Abstract
Macrophage migration inhibitory factor (MIF) is a multifunctional cytokine that is overexpressed in lung cancer. The MIF receptor was recently discovered and found to be the invariant chain of the HLA class II molecule, CD74. We hypothesized that the expression of this receptor-ligand pair in lung cancer is associated with the angiogenic activity and level of CXC chemokine expression in human specimens of non-small cell lung cancer. We, therefore, performed immunolocalization of CD74 and compared it with the localization of MIF in non-small cell lung cancer to determine their respective locations, as well as the relationship between the co-expression of MIF-CD74 and angiogenic CXC chemokines with tumor angiogenesis. We found intense CD74 expression by immunohistochemistry in 57 of 70 tumors with minimal to no staining in the remaining 13 tumors. Comparing the localization of CD74 with its putative ligand, MIF, we found that CD74 and MIF were co-expressed in tumors in close proximity, and that co-expression of the MIF-CD74 pair was associated with both higher levels of tumor-associated angiogenic CXC chemokines (ie, the ELR score) and greater vascularity compared with tumors in which MIF-CD74 co-expression was not present. We also found that MIF induced angiogenic CXC chemokine expression in an autocrine manner in vitro, a function that was specifically inhibited by antibodies to CD74.
Lung cancer, like all cancers, is characterized by pathological angiogenesis. We have shown that macrophage migration inhibitory factor (MIF) induces expression of angiogenic CXC chemokines by tumor-associated monocytes, and that MIF-dependent expression of angiogenic CXC chemokines is one of several major pathways by which lung cancer tumors induce an angiogenic environment. We and others have shown that MIF is markedly overexpressed in lung cancer1,2,3 and nearly all other common solid tumors; eg, breast,4,5,6,7,8 ovarian,9,10 prostate,6,7,8 bladder,11,12 and colon cancer,13 as well as melanoma.14,15 In lung cancer MIF expression is associated with increased production of angiogenic CXC chemokines both in vitro,16 and in vivo.2 High expression of MIF in patients with lung cancer is also associated with a worse prognosis for disease-free and overall survival.2,3,17 MIF was one of the first cytokines ever to be described,18,19 yet until it was cloned in 1989,19 many of its biological activities in health and disease remained unknown. It is a unique cytokine with unusual physicochemical properties, and varied biological properties that, in addition to its role in angiogenesis, include antagonism of p53,20,21 inhibition of Rb function,22,23 and activation of Akt.24 Others have shown that MIF can directly induce angiogenesis in vivo as well.25 This combination of properties suggests that MIF may play a pivotal role in tumor biology.
Until recently the cell surface receptor for MIF remained unknown. Leng and colleagues26 screened a cDNA library for genes capable of conferring MIF binding and discovered that the invariant chain of the HLA class II peptide (CD74) was the cell surface receptor for MIF.26 Despite the role played by MIF in lung cancer, there are few studies demonstrating a role for CD74 in lung cancer. Two studies examined the expression of CD74 in gastric carcinogenesis induced by Helicobacter pylori, and found that it was associated with poorer overall survival.27,28 One study done to examine the reactivity of a panel of monoclonal antibodies in 25 lung cancer specimens included the LN2 monoclonal antibody against CD74 and found that this was among a panel of lymphoid antibodies that could distinguish small cell from non-small cell lung cancer (NSCLC).29 This study predated the understanding that CD74 may play a role in MIF signaling.
We sought to confirm whether CD74 was present in human lung cancer specimens and reasoned that the distribution of CD74 might yield clues as to the role of MIF in lung cancer. For example, if CD74 is primarily expressed in stromal cells, it would suggest that the effects of MIF in these tumors is primarily through influencing this cell population, whereas CD74 expression primarily on the malignant cells in the tumor would suggest that MIF might be working through antagonism of apoptotic pathways, or by autocrine regulation of angiogenic factor expression. We performed the current study to determine the presence and distribution of CD74 in human lung cancer, to compare the distribution of MIF with its putative receptor CD74, and to determine the relationship of these two factors to angiogenesis in their respective tumors.
Our results demonstrate that CD74 is expressed in the majority of lung cancer tumors, with expression being found primarily in stromal compartments in some tumors, whereas others show mixed stromal and malignant epithelial expression. We also observed co-expression of CD74 in close proximity to the ligand MIF and found that CD74 co-expression with MIF was associated with higher levels of angiogenic CXC chemokines and greater tumor vascularity, as measured by factor VIII staining. We further found that in vitro inhibition of MIF or its receptor resulted in reduced production of angiogenic CXC chemokines by human lung cancer cells.
Materials and Methods
Tumor Tissue
All patients gave informed consent for collection of tumor and normal lung tissue at the time of thoracotomy for known or suspected lung cancer. Tissue acquisition and tumor processing methods have been described previously.2 All studies were approved by the University of Michigan Institutional Review Board.
Antibodies Used in Immunohistochemistry
Mouse anti-human CD74 was purchased from Abcam (Cambridge, MA) (LN2 monoclonal). Goat anti-human MIF was purchased from R&D Systems (Minneapolis, MN). We also produced a chicken IgY antibody that recognizes both human and murine MIF. For production of this antibody, the murine MIF (mMIF) cDNA was initially amplified by polymerase chain reaction (PCR) from WEHI 274.1 monocytic cells with the following primers: sense, 5′-CATGCCTATGTTCATCGTGAA-3′, and anti-sense 5′-GTAAGTGGATCCAGGACTCAA-3′. We cloned the resulting product into the Xa expression vector (Promega, Madison WI) and confirmed the identity of the cDNA by sequencing as 100% homologous to mMIF. We then subjected this plasmid to a second round of PCR to introduce XbaI and ApaI restriction sites at the 5′ and 3′ ends, respectively. After digestion, we cloned this product into the p-tetO expression cassette, which consists of seven tetO repeats, a CMV minimal promoter, and a bovine growth hormone polyadenylation sequence (a kind gift from Dr. Jeffery Whitsett and Dr. J. Tichelaar, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), and designated this vector as pTet-MIF. To generate his-tagged MIF we digested the pTet-MIF vector with XhoI and ApaI and cloned this product containing the MIF cDNA into the PROTet 6xHN bacterial expression system (Vector 3; BD Biosciences, Palo Alto, CA). This Escherichia coli-expressed recombinant histidine-tagged MIF was purified using the TALON affinity metal resin (BD Biosciences). Antibody production was performed in contract with GenWay Biotech (San Diego, CA). His-tagged MIF in complete Freund’s adjuvant was injected intramuscularly into laying hens (Leghorn and Rhode Island). Boost injections were performed at 2- to 3-week intervals in incomplete Freund’s adjuvant for three times. Antibodies were isolated from egg yolks using a polyethylene glycol precipitation method according to Polson and colleagues.30 The antibodies were further purified by the affinity chromatography method using the immunogen as the affinity ligand. The resulting antibody was provided as an affinity-purified antibody and reactivity against recombinant murine MIF was confirmed in Western blot against the immunogen. We confirmed that this antibody reacted specifically with human MIF using recombinant human MIF (R&D Systems) in Western blot, and subsequently documented MIF immunoreactivity with human MIF in histological sections previously stained with commercially available antibody (data not shown).
MIF Inhibition Experiments in Vitro
We plated A549 and Calu 6 cells in six-well plates and grew them to 80% confluence before changing to serum-free media. One of the following inhibitors were then added: 2 μg/ml anti-CD74 or isotype IgG, 2 μg/ml anti-MIF, or IgY control, 100 nmol/L (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1, a small molecular inhibitor of MIF; a kind gift of Dr. Yousef Al Abed, The Feinstein Institute for Medical Research, North Shore-LIJ Research Institute, Manhasset, NY) with dimethyl sulfoxide as a control. We harvested 48-hour serum-free conditioned media and froze it for later analysis by enzyme-linked immunosorbent assay.
Enzyme-Linked Immunosorbent Assay
Purified and biotinylated antibody for human CXCL8/interleukin-8, CXCL5/ENA-78, CXCL1/GRO-α, MIF, and vascular endothelial growth factor were obtained from R&D Systems, Inc. Antibodies for CXCL3/GRO-γ were obtained from PeproTech Inc. (Rocky Hill, NJ). Secondary antibodies for immunohistochemistry were rabbit IgG, biotinylated and directed against the immunoglobulin of the species of primary antibody (Vector Laboratories, Burlingame, CA). Vectastain Elite ABC reagent was used for enzymatic detection with diaminobenzidine used at the chromogen. Tumor-associated levels of CXC chemokines [interleukin-8 (CXCL8), ENA-78 (CXCL5), GRO-α (CXCL1), MIG (CXCL9) and IP-10 (CXCL10)], were measured using a double-ligand method previously described.2 Standards were prepared as ½ log serial dilutions of purified recombinant cytokines, from 100 ng/ml to 0.001 ng/ml per well.
Immunohistochemistry
Microwave antigen retrieval was performed for all immunostaining, using an antigen unmasking solution (Vector Laboratories) with the supplier’s protocol. Immunohistochemistry for factor VIII-related antigen and MIF staining has been described previously.2 Tissue sections were 5 μm thick and dewaxed through two washes in xylene, followed by rehydration through graded concentrations of ethanol [100% ×2, 90% ×2, 70% ×2, then phosphate-buffered saline (PBS) in distilled water]. Tissue sections were blocked with normal rabbit serum (1:50 in PBS) for 30 minutes. Primary antibodies were applied and diluted in PBS (goat anti-human MIF, 1:500; chicken IgY anti-MIF, 1;250; or LN2 monoclonal anti-CD74, 2 μg/ml) for 1 hour. Control sections were stained with nonspecific isotype-matched immunoglobulin from the same species as the primary. Tissue sections were considered positive for CD74 if specific staining was present in the CD74-stained sections without nonspecific staining in the control stained sections. Tissue with no evident staining for CD74 was considered negative. We did not assign a quantitative value to the qualitative determination of whether CD74 was present. Biotinylated secondary antibodies were rabbit anti-goat, -chicken, or -mouse applied for 30 minutes in PBS (1:50 dilution of stock, purchased from Vector Laboratories). Immunohistochemistry for factor VIII-related antigen was performed with DAKO-EPOS Factor-VIII antibody (DAKO, Carpinteria, CA) as previously described.2 Vectastain ELITE ABC kits were used for immunolocalization with diaminobenzidine as the chromogen.
For fluorescence immunostaining, we treated the slides to reduce background autofluorescence. During dewaxing and rehydration of the samples (graded 2-minute ethanol washes in 100% ×2, 90% ×2, and 70% ×2) a third wash in 70% alcohol with 0.25% NH3 was added. After a final wash in 50% EtOH, we transferred the slides to modified Hanks’ buffer [MHB: Hanks’ buffer without calcium, containing 2 mmol/L EGTA and 5 mmol/L MES (2-morpholino-ethanesulfonic acid), pH 6.2 to 6.4]. Samples were incubated in ice-cooled freshly prepared MHB supplemented with 10 mg/ml of borohydride for 40 minutes, and washed three more times in MHB.31 Sections were blocked with Universal protein block (Biogenex, San Ramon, CA) for 30 minutes. Primary antibodies were used as above, and secondary antibodies were 1:50 dilutions of Texas Red (tetramethyl-rhodamine isothiocyanate)-conjugated rabbit anti-goat IgG, or fluorescein isothiocyanate (FITC)-conjugated goat anti-chicken IgY (both antibodies from Santa Cruz Biotechnology, Santa Cruz, CA). Sections were counterstained with DAPI mounting medium, and photographed under epi-illumination with a SPOT RT Slider camera, interfaced with a Nikon (Melville, NY) E600 microscope with appropriate FITC, Texas Red, and DAPI filters.
Flow Cytometry
To determine whether surface staining of CD74 was present in tumor cells, we performed flow cytometry on freshly resected tumor specimens or on cultured tumor cell lines (A549 and Calu 6). Tumors were first minced in a DAKO Medimachine, and filtered through 70-μm filters (DAKO). Cells were then counted and suspended at 1 × 106 cells per ml in fluorescent antibody buffer with 0.01% sodium azide (Sigma, St. Louis, MO), and blocked with 2% normal goat serum. In some specimens we first performed two serial negative selections on fresh tumor digests to deplete the specimens of macrophages with CD45-coated magnetic beads (Miltenyi Biotec, Auburn, CA). We then stained the CD45-depleted and control-depleted fractions separately for CD74. For cultured cells, we first harvested confluent A549 or Calu 6 cells with 0.01% trypsin, and washed with PBS supplemented with 0.01% sodium azide and 2% normal goat serum. Primary antibody was mouse monoclonal LN2 (anti-CD74), and secondary antibody was FITC-conjugated goat anti-mouse IgG. Each staining step was performed for 30 minutes on ice, with two washes of fluorescent antibody buffer in between. Specimens were delivered to the University of Michigan Cancer Center Flow Cytometry Core where they were run on a Coulter (Hialeah, FL) Epics XL cytometer, with gating based on unstained and control stained specimens (primary antibody omitted).
Statistical Analysis
Two group comparisons (Table 1) were completed with Student’s t-test, and for three group comparison (Table 2) analysis of variance was used to test for significant differences.
Table 1.
Demographic Characteristics and Stage Distribution of 70 Patients with Non-Small Cell Lung Cancer, with Groups Separated by the Presence or Absence of CD74 Immunoreactivity
| CD74-positive (n = 57) | CD74-negative (n = 13) | P value | |
|---|---|---|---|
| Age | 64.2 ± 8.6 | 62.4 ± 9.5 | NS |
| Sex (% male) | 54% | 55% | NS |
| Stage | NS | ||
| I | 32 | 11 | |
| II | 1 | 1 | |
| III | 7 | 1 | |
| IV | 4 | 0 | |
| % Nonsmokers | 16% (9 of 57) | 15% (2 of 13) | NS |
| Adenocarcinoma | 35 | 8 | NS |
| Squamous | 19 | 5 | NS |
| Other (two large cell neuroendocrine, one small cell) | 3 | 0 | NS |
| Vessel density (vessels per high-power field, mean ± SE) | 36.1 ± 3.2 | 22.9 ± 3.8 | 0.02 |
| Angiogenic CXC chemokines (ELR score; ng/mg total protein) | 95.6 ± 34.4 | 8.8 ± 3.2 | 0.05 |
Vessel density and levels of angiogenic ELR CXC chemokines in lung cancer tumors are higher in tumors where CD74 was present by immunohistochemistry. NS = nonsignificant.
Table 2.
Vessel Density and Levels of Angiogenic ELR CXC Chemokines in Lung Cancer Tumors Stratified by the Pattern of CD74 Staining
| CD74 pattern | Mixed (n = 38) | Stromal (n = 19) | Negative (n = 13) | P value (analysis of variance) |
|---|---|---|---|---|
| Vessel density (mean ± SE) | 39.9 ± 4.5 | 28.5 ± 3.2 | 22.9 ± 3.8 | 0.02 |
| Angiogenic CXC chemokines (ELR score; ng/mg total protein) | 117.6 ± 31 | 54.0 ± 19 | 8.8 ± 3.2 | 0.05 |
Results
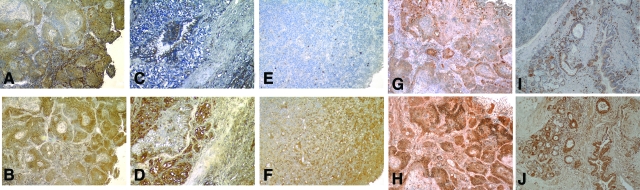
We routinely collected tissue specimens from patients undergoing resection of known or suspected lung cancer. We randomly selected cell blocks from our tissue bank and cut 5-μm-thick sections to stain for the presence of CD74. We stained a total of 85 specimens. After unblinding the identity of the tissue sections, 15 samples were excluded because they were granulomas (n = 5), carcinoid tumors (n = 7), metastasis from other organs (n = 2), or hamartoma (n = 1). Of the 70 remaining tumors, 57 (81%) stained positively for CD74 with the distribution of CD74 immunoreactivity varying from purely restricted to the stromal cells, to mixed tumor-stromal immunoreactivity (Table 1). The demographics of the patients from whom the tumors were evaluated is also shown in Table 1, and this cross section is representative of the demographic characteristics of all patients treated at our institution for lung cancer throughout this time period. Figure 1 shows representative slides demonstrating the CD74 staining pattern observed in NSCLC tumors. CD74 immunoreactivity was present in the stromal cells in most tumors (arrows in Figure 1E for example). However, in many tumors (38 of 57, 67%) the malignant cells themselves also strongly expressed CD74. We observed no variation in staining pattern (negative, stromal only, or mixed) after stratifying by sex, or smoking status (P > 0.25 for all adjustments). A larger proportion of CD74-negative tumors were stage I-II (9 of 11), compared with CD74-positive tumors (32 of 57), but this difference was not statistically significant (P = 0.07). Normal lung tissue stained for CD74 demonstrates reactivity only in alveolar macrophages (data not shown).
Figure 1.
Immunolocalization of CD74 in human NSCLC tumors. A, C, and E are stained with mouse anti-human CD74 IgG. B, D, and F are control staining with preimmune isotype-matched mouse IgG. Original magnifications: ×200 (A, C); ×100 (E).
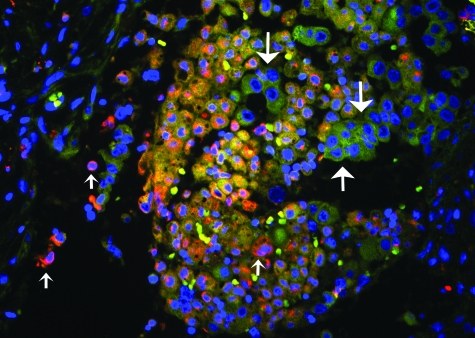
We wanted to compare the distribution of CD74 with its ligand MIF within the tumor microenvironment. We have previously shown that MIF staining is universally present in NSCLC,2 and as part of this study we stained additional tumors for MIF in adjacent sections of tumors stained for CD74. When we compared the sequential sections of tumors stained for both CD74 and MIF we observed a pattern of MIF expression in malignant cells (Figure 2, B, D, F, H, and J) surrounded by stromal cells expressing CD74 (Figure 2, A, C, E, G, and I). This suggested a paracrine effect of MIF in these tumors consistent with our previous findings in vitro.16 However, MIF-expressing malignant cells could also be observed to stain positively for CD74 in adjacent sections (Figure 2, A and B, G and H, for example). To confirm co-localization of ligand and receptor, we performed double-immunofluorescent staining with anti-MIF (FITC, green) and CD74 (Texas Red) in tumors. Immunofluorescence confirmed the co-localization of MIF and CD74 in the tumor microenvironment (Figure 3).
Figure 2.
Adjacent tumor sections stained for CD74 (A, C, E, G, and I) and MIF (B, D, F, H, and J) demonstrating that MIF and its receptor are expressed in close proximity in the tumor microenvironment. Original magnifications: ×100 (A, B, I, J); ×200 (C--H).
Figure 3.
Double-immunofluorescence staining for MIF (FITC; green, large arrows) or its receptor CD74 (Texas Red, small arrows) in a lung cancer specimen. In the center of the photomicrograph, a cluster of cells shows co-localization of the fluorochromes, presumably reflecting bound receptor and ligand. Original magnification, ×400.
To gain further insight into the role played by the MIF-CD74 interactions in these tumors, we compared the levels of angiogenic CXC chemokines in tumors where we observed expression of CD74 with those in which expression was absent. In tumors where CD74 expression was observed, angiogenic CXC chemokine expression (as reflected by the ELR score)2 was significantly increased (103 ± 37 ng/mg) relative to tumors where CD74 immunoreactivity was not seen (9.1 ± 3.4 ng/mg, P < 0.01; Table 1). We also compared tumor vessel density between tumors with (36.6 ± 3.5 vessels/hpf) and without (22.0 ± 3.6 vessels/hpf) CD74 immunoreactivity and found that CD74-positive tumors had significantly greater vascularity (P = 0.05, Table 1). We stratified the levels of tumor-associated CXC chemokines (ELR score) by the pattern of staining for CD74 (stromal only, mixed tumor and stromal staining, and negative), and found that tumors where CD74 was present on both tumor cells and host stromal cells had the highest level of angiogenic CXC chemokines (Table 2).
This suggested that (in addition to the paracrine induction of angiogenic CXC chemokines we have previously shown) MIF could be acting in an autocrine manner to increase tumor-associated CXC chemokine expression. To further explore this, we compared the expression of angiogenic CXC chemokines between NSCLC cell lines treated with MIF inhibitors (anti-MIF antibody, anti-CD74 antibody, or small molecule MIF inhibitor with appropriate controls). A549 and Calu 6 cells both produce significant quantities of MIF, as we have previously shown.16 Angiogenic CXC chemokine expression was reduced in the presence of all three types of inhibitors (Figure 4, A and B). We did not observe induction of the anti-angiogenic interferon-inducible CXC chemokines CXCL9 or CXCL10 (data not shown).
Figure 4.
Production of angiogenic CXC chemokines CXCL5, and CXCL8 by A549 (A) or Calu 6 (B) cells in the presence of antibody to CD74 (aCD74) or control (IgG), antibody to MIF (aMIF) or control (IgY), and ISO-1 (MIF inhibitor) or control (DMSO). A: P < 0.02 for CXCL8 and P < 0.004 for CXCL5. B: P < 0.05 for CXCL5 and P < 0.01 for CXCL8.
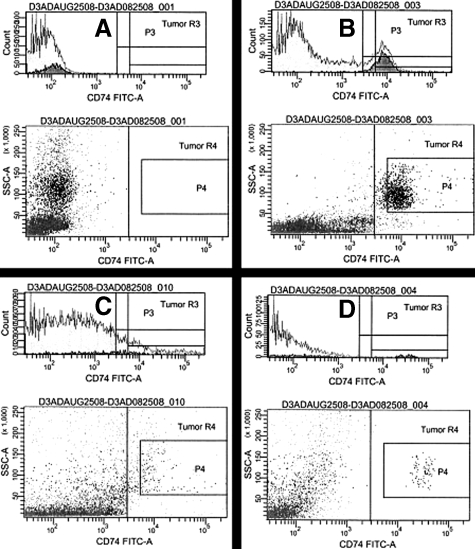
Although immunohistochemistry confirms the presence of the receptor for MIF in human lung cancer tumors, for extracellular MIF signaling to be mediated by CD74 in vivo, it must be present on the cell surface. Therefore we analyzed fresh human NSCLC tumors digested to single cell suspensions to determine whether surface expression of CD74 was present. We detected CD74 by flow cytometry in all five specimens so analyzed, with the percentage of CD74+ cells varying from 8 to 30% (Figure 5, A–C). To determine whether nonmyeloid cells in the tumor also expressed CD74, we performed serial magnetic depletion through two passages of single cell digests with CD45 or control microbeads to remove macrophages. Flow cytometry still yielded CD74+ cells in the remaining population (Figure 5D). Flow cytometry analysis of two NSCLC cell lines (A549 and Calu 6) revealed a detectable but low percentage of CD74+ cells as well (Figure 6). We determined the median disease-free survival for patients in this cohort, stratified for the presence or absence of CD74 staining in their tumors, but observed no difference in the median disease-free survival (data not shown) or overall stage distribution between CD74-positive or -negative tumors.
Figure 5.
Representative histograms from two of five NSCLC tumors. Flow cytometric analysis of unstained tumor specimen (A), as a control for CD74-stained tumor specimen (B) showing 15.4% of gated cells expressing CD74. C: Another CD74-stained tumor specimen, subject to two passages with control antibody showing 8.5% of CD74+ cells. D: The same specimen as in C, after two passages with anti-CD45-coated magnetic beads to remove myeloid cells, showing 4.1% of remaining cells positive for CD74.
Figure 6.
Flow cytometric analysis of A549 cells (top) and Calu 6 cells (bottom) stained with control antibody (left) or CD74 antibody (right), showing surface expression of CD74 in a fraction of the cells. Cells were harvested with trypsin, and with the gating shown, 26% of A549 cells and 28% of Calu 6 cells were positive for CD74. Data are representative of two experiments.
Discussion
We and others have shown that MIF is markedly overexpressed in lung cancer1,2,3 and nearly all other common solid tumors; eg, breast,4,5,6,7,8 ovarian,9,10 prostate,6,7,8 bladder,11,12 and colon cancer,13 as well as melanoma.14,15 In lung cancer MIF expression is associated with increased production of angiogenic CXC chemokines both in vitro,16 and in vivo.2 High expression of MIF in patients with lung cancer is also associated with a worse prognosis for disease-free and overall survival.2,3,17 Because CD74 was identified as a cell surface receptor for MIF, it is important to characterize its expression in lung cancer. This is especially true given the diverse biological activities attributed to MIF.32,33 Consistent with what we have found with respect to MIF and lung cancer, we observed a relationship between the presence of CD74 by immunohistochemistry, and levels of angiogenic CXC chemokines, as well as with tumor vessel density in this study. On the other hand, we did not observe a relationship between CD74 immunohistochemistry and disease-free survival.
Although MIF was one of the earliest cytokine activities to be described in 1966, it was not until it was cloned in the late 1980s that more became known about its diverse properties which include the ability to antagonize corticosteroid activity, block p53-dependent apoptosis,20,21,34 antagonize the Rb tumor suppressor,22 promote angiogenesis,16,25,35,36 and even mediate a tautomerase enzymatic activity.37 Many of these properties suggest that MIF could play a role in tumor biology, but further study of this was limited by the lack of a known receptor for MIF. One report showed that MIF had an intracellular binding target, called Jnk-activation domain-binding protein 1 (Jab1) which is a co-activator of the AP-1 transcription factor. MIF was found to inhibit AP-1-dependent transcription. However, this did not explain the ability of MIF to act as a potent stimulator of sustained Erk1/2 phosphorylation. More recently, one report described the finding that MIF can signal through the CXC chemokine receptor CXCR2.38 However, this study did not account for the fact that MIF potently induces the expression of CXCR2 ligands, the angiogenic CXC chemokines.
A screen of cDNA libraries for clones that conferred extracellular MIF binding identified CD74 as the surface receptor for MIF. CD74 designates the invariant chain of HLA class II (Ii, or in the mouse Ia). The main function of invariant chain was believed to be as a chaperone molecule, directing the assembly of class II α and β chains in the Golgi compartment, and transporting them to the cell surface.39 However, a small fraction of invariant chain can become membrane-associated, as reported by Wraight and colleagues.40 Overall, there is substantial evidence from in vitro studies and animal models that CD74 is the receptor through which protumor properties of MIF are mediated.11,24,41,42,43 CD74-dependent signaling of extracellular MIF has been identified in gastric,41 prostate,42 and breast24 carcinoma cells in vitro. A study done by Ioachim and colleagues29 intended to examine the reactivity of a panel of lymphoid monoclonal antibodies against 25 lung cancer specimens, in which the LN2 monoclonal antibody (against CD74) was one of the antibodies which demonstrated reactivity with NSCLC (but not small cell, SCLC). These authors postulated that this panel of antibodies (including LN2) could be useful to distinguish NSCLC from SCLC. This finding predated the discovery that CD74 was a potential receptor for MIF.26,29
We reasoned that if CD74 was the cell surface receptor for MIF in lung cancer, its expression should be readily identified, particularly in areas where MIF was present. We also anticipated that the distribution of CD74 might indicate whether the primary role of MIF in lung cancer was as a paracrine factor (such as inducing the expression of angiogenic CXC chemokines in tumor-associated macrophages), or as an autocrine factor to promote cell cycle activity,44 or antagonize key tumor suppressor pathways.20,21,22,24 Our results confirm that CD74 is present in the large majority of lung cancer specimens. In some specimens it is present only in stromal cells of the surrounding tumor microenvironment (Figure 2E for example). This supports our initial assumption that the role of MIF in NSCLC is to induce the expression of angiogenic CXC chemokines from tumor-associated stromal cells.16 Flow cytometry confirms that CD74 expression is indeed present on the surface of cells within the tumor, and deletion of myeloid cells with anti-CD45 microbeads did not completely remove all of the CD74-positive cells from tumor digests. We cannot say what the remaining cells were in the tumor digests after depletion of CD45+ cells, but it is likely a mixture of malignant epithelial cells and myofibroblasts. The percentage of tumor cells staining for CD74 in flow cytometry is less than the proportion one might guess from looking at immunohistochemistry alone, and this would suggest that a proportion of the CD74 observed in tissue sections is intracellular. Nevertheless, the presence of CD74 on cultured NSCLC cell lines, and the ability of anti-CD74 antibody to reduce the expression of angiogenic CXC chemokines in vitro in our study, and in others’ hands,45 suggests that cell surface CD74 in NSCLC is an important mediator of the biological effects of MIF in lung cancer. The majority of tumors showed immunoreactivity for CD74 on both tumor and stromal cells, suggesting an autocrine role of tumor cell-derived MIF. Indeed, we found that the levels of angiogenic CXC chemokines were higher in these tumors than in tumors where CD74 was restricted to the stromal cells. This suggests that tumor cell-derived MIF might act on both malignant cells as well as stromal cells to increase the expression of angiogenic CXC chemokines. In vitro studies confirmed that MIF, likely acting via its receptor CD74, can induce expression of angiogenic CXC chemokines in an autocrine manner as well.
In summary, we report finding CD74 expression in the large majority of NSCLC samples by immunohistochemistry. Consistent with prior observations reported by our laboratory, the co-expression of MIF and its putative receptor CD74 in NSCLC is associated with greater tumor vascularity and greater levels of angiogenic CXC chemokines. CD74 may be a valuable therapeutic target for anti-angiogenic therapy.
Footnotes
Address reprint requests to Douglas Arenberg, M.D., Division of Pulmonary and Critical Care Medicine, University of Michigan Medical Center, 1150 W. Medical Center Dr., 6301 MSRB III, Ann Arbor, MI 48109-0642. E-mail: darenber@umich.edu.
Supported by the National Institutes of Health (grant R01-CA94121 to D.A.), the Sidney Kimmel Foundation (grant to D.A.), the Flight Attendants Medical Research Institute, and the Michigan Tri-Technology Corridor (grant GR-687 for the Proteomics Alliance for Cancer Research).
References
- Kayser K, Nwoye JO, Kosjerina Z, Goldmann T, Vollmer E, Kaltner H, Andre S, Gabius HJ. Atypical adenomatous hyperplasia of lung: its incidence and analysis of clinical, glycohistochemical and structural features including newly defined growth regulators and vascularization. Lung Cancer. 2003;42:171–182. doi: 10.1016/s0169-5002(03)00289-7. [DOI] [PubMed] [Google Scholar]
- White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–860. [PubMed] [Google Scholar]
- Kamimura A, Kamachi M, Nishihira J, Ogura S, Isobe H, Dosaka-Akita H, Ogata A, Shindoh M, Ohbuchi T, Kawakami Y. Intracellular distribution of macrophage migration inhibitory factor predicts the prognosis of patients with adenocarcinoma of the lung. Cancer. 2000;89:334–341. [PubMed] [Google Scholar]
- Bando H, Matsumoto G, Bando M, Muta M, Ogawa T, Funata N, Nishihira J, Koike M, Toi M. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res. 2002;93:389–396. doi: 10.1111/j.1349-7006.2002.tb01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261:147–157. doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler K. Macrophage migration inhibitory factor increases MMP-2 activity in DU-145 prostate cells. Cytokine. 2000;12:914–921. doi: 10.1006/cyto.2000.0682. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler K, Hudson PB. Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology. 1996;48:448–452. doi: 10.1016/S0090-4295(96)00207-5. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler KL, Iczkowski KA, Vera PL. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC Cancer. 2005;5:73. doi: 10.1186/1471-2407-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Whang DH, Alvero AB, Visintin I, Lai Y, Segal EA, Schwartz P, Ward D, Rutherford T, Mor G. Macrophage migration inhibitory factor expression in ovarian cancer. Am J Obstet Gynecol. 2007;196:e1–e5. doi: 10.1016/j.ajog.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. 2007;6:1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, III, Kuchel GA, Hegde P, Voznesensky OS, Claffey K, Tsimikas J, Leng L, Bucala R, Pilbeam C. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer. 2007;7:135. doi: 10.1186/1471-2407-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, Todo S. Macrophage migration inhibitory factor promotes tumor invasion and metastasis via the Rho-dependent pathway. Clin Cancer Res. 2005;11:1050–1058. [PubMed] [Google Scholar]
- Culp WD, Tsagozis P, Burgio M, Russell P, Pisa P, Garland D. Interference of macrophage migration inhibitory factor expression in a mouse melanoma inhibits tumor establishment by up-regulating thrombospondin-1. Mol Cancer Res. 2007;5:1225–1231. doi: 10.1158/1541-7786.MCR-07-0229. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264:751–758. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- White ES, Strom SRB, Wys NL, Arenberg DA. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity. J Immunol. 2001;166:7549–7555. doi: 10.4049/jimmunol.166.12.7549. [DOI] [PubMed] [Google Scholar]
- Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin Cancer Res. 2002;8:3755–3760. [PubMed] [Google Scholar]
- David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser WY, Temple PA, Witek-Giannotti JS, Remold HG, Clark SC, David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1989;86:7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Muller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci USA. 2003;100:9354–9359. doi: 10.1073/pnas.1533295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko O, Moll UM. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol Cell. 2005;17:225–236. doi: 10.1016/j.molcel.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Fingerle-Rowson G, Petrenko O. MIF coordinates the cell cycle with DNA damage checkpoints. Lessons from knockout mouse models. Cell Div. 2007;2:22. doi: 10.1186/1747-1028-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Luscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun CT, Lin JT, Huang SP, Lin MT, Wu MS. Expression of macrophage migration inhibitory factor is associated with enhanced angiogenesis and advanced stage in gastric carcinomas. World J Gastroenterol. 2005;11:3767–3771. doi: 10.3748/wjg.v11.i24.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick EJ, Pinchuk IV, Minch K, Suarez G, Sierra JC, Yamaoka Y, Reyes VE. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect Immun. 2006;74:1148–1155. doi: 10.1128/IAI.74.2.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim HL, Pambuccian SE, Hekimgil M, Giancotti FR, Dorsett BH. Lymphoid monoclonal antibodies reactive with lung tumors: diagnostic application. Am J Surg Pathol. 1996;20:62–71. doi: 10.1097/00000478-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Polson A, von Wechmar MB, van Regenmortel MH. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9:475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J Histochem Cytochem. 2001;49:1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26:281–285. doi: 10.1016/j.immuni.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor (MIF). Semin Cancer Biol. 2000;10:359–366. doi: 10.1006/scbi.2000.0328. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–191. [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Nishihira J, Sato Y, Kondo M, Takahashi N, Oshima T, Todo S. An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine. 2000;12:309–314. doi: 10.1006/cyto.1999.0562. [DOI] [PubMed] [Google Scholar]
- Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–291. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- Wraight CJ, van Endert P, Moller P, Lipp J, Ling NR, MacLennan IC, Koch N, Moldenhauer G. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990;265:5787–5792. [PubMed] [Google Scholar]
- Beswick EJ, Pinchuk IV, Suarez G, Sierra JC, Reyes VE. Helicobacter pylori CagA-dependent macrophage migration inhibitory factor produced by gastric epithelial cells binds to CD74 and stimulates procarcinogenic events. J Immunol. 2006;176:6794–6801. doi: 10.4049/jimmunol.176.11.6794. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, Leng L, Goldenberg DM, Shvidel L, Berrebi A, Bucala R, Shachar I. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104:13408–13413. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Bucala R, Mitchell RA. Adhesion-dependent signaling by macrophage migration inhibitory factor (MIF). J Biol Chem. 2003;278:76–81. doi: 10.1074/jbc.M208820200. [DOI] [PubMed] [Google Scholar]
- Coleman AM, Rendon BE, Zhao M, Qian MW, Bucala R, Xin D, Mitchell RA. Cooperative regulation of non-small cell lung carcinoma angiogenic potential by macrophage migration inhibitory factor and its homolog, D-dopachrome tautomerase. J Immunol. 2008;181:2330–2337. doi: 10.4049/jimmunol.181.4.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]