants under our assay conditions (not shown). These results indicate that activation of Rac1-Erk1/2 pathway contributes to up-regulation of MT1-MMP expression and function, as revealed by MMP-2 activation and enhanced invasion.
Figure 5.
Rac-Erk1/2 signaling controls up-regulation by CXCL12 of MT1-MMP expression. A: Cells were incubated for 24 hours with CXCL12 in the absence (control) or presence of LY (20 μmol/L) or UO126 (5 μmol/L), and tested with Western blot analyses for MT1-MMP expression. BLM melanoma cells were transfected with control or Rac1 siRNA (B), or with GFP-fused wild-type (wt) or constitutively activated (CA) forms of Rac (C). Transfectants were subsequently incubated for 24 hours with CXCL12 and subjected to Western blotting for expression of MT1-MMP, phospho-Erk1/2, total Erk1/2, and Rac1. D: Transfectants expressing Rac wt or Rac CA were subjected to Matrigel invasion assays toward CXCL12, in the absence or presence of UO126 and control or anti-MT1-MMP mAb (left). Supernatants from these invasions were tested by gelatinolytic zymography for MMP-2 activity (right).
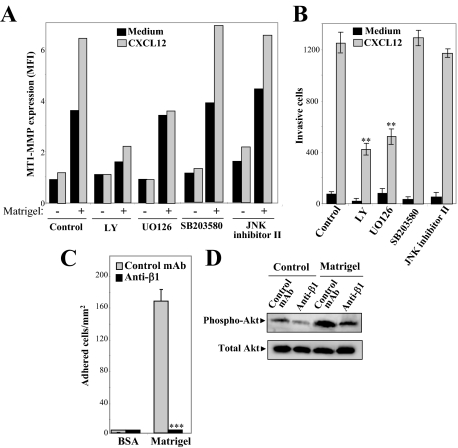
Interestingly, when nonstimulated or CXCL12-activated transfectants were treated with LY for 2.5 hours previous to the 45-minute period of incubation on Matrigel, induction of MT1-MMP cell membrane expression was largely reduced (Figure 6A). In contrast, no such inhibition was seen in cells treated with UO126, and because this inhibitor blocks up-regulation by CXCL12 of total MT1-MMP expression, surface levels of this metalloproteinase on chemokine-incubated cells exposed to Matrigel were similar to unstimulated counterparts. When we examined invasiveness, we found that LY and U0126 partially inhibited BLM cell invasion to CXCL12, whereas the p38 and JNK MAP kinase inhibitors SB203580 and JNK inhibitor II, did not affect invasion or MT1-MMP redistribution to the cell surface (Figure 6, A and B). Together, these results suggest that CXCL12 stimulates intracellular MT1-MMP accumulation through an Erk1/2 MAP kinase-dependent pathway, and that subsequently this metalloproteinase redistributes to the cell membrane after exposure to laminin-containing basement membranes in a PI3-K-dependent manner, a process that contributes to cell invasion.
Figure 6.
Role of phosphatidylinositol 3-kinase and Erk1/2 on melanoma cell surface expression of MT1-MMP and cell invasion. A: Mock cells were incubated for 24 hours with or without CXCL12, in the absence or presence of UO126, SB203580 (13 μmol/L), or JNK inhibitor II (30 μmol/L), whereas LY was added for the last 2.5 hours of this incubation. Subsequently, cells were exposed to Matrigel or medium alone, and subjected to flow cytometry to determine MT1-MMP expression. A representative result from three independent experiments is shown. B: BLM cells were subjected to Matrigel invasion assays in the presence of the indicated inhibitors. Invasion was significantly inhibited, **P < 0.01 (n = 3). C: Mock cells were subjected to adhesion assays to Matrigel, in the presence of blocking anti-β1 mAb Lia1/2.1 or control nonblocking anti-α5 mAb. Basal adhesion to BSA is also shown. Adhesion was significantly inhibited, ***P < 0.001. D: Mock cells were incubated for 30 minutes with or without Matrigel, in the presence of anti-β1 or control anti-α5 mAb, and subsequently collected and tested by immunoblotting with antibodies against phospho-Akt or Akt.
Because laminin-1 is a ligand for β1 integrins and β1 integrin-mediated cell adhesion induces PI3-K activation,29 we tested whether melanoma cell incubation on Matrigel triggered β1-mediated PI3-K activation, which might represent a mechanism accounting for MT1-MMP cell membrane expression. Anti-β1 integrin antibodies blocked melanoma cell attachment to Matrigel, and inhibited Matrigel-dependent activation of Akt, a downstream PI3-K effector (Figure 6, C and D), suggesting that this mechanism could indeed mediate the redistribution of MT1-MMP to the cell membrane.
Discussion
The distinctive homing of tumor cells to organs and tissues during metastasis is contributed by specific attracting molecules, which recruit and promote migration of circulating tumor cells, and by tumor proteolytic enzymes that remodel pericellular ECM allowing cell invasion across tissue barriers to establish new metastatic sites.1 Chemokines and metalloproteinases have been proposed to mediate these processes, leading to tumor cell dissemination into target organs.2,30 Studies addressing the individual roles of the chemokine receptor CXCR4 and the metalloproteinase MT1-MMP during in vivo metastasis of melanoma have shown that their overexpression on the murine B16 melanoma model leads to lung metastasis.28,31 However, characterization of CXCR4 and MT1-MMP involvement at different steps of melanoma cell homing into lungs, whether they are mutually required during metastasis, as well as whether they establish mechanistic relationships, has not yet been investigated. By generating in the same human melanoma cell line transfectants either silenced for CXCR4 or MT1-MMP expression, overexpressing these proteins, or having a combination of their silencing and overexpression, we demonstrate here using xenograft models that CXCR4 strongly contributes to early migratory steps in melanoma cell homing to lungs. Instead, MT1-MMP is dispensable for the initial phases, but together with CXCR4, it promotes subsequent invasiveness for efficient lung dissemination, indicating that these proteins are mutually required during different steps of melanoma cell metastasis.
In vitro transfectant characterization already revealed that invasive cell responses involving CXCR4 and MT1-MMP individually were not sufficient to promote invasion, but rather their coordinated activities were necessary. Subsequent in vivo approaches demonstrated that CXCR4 expression provides melanoma cells with metastatic advantage over CXCR4 knockdown cells during early steps of homing into lungs. This conclusion is based on data from nested PCR experiments that revealed that CXCR4hi melanoma cells accumulated faster in the lungs in the first hours after intravenous inoculation than CXCR4lo counterparts. In addition, adhesion assays showed that CXCR4hi cells developed stronger attachment under shear stress on endothelial VCAM-1 co-immobilized with CXCL12 compared with CXCR4lo counterparts. This in vitro result could mimic the melanoma adhesive steps in the lung endothelium, where CXCL12 might be exposed on VCAM-1-rich areas of endothelium, being capable of up-regulating α4β1-mediated melanoma cell attachment leading to firm arrest. PET-CT analyses together with visual examination of melanoma lung metastases indicated that rapid and more efficient accumulation of CXCR4hi cells in the lungs led to a posterior faster organ colonization, causing a notable survival shortening, as compared with CXCR4lo mice.
In contrast to CXCR4, we found that MT1-MMP was not needed in early phases of melanoma cell homing into lungs. However, the activity of this metalloproteinase together with CXCR4 was required for subsequent dissemination of the tumor because CXCR4hiMT1lo melanoma cells displayed a significant reduction in their aggressiveness in lungs, which was associated with prolonged mice survival. Additional evidence of mutual requirement between CXCR4 and MT1-MMP for efficient lung metastasis was provided by the observation that subcutaneously-inoculated CXCR4hiMT1lo and CXCR4loMT1hi melanoma cells displayed a notable decrease in lung colonization compared with CXCR4hi and MT1hi single counterparts. The expression of MT1-MMP on the invading tumor front in melanoma,21 its reported localization on melanoma invadopodia20 together with our present in vivo data support an important role for MT1-MMP in melanoma lung colonization. Although our results strongly indicate that CXCR4 and MT1-MMP activities are functionally coordinated during different steps of melanoma cell trafficking into lungs, we cannot exclude that CXCR4hi melanoma cells might take advantage of CXCL12-dependent growth properties32,33 to proliferate in the lungs with higher rates than CXCR4lo counterparts, based on the shorter doubling times of CXCR4hi cells in the presence of the chemokine.
Mutual requirement between CXCR4 and MT1-MMP could take place independently from each other’s activity, but there might also be molecular cross-talks between them. Indeed, we previously reported that CXCL12 up-regulates MT1-MMP expression in melanoma cells, involving Vav-Rho GTPase activation.12,13 Here we have further characterized this signaling, and thus we show that Erk1/2 MAP kinase represents a Vav-Rac1-activated downstream target that is required for stimulation of the expression and function of MT1-MMP in melanoma cells. It is well established that the Rac-PAK pathway contributes to the activation of MEK-Erk1/2 signaling,34,35 and therefore our data suggest that CXCL12-activated Vav-Rac1-PAK-Erk1/2 pathway can promote enhancement of MT1-MMP expression in melanoma cells.
Cell membrane expression levels of MT1-MMP are generally low, with most of this metalloproteinase residing intracellularly, likely reflecting a tight cell regulation to avoid excessive pericellular proteolysis. In agreement with this expression pattern, MT1-MMP on BLM melanoma cells predominantly accumulates intracellularly, even if its expression is elevated by CXCL12. Interestingly, we have found that when cells come in contact with components of Matrigel basement membranes, especially with laminin, a phosphatidylinositol 3-kinase-dependent transient increase in MT1-MMP cell surface expression is detected, this increase being higher in CXCL12-incubated CXCR4hi cells. These data indicate that melanoma cells control their MT1-MMP cell surface expression after contact with specific ECM proteins. Integrins transmit outside-in signals for PI3-K activation after interaction with their ECM ligands,33 and thus are potential candidates to contribute to up-regulation of cell surface MT1-MMP expression. We show here that β1 integrins mediate melanoma cell attachment to Matrigel ECM proteins, which causes Akt activation, suggesting that β1 integrin-dependent PI3-K activation represents a mechanism likely regulating MT1-MMP cell membrane expression.
Involvement of PI3-kinase activity in protein trafficking, both in regulated exocytosis and endocytosis, has been demonstrated.36,37,38,39 In particular, class I and III (hVPS34) PI3-kinases regulate redistribution of surface proteins together with key members of the exocytic and endocytic pathways. Expression of MT1-MMP on the cell surface has been reported to be regulated, among other processes, by internalization,40,41,42 recycling,43 autocatalysis,44 and exocytosis.45,46 At present, it is not known whether PI3-K-mediated stimulation of exocytosis or inhibition of internalization account for the increase in MT1-MMP melanoma cell surface expression. Further studies are needed to better characterize the role of this kinase in MT1-MMP trafficking to the cell membrane, where it exerts its ECM pericellular remodeling.
Together, these data highlight the cross talk between CXCR4 and MT1-MMP for efficient melanoma cell invasion. Thus, expression of MT1-MMP is stimulated by CXCL12 leading to its intracellular accumulation and subsequent redistribution to the cell surface after cell contact with basement membrane proteins, a mechanism contributing to a greater invasiveness. Accordingly, changes in cell surface expression of CXCR4 on melanoma should strongly affect its coordinated activity with MT1-MMP during lung metastasis. Expression of CXCR4 in melanoma and in non-small cell lung cancer can be stimulated by transforming growth factor-β1 and epidermal growth factor, respectively,12,47 whereas its expression in breast cancer cells can be increased by HER2 signaling leading to lung metastasis.48 Additional characterization of the mechanisms controlling CXCR4 expression will contribute to improve our knowledge of its metastasis-promoting properties.
Collectively, the results from the present work dissect independent and coordinated roles for CXCR4 and MT1-MMP during the metastasis of melanoma cells into lungs, establishing molecular cross-talks between these molecules, and suggest that combination therapies targeting CXCR4 and MT1-MMP should benefit and contribute to ameliorate the limitations of the current therapies in metastatic melanoma.
Supplementary Material
Acknowledgments
We thank Manuel Moreno, Maria Herrera, and Nohemí Arellano, from the Centro de Investigaciones Biológicas Animal Facility, and Isabel Treviño for expert technical assistance; Drs. Goos N.P. van Muijen and Angeles García-Pardo for cells and reagents; and Dr. Ana C. Carrera for helpful discussions.
Footnotes
Address reprint requests to Joaquin Teixidó. Centro de Investigaciones Biológicas, Department of Cellular and Molecular Physiopathology, Ramiro de Maeztu 9, 28040 Madrid, Spain. E-mail: joaquint@cib.csic.es.
Supported by the Ministerio de Educación y Ciencia (grants SAF2005-02119 to J.T., SAF2006-08615 to P.S.M., and TEC2004-07052-C02-01/TCM to M.D.), the Fundación de Investigación Médica Mutua Madrileña (to J.T.), the Red Tematica de Investigacion Cooperativa en Cancer (RTICC) (grant RD06/0020/0011 to J.T.), the Ministerio de Sanidad y Consumo (CIBER CB06/01/0079 to M.D. and grants FIS PI030300 and G030173 to R.D.), the Ministerio de Industria (Programa Consorcios Estratégicos Nacionales de Investigación Técnica to M.D.).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
Present address of M.E.M.-C.: Unidad de Investigación, Hospital Universitario Santa Cristina, Madrid, Spain.
References
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Robledo MM, Bartolome RA, Longo N, Rodriguez-Frade JM, Mellado M, Longo I, van Muijen GN, Sanchez-Mateos P, Teixido J. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–45105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, Hwang ST. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- Saur D, Seidler B, Schneider G, Algul H, Beck R, Senekowitsch-Schmidtke R, Schwaiger M, Schmid RM. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237–1250. doi: 10.1053/j.gastro.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- Longo-Imedio MI, Longo N, Trevino I, Lazaro P, Sanchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. 2005;117:861–865. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- Bartolomé RA, Galvez BG, Longo N, Baleux F, Van Muijen GN, Sanchez-Mateos P, Arroyo AG, Teixido J. Stromal cell-derived factor-1alpha promotes melanoma cell invasion across basement membranes involving stimulation of membrane-type 1 matrix metalloproteinase and Rho GTPase activities. Cancer Res. 2004;64:2534–2543. doi: 10.1158/0008-5472.can-03-3398. [DOI] [PubMed] [Google Scholar]
- Bartolomé RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–258. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Kurschat P, Zigrino P, Nischt R, Breitkopf K, Steurer P, Klein CE, Krieg T, Mauch C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274:21056–21062. doi: 10.1074/jbc.274.30.21056. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida J, Wilhelmson KL, Price MA, Wilson CM, Pei D, Furcht LT, McCarthy JB. Membrane type-1 matrix metalloproteinase promotes human melanoma invasion and growth. J Invest Dermatol. 2004;122:167–176. doi: 10.1046/j.0022-202X.2003.22114.x. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen WT. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000;191:245–256. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH632>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- García-Bernal D, Wright N, Sotillo-Mallo E, Nombela-Arrieta C, Stein JV, Bustelo XR, Teixido J. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin {alpha}4{beta}1. Mol Biol Cell. 2005;16:3223–3235. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Voorhoeve PM, Agami R. The tumor-suppressive functions of the human INK4A locus. Cancer Cell. 2003;4:311–319. doi: 10.1016/s1535-6108(03)00223-x. [DOI] [PubMed] [Google Scholar]
- Yang S, Delgado R, King SR, Woffendin C, Barker CS, Yang ZY, Xu L, Nolan GP, Nabel GJ. Generation of retroviral vector for clinical studies using transient transfection. Hum Gene Ther. 1999;10:123–132. doi: 10.1089/10430349950019255. [DOI] [PubMed] [Google Scholar]
- Mañes S, del Real G, Lacalle RA, Lucas P, Gomez-Mouton C, Sanchez-Palomino S, Delgado R, Alcami J, Mira E, Martinez AC. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Savinov AY, Golubkov VS, Tomlinson S, Strongin AY. Interference with the complement system by tumor cell membrane type-1 matrix metalloproteinase plays a significant role in promoting metastasis in mice. Cancer Res. 2006;66:6258–6263. doi: 10.1158/0008-5472.CAN-06-0539. [DOI] [PubMed] [Google Scholar]
- Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304–6311. [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengholm A, Meyer T. A PI3-kinase signaling code for insulin-triggered insertion of glucose transporters into the plasma membrane. Curr Biol. 2002;12:1871–1876. doi: 10.1016/s0960-9822(02)01223-x. [DOI] [PubMed] [Google Scholar]
- Chieregatti E, Meldolesi J. Regulated exocytosis: new organelles for non-secretory purposes. Nat Rev Mol Cell Biol. 2005;6:181–187. doi: 10.1038/nrm1572. [DOI] [PubMed] [Google Scholar]
- Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–614. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- Backer JM. The regulation and function of class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci USA. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekita T, Itoh Y, Yana I, Ohno H, Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci. 2003;116:3905–3916. doi: 10.1242/jcs.00710. [DOI] [PubMed] [Google Scholar]
- Stanton H, Gavrilovic J, Atkinson SJ, d'Ortho MP, Yamada KM, Zardi L, Murphy G. The activation of ProMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell Sci. 1998;111:2789–2798. doi: 10.1242/jcs.111.18.2789. [DOI] [PubMed] [Google Scholar]
- Zucker S, Hymowitz M, Conner CE, DiYanni EA, Cao J. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab Invest. 2002;82:1673–1684. doi: 10.1097/01.lab.0000041713.74852.2a. [DOI] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem. 2005;280:22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.