Abstract
There are conflicting data regarding the effects of transplantation of bone marrow-derived cells (BMDCs) on the severity of diabetes. We therefore inquired whether the competence of BMDCs is preserved on adoptive transfer into diabetic (db/db) mice and how the adoptive transfer of BMDCs affects vascular and metabolic abnormalities in these mice. Recipient db/db mice received infusions of BMDCs prepared from either db/db or non-diabetic heterozygout mice (db/m) mice and effects on endothelium-dependent relaxation, insulin sensitivity, and renal function were evaluated. Recipients of BMDCs from db/m, but not db/db donors showed better glucose control, exhibited striking improvement in endothelium-dependent relaxation in response to acetylcholine, and had partially restored renal function. Improved glucose control was due to enhanced insulin sensitivity, most likely secondary to improved vascular function. Enhanced apoptosis of endothelial progenitor cells under oxidative stress, as well as decreased endothelial progenitor cell numbers were responsible for the apparent functional incompetence of BMDCs from db/db donors. Treatment of db/db mice with Ebselen restored the resistance of both BMDCs and endothelial progenitor cells to oxidative stress, improved acetylcholine-induced vasorelaxation, and reduced proteinuria in db/db recipients of BMDC transplantation. In conclusion, infusion of BMDCs obtained from db/m donors to db/db recipient mice benefited macrovascular function, insulin sensitivity, and nephropathy. BMDCs obtained from db/db mice were functionally incompetent secondary to the increased proportion of apoptotic cells on oxidative stress challenge; their competence was restored by Ebselen therapy.
Transplantation of bone marrow-derived cells (BMDCs) has emerged as a promising tool in regenerative medicine. This heterogeneous cell population, consisting of hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and endothelial progenitors, has the capacity to differentiate into cells of endothelial, epithelial, cardiomyocyte, and neuronal lineage.1,2,3,4 In a model of hindlimb ischemia, implantation of autologous BMDCs resulted in therapeutic angiogenesis and improved vascularization of the affected limb in both non-diabetic and diabetic rats.5 In ApoE-deficient mice, transplantation of BMDCs resulted in the restoration of vascular functions.6 There are conflicting data on the effect of transplantation of BMDCs on the severity of diabetes7,8,9,10 and on the renal pathology and dysfunction in the murine model of renal fibrosis and ischemia-reperfusion injury.11,12 Furthermore, recent data indicate that BMDC may become incompetent with regard to their ability to regenerate various tissues and organs.6,13 Taking into account multiple macro- and microvascular complications of diabetes, we inquired a) whether transplantation of BMDCs may affect some of these functional abnormalities, and b) whether the competence of BMDCs in diabetic mice is preserved. Here, we report a dramatic improvement of macrovascular dysfunction and insulin sensitivity in db/db mice recipients of syngeneic BMDC isolated from the non-diabetic mice (but not from their diabetic counterparts), and provide evidence for BMDC incompetence in diabetic animals.
Materials and Methods
Animals, Experimental Design, and Bone Marrow Adoptive Transfer
The animal study protocol was in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services Public Health Services, NIH, NIH Publication No. 86–23, 1985) and approved by the Institutional Animal Care and Use Committee. The type II diabetic murine model db/db mice and db/m mice were obtained from Jackson Laboratory (Bar Harbor, Maine; C57BL/6 background). The body weight and blood glucose level of mice between ages of 8 to 16 weeks were monitored throughout the study. Briefly, bone marrow from male donor db/db and db/m mice was flushed under sterile conditions with Hank’s balanced salt solution (HBSS) from the medullary cavities of tibiae and femurs using a 21-gauge needle. Whole bone marrow single cell suspension was fractionated using Histopaque-1077 solution (Sigma) gradient separation. Mononuclear cells were collected, washed, and checked for viability using trypan blue exclusion technique.8 BMDCs were labeled with Cell Tracker (CM-DiI) (Invitrogen, Eugene, OR). In three independent experiments, male recipient db/db mice (age 16 weeks) received approximately 106 BMDC by tail vein injection. The db/db mice that received the BMDC from db/m mice are designated as dbTxm. The db/db mice that received the BMDC from db/db mice are designated as dbTxdb. The same transfusion procedure was repeated three times every 10 days. In additional series of experiments, db/db mice were treated with Ebselen by gavage, twice a day at 5 mg/kg/day, dissolved in 5% carboxymethyl (CM) cellulose suspension. This group of donor mice was labeled as dbEbs-in vivo and its corresponding recipient db/db mice were designated as TxdbEbs-in vivo. Control db/db mice received only CM cellulose (Sigma, St. Louis, MO) suspension (vehicle treatment; designated as dbCM and its corresponding BMDC recipient db/db mice designated as TxdbCM.) Another group of recipient db/db mice was transfused with BMDC of db/db origin, but treated with Ebs (1 μg/ml) overnight in full Dulbecco’s Modified Eagle Medium (DMEM) medium at 37°C in CO2 incubator before transfusion. This group of recipient mice was designated as TxdbEbs-ex vivo. Because all animals had the same C57BL/6 background, no alloimmune or graft-versus-host response was expected (nor observed). Mice were euthanized 20 days after the final transfusion (at age 23 weeks) by intraperitoneal injection of ketamine/xylazine (60/7.7 mg/kg, respectively). A mid-laparotomy was performed and blood, thoracic aorta, kidney, and pancreas were harvested for further analyses.
Acetylcholine-Induced Vasorelaxation
Thoracic aortas were cleared of periadventitial tissue and cut transversely into rings 1.5 to 2.0 mm in diameter. Vascular rings, handled carefully to avoid damage to the inner surface, were mounted on wires in the chambers of a multivessel myograph (J.P. Trading, Aarhus, Denmark) and bathed in Krebs’ buffer. The medium was gassed with 95% O2 and 5% CO2 and maintained at 37°C (pH 7.4). After equilibration (30 minutes), the rings were set to an internal circumference equivalent to 90% of full relaxation under a transmural pressure of 100 mm Hg and allowed to stabilize for 20 to 30 minutes. The rings were then depolarized with potassium chloride (60 mmol/L) to evaluate maximal contraction. After washing with a Krebs’ buffer, the vascular preparations were contracted with phenylephrine (10−6 mol/L), and when the contractile response was stabilized (steady-state phase, 12 to 15 minutes), vasorelaxing responses to cumulative increments in the concentration of acetylcholine or NONOate were examined.14
Blood Glucose, Insulin Tolerance, and Homeostasis Model Assessment Index
Plasma glucose was measured using glucometer (OneTouch Ultra, Lifescan) by collecting 2 μl blood through nicking the end of the tail. To estimate insulin resistance, we conducted insulin tolerance test and homeostasis model assessment (HOMA) index analysis, as previously described.15,16 Briefly, for the insulin tolerance test, animals were fasted for 3 hours. The mice were weighed and 1.5 units/kg body weight of diluted regular human insulin 1:1000 (0.1 inits/ml) was injected intraperitoneally. At 90 and 180 minutes, blood glucose was sampled. HOMA index was calculated by the formula: fasting plasma insulin × fasting plasma glucose/405.
Measurement of Cytokines/Chemokines and Insulin
The Luminex multiplex assay (cat#: MCYTO-70K-PMX) was used for simultaneous quantification of the following mouse cytokines/chemokines in the plasma: interleukin (IL)-1α and β, IL-6, IL-9, IL-10, interferon (IFN)γ, interferone-gamma-inducible protein (IP-10), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor (TNFβ), keratinocyte chemoattractant (KC), monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein (MIP-1β) and mRANTES. A mixture of beads was incubated with standards or plasma samples, followed by the appropriate biotinylated antibody and streptavidin-phycoerythrin reporter. Beads were analyzed using Luminex-100. Plasma insulin, amylin, glucagon, and leptin concentration were simultaneously measured using the same technique (cat#: MENDO-75K-05).
Immunohistochemical and Immunofluorescence Staining and Analysis
Tissue samples of kidney and pancreas were fixed in a 4% paraformaldehyde solution (Electron Microscopy Sciences, Hatfield, PA) overnight at 4°C, followed by sequential incubation in 15% and 30% sucrose overnight at 4°C each. Embedding was performed in an optimal cutting temperature compound (Tissue-Tek, Torrance, CA), and embedded samples were stored at −80°C. Frozen samples were cut into 10-μm-thick sections (Cryomicrotom CM 1850, Leica Microsystems, Bannockburn, IL). Nonspecific protein binding was blocked by 1-hour incubation with PBS-bovine serum albumin (1%). The following primary antibodies were used: anti-mouse insulin (Santa Cruz Biotechnology, Santa Cruz, CA), CD31 (BD Pharmingen, San Jose, CA), and CD68 (Serotec, Oxford, UK). For CD-68 staining, horseradish peroxidase conjugated goat anti-rat IgG was used as the secondary antibody. Peroxidase activity was blocked by 15 minutes of incubation with peroxidase block solution (1:10; DakoCytomation, Glostrup, Denmark). To visualize the positive immunoreaction, the peroxidase substrate 3,3′-diaminobenzidine chromogen was used. Hematoxylin solution was used for counterstaining. Negative controls for all immunolabeling procedures were accomplished by incubation with 1% PBS-bovine serum albumin instead of the primary antibody. For immunofluorescence staining, fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used. Incubations with primary antibodies were performed overnight at 4°C and incubations with secondary antibodies were performed for 1 hour at room temperature. Control samples were stained with secondary antibodies only. To visualize the nuclei, tissue sections were counterstained with 4,6-diamidino-2-phenylindole (Molecular Probes). Sections were examined using a Nikon inverted fluorescence microscope (Eclipse TE2000-U) equipped with a digital camera (Spot model 4.2; Diagnostic Instruments, Sterling Heights, MI).
For histological examination of kidneys, paraffin-embedded tissue samples were cut into 3-μm-thick sections and stained with H&E, periodic acid-Schiff, or Masson’s trichrome (American MasterTeck, Lodi, CA). Slides were examined and scored for abnormalities by two nephropathologists. For detection of tubular necrosis, the scoring range from 0 to 3 was used to define noticeable cell damage in the form of hydropic change, cast formation, necrosis or apoptosis in the tissue area. The scoring criteria were defined as follows: score 0 = no noticeable cell damage; 1 = noticeable cell damage in tissue area <10%; 2 = noticeable cell damage in tissue area between 10 to 50%; 3 = noticeable cell damage in tissue area >50%. Additional 3 μm-thick periodic acid-Schiff-stained paraffin sections of kidneys were evaluated for diabetic nephropathy from 4 different groups (n = 3 in each group): controls (m), diabetic (db), stem cell transplantation from control mice (Tx M) and from db mice (Tx db). Slides were examined by Olympus BX41 microscope under ×40 magnification. All glomeruli were counted in a single cross section as well as the numbers of lesioned microvessels, which were expressed as per 100 glomeruli. The normal mesangial area in the control animals was assigned 0 score, with scores 1+ if these were twice the size of the control, 2+ and 3+ when expanded to 3 and 4 times, respectively.
Fluorescence-Activated Cell Sorting
Bone marrow (BM)-derived mononuclear cells were analyzed for an array of markers, including FITC or phycoerythrin (PE) conjugated anti-mouse CD117 (c-kit), CD150, sca-1, c-kit, CD34, CD31, CD44, CD45, flk,−1, and unconjucated anti-mouse vimentin, and nestin that paired with corresponding fluorescent secondary antibody (Jackson ImmunoResearch Laboratories). All primary antibodies were produced by BD Biosciences (Rockville, MD). Data were acquired using a FACScan cytometer equipped with a 488-nm argon laser and a 620-nm red diode laser and analyzed using CellQuest software (Becton Dickinson, San Jose, CA). The setup of FACScan was performed using unstained and single antibody-stained cells.
BM-Derived MSC and Endothelial Progenitor Cell Isolation
To isolate MSCs from the bone marrow of db/db and db/m mice, the fresh BMDC preparations were re-suspended in complete MSC culture medium (StemCell Technologies Inc, Canada) and seeded into 6-well plates. The cells were than kept 3 days at 37°C in a CO2 incubator, fresh medium was changed, and the adherent layer was re-fed at 7 days. For analysis of apoptosis cells from 1 to 2 passages were used. To isolate endothelial progenitor cells (EPCs), BMDC were re-suspended in mouse EPC medium (Celprogen, San Pedro, CA) supplemented with 10% fetal bovine serum. Seven days after initiation of cultures on 4-well chamber slides (Nalge Nunc International) coated with Vitronectin (10 μg/ml), EPCs were assayed by costaining with acetylated LDL (acLDL)-Dil (Biomedical Technologies) for 3 hours at 37°C and FITC-conjugated Ulex europeaus Lectin (Sigma) for 30 minutes at 37°C, both characteristically staining cells of endothelial lineage.17 Double-positive cells were counted as EPC in eight randomly selected fields of each slide. The proportion of apoptotic cells under basal and oxidative stress conditions, as detailed in Results, was examined using annexin V (BD pharmingen) and activated caspases detection using FITC-VAD-FMK (Calbiochem, La Jolla, CA).
Statistical Analysis
Results were summarized from three independent BM transfusion experiment and the numbers of mice for each study group were totaled: db/m n = 24, db/db control mice n = 20, dbCM n = 4, dbEbs-in vivo n = 5, dbTxm mice n = 10, dbTxdb mice n = 9, TxdbCM n = 4, TxdbEbs-in vivo n = 5, and TxdbEbs-ex vivo n = 5. The results were expressed as means ± SD The means of two populations were compared by Student’s t-test. For multiple comparisons analysis of variance was used. Differences were considered significant at P < 0.05.
Results
Defective Vasorelaxation in db/db Mice is Dramatically Improved by Transfusion of Syngeneic BMDCs
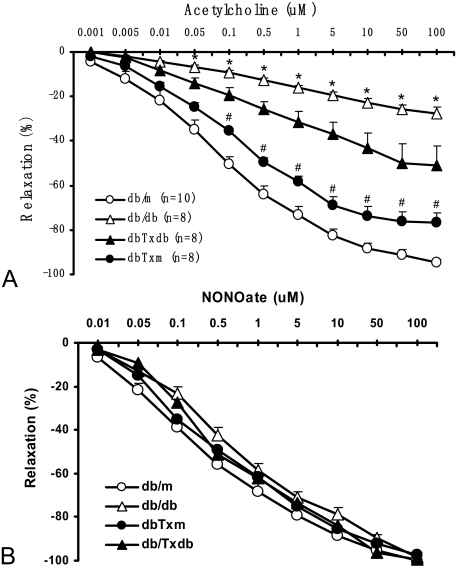
We have previously demonstrated that conspicuous defects in endothelium-dependent vasorelaxation of aortic rings in db/db mice could be detected as early as 9 to 10 weeks of age, ie, just 2 weeks after establishment of a persistent hyperglycemia,14 in association with the increased numbers of prematurely senescent endothelial cells. Therefore, we pursued these studies of the macrovascular dysfunction in db/db mice recipients of BMDC transplants using acetylcholine-induced vasorelaxation assay. Aortic rings obtained from db/db mice showed a profound impairment of relaxation in response to the application of acetylcholine (Figure 1A). Maximal concentration of acetylcholine (100 μmol/L) elicited only a 29% relaxation of aortic rings compared with db/m mice. In contrast, db/db mouse-recipients of BMDC from db/db donors (dbTxdb group) showed a mild-to-moderate improvement of aortic relaxation (maximal relaxation of 56%), whereas the db/db mice receiving BMDC from their db/m littermates (dbTxm group) exhibited a dramatic improvement of aortic vasorelaxation with the maximal values achieving 81% of control db/m mice. Notably, all vessels responded to nitric oxide (NO) donor NONOate with equal relaxation (Figure 1B), thus indicating that the impaired responses to acetylcholine were due to defective endothelium-dependent relaxation.
Figure 1.
Acetylcholine-induced vasorelaxation of aortic rings. A: Cumulative dose-response curves of acetylcholine-induced vasorelaxation in phenylephrine-preconstricted aortic rings. Significant improvement in acetylcholine-induced vasorelaxation was documented for db/db mice after transfusion of BMDC, especially in db/db recipients of BMDC isolated from db/m counterparts. B: Cumulative dose-response curves of NONOate-induced vasorelaxation in denuded phenylephrine-preconstricted aortic rings showed comparable responsiveness among all different groups. *P < 0.01 vs db/m, dbTxm; #P < 0.05 vs dbTxdb.
BM Adoptive Transfer Improves Fasting Glucose Level and Insulin Sensitivity
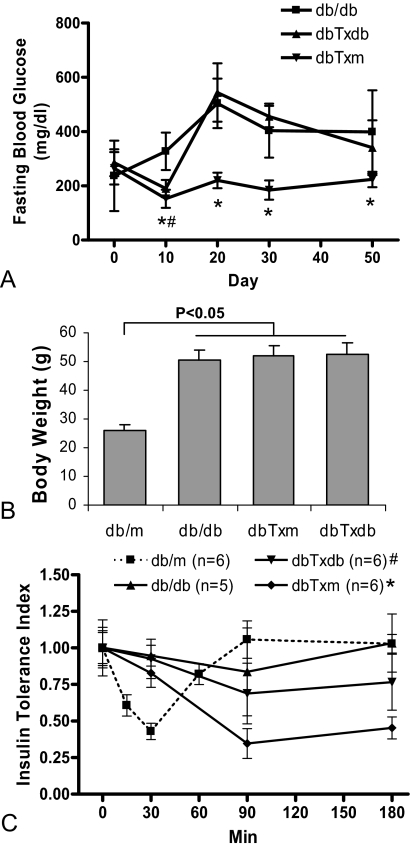
Monitoring blood glucose levels in db/db recipients of BMDC infusions (10 days after each infusion and 20 days after the last infusion) indicated a significant improvement of fasting blood glucose level in recipients of BMDC from db/m donors (dbTxm group) (Figure 2A). In mice that received BMDC from db/db donors (dbTxdb group), the improvement in fasting blood glucose level was transient and occurred only after the first transfusion, when the BMDC donor db/db mice were 8 weeks old (this is the age when hyperglycemia commences in db/db mice). Hyperglycemia resumed after the subsequent transfusions and was indistinguishable from non-treated db/db mice of equivalent age.
Figure 2.
Fasting blood glucose level and insulin tolerance test. A: Improvement of fasting glucose level was documented in the dbTxm group following each infusion of the BMDC, compared with untreated db/db mice. In the dbTxdb group, improvement of fasting glucose level was documented only after the first infusion. *#P < 0.05 vs db/db. B: Body weight measurement indicated that the diabetic mice were all obese compared with db/m, but showed no difference between db/db, dbTxm, and dbTxdb groups. C: Insulin tolerance index in BM transplanted group showed significant improvement in insulin sensitivity over db/db mice, especially in the dbTxm group. *P < 0.01 compared with db/db at 90 and 180 minutes, P < 0.05 compared with dbTxdb group; #P < 0.05 compared with db/db at 90 and 180 minutes (n = 6 for db/m, dbTxm, and dbTxdb groups, n = 5 for db/db).
Treatment of db/db mice with BMDC of either origin did not affect their body weight and all animals remained equally obese (Figure 2B). The results of the insulin tolerance test performed 20 days after the last BM transfusion showed that the sensitivity of mice to the injected insulin in the dbTxm group was improved compared with db/db control and dbTxdb group, although hypoglycemic responses remained equally delayed (Figure 2C), suggesting that the observed improvement of fasting blood glucose level in db/db recipients of BMDC from db/m donor mice was secondary to improved insulin sensitivity rather than reduced obesity.
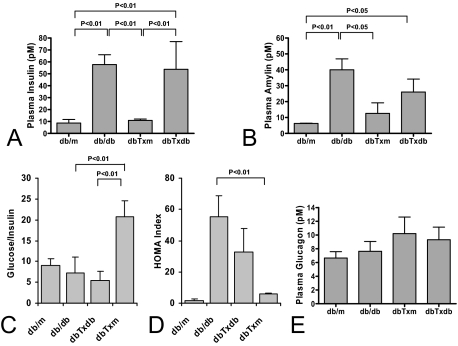
To further address the possibility that BM adoptive transfer can improve insulin sensitivity in the recipient diabetic mice, we measured their fasting plasma insulin, amylin, and glucagon level. Compared with db/db mice that exhibited elevated plasma insulin and amylin level, mice in the dbTxm group showed normalization of hyperinsulinemia and hyperamylinemia, whereas dbTxdb mice showed no improvement in insulin and only a partial improvement in amylin level (Figure 3, A and B). In accord with these results, the calculated glucose/insulin ratio and HOMA index showed significant improvement in the dbTxm group compared with db/db control and dbTxdb mice (Figure 3, C and D). Plasma glucagon measurements showed no significant differences between all of the experimental groups (Figure 3E). In aggregate, having excluded the contribution of other factors to the improved insulin sensitivity, the most plausible explanation is found in the BMDC-induced alleviation of endothelial dysfunction and improvement of microcirculation.
Figure 3.
Analysis of hormones regulating glucose level. A, B, and E: comparison of plasma insulin, amylin, and glucagon level among experimental groups. C and D: analysis of glucose/insulin ratio and HOMA index showed improved insulin sensitivity in dbTxm group compared with db/db control. Results are based on the study of n = 6 for db/m, dbTxm, and dbTxdb groups; n = 5 for db/db.
Cytokine Profile of db/db Mice after BM Adoptive Transfer Suggest an Inflammation Independent Mechanism for the Observed Benefits
Another possible explanation for the observed improved insulin sensitivity was related to the modulation of pro-inflammatory mediators.18 To explore this possibility we measured the plasma concentration of 14 pro- and anti-inflammatory cyto- and chemokines (IL-1α and β, IL-6, IL-9, IL-10, IFNγ, IP-10, G-CSF, GM-CSF, TNFα, KC, MCP-1, MIP-1α, and mRANTES) following BM transfusion. The results showed elevated levels of IL-1α and G-CSF after BM transfusion regardless of the donors, elevated levels of IP-10 in dbTxm and elevated IL-10 levels in recipients of db/db BMDC (supplemental Figure 1, see http://ajp.amjpathol.org).
In addition, we analyzed the extent of macrophage/mononuclear infiltration of the pancreatic and kidney parenchyma (supplemental Figure 2A, see http://ajp. amjpathol.org). Immunohistochemical staining showed that the CD68-positive cells were rare and scattered evenly in the pancreas and kidney sections with no differences detectable among the studied groups (supplemental Figure 2B–C, see http://ajp.amjpathol.org).
The above analyses indicated the existence of low-grade pro-inflammatory conditions following the infusion of BMDC. These data ruled out the possibility that the improved insulin sensitivity following BM transfusion was due to the improved profile of pro-inflammatory cytokines.
No Evidence of Trans-Differentiation of the Engrafted Donor BMDC to Insulin-Producing Cells in the Pancreas
To examine the possibility of trans-differentiation of engrafted donor BMDC to insulin-producing cells and evaluate its contribution to the observed benefits following BM transfusion, we studied frozen sections of the pancreas. Fluorescent microscopy confirmed the presence of CM-Dil positive donor BMDC in the recipient’s pancreas (supplemental Figure 2D, see http://ajp.amjpathol.org). These scattered cell tracker-labeled cells were rare and showed no difference in frequency between dbTxm and dbTxdb group. Examination of the pancreas co-stained with anti-mouse insulin antibody failed to show insulin-positive staining of engrafted donor BMDC (data not show). Immunohistochemical staining of insulin showed comparable density and normal morphology of the islets among db/m, db/db, and BMDC-treated diabetic mice (data not show). Quantification of the islets showed equal levels among different experimental groups (supplemental Figure 2E, see http://ajp.amjpathol.org). Our results suggested that a direct trans-differentiation to insulin-producing cell was not evident in the pancreas, and BMDC transfusion did not influence pancreatic islands structure and density.
Renal Function Improves in a Subgroup of db/db Mice after Treatment with BMDC from db/m Donors; Risk of Tubular Necrosis in Bone Marrow Transplantation
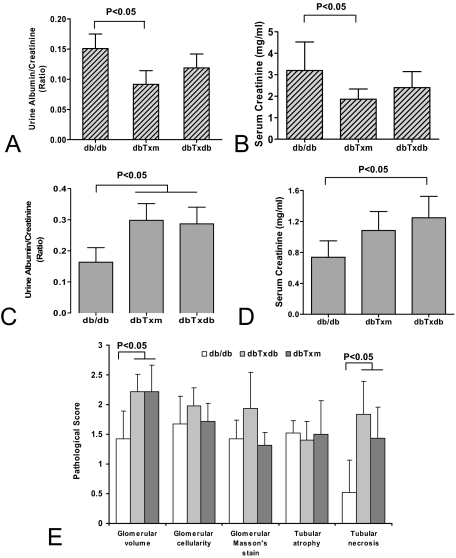
Effects of BMDC adoptive transfer on renal function in db/db mice were examined. The recipients of BMDC from db/m donors exhibited improved renal function (Figure 4, A and B) judging from urinary protein/creatinine ratio and plasma creatinine level. Glomeruli showed mild to moderate hypertrophy in all db/db animals compared with controls and in addition displayed mesangial expansion (range, 2 to 2.5; average 2.3+) and arteriolar hyalinosis (range, 8% to 18%; average 9%; supplemental Figure 3, see http://ajp.amjpathol.org). An occasional lesion of focal and segmental glomerulosclerosis, containing extracellular lipid droplets, was also evident (1/3 animals) in db/db mice. Kidneys from dbTxm group showed mild decrease in mesangial expansion (range 1.5 to 2.0; average 1.66+) and in areteriolar hyalinosis (range, 2% to 4%; average 3.3%). No lesions of focal and segmental glomerulosclerosis were present in any of these sections obtained from dbTxm mice. The decline in mesangial expansion (range, 2 to 2.5; average 2.17+) and in areteriolar hyalinosis (range, 2% to 7%; average 4.66%) were less impressive in the dbTxdb animals. An occasional lesion of focal and segmental glomerulosclerosis was evident in one of the three animals as well.
Figure 4.
Analysis of renal function following BM transfusion. Urine protein/creatinine ratio (A) and serum creatinine concentration (B) measurements in the recipients of BMDC from db/m donor mice showed improvement in these parameters. In approximately 40% of animals, however, analysis of the urine protein/creatinine ratio (C) and serum creatinine level (D) showed deterioration in recipients of db/m or db/db BMDC infusion. Results of the analysis of pathology scores in this subgroup (E) showed increased level of tubular necrosis following BMDC infusion. The number of animals studied in (A, B, and C) = db/db n = 5, dbTxm n = 6, dbTxdb n = 6; and, (D and E) = db/db n = 3, dbTxm n = 4, dbTxdb n = 4.
Of note, these changes were not universal and analysis showed that there was a subgroup, which was analyzed separately (40%), that showed the deterioration of proteinuria and plasma creatinine level after BM transfusion from either donor group. (Figure 4, C and D). Consistent with these findings, histological examination of kidney sections revealed elevated pathological score of tubular necrosis in this subgroup along with an increased glomerular volume (Figure 4E). These findings are not inconsistent with multiple clinical observations that pointed out the risk of acute kidney injury in 53% to 92% after BM transplantation.19,20,21 Despite the syngeneic nature of transplanted cells, it is not excluded that the procedure itself carries a risk of acute tubular necrosis, probably due to the formation of cellular clamps in the injectate. The performed “grouping” presented an attempt to objectively analyze beneficial and adverse actions of transplantation of BMDC in the kidney.
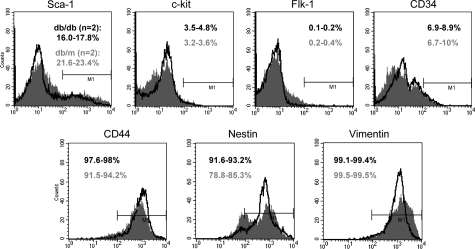
Profiling of Cell Markers for BMDC of the Donor db/db and db/m Mice
Some of the detected beneficial effects of the adoptive transfer of BMDC in db/db mice could be secondary to the infusion of the progenitor/stem cell population present in the isolated donor BMDC. Differences in composition could hence affect their beneficial outcomes, which may also reflect the existed changes in the BM progenitor/stem cell population under normal versus disease condition. We used an array of cell surface markers to profile the freshly isolated BMDC (pooled from at least four animals for each group) using FACS analysis (Figure 5), including sca-1, c-kit, CD34, flk-1, CD44, vimentin, and nestin. The results from two independent experiments showed no substantial differences in the expression of detected markers between db/db and db/m mice, except for some noticeable discrepancy in sca-1 and nestin. BMDC used for transfusion were represented mostly by mesenchymal and hematopoietic stem cells nearly equally represented numerically in db/m and db/db bone marrow, but proportional differences in some markers do exist.
Figure 5.
FACS analysis of cell markers in BMDC. Freshly isolated BMDC were analyzed for their composition using a panel of markers related to BM stem/progenitor cells. No substantial differences were observed in the expression of detected markers between db/db and db/m mice, except for some noticeable distinctions in Sca-1 and nestin. The results for db/db mice are shown in black, db/m mice in gray. Ranges are given for two independent experiments.
Functional Analysis of BMDC and their Progenitor/Stem Cell Population in the Donor db/db and db/m Mice
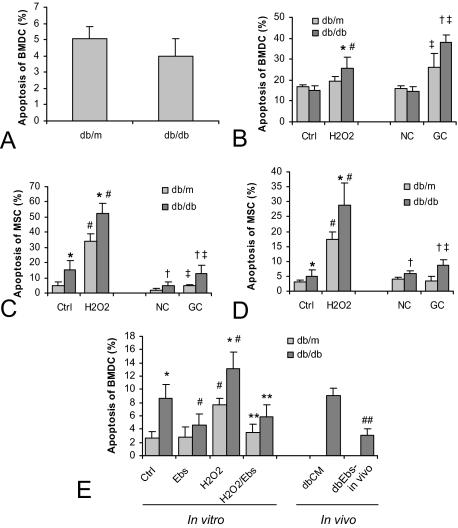
Although the surface markers of cells in the transfused BMDC of either type of the donor, db/db or db/m, appeared to be little unchanged, the end-effects of their transfusion were much different, as shown in Figures 1 and 2. To evaluate the possibility that the infused BMDC differed qualitatively, we analyzed the viability of BMDC and their progenitor/stem cell population by study the markers of apoptosis under basal and stressed conditions. FACS analysis of Annexin V staining showed comparable percentages of positive cell in freshly isolated db/db and db/m mice BMDC (Figure 6A). This result was confirmed with Hoechst staining. We then tested the resistance of the isolated BMDC to challenge with H2O2 (50 μmol/L) or glycated collagen I (GC, 50 μg/ml, a mimic of diabetic microenvironment; Figure 6B). Though the frequency of apoptosis was comparable between db/db and db/m groups under the basal conditions and after exposure to native collagen (NC, 50 μg/ml), the rate of apoptosis was significantly elevated in the db/db mice on exposure to H2O2 or GC stress.
Figure 6.
Analysis of apoptosis in BMDC and BM-derived MSC. The population of apoptotic cells among freshly isolated BMDC showed no significant differences between db/db and db/m mice (A). However, an overnight culture in the presence of H2O2 (50 μmol/L) or glycated long-lived protein, collagen I (GC, 50 μg/ml) revealed a much higher proportion of apoptotic cells in the BMDC in the db/db mice compared with db/m counterparts (B). Analysis of the BM-derived MSC in overnight (C) and 3-day (D) culture showed an increased number of apoptotic cells in db/db mice even under the basal culture condition. Challenge with H2O2 and GC enhanced the rate of apoptosis, especially in the MSC isolated from db/db. The addition of ebselen (Ebs) to BMDC culture medium prevented apoptosis under basal and H2O2 stress conditions (E). Apoptosis was detected using annexin V and Hoechst staining. *†P < 0.05 compared with db/m within each treatment; #‡P < 0.05 compared with each corresponding control; **P < 0.05 compared with H2O2 treatment; ##P < 0.05 compared with dbCM. The number of animals: n = 4 for db/m, db/db, and dbCM groups; n = 5 for dbEbs-in vivo group.
In addition to the freshly isolated BMDC, we also characterized stress resistance of MSCs isolated from db/db and db/m mice (Figure 6, C and D). The results summarized from both the short overnight stress (C) and prolonged 3 days stress (D) indicated a significantly higher apoptotic population among MSC isolated from db/db mice compared with that of db/m counterparts, even under the basal culture condition (control and NC). These differences in the rate of apoptosis were further markedly increased in both groups of mice on H2O2 and GC challenge, but were significantly higher in the db/db group mice.
Ex Vivo and in Vivo Ebselen Treatment Corrects Stem Cell Competence
We have previously demonstrated beneficial vascular effects of a selenoorganic peroxynitrite scavenger and antioxidant, Ebselen, in Zucker diabetic rats14 and, therefore, tested its potential to improve the resistance to stress of BMDC and their functional competence. The addition of Ebselen (Ebs) to BMDC culture medium prevented apoptosis under basal and H2O2 stress conditions, as detected by the activated caspases using FITC-VAD-FMK probe (Figure 6E). Compared with vehicle-treated control, in vivo chronic administration of Ebs by gavage decreased the rate of apoptosis in BMDC from db/db mice.
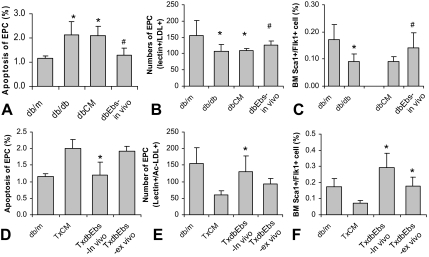
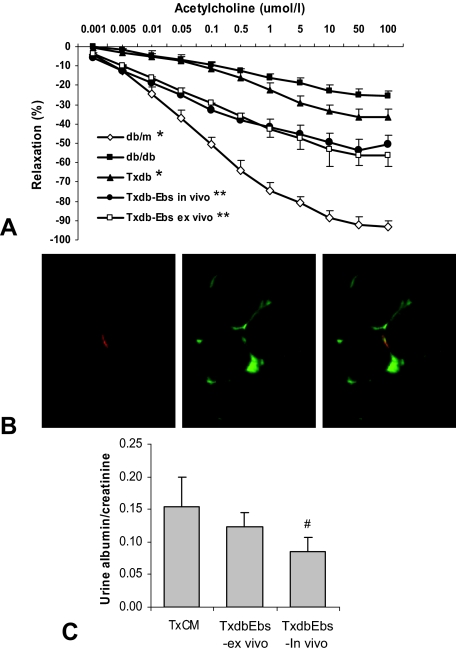
In view of the differences encountered during ScaI and Flk1 profiling (Figure 5), we further tested the possibility that the EPCs in BMDC population may vary between db/db and db/m mice. FACS analysis showed that db/db BMDC contained less ScaI+/Flk1+ cells (Figure 7C). EPCs cultured from db/db mice also showed decreased number and elevated apoptosis rate (Figure 7, A and B). In vivo treatment of db/db mice with Ebs restored viability and increased the EPC population. The recipients of the BMDC obtained from in vivo Ebs-treated db/db mice showed an increased BM EPC population and decreased apoptotic rate in EPCs (Figure 7, D–F). BMDC from db/db mice treated with Ebs ex vivo (overnight) also elicited improved endothelium-dependent relaxation after transplantation to db/db recipient mice, which was accompanied by increased EPC number within their BMDC population. In parallel, improvement of the endothelium-dependent relaxation in response to acetylcholine (Figure 8A; compare with Figure 1A) ensued. Specifically, in vivo and ex vivo therapy with Ebs resulted in maximal relaxation to acetylcholine reaching 55% and 61% of control db/m mice, respectively, compared with 38% of maximal relaxation in vehicle-treated group.
Figure 7.
Analysis of BM-derived EPC. FACS analysis and direct cell culture of BMDC showed decreased EPC numbers (A, B) and increased EPC apoptosis (C) in db/db mice. In vivo treatment of db/db mice with Ebs increased their BM EPC population and normalized their apoptosis rate. Compared with the recipient of BMDC of vehicle treatment, the db/db mice transferred with BMDC of Ebs treated db/db donor mice (in vivo and/or ex vivo) showed increased BM EPC number (D, E) and decreased apoptosis (F). *P < 0.05 compared with db/m or TxCM; #P < 0.05 compared with dbCM. The number of animals: n = 4 for db/m, db/db, dbCM, and TxCM groups; n = 5 for dbEbs-in vivo, TxdbEbs-in vivo, and TxdbEbs-ex vivo groups.
Figure 8.
Analysis of Acetylcholine-induced vasorelaxation of aortic ring, CD31 staining of kidney section, and urine albumin/creatinine level. A: Cumulative dose-response curves of acetylcholine-induced vasorelaxation in phenylephrine-preconstricted aortic rings. Compared with BMDC of db/db mice (Txdb group), better improvement in acetylcholine-induced vasorelaxation was documented for recipient mice after transfusion of BMDC of donor db/db mice that treated with Ebs both in vivo and ex vivo (Txdb-Ebs in vivo and Txdb-Ebs ex vivo). Cumulative dose-response curves of NONOate-induced vasorelaxation in denuded phenylephrine-preconstricted aortic rings showed comparable responsiveness among all different groups (not show). *P < 0.05 compared with db/db in all tested acetylcholine level above 0.005 umol/L for db/m and 5 umol/L for Txdb; **P < 0.05 compared with db/db and Txdb in all tested acetylcholine level above 0.005 umol/L. The number of animals: n = 8 for db/m, db/db, and Txdb groups; n = 6 for TxdbEbs-in vivo and TxdbEbs-ex vivo groups. B: Ultrathin deconvoluted images (0.625 μm) showed that some of the engrafted CellTracker-labeled cells (infused BMDC) co-expressed CD31 (an endothelial marker) and could be localized to the renal microvasculature. C: The recipient of BMDC in in vivo Ebs-treated mice showed improved urine albumin/creatinine levels indicating the improvement of renal function. #P ≤ 0.05 as compared with TxCM.
To evaluate the possible engraftment to the microvasculature, we stained the kidney sections with CD31 monoclonal antibody (Figure 8B). Fluorescence microscopy followed by deconvolution analysis indicated that some of the engrafted CellTracker-labeled infused BMDC co-expressed CD31 and could be localized to the renal microvasculature. While this double-staining was a rarity in recipients of BMDC from db/db mice, such double-stained cells were readily detectable in the recipients of BMDC from db/m mice.
The urine albumin/creatinine ratio of recipients of BMDC obtained from Ebs-treated donor db/db mice also showed improvement compared with recipients of BMDC obtained from vehicle-treated mice (Figure 8C). In conclusion, Ebselen treatment both in vivo and ex vivo restored competence to BMDC, improved EPC resistance to oxidative stress and was associated with improved vascular and renal function. These findings suggest that targeting the competence of endogenous BMDC may represent an alternative strategy in managing complications of type II diabetes and metabolic syndrome.
Discussion
Strategy of bone marrow transplantation has recently been adapted as an important procedure to pinpoint novel therapeutic approaches and to reveal new pathogenic mechanisms for many insidious human diseases. Type I diabetes models have been used in several recent studies to evaluate the existence of adult progenitor/stem cells in the BM not only for merely mechanistic insights but also the development of new curative strategies.8 Implantation of autologous BMDC in the ischemic hindlimb of diabetic rats enhanced the angiogenesis and improved vascularization that contributed to faster recovery.5 Transplantation of syngeneic BMDC after acute injury also promoted β-cell regeneration in pancreas.10 However, the models of type II diabetes have been scarcely explored with regard to the effects of BMDC transplantation.7
In the present study using db/db mice, a well recognized animal model of metabolic syndrome and type II diabetes, we made two important observations using BMDC adoptive transfer. Firstly, infusion of BMDC obtained from the db/m littermates dramatically improved the macrovascular function (acetylcholine-induced relaxation of aortic rings) and insulin sensitivity in the recipient db/db mice. Secondly, these observed beneficial effects of treatment were nearly absent when the BMDC obtained from diabetic donor mice were used. The mechanism(s) for these observed beneficial metabolic outcomes of BMDC infusion were not apparent. After an extensive search, we were able to exclude several potential candidates, such as changes in the degree of obesity (body weight remained stable), improvement of pro-inflammatory cytokines profile (in fact there was elevation of G-CSF and IL-1α, although anti-inflammatory IL-10 levels also increased, especially in the dbTxdb group), and disparate engrafting ratio of the transfused BMDCs (comparable number of engrafted CM-DiI positive cell were found). These data, in conjunction with dramatic improvement of vascular function in dbTxm mice argue in favor of a conclusion that circulatory mechanisms underlie the observed correction of insulin sensitivity. The possibility of changes in adiponectin levels being responsible for metabolic benefits in dbTxm group remains to be evaluated. It is also not clear whether the improved glycemic control in dbTxm group is responsible for amelioration of vascular and renal complications in db/db mice or BMDC transplantation has separate effects on metabolic, vascular and renal manifestations.
The differences in the study outcome using BMDCs that originated from normal or diabetic animal are striking. The possibility that it may reflect the alterations in cell composition and competence, both in general and progenitor/stem cell population, as well as alternations in cell functionality appear to deserve credence. The existence of dysfunctional EPCs originating from BM has been documented in atherosclerosis,6,22 essential hypertension,23,24 preeclampsia,25 hyperglycemia,26 smoking24,27 and type I and type II diabetes patients.28,29,30 It has recently been shown that diabetic state promotes aging of cardiac stem cells, a tissue resident stem cell population, and contribute to the heart failure.31
We explored this hypothesis further in our BMDC transplant setting. Referring to the initial finding that the ScaI- or Flk1-positive cell were decreased in BMDC of db/db mice, we further analyzed the cell population double-positive for ScaI and Flk1, the proposed EPC population. A decreased percentage of BM ScaI+/Flk1+ cells was documented in db/db mice by FACS analysis. This finding was supported by experiments on cultured EPC and is consistent with the results from others.28,29,30,32,33 Next, we qualitatively tested their viability and resistance to oxidative stress in cell culture. An increase in apoptosis under basal culture conditions was detectable in BMDC of db/db origin, as well as in the MSC and EPC. Challenging BMDC and MSC with oxidative stress (H2O2) or a glycated long-lived protein (GC) disclosed alterations in db/db mice. Proportion of apoptotic cells after H2O2 or GC treatment was significantly higher in the BMDC and MSC prepared from diabetic mice.
Having demonstrated functional incompetence of BMDC obtained from db/db mice, we attempted to correct it using the selenoorganic antioxidant and peroxynitrite scavenger Ebs. Ex vivo treatment of cultured BMDC with Ebselen resulted in a significant improvement of their resistance to oxidant stress and reduced rate of apoptosis. In vivo therapy of db/db mice with Ebs also reduced the rate of apoptosis in BMDC and EPC in culture and normalized the number of EPC. Furthermore, adoptive transfer of the BMDC from db/db mice treated with Ebs in vivo or ex vivo produced striking reduction of vasculopathy and improvement of renal function in recipient db/db mice. These functional improvements in recipient db/db mice were associated with much improved resistance to apoptosis and elevated EPC numbers in their BM. This finding may explain at least in part the compromised state of BMDC isolated from db/db mice. However, these data also added another interesting layer of complicity to the mechanisms for the benefits following adaptive transfer. In contrast to the improved vasculopathy and renal dysfunction, the beneficial effect on fasting glucose level and insulin sensitivity were absent in the recipients of BMDC transfer from db/db mice treated with Ebs (data not shown). This disparity suggests that the variety of beneficial effects in db/db recipients of db/m BMDC transfer may be well mediated through multiple mechanisms, which are all compromised in db/db mice. Some of these compromised mechanisms in db/db mice are improved with the use of antioxidant therapy, but some are not.
In contrast to renal functional improvement in the majority of recipient animals in the present study, the deterioration of renal dysfunction in a subgroup stands apart. Our own data obtained in mice with acute kidney injury,34 as well as previous findings by Prockop’s group35 in streptozotocin-diabetic mice all showed beneficial effect of infused MSC on renal function. In another study reported by Cook’s group, transplantation of wild-type BMDC improved renal function in Col4α3−/− mice through, in part, a mechanism involving regeneration of padocytes without the gene defect.36 These distinct outcomes may be due to severity of hyperlipidemia in db/db mice or to circulating cells as referred to in the previous work by Striker’s group.37 Secondly, these findings may be linked to the BMDC transplantation per se. The decades of experience with bone marrow transplantation buttress these findings. The incidence of renal dysfunction of various degrees ranges from 53% to 92% with 24% of patients requiring dialysis.19,20,21 Development of proteinuria was found to be near-universal after bone marrow transplantation.38 In our experimental setting, despite the fact that a syngeneic transplantation was performed, the possibility of microembolism due to cell clamping, both in BMDC from db/db and db/m donors, cannot be ruled out and was presumably the cause of the observed tubular necrosis.
In conclusion, infusion of BMDC obtained from db/m donors to db/db recipient mice benefited macrovascular function, insulin sensitivity, and, in majority of cases, renal function. The BMDC obtained from db/db mice were functionally incompetent partly secondary to their decreased viability under increased oxidative stress challenge. Our work emphasizes benefits and risks of cell therapy, and reduced competence of BMDC in db/db mice, and suggests that antioxidant targeting of BMDC, both in situ or ex vivo, may represent an alternative strategy in managing complications of type II diabetes and metabolic syndrome.
Supplementary Material
Acknowledgments
We are grateful to Dr. Michael Wolin and Dr. Qun Gao for their help with deconvolution fluorescence microscopy analysis.
Footnotes
Address reprint requests to Address correspondence/reprint requests to: Jun-Chen@nymc.edu or, Michael_Goligorsky@nymc.edu.
Supported by the American Heart Association Scientist Development award 0430255N (J.C.) and NIH grants DK052783, DK45462, and DK054602 (M.S.G.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Hirata K, Li TS, Nishida M, Ito H, Matsuzaki M, Kasaoka S, Hamano K. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol. 2003;284:H66–H70. doi: 10.1152/ajpheart.00547.2002. [DOI] [PubMed] [Google Scholar]
- Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- Than S, Ishida H, Inaba M, Fukuba Y, Seino Y, Adachi M, Imura H, Ikehara S. Bone marrow transplantation as a strategy for treatment of non-insulin-dependent diabetes mellitus in KK-Ay mice. J Exp Med. 1992;176:1233–1238. doi: 10.1084/jem.176.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Ogihara T, Yamada T, Ishigaki Y, Imai J, Uno K, Gao J, Kaneko K, Ishihara H, Sasano H, Nakauchi H, Oka Y, Katagiri H. Bone marrow (BM) transplantation promotes beta-cell regeneration after acute injury through BM cell mobilization. Endocrinology. 2007;148:2006–2015. doi: 10.1210/en.2006-1351. [DOI] [PubMed] [Google Scholar]
- Roufosse C, Bou-Gharios G, Prodromidi E, Alexakis C, Jeffery R, Khan S, Otto WR, Alter J, Poulsom R, Cook HT. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol. 2006;17:775–782. doi: 10.1681/ASN.2005080795. [DOI] [PubMed] [Google Scholar]
- Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, Goligorsky MS. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004;94:377–384. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- Francisco G, Hernandez C, Galard R, Simo R. Usefulness of homeostasis model assessment for identifying subjects at risk for hypoglycemia failure during the insulin hypoglycemia test. J Clin Endocrinol Metab. 2004;89:3408–3412. doi: 10.1210/jc.2003-031883. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Zager RA, O'Quigley J, Zager BK, Alpers CE, Shulman HM, Gamelin LM, Stewart P, Thomas ED. Acute renal failure following bone marrow transplantation: a retrospective study of 272 patients. Am J Kidney Dis. 1989;13:210–216. doi: 10.1016/s0272-6386(89)80054-x. [DOI] [PubMed] [Google Scholar]
- Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443–1458. doi: 10.1038/ki.1994.417. [DOI] [PubMed] [Google Scholar]
- Parikh CR, McSweeney PA, Korular D, Ecder T, Merouani A, Taylor J, Slat-Vasquez V, Shpall EJ, Jones RB, Bearman SI, Schrier RW. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62:566–573. doi: 10.1046/j.1523-1755.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H, Yaegashi N, Okamura K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90:5329–5332. doi: 10.1210/jc.2005-0532. [DOI] [PubMed] [Google Scholar]
- Krankel N, Adams V, Linke A, Gielen S, Erbs S, Lenk K, Schuler G, Hambrecht R. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25:698–703. doi: 10.1161/01.ATV.0000156401.04325.8f. [DOI] [PubMed] [Google Scholar]
- Gennaro G, Menard C, Michaud SE, Deblois D, Rivard A. Inhibition of vascular smooth muscle cell proliferation and neointimal formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation. 2004;110:3367–3371. doi: 10.1161/01.CIR.0000147773.86866.CD. [DOI] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- Awad O, Jiao C, Ma N, Dunnwald M, Schatteman GC. Obese diabetic mouse environment differentially affects primitive and monocytic endothelial cell progenitors. Stem Cells. 2005;23:575–583. doi: 10.1634/stemcells.2004-0185. [DOI] [PubMed] [Google Scholar]
- Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ, Seibold JR, Uitto J, Dorwart BB. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–2455. doi: 10.1634/stemcells.2006-0201. [DOI] [PubMed] [Google Scholar]
- Cornacchia F, Fornoni A, Plati AR, Thomas A, Wang Y, Inverardi L, Striker LJ, Striker GE. Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors. J Clin Invest. 2001;108:1649–1656. doi: 10.1172/JCI12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kone BC, Whelton A, Santos G, Saral R, Watson AJ. Hypertension and renal dysfunction in bone marrow transplant recipients. Q J Med. 1988;69:985–995. [PubMed] [Google Scholar]
- Chen J, Park H, Pelger E, Li H, Plotkin M, Goligorsky MS. Kidney-derived mesenchymal stem cells participate in vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.